Abstract
Objective:
Impairment of the arousal process from sleep is thought to be involved in the pathogenesis of sudden infant death syndrome (SIDS). We hypothesized that a greater propensity for cortical arousal in the prone position may, in a normal infant, be a protective mechanism to promote complete arousal in a vulnerable sleeping position, a protection that is absent in SIDS victims. We aimed to examine the arousal process in a group of infants exposed to maternal smoking, who are thus at higher risk for SIDS.
Design:
Twelve healthy, full-term infants born to smoking mothers were studied using daytime polysomnography at 2 to 4 weeks, 2 to 3 months and 5 to 6 months postnatal age. Data were compared with 13 healthy infants born to nonsmoking mothers. Arousal was induced by pulsatile air-jet stimulation to the nostrils during active and quiet sleep, in both supine and prone positions. For each stimulus, physiologic and electroencephalogram changes were visually assessed and arousal responses were classified as sub-cortical activation or cortical arousal.
Results:
In smoke-exposed infants, the progression from sub-cortical activation to cortical arousal was depressed at 2 to 4 weeks and 5 to 6 months. There was no effect of maternal smoking observed at 2 to 3 months; however, a significant dose-dependent relationship between cortical activation proportions and urinary cotinine levels was present in both supine and prone positions.
Conclusion:
We have shown that maternal smoking is associated with impaired arousal processes to trigeminal stimulation that may increase the risk for SIDS. This further highlights the importance of public education of the risks of maternal smoking.
Citation:
Richardson HL; Walker AM; Horne RSC. Maternal smoking impairs arousal patterns in sleeping infants. SLEEP 2009;32(4):515-521.
Keywords: Smoking, SIDS, arousal, sleep
THE PATHOGENESIS OF SUDDEN INFANT DEATH SYNDROME (SIDS), THE THIRD LEADING CAUSE OF INFANT MORTALITY IN THE UNITED STATES,1 IS CONSIDERED to be multifactorial. Although exact mechanisms remain uncertain, an impairment of the arousal process from sleep in response to a life-threatening stressor is commonly thought to be involved.2 In support of this idea, autopsy studies of SIDS victims have revealed brainstem abnormalities in key areas required for arousal and cardiorespiratory control.3, 4 More recently, Paterson et al5 described irregularities in the medullary serotonergic (5-HT) system in SIDS victims that have the potential to compromise critical protective reflexes such as arousal or head turning. Furthermore, we and others have previously demonstrated that arousability from sleep in otherwise healthy infants is depressed by major risk factors for SIDS, such as prone sleeping6–8 and exposure to maternal smoking.9–11
Studies have identified a stereotypical sequence of infant arousal behaviors, from sub-cortical responses, such as sighs, startles, and limb movements, to full awakening with crying.12 It has been suggested that both spontaneous and stimulus-induced arousal responses involve these pathways of central nervous system activation from spinal to sub-cortical levels, before progressing to the cortex13,14; this progression has been used to define infant arousals as sub-cortical activations (SCA) or full cortical arousals (CA) characterized by electroencephalogram (EEG) changes.15 One study that investigated spontaneous arousals from sleep in infants who subsequently died of SIDS observed fewer CA and more SCA than in age-matched controls, suggestive of an incomplete arousal process, perhaps due to pre-existing abnormalities in brainstem-cortex arousal pathways.16
A distinct peak in SIDS prevalence occurs between 2 and 4 months of age,17 which is a critical period for the maturation and organization of sleep states and thus may represent a developmental window of vulnerability.18 We have previously shown that healthy, full-term infants have alterations in the nature of arousal responses at this age; during both active sleep (AS) and quiet sleep (QS), infants exhibit increased proportions of CA when sleeping prone compared with supine.19 We hypothesized that this greater propensity for CA in the prone position may, in a normal infant, be a protective mechanism to promote complete arousal in a vulnerable sleeping position, a mechanism that is absent in SIDS victims. The aim of this study was to investigate arousal patterns in a group of infants exposed to both maternal smoking and prone sleeping, and who therefore are at increased risk for SIDS. We predicted that these infants would exhibit increased SCA and decreased CA when prone, similar to the modified arousal responses of SIDS victims, and that this effect would be most marked during the high-risk period for SIDS.
