Abstract
Interventional and fluoroscopic imaging procedures for pediatric patients are becoming more prevalent because of the less-invasive nature of these procedures compared to alternatives such as surgery. Flat-panel X-ray detectors (FPD) for fluoroscopy are a new technology alternative to the image intensifier/TV (II/TV) digital system that has been in use for more than two decades. Two major FPD technologies have been implemented, based on indirect conversion of X-rays to light (using an X-ray scintillator) and then to proportional charge (using a photodiode), or direct conversion of X-rays into charge (using a semiconductor material) for signal acquisition and digitization. These detectors have proved very successful for high-exposure interventional procedures but lack the image quality of the II/TV system at the lowest exposure levels common in fluoroscopy. The benefits for FPD image quality include lack of geometric distortion, little or no veiling glare, a uniform response across the field-of-view, and improved ergonomics with better patient access. Better detective quantum efficiency indicates the possibility of reducing the patient dose in accordance with ALARA principles. However, first-generation FPD devices have been implemented with less than adequate acquisition flexibility (e.g., lack of tableside controls/information, inability to easily change protocols) and the presence of residual signals from previous exposures, and additional cost of equipment and long-term maintenance have been serious impediments to purchase and implementation. Technological advances of second generation and future hybrid FPD systems should solve many current issues. The answer to the question ‘how much better are they?–is ‘significantly better– and they are certainly worth consideration for replacement or new implementation of an imaging suite for pediatric fluoroscopy.
Keywords: Flat-panel detectors, Fluoroscopy, Interventional radiology
Introduction
Real-time fluoroscopic imaging has been available for medical diagnostic purposes since the beginning of X-ray applications in medicine, before the turn of the 20th century [1]. A light image derived from the conversion gain of X-rays into visible light photons formed the real-time display for the diagnostician. Technical advances through the next 40 years improved the X-ray to light conversion factor of a standard Patterson fluoroscopic screen, a scintillator without any secondary gain, but light levels were still low, requiring dark adaptation of the human visual response and high radiation dose per frame. In this situation, the diagnostician had to render a diagnosis with less visual acuity, using the dark-sensitive rods of the eye as opposed to the higher acuity cones of the eye that function only in higher intensity light. A breakthrough in fluoroscopy technology occurred with the introduction of the X-ray image intensifier (II), introduced in the late 1940s; the II eventually revolutionized real-time X-ray fluoroscopic imaging as we know it. This device provided an output of non-limiting brightness and smaller (‘minified– image size, achieved by conversion of X-rays to light to electrons to light, with the key being the acceleration of electrons and minification of the image for signal amplification. Finally, the radiologist was able to do work without dark adapting to low light levels, thus improving acuity and image information transfer. Subsequent improvements were the optical coupling of the output image to a television (TV) camera and closed-circuit viewing system for real-time video image display in the fluoroscopy room. Even though the image was significantly degraded by the limited resolution and bandwidth of the TV system, the convenience of viewing, adjusting contrast, and storing images on videotape were significant. Incremental improvements in image quality and image size occurred throughout the 1960s and 1970s.
Revolutionary change in fluoroscopy occurred in the mid-1970s with the introduction of high-speed digitization of the analog video signal. Using rapid image processing, real-time subtraction of pre- and postcontrast images led to the implementation of digital subtraction angiography (DSA), certainly a very important event in the history of interventional angiography. Enhanced video memory and computer technology together with low-dose rapid switching X-ray tubes brought the introduction of ‘pulsed fluoroscopy–to the clinical environment in the mid-1980s. Pulsed fluoroscopy demonstrated the ability to trade-off temporal resolution for lower dose. Even today, some 20 years later, there is a need to understand the appropriate configuration settings for the image quality versus reduced dose for continuous versus still frame (last-image hold) [2]. Scientific-grade charge-coupled device (CCD) cameras as a replacement for vidicon-type analog cameras provided another leap in performance, because of greater temporal stability and low electronic noise. With the current state of the art II/TV system, improvements in image quality for the II have largely flattened.
In the late 1990s, the monetary and technological investments in flat-panel displays led to the implementation of the flat-panel detector (FPD) for radiography by many manufacturers. Soon after, FPD fluoroscopy operation soon began, and with several years of refinement and technological advances, is now poised to compete with the II in the clinical marketplace. The questions regarding its capabilities, however, have not been completely answered, and FPD implementations for fluoroscopy thus far have had a mixed review, particularly as it pertains to image quality at low exposure levels. The intent of this article is to review the II and FPD technologies, discuss the advantages and disadvantages of each, and to remark on some of the future detectors that might indicate the end of the II/TV system as we know it.
Image intensifier technology
The need for image intensification to overcome the limitations of the human visual system under conditions of low light levels typical of early fluoroscopy in terms of lack of acuity and dark adaptation was first described by Chamberlain [3]. The technology to meet these needs, the II, was initially developed by Coltman [4]. A single Patterson scintillator screen commonly used for real-time imaging [5] simply did not have sufficient light output, and it required the radiologist to be dark-adapted (10–0 min sitting in subdued light and/or wearing red goggles throughout the day) for optimal information transfer prior to performing a study. The II was constructed with a three-stage conversion and amplification process to create a light image of much greater intensity instead of relying on one conversion and amplification stage (X-rays to light) as with the simple scintillator. This device revolutionized the use of fluoroscopy in clinical diagnosis.