METHODS
Participants
Ethical approval was obtained from the Southern Health (Monash Medical Centre) and Monash University Human Research Ethics Committees. Participation was voluntary, with no monetary incentive provided. Written informed parental consent was obtained prior to study commencement.
Twenty-five healthy term infants born at 38 to 42 weeks gestation with normal Apgar scores (median 9 [range 5-10] at 1 and 5 minutes) were recruited. Thirteen (7 girls/6 boys) were born to nonsmoking mothers and 12 (9 girls/3 boys) to mothers who smoked during pregnancy. All infants routinely slept supine at home.
Maternal Smoking
Maternal smoking during pregnancy was identified from medical records prior to recruitment. This was later confirmed by a maternal questionnaire detailing smoking habits and analysis of infant urinary cotinine (nicotine metabolite) concentrations at 2 to 3 months, as published previously.9
Mothers in the smoking group reported that they smoked 15 ± 6 (range 3-20) cigarettes per day. Two ceased smoking after 6 and 7 weeks of pregnancy; however, both resumed smoking 10 to 15 cigarettes per day after giving birth. When urinary cotinine was measured, 1 of these infants had less than 10 ng/mL; however, the remaining infants ranged between 10.6 to 286 ng/mL (mean ± SEM, 82.5 ± 29.2 ng/mL). In the nonsmoking group, there were no reports of smoking by mothers or anyone in the household during or after the pregnancy, and urinary cotinine levels were all less than 10ng/mL.
Polysomnography
Daytime polysomnography was performed on 3 occasions—at 2 to 4 weeks, 2 to 3 months, and 5 to 6 months postnatal age—in a sleep laboratory where temperature was 21°C to 24°C and outside noise was minimal. Physiologic variables were recorded using a 16-channel polygraph (Model 78A, Grass Instrument Co., Quincy, MA). Gold-plated electrodes were attached for 2 scalp EEG channels (C4/A1 and O2/A1 were recorded between 0.3 to 30 Hz at a sampling rate of 500 Hz). Left and right electrooculograms, submental electromyogram, electrocardiogram, instantaneous heart rate, and rectal and abdominal skin temperatures (YSI 400 Series Thermistor, Mallinckrodt, Melbourne, VIC, Australia) were also recorded. Thoracic and abdominal breathing movements were monitored using piezoelectric sensors (Resp-ez, EPM Systems, Midlothian, VA). Blood oxygen saturation was measured at a 3-second averaging time (BIOX 3700e pulse oximeter, Ohmeda, Louisville, CO). Recording electrodes were attached during a feeding; infants were then placed in a bassinet, and the study began when the infant was asleep. Using standard criteria, sleep state was defined as AS, QS, or indeterminate sleep.20
Protocol
Infants slept both supine and prone, with starting positions randomized for the first study, then alternated for subsequent studies. Sleep position was changed between morning and afternoon naps, generally after a feeding. Arousability was assessed using trigeminal stimulation during AS and QS in both positions. As described previously, a pulsatile air jet (3 Hz, for 5 seconds) was delivered via a hand-held cannula at the infants' left and right nostrils, alternately.9 Stimulus-driving pressures were increased in 50- to 100 cmh2o increments until arousal criteria were met; then pressures were decreased.9 The maximum driving pressure used was 950 cmh2o, equivalent to 1.6 cmh2o received by the infant at the distal end of the cannula. This staircase method was continued until infants awakened or changed sleep state, following which a new series of stimuli was commenced.
Arousal Definition
Baseline data were obtained from 10 seconds directly preceding each air-jet stimulus. Polysomnographic recordings and direct observations were used to visually identify infants' arousal responses.15 SCA was defined by the presence of at least 2 of 3 criteria: (1) a gross body movement; (2) a heart rate increase of at least 10% above baseline; and (3) during QS, a respiratory change in either amplitude or frequency or, during AS, an increased electromyogram amplitude. CA was scored when an abrupt change in EEG frequency was observed (for at least 3 seconds in duration) in association with a SCA. To reduce the probability that arousals were spontaneous, these changes were analyzed only if they first occurred within 7 seconds of stimulus initiation. Full awakenings (scored by a stable and concordant cortical change for ≥ 1 minute) were infrequent because the objective of the air-jet protocol was to induce arousals without compromising the infants' natural sleep cycles.
Statistical Analysis
Statistical analyses were performed using SigmaStat (Systat Software Inc, Richmond, CA). χ2 statistics (2 × 3 table) were employed to investigate positional effects on nonarousals, SCA, and CA, expressed as percentages of total air-jet stimuli. To further elucidate where differences lay within the data, odds ratio analysis was performed, assessing the strength of each level of arousal (SCA and CA) relative to nonarousal.21
Secondary analyses controlled for known sleep state and positional differences in overall arousability6 by investigating the proportions of SCA and CA as a percentage of total arousals (SCA + CA). χ2 tests were also used to investigate position differences and maturation changes in arousal proportions and to compare smoking and nonsmoking groups. To ensure that each infant was represented equally, arousal responses were reanalyzed to control for the number of stimuli presented to each individual infant; results matched those from the above methods and, hence, are not presented in this paper. Regression analysis of infant urinary cotinine concentrations and CA proportions at 2 to 3 months was also performed for both supine and prone positions.
RESULTS
Overall arousal thresholds and probability of arousal in these infants have previously been reported9; however, because these studies were carried out prior to the recently published concensus on arousal definitions for infants,15 EEG changes were not analyzed, and no assessment could be made of the progression of SCA to CA. In the present study, EEG characteristics were examined, and arousal responses were reassigned as either SCA or CA, according to these definitions, thus allowing an investigation of the arousal process itself. Sleep information and arousal data have previously been published for only the nonsmoking control group. This paper reports the new findings in the infants exposed to maternal smoking and compares them with the findings from the nonsmoking controls.19 Between smoking and nonsmoking groups, there were no differences in gestational age at birth, birth weight, total sleep time, or age or weight recorded at each study (Table 1).
Table 1.
Number, Age, Weight, and Total Sleep Time of Infants in Smoking and Nonsmoking Groups at Birth and the 3 Ages Studied
| Birth | 2-4 wk | 2-3 mo | 5-6 mo | |
|---|---|---|---|---|
| Infants, no (boys/girls) | ||||
| Smoking | 12 (6/6) | 11 (6/5) | 7 (3/4) | |
| Nonsmoking | 12 (3/9) | 11 (3/8) | 8 (3/5) | |
| Age | ||||
| Smoking | 40 ± 0.4 wk | 15 ± 1 d | 75 ± 2 d | 176 ± 5 d |
| Nonsmoking | 40 ± 0.3 wk | 14 ± 1 d | 74 ± 2 d | 189 ± 5 d |
| Weight, gm | ||||
| Smoking | 3598 ± 129 | 3886 ± 140 | 5805 ± 227 | 7935 ± 223 |
| Nonsmoking | 3498 ± 102 | 3709 ± 129 | 5453 ± 213 | 7865 ± 359 |
| Study Sleep Time, min | ||||
| Smoking | 178 ± 8 | 154 ± 13 | 72 ± 15 | |
| Nonsmoking | 157 ± 10 | 136 ± 12 | 96 ± 9 |
Data are presented as mean ( ± SEM) except group assignments, which are total number (boys/girls). Age at birth is gestational age.
In the cohort of smoke-exposed infants, 1 infant was unavailable after the first study, another did not complete the third study, and some files were corrupted by technical complications following the studies; hence, arousal analysis was performed for 12 infants at 2 to 4 weeks, 11 infants at 2 to 3 months, and 7 infants at 5 to 6 months. A total of 2627 air-jet stimuli administered (22 ± 1 per infant for each sleep state, position, and age) induced 1021 arousal responses for further analysis (567 in AS; 454 in QS). All data were included from the 2 infants whose mothers stopped smoking during the first trimester because their sleep and arousal responses did not differ from those of the rest of the group.