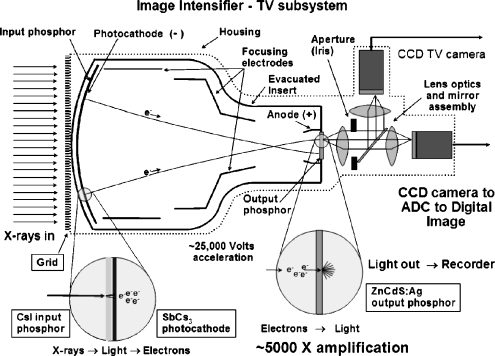
Largely unchanged from early designs, the function of the modern X-ray II is illustrated in the cross-sectional diagram in Fig. 1. X-rays are detected and converted to light photons by the input phosphor scintillator, a corresponding number of electrons are released by the photocathode, the electrons are accelerated and focused using cylindrical electronic focusing gradients to an output phosphor, and light photons are produced at the output phosphor. Optical lenses couple the light emitted from the output phosphor to the TV camera, creating a video signal subsequently re-rendered on a video monitor in a closed-circuit configuration for viewing the X-ray fluoroscopy sequence.
Fig. 1.
Cross-section of the II system illustrates the function of the input phosphor (convert X-rays to light), the photocathode (convert light to electrons), the cylindrical focusing lens and applied voltage (accelerate and focus electrons onto the output phosphor), the output phosphor (create very bright light image from electrons impinging on output phosphor), the conjugate lens system and aperture (refocus light onto light-sensitive TV camera target and adjust intensity of light), the TV camera (produce a variable video voltage amplitude corresponding to the incident X-ray flux), and the analog to digital converter to convert the continuous video voltage into a corresponding digital value stored in a matrix
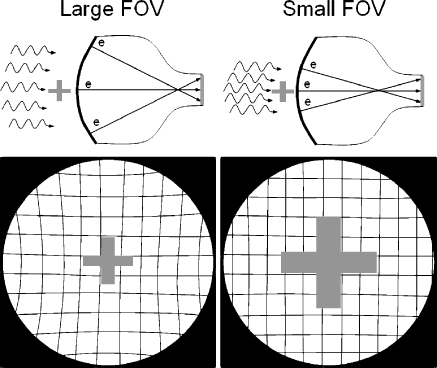
II amplification gain is mainly achieved by acceleration of the electrons under an applied potential difference of about 25,000 V from the cathode to the anode (electronic gain), as well as by the minification of the electron image emitted by the photocathode and compressed to the output phosphor area (minification gain). The overall conversion gain is a product of the electronic and minification gains and is on the order of about 5000, meaning that substantial numbers of light photons are produced for each X-ray absorption event, and statistics of the conversion process are dominated by the number of X-ray photons absorbed. Of importance for ALARA in pediatric fluoroscopy is the loss of minification gain that occurs when the field-of-view (FOV) is reduced to a smaller input phosphor area. Commonly known as ‘magnification mode– objects within the smaller FOV are mapped to the total output phosphor area, thus increasing their rendered size relative to the output phosphor and to the TV camera/video monitor and improving spatial resolution (Fig. 2, cross-pattern). A trade-off is a loss of minification gain with consequent reduced brightness at the output phosphor by the ratio of the small FOV input phosphor area to the large FOV input phosphor area, or by the ratio of the square of their respective diameters. Under ‘automatic brightness control–(ABC) operation, the fluoroscopy system attempts to maintain a constant amount of light produced at the output phosphor, and does so by electronic feedback to the X-ray generator to vary the kV and/or the mA accordingly. In the case of magnification mode, this increases the dose to the patient, the magnitude of which depends on the FOV, the lens aperture/iris within the optical lens system, and the electronic gain of the TV camera. Typically, the system adjusts the relative incident radiation exposure to the II input phosphor as FOV− (instead of FOV− if the aperture or other gains were not adjusted). For instance, if a 30-cm FOV requires 1 μGy of incident air kerma, and a magnification mode FOV of 20-cm diameter is subsequently employed, then the adjusted air kerma would be approximately 30/20, or 1.5 μGy, a 50% increase in patient dose (of course there would be an increase in spatial resolution). Because of the high acceleration and minification gain of the II, even with the use of high magnification modes, essentially ‘noiseless–gain, can be achieved by the II at extremely low fluoroscopy exposure rates. (By noiseless gain, it is implied that the noise variations in the image are chiefly caused by X-ray statistics, which is the ideal situation).
Fig. 2.