Total Responses to Air-jet Stimuli
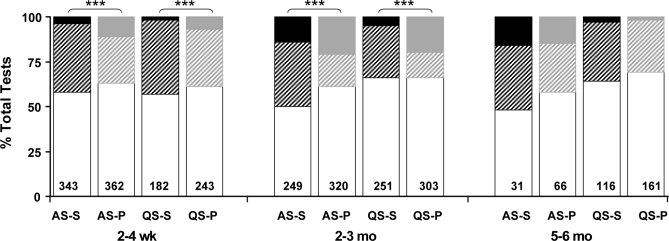
Frequencies of nonarousals, SCA, and CA, calculated as proportions of the total number of air-jet stimuli, are shown in Figure 1. Overall differences were found in the relative frequencies of nonarousal, SCA, and CA between prone and supine sleeping positions (P < 0.001) for both sleep states at 2 to 4 weeks and 2 to 3 months. Odds ratio analysis showed that overall differences were due to a combination of significantly increased CA, decreased SCA and increased non-arousal frequencies in the prone position compared with the supine position (P < 0.05).
Figure 1.
Nonarousals (white bars), sub-cortical activations (striped bars), and cortical arousals (solid bars) of smoke-exposed infants in response to air-jet stimulation at 2-4weeks, 2-3 months, and 5-6 months, during quiet sleep (QS) and active sleep (AS) in both prone (P) and supine (S) sleeping positions. Data are expressed as proportions of total air-jet tests, with total stimuli numbers shown within the histograms. ***P < 0.001 between prone and supine positions (χ2 analysis).
Stimulus Intensity
There was no difference in stimulus-driving pressures that induced SCA or CA; this finding was consistent in both sleep states and sleeping positions at all ages studied (Table 2).
Table 2.
Pressure Thresholds for Sub-Cortical Activations and Cortical Arousals in the Smoke-Exposed Infants, for Each Sleep State and Position at 2 to 4 Weeks, 2 to 3 Months and 5-6 Months Postnatal Age in the Supine and Prone Positions During AS and QS.
| Sleep State - Position | Age |
|||||
|---|---|---|---|---|---|---|
| 2-4 w |
2- 3 mo |
5-6 mo |
||||
| SCA | CA | SCA | CA | SCA | CA | |
| AS-Supine | 193 ± 22 | 130 ± 43 | 113 ± 18 | 138 ± 27 | 150 ± 61 | [233 ± 136] |
| AS-Prone | 252 ± 35 | 200 ± 26 | 187 ± 41 | 188 ± 28 | 208 ± 120 | [133 ± 44] |
| QS-Supine | 235 ± 31 | 300 ± 153 | 401 ± 68 | 512 ± 138 | 347 ± 107 | [713 ± 130] |
| QS-Prone | 310 ± 46 | [443 ± 70] | 448 ± 73 | 481 ± 52 | 597 ± 76 | 767 ± 88 |
Values in brackets indicate numbers that were excluded from statistical analysis due to low numbers of cortical arousals observed (n < 3). Pressure thresholds are in cmh2o. SCA, sub-cortical activations; CA, cortical arousals; AS, active sleep; QS, quiet sleep.
Arousal Proportions
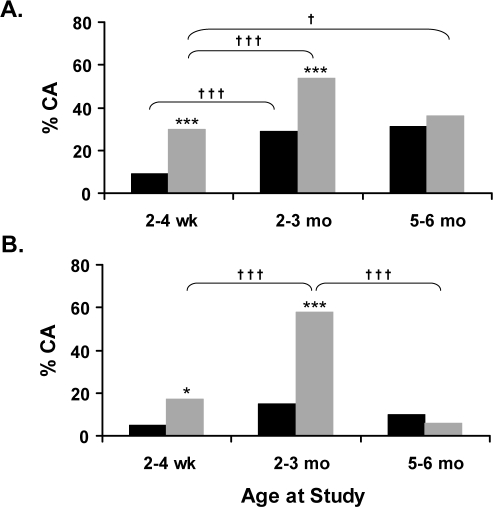
At 2 to 4 weeks and 2 to 3 months, the proportions of CA were significantly increased in the prone position compared with supine, during both AS (Figure 2A) and QS (Figure 2B). There were no positional differences found at 5 to 6 months in either sleep state.
Figure 2.
Cortical arousals (CA) observed in the smoke-exposed infants, expressed as proportions of total arousals, during (A) active sleep (AS) and (B) quiet sleep (QS) in supine (black) and prone (grey) positions at 2-4 weeks, 2-3 months, and 5-6 months. Symbols represent statistically significant differences; *P < 0.05, ***P < 0.001 between prone and supine positions; †P < 0.05, †††P < 0.001 between postnatal ages.
Maturational changes in the proportions of CA from total arousals throughout the first 6 months of life are also illustrated in Figure 2. During AS (Figure 2A), the proportion of CA increased significantly between 2 to 4 weeks and 2 to 3 months in both sleeping positions (P < 0.001) and, when supine, remained elevated at 5 to 6 months (P < 0.05). During QS (Figure 2B), a similar peak in CA was evident at 2 to 3 months when infants slept prone (P < 0.001); in the supine position, values remained similar across all ages.
Nicotine Exposure
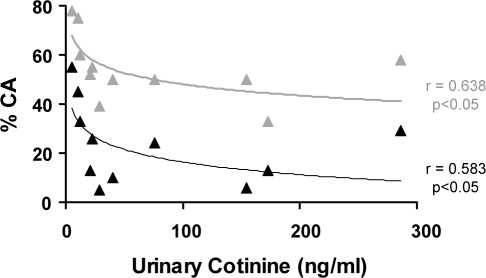
With AS and QS data combined, Figure 3 shows CA proportions and the corresponding urinary cotinine concentrations for the smoke-exposed infants at 2 to 3 months. Logarithmic relationships were observed in both prone and supine positions; with increasing cotinine levels, CA proportions rapidly decreased initially, then plateaued. There was no such relationship found between CA proportions and the reported number of cigarettes smoked daily.
Figure 3.
Regression analysis of cortical arousal (CA) proportions (of total arousals) and urinary cotinine concentrations for each of the smoke-exposed infants at 2 to 3 months. Note the significant logarithmic relationships in both supine (black) and prone (grey) sleeping positions.
Comparison between Smoking and Nonsmoking Groups
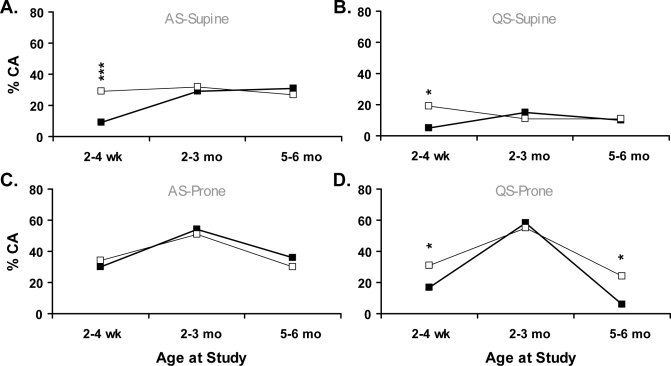
The proportions of CA induced by air-jet stimulation are compared between smoking and nonsmoking groups in Figure 4. When sleeping supine at 2 to 4 weeks, the smoking group exhibited significantly decreased proportions of CA in both sleep states, with similar arousal proportions observed between groups at both 2 to 3 months and 5 to 6 months. When prone during QS, the smoking group showed decreased proportions of CA at 2 to 4 weeks and 5 to 6 months (P < 0.05); at 2 to 3 months, both groups exhibited a peak in CA to approximately 60% of total arousals. No effects of maternal smoking were observed during prone AS.