Electronic focusing allows the II to change the FOV and magnify the anatomy. The upper and lower left drawings illustrate the II/TV in the large FOV mode, providing good coverage and high gain as a result of electron minification. Note the presence of geometric ‘pin-cushion–distortion in the periphery of the image. This is caused by mapping the spherical input phosphor electron image onto the planar output phosphor. In magnification mode (smaller FOV), shown in the upper and lower right drawings, objects are magnified and spatial resolution is improved; however, this is at the expense of lower gain (less minification) and higher patient dose. Geometric pin-cushion distortion is reduced as there is less ‘warping–of the image onto the output phosphor from the central area of the input phosphor
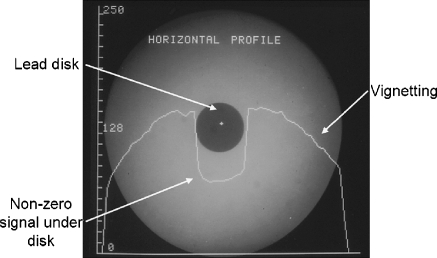
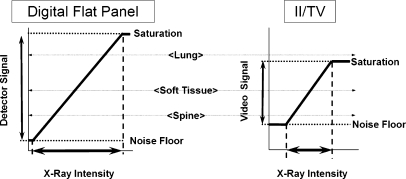
Other attributes of the II image, however, are less than ideal. A highly evacuated tube envelope requires that the input phosphor be spherically configured to withstand immense external atmospheric pressure. Electrons are accelerated from the curved photocathode to a planar output phosphor surface, resulting in a warping of the image mainly at the periphery, known as pin-cushion distortion (Fig. 2, square mesh background illustration, lower left). This can result in distance measurements with errors of 10% to 15% in peripheral areas of large FOV images compared to the measurements in the central area (in magnification mode, the distortions are fewer). Electrostatic and magnetic cylindrical focusing gradients sometimes interact with the earth’s magnetic field, causing transient ‘S–distortions, which can be problematic for rotational angiography and cone-beam reconstructions. Vignetting, a process describing the loss of light intensity at the periphery of the image because light scattered away from the output phosphor, results in a significant drop-off and non-uniform image profiles of the output image. Veiling glare, a long-range light-scattering event, occurs chiefly at the output phosphor and reduces the overall image contrast as shown by the large signal appearing under otherwise radioopaque lead attenuators (Fig. 3). Non-ideal light-loss and light-scatter responses within the II have been mitigated somewhat by modern technology, but they are still an issue. The light intensity response of the II is linear over a very wide range of incident exposures; however, the TV camera does not respond similarly during the conversion of the incident light to a corresponding video signal amplitude or digital value (nor does the video display monitor, for that matter). Often, some of the image information content is lost by saturation or threshold of the signals (Fig. 4). This can be mitigated somewhat with the light-adjusting aperture. Nevertheless, with more than 50 years of experience, the II/TV system, particularly that outfitted with low-noise and wider dynamic range CCD cameras, provides excellent image quality for both low-dose fluoroscopy and higher-dose studies such as DSA and electronic spot imaging. This is achieved by the flexibility afforded by the conjugate optical lens system and the aperture/iris control to control the ‘optimal–detector speed and to ensure that the critical anatomy signal falls within the dynamic range of the TV camera response. What are options for overcoming the limitations?
Fig. 3.
Light scatter within the II reduces contrast in the image. Shown is an image of a radioopaque lead disk and a substantial signal underneath, as illustrated by the digital horizontal profile values plotted across the image through the center of the disk. Ideally, the signal would be at zero, while in the figure a value of about 60 occurs. This has a deleterious impact on image contrast and quantitative accuracy. Vignetting is also illustrated; it is the fall-off of light intensity at the periphery of the image relative to the center, caused by light scatter loss at the edge of the image
Fig. 4.
X-ray intensity (incident exposure) produces proportional signals in the detector. For FPD systems, the response is linear over a wide range, as illustrated on the left. In II/TV systems, even though the II produces light linearly proportional to the X-ray intensity over a range similar to the FPD, the TV camera response is limited (as is the video monitor response). For radiographic or fluoroscopic studies that produce studies with a wide range of transmitted intensity (e.g., when using low kVp), the limited II/TV dynamic range can result in loss of some anatomical detail because of saturation or thresholding (right illustration)
Flat-panel detectors for X-ray imaging: thin film transistor arrays
Amorphous silicon (a-Si) thin-film-transistor (TFT) arrays represent the technological research and monetary investment in the creation of compact displays for laptop computers, begun in the late 1980s. In the mid-1990s, feasibility studies for producing an X-ray detector demonstrated that the same technology could be used for acquiring a two-dimensional (2D) projection X-ray image, and subsequently a ‘real-time–fluoroscopy sequence. Currently, in 2006, the technology has advanced to clinical mainstream applications in both radiography and fluoroscopy. Clinical radiography results have demonstrated the clear superiority of FPD systems over screen-film radiography and other digital radiography devices [6, 7], but the question of how much better the FPD is for fluoroscopic applications still requires investigation. Some reports of FPD fluoroscopy capabilities are reviewed below.
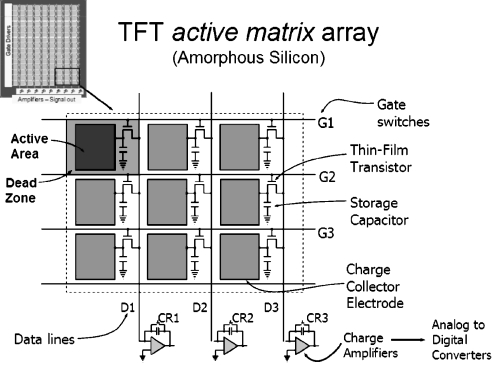
Common to both the indirect and direct X-ray conversion technologies, the basic architecture of an a-Si TFT device is arranged as a row and column array of detector elements (Fig. 5). Within each detector element are the TFT, a charge collection electrode and a charge collection capacitor. Interconnecting each element via the TFT and capacitor are ‘gate–and ‘drain–lines. By keeping the TFT switch closed during the exposure, incident X-rays interact with the converter and produce a corresponding charge that is stored in the local capacitor. When the X-ray exposure is terminated, one gate line at a time is set high to activate all connected TFTs along the row, where the charge flows from the local capacitors through the transistors and down the drain lines in parallel to the output charge amplifiers at each column of the matrix. Digitization of the output signal occurs and the digital image is built up one row at a time. Deactivating the gate line resets the TFTs for the next exposure, and the adjacent gate line is activated for the next row of data, with the process continuing until the whole array is analyzed. For real-time fluoroscopic imaging, the readout procedure must occur fast enough to acquire data from all detector elements over a period of 33 ms or 30 frames per second, which places high demands on the switching characteristics of the TFTs, charge/discharge rate of the capacitors, and the speed of the charge amplifiers and digitizers of the output stage.
Fig. 5.
The TFT active matrix array is composed of millions of individual detector elements, each of which contains a transistor, charge collector electrode and storage capacitor, all arranged on an amorphous silicon substrate. Individual elements are connected by gate lines along rows (operating the TFT), by drain lines along columns (connected to the TFT output), and charge amplifiers connected to the drain lines to receive the charge from specific detector elements. In operation, local charge created by local X-ray absorption is stored at each detector element and actively read by turning row gate lines on one at a time, allowing charge to pass from the local storage capacitor through the TFT, down the drain line to the charge amplifier. Each transistor is reset and ready for the next exposure. The image is created sequentially, row by row. For real-time (30 frame per second) fluoroscopy, all of the detector rows must be read in 33 ms or less. The upper left detector element illustrates the concept of ‘fill-factor–with TFT arrays, caused by less than 100% geometric capture efficiency of X-rays that fall upon inactive areas of the TFT matrix
The ‘fill-factor–is a characteristic of the TFT and represents the fraction of each detector element that efficiently collects charge from the energy deposited by the absorbed X-ray signal in the converter material above it. Dead areas of the element include the gate, drain, TFT, and capacitor electronics. As the detector element gets smaller, the fill factor gets smaller and less efficient, ultimately setting a lower limit on the achievable spatial resolution. Typical fill factors (1 is ideal) range from about 0.5 to 0.8 for indirect detectors and are larger for direct detectors because of the ability to redirect the charge using ‘mushroom–electrodes [8].
X-ray signal detection: indirect and direct conversion
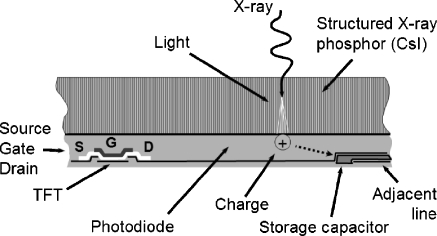
Although the TFT array and associated electronics are common to FPD systems, significant differences in the X-ray detection and signal conversion exist. Indirect detectors use a phosphor (scintillator) material that absorbs X-rays and produces a proportionate number of light photons that subsequently interact with a photodiode electrode on the TFT array, to produce the corresponding charge in the detector element capacitor. Phosphors are either unstructured, such as gadolinium oxysulfide (Gd2O2S), or structured, such as cesium iodide (CsI). Benefits of the unstructured phosphors are low cost and inert physical characteristics; however, the classic tradeoff of spatial resolution versus absorption efficiency is a distinct disadvantage. To achieve good absorption efficiency typically requires a thick phosphor, but to achieve good spatial resolution requires a thin phosphor, and thus a compromise must be struck between these two opposite factors. Benefits of the structured phosphor are the ability to confine the light photons in the needle structure, thus limiting lateral light spread and providing high resolution, while at the same time being able to deposit a thick phosphor layer, thus achieving good absorption efficiency. Fragility of the CsI phosphor and slight hygroscopic (water absorbing) characteristics are disadvantages. Because of good absorption and good resolution, the structured phosphor is widely employed. A cross-section of the indirect FPD is illustrated in Fig. 6.
Fig. 6.