Figure 4.
Air-jet–induced cortical arousals, as percentage of total arousals during active sleep (AS) and quiet sleep (QS) in supine and prone positions; (A) AS-Supine, (B) QS-Supine, (C) AS-Prone, (D) QS-Prone. Symbols represent significant differences between infants exposed to maternal smoking (■) and controls (□); *P < 0.05, ***P < 0.001.
DISCUSSION
This is the first investigation of the effects of maternal smoking on infant arousal processes, distinguishing responses between SCA and CA. We have shown that maternal cigarette smoking is associated with modified arousal processes to somatosensory stimulation in otherwise healthy term infants. Infants born to smoking mothers exhibited decreased CA proportions (hence increased SCA proportions) when compared with the nonsmoking group. This change is potentially important because decreased spontaneous CA have been observed in victims of SIDS prior to death.16 Interestingly, our study also showed that, despite altered arousal proportions in the smoking group, the difference between prone and supine positions remained. Like controls, smoke-exposed infants exhibited higher CA proportions when prone during both sleep states, at 2 to 4 weeks and 2 to 3 months of age; hence, position appears to have a powerful biologic impact on the arousal process.
In Western countries, 15% to 25% of pregnant women smoke throughout pregnancy,22 and maternal smoking has replaced prone sleeping as the greatest modifiable risk factor for SIDS.23 In one study, 79% of SIDS victims had been exposed to maternal cigarette smoke.24 We previously demonstrated that, in the supine position, arousability was lower in smoke-exposed infants than in a nonsmoking control group; notably, this difference was significant during QS at 2 to 3 months, the age at which SIDS risk is greatest.9 In addition, other studies have found blunted behavioral responses to auditory10, 11 and respiratory25 stimuli at this high-risk age in infants who were exposed to cigarette smoke in utero. These previous reports of depressed total arousability in infants of smoking mothers, together with the modifications to arousal processes observed in this study, support the recent proposal that infants who later succumb to SIDS may have subtle abnormalities that lead to impaired or incomplete arousal responses.16,26
Autopsy studies have revealed numerous anatomic and physiologic abnormalities in SIDS infants, the majority of which are related to impaired brain and/or brainstem development; these include decreased binding of muscarinic cholinergic receptors and multiple irregularities in the serotonergic system of the medulla oblongata.3,5 Although, in one study, binding of nicotinic acetylcholine receptors (nAchR) was found to be unaltered in the brains of SIDS victims compared with those of controls,27, 28 a later study from the same laboratory suggested that the radioligand used (3H-nicotine) may have masked a specific nAChR subunit abnormality.29 Furthermore, the regulatory response to maternal smoking appeared defective in SIDS victims.27 One could speculate that the above-described brainstem abnormalities may be associated with compromised vital protective responses, such as movement away from dangerous stimuli and arousal. It has been shown that the removal of ascending cholinergic projections during early gestation in rats lead to marked and long-lasting EEG alterations later in life.30
Numerous studies have shown that prenatal exposure to the nicotine in cigarette smoke disturbs fetal development by affecting cholinergic and serotonergic transmitter systems.31–33 Nicotine readily crosses to the fetal circulation via the placenta and binds directly to the nAchRs distributed throughout the brain.22 High-density binding sites have been observed in brainstem areas related to cardiopulmonary integration, arousal, and sleep.31,34 Our present finding of decreased proportions of CA in smoke-exposed infants is supported by another study that showed that prenatal nicotine exposure in mice preferentially decreased cortical rather than brainstem responses to hypoxia after birth; desensitization of nAChRs was suggested as a possible cause.35
Although the exact timing that nAchRs appear during human development is unknown, expression of some receptor subunits (α4 and α7) has been observed in the medulla oblongata, pons, and cerebellum as early as 5 weeks of gestation.31 Binding of nicotinic receptors is reported to be highest at midgestation and to subsequently decrease, leading to the suggestion that this phenomenon indicates a period of increased vulnerability to the adverse affects of nicotine.34 Though 2 mothers in our study stopped smoking at 6 to 7 weeks of pregnancy, before this “vulnerable” period, there was no evident improvement or restoration in the nature of arousal responses observed in these infants. Our finding is supported by those of Falk et al, who showed that smoking during the first trimester can still influence nAChR expression.31 A larger sample size would be needed to confirm the timing of the effects of maternal smoking on arousal pathways. It is important to acknowledge that, in the present study, all the smoking mothers continued to smoke after giving birth; thus, it is impossible to ascertain whether the observed arousal impairment occurred due to prenatal or postnatal cigarette exposure.