Cross-section of an indirect TFT detector using CsI structured phosphor shows the conversion of X-rays first into light, traveling through the structured phosphor to a photodiode etched on the TFT array, and the creation of a proportional charge stored in the local capacitor
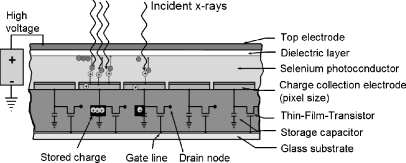
Direct detectors use a semiconductor material sandwiched between two electrodes to absorb and convert the X-ray energy directly into ion pairs (electrons and positively charged entities). Currently, amorphous selenium (a-Se) is the only clinical choice for a direct FPD, though there are other semiconductors under investigation in research laboratories [9]. To collect the charge confined to the detector element with minimal spread, and to keep the ion pairs from recombining, a large voltage bias is placed between the electrodes. Active collection of the X-ray-induced charges allows a relatively thick substrate material with reasonable absorption efficiency despite the low atomic number of selenium. Advantages of the direct conversion FPD include the simpler TFT structure (no photodiode is necessary but just a charge collection electrode, simplifying the production process), and high intrinsic spatial resolution achieved by the active collection of ion pairs under a high voltage with minimal resolution-reducing lateral spread. Disadvantages are (1) charge-trapping within the thick a-Se absorber, which reduces absorption efficiency, increases signal retention, and causes a greater amount of lag, and (2) potential destruction of a TFT in a detector element by overcharging caused by high X-ray exposures. A cross-section of the direct conversion FPD is illustrated in Fig. 7.
Fig. 7.
Cross-section of a direct TFT detector using a thick a-Se semiconductor layer under high voltage shows the creation of ion pairs directly by X-ray absorption, the separation and collection of the opposite charges at the electrodes, and the storage of the charge in the local capacitors
Until recently, indirect detection methods have been the only flat-panel technology available for clinical fluoroscopy. Direct FPD systems for fluoroscopy are now available because technological advances have reduced the effects of lag caused by charge trapping in the semiconductor and because overcharging protection circuits have been implemented.
Image intensifier/TV versus flat-panel detector for fluoroscopy
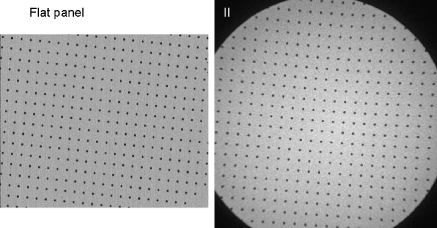
Are FPD systems better than II/TV systems, despite their higher cost? Can they be used to advantage in pediatric fluoroscopic applications to achieve ALARA doses? Certainly, from qualitative image presentation, the FPD has distinct advantages over the II with respect to lack of geometric distortion (Figs. 2 and 8), excellent image uniformity and flat-field capabilities (Fig. 9), no veiling glare or vignetting as with II/TV systems (see Fig. 3), rectangular FOV and full use of the image monitor (Fig. 10), wide dynamic range response (Fig. 4), small compact design and improved patient access (Fig. 11). A comparison of the features of II/TV and FPD systems is presented in Table 1.
Fig. 8.
Images of an array of equally spaced radioopaque spheres as imaged by a flat panel fluoroscopy system (left) and an II/TV system (right) show the lack of geometric distortion by the FPD system
Fig. 9.
Left ‘Raw–uncorrected flat-panel image of step wedges.Center left Image uniformity corrections for a FPD are possible by first mapping non-functional detector elements, columns and rows with replacement of nearest-neighbor average values and the creation of an inverted correction ‘flat-field–mask of normalized values. Center right Application of the flat-field mask to the uncorrected image produces the ‘for-processing–image. Right Image processing through contrast and spatial resolution enhancement results in the ‘for-presentation–image
Fig. 10.
Left An II (and TV) image is inherently circular, which results in inefficient use of a rectangular format image.Right A flat panel image provides full FOV, a decided advantage, but circular collimators can (and should) be used with FPD systems to reduce irradiated volume
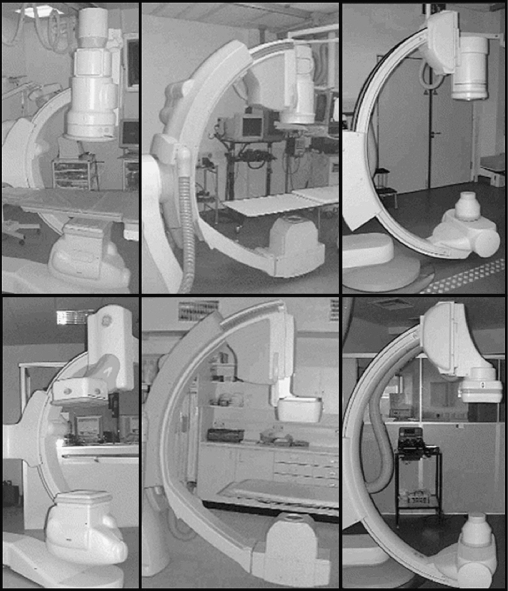
Fig. 11.
A comparison of II/TV and corresponding FPD systems for interventional imaging by three manufacturers shows the significant reduction in detector bulk and size, providing improved patient access and easier maneuverability
Table 1.
Feature comparison of II/TV and FPD systems
| Feature | Digital flat panel | Conventional II/TV |
|---|---|---|
| Dynamic range | Wide, about 5,000:1 | Limited by TV, about 500:1 |
| Geometric distortion | None | Pin-cushion and ‘S–distortion |
| Detector size (bulk) | Thin profile | Bulky, significant with large FOV |
| Image area FOV | 41×41 cm | 40 cm diameter (25% less area) |
| Image quality | Better at high dose | Better at low dose |
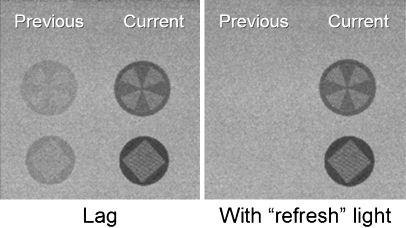
Quantitatively, from detection efficiency metrics, the FPD holds up quite well at high exposures encountered in cine-radiography and interventional DSA studies. Detective quantum efficiency (DQE), a measure of a detector’s ability to preserve information in the image relative to the incident X-ray information presented at the phosphor, is higher for the FPD relative to the II except at low exposures typically encountered in continuous fluoroscopy (30 frames per second). At higher exposure levels typical of radiography and DSA, the relatively large signal produced by the absorbed X-rays allows the gain of the output charge amplifiers of the FPD to be low. However, at fluoroscopy exposure levels, the necessary low exposure per image requires significant gain amplification to achieve a reasonable signal level for digitization. Not only is the signal amplitude increased, but electronic and other noise sources from the FPD are increased, as well, resulting in an image with low signal-to-noise ratio. The output image is no longer ‘quantum noise limited–(X-ray statistics are not the dominant noise source in the image), low contrast resolution is reduced, and image quality suffers. At low exposure levels, detector lag also plagues these detectors during readout (similar to residual lag signal in vidicon TV cameras [10]). Ideally, all of the charge from the previous image frame is removed from each detector element in the TFT array, but residual (trapped) charge contributes to signal retention, producing undesirable image lag, reduced temporal and spatial resolution of moving anatomy, and image artifacts. In imaging situations with little or no anatomical motion, lag helps reduce quantum mottle but is undesirable otherwise. Manufacturers are discovering methods to reduce or eliminate lag by using detector backlighting [11], as illustrated in Fig. 12, and tightened specifications for TFT panel manufacturing tolerances. If a certain amount of ‘lag–is desired for reducing noise, the best solution is to start with an image detector with no lag and employ image processing methods such as real-time recursive filtration to optimize the trade-off between needed temporal resolution and low image noise.
Fig. 12.
Image lag results from residual signals from previous exposures being superimposed on the current image. The magnitude of the lag can be quite extensive (e.g., 5–0% of the previous signal) and easily observed (left figure). Poor temporal resolution and potential artifacts are drawbacks. Methods such as detector ‘backlighting–are being implemented to reduce or eliminate the residual lag (image from Philips Medical Systems website [11])
Anecdotal reports of poor image quality rendered by FPD fluoroscopy are, in part, a result of the lack of experience, inappropriate configuration (e.g., radiation dose levels, image postprocessing), and inadequate training regarding the optimal use of these detector systems. First generation FPD systems did not have many of the table-side controls found on a corresponding II/TV system, making the transition difficult (personal communication, Phil Rauch, Henry Ford Hospital, Detroit, Mich., July 2005). Often, disconnect between the radiologist needing to achieve low contrast resolution in the fluoroscopy image and the aggressive low-dose configuration of the FPD system by the system installer has resulted in disgruntled users of such systems (personal communication, Paul Brown, Oregon Health Sciences University, Portland, Ore., February 2006). Ineffective or improper setting of pulsed fluoroscopy rates and dose per frame can lead to inadequate image presentation, reduced temporal resolution, poor image quality and needless overexposure rather than a reduced dose. This is particularly true for current generation FPD systems that do not have the gain characteristics of the II/TV systems. Certainly, for pulsed fluoroscopy, proper dose adjustment per frame and appropriate frame rates are essential to ensure that the optimal image quality is achieved [2], and with careful adjustments, the chief limitation of FPD systems, namely that of electronic noise, can largely be overcome.
Quantitative evaluation of FPD systems compared to II/TV systems for fluoroscopy in the laboratory setting indicates equivalent or superior performance of the flat-panel system at high and intermediate air kerma rates but poorer performance at low air kerma rates in under-penetrated areas of an image with less than 0.1 μGy per frame using qualitative low-contrast sensitivity phantoms [12]. This is corroborated by data from other investigators using quantitative methods to measure the DQE as a function of spatial frequency (object size) for levels of incident exposure from high to extremely low for both indirect and direct FPD systems [13–16]. Often cited in articles are incident air kerma of 1 μGy per frame (typical fluoro rate) for a uniform exposure, but much of the image will be less than one-tenth of that level (e.g., 0.1 μGy per frame) where current flat panels have problems with electronic and non-quantum noise sources. What strategies are being considered to overcome this limitation? Already mentioned was lower frame rate pulsed fluoroscopy, to allow increased incident exposure per frame while keeping the overall exposure rate to the patient lower than continuous mode operation. Anti-scatter grids, used for both FPD and II/TV fluoro systems, must be easily removable by the operator prior to imaging smaller pediatric patients to eliminate the dose penalty (grid Bucky factor) for fluoroscopic and radiographic procedures. Another possibility is the implementation of a FPD that provides programmable ‘noiseless–gain to signals dependent on incident (low) exposure; one such device is now under investigation and described in the next section.
Flat-panel technology: variable gain hybrid design
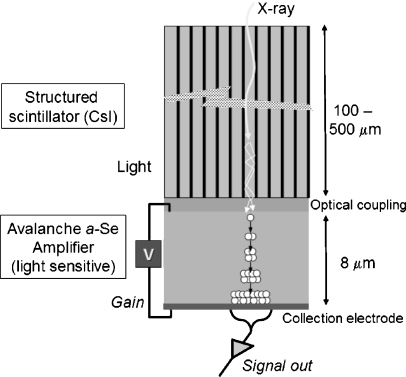
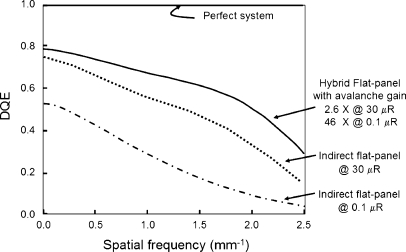
A very interesting hybrid design FPD is based upon the coupling of a thick CsI structured scintillator (e.g., 500 μm for good X-ray absorption efficiency) to a thin (8 μm), photosensitive a-Se semiconductor [16]. The thin semiconductor layer produces photoinduced electron-hole pairs at the top interface from incident light photons, and under an applied voltage, holes propagate toward the bottom surface. With sufficiently high voltage, the holes in traveling through the semiconductor undergo avalanche multiplication and create more holes and electrons (Fig. 13). The gain of the signal ranges from 1 to about 1,000, depending on the magnitude of the programmable applied voltage (typical amplification gains used in prototype systems are from about 5 to about 50). The amplified output signals are collected by the detector element electrodes/capacitors in the TFT array. Compared to conventional indirect detection TFT arrays, potential advantages of the hybrid approach are improved noise performance at much lower (fluoroscopy) radiation levels, better temporal performance with less lag and ghosting as a result of less trapping density in the very thin a-Se layer, improved compatibility with the TFT manufacturing process (no need to have on-board photodiode electrodes nor suffer the ‘fill-factor penalty–for small detector elements in an indirect conversion FPD) and ability to use higher-resolution CsI scintillators with less light output (gain can be increased to compensate). Compared to conventional direct detection TFT arrays, the hybrid design advantages are programmable gain and low probability of charge-trapping with the thin a-Se layer (unlike the very thick a-Se layers needed for X-ray absorption in direct detection designs). Although a full-scale detector is not yet functional, implementation appears achievable within a multiple-year time frame, with a promise of low gain for radiographic applications, intermediate gain for interventional and cine radiography applications, and high gain operation for low-dose fluoroscopy, thus optimizing detection efficiency and image signal-to-noise ratio at radiation dose levels typical of each study. Quantitative results from a prototype system (Fig. 14) illustrate the significant improvements possible at high and low doses [16]. This technological implementation could have a distinct positive impact on achieving ALARA for pediatric imaging in fluoroscopy.
Fig. 13.
Cross-sectional illustration of a ‘hybrid–flat-panel detector system under development shows the CsI scintillator to convert X-rays to light, an optical coupling medium to efficiently transfer light from the CsI to a thin a-Se ‘avalanche–detector under a high voltage. Light photons create electron-hole pairs, and as the holes accelerate to the negative electrode under high voltage, more ion pairs are created and are collected at the collection electrode. The amplification gain (1 to about 1,000) is controlled by the applied voltage (adapted from Zhao et al. [16])
Fig. 14.
DQE comparison of prototype hybrid system and conventional flat panel (indirect) detector systems at low (0.1 μR) and high (30 μR) incident exposures are plotted (data extracted from references [13, 16])
Quality assurance and quality control
An imaging system is only as good as its weakest link, and in the case of real-time fluoroscopic imaging, there are many cascading processes during image formation that can affect the overall image quality and ALARA dose efficiency. Additionally, care must be taken to consider installation of FPD peripheral equipment and heating, ventilation, and air-conditioning requirements that are often overlooked and/or assumed to be insignificant compared to older II/TV systems, when in fact they are not [17]. In order to establish baseline operation values and to ensure optimal functionality, acceptance testing by a qualified medical physicist is strongly suggested during installation and initial implementation. Performance metrics that should be evaluated are reviewed by Rauch [17], though there are no formal recommendations for FPD systems beyond that of II/TV systems at this time. Additionally, periodic quality control testing and verification of proper system functionality at a frequency relevant to discovering possible equipment problems is important, as is plotting test results to reveal trends and intervene before problems are actually manifested. An excellent resource for additional information on acceptance testing, quality control, and operational issues for fluoroscopic imaging is highly recommended as a starting point [18].
Conclusions
FPDs designed specifically for fluoroscopic use provide image quality and dose efficiency generally superior to the II systems that they replace, except at the lowest fluoro dose levels. Advantages include excellent image uniformity, no geometric distortion, no veiling glare or vignetting, and small, thin physical size for improved patient accessibility, an important consideration for pediatric imaging. The key disadvantage is less efficiency and performance at the lowest exposure levels; however, with judicious use and configuration knowledge of pulsed fluoroscopy and removable anti-scatter grids, this can be mitigated to a large extent with current technology. As with all technological innovations, there is a learning curve for implementation, adjustment, and optimal use of the technology, with significant room for ongoing improvement. Long-term reliability, radiation tolerance, and replacement costs, currently unknown, will take time to determine. On the horizon are technological advances that promise an ability to optimize image quality at all radiation exposure levels and portend the inevitable demise of the venerable II/TV fluoroscopy system, but not just yet. For pediatric fluoroscopic imaging and the ALARA principle, whether using II/TV systems or FPD systems, always important is the vigilance and understanding by the operator of the fluoroscopic procedure and equipment and ways to minimize study times, exposure times and radiation dose for the benefit of our patients.
References
- 1.Eisenberg RL (1992) Radiology, an illustrated history. Chapter 10: dark adaptation and image intensification. Mosby Year Book, St. Louis, pp 149–55
- 2.Strauss K (2006) Pediatric interventional radiography equipment: safety considerations. Pediatr Radiol Suppl DOI 10.1007/s00247-006-0220-4 [DOI] [PMC free article] [PubMed]
- 3.Chamberlain WE (1942) Fluoroscopes and fluoroscopy. Radiology 38:383–12
- 4.Coltman JW (1948) Fluoroscopic image brightening by electronic means. Radiology 51:359–67 [DOI] [PubMed]
- 5.Fuchs AW (1933) Radiographic recording media and screens. In: Glasser O (ed) The science of radiology. Charles C. Thomas, Springfield, pp 97–19
- 6.Rong XJ, Shaw CC, Liu X, et al (2001) Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems—a contrast-detail phantom study. Med Phys 28:2328–335 [DOI] [PubMed]
- 7.Floyd CE, Warp RJ, Dobbins JT, et al (2001) Imaging characteristics of an amorphous silicon flat-panel detector for digital chest radiography. Radiology 218:683–88 [DOI] [PubMed]
- 8.Yaffe MJ, Rowlands JA (1997) X-ray detectors for digital radiography. Phys Med Biol 42:1–9 [DOI] [PubMed]
- 9.Street RA, Ready SE, Van Schuylenbergh K, et al (2002) Comparison of PbI2 and HgI2 for direct detection active matrix x-ray image sensors. J Appl Phys 91:3345–355 [DOI]
- 10.Bushberg JT, Seibert JA, Leidholdt EM, et al (2002) Essential physics of medical imaging. Chapter 9, Fluoroscopy, 2nd edn. Lippincott Williams & Wilkins, Baltimore, pp 231–54
- 11.Philips Medical Systems (2006) Philips flat detector technology. Philips Medical Systems, Bothell, WA. http://www.medical.philips.com/us/products/cardiovascular/philips_flat_detector_technology.html. Accessed 8 February 2006
- 12.Davies AG, Cowen AR, Kengyelics SM, et al (2001) Threshold contrast detail detectability measurement of the fluoroscopic image quality of a dynamic solid-state digital X-ray detector. Med Phys 28:11–5 [DOI] [PubMed]
- 13.Granfors PR, Aufrichtig R, Possin G, et al (2003) Performance of a 41×41 cm2 amorphous silicon flat panel x-ray detector designed for angiographic and R&F imaging applications. Med Phys 30:2715–726 [DOI] [PubMed]
- 14.Hunt DC, Tousignant O, Rowlands JA (2004) Evaluation of the imaging properties of an amorphous selenium-based flat panel detector for digital fluoroscopy. Med Phys 31:1166–175 [DOI] [PubMed]
- 15.Vano E, Geiger B, Schreiner A, et al (2005) Dynamic flat panel detector versus image intensifier in cardiac imaging: dose and image quality. Phys Med Biol 50:5731–742 [DOI] [PubMed]
- 16.Zhao W, Li D, Reznik A, et al (2005) Indirect flat-panel detector with avalanche gain: fundamental feasibility investigation for SHARP–AMFPI (scintillator HARP active matrix flat panel imager). Med Phys 32:2954–966 [DOI] [PubMed]
- 17.Rauch P (2005) Fluoroscopy acceptance testing: technical considerations for image quality and dose. Presentation at the 2005 AAPM Annual Meeting, Seattle, WA. http://aapm.org/meetings/05AM/PRAbs.asp?mid=18&aid=2628. Accessed 19 Jan 2006
- 18.Balter S, Chan R, Shope T (2002) Intervascular brachytherapy/fluoroscopically guided interventions. Medical Physics Monograph no. 28. Medical Physics Publishing, Madison