We have previously proposed that, in healthy infants, the promotion of full CA may be protective, to ensure an adequate response when required during a vulnerable developmental period.19 An interesting finding of this present study was that both nonsmoking- and smoking-exposed infants showed similar responses with respect to maturation changes across the 3 ages studied; in the prone position, proportions of induced CA were increased significantly at 2 to 3 months, to 51% to 58% of total arousals from 17% to 34% at 2 to 4 weeks. Although the smoking group exhibited decreased proportions of CA at 2 to 4 weeks (in both positions and sleep states) and 5 to 6 months (during prone QS) when compared with the nonsmoking control group, it is possible that nicotine exposure in these infants was not high enough to impair the protection of increased CA at 2 to 3 months. Our finding of decreasing CA proportions with increasing urinary cotinine concentrations at this age supports the concept of a dose-dependent effect of nicotine exposure. Similar dose-response associations have been described with increased risk of SIDS36 and severity of brainstem gliosis in SIDS victims.37 Altered sleep architecture in preterm infants has also been associated with prenatal cigarette exposure, though only when mothers smoked more than 10 cigarettes per day.38 Despite well-known relationships of maternal cigarette smoking with intrauterine growth restriction and low birth weight,39 mean birth weights in this study did not differ between the nonsmoking- and smoking-exposed infants, nor did total sleep time or time spent in each sleep state. Mothers in the smoking group reported smoking between 3 and 20 cigarettes per day, thus we may have observed significant differences in birth weight and sleep architecture in a study population with more severe smoke exposure.
CONCLUSIONS
We have demonstrated that not only total arousability, but also the arousal process itself (ie, the progression from SCA to full CA) is altered by maternal smoking. The incidence of SIDS has decreased dramatically throughout the world with the introduction of campaigns to promote supine sleeping. To further reduce the incidence of SIDS, public education about the risks associated with maternal smoking and the potential detrimental effects on the infant, particularly during critical developmental periods, are urgently required, and this study provides new evidence to support these campaigns.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kung H, Hoyert DL, Xu J, Murphy SL. Deaths: Final Data for 2005. Natl Vital Stat Rep. 2008:56. [PubMed] [Google Scholar]
- 2.Kahn A, Sawaguchi T, Sawaguchi A, et al. Sudden infant deaths: from epidemiology to physiology. Forensic Sci Int. 2002;130S:S8–S20. doi: 10.1016/s0379-0738(02)00134-2. [DOI] [PubMed] [Google Scholar]
- 3.Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–50. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- 4.Biondo B, Magagnin S, Bruni B, Cazzullo A, Tosi D, Matturri L. Glial and neuronal alterations in the nucleus tractus solitarii of Sudden Infant Death Syndrome victims. Acta Neuropathol. 2004;108:309–18. doi: 10.1007/s00401-004-0895-2. [DOI] [PubMed] [Google Scholar]
- 5.Paterson DS, Trachtenberg FL, Thompson EG, et al. Multiple serotonergic brainstem abnormalities in Sudden Infant Death Syndrome. JAMA. 2006;296:2124–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 6.Horne RSC, Ferens D, Watts A-M, et al. The prone sleeping position impairs arousability in term infants. J Pediatr. 2001;138:811–6. doi: 10.1067/mpd.2001.114475. [DOI] [PubMed] [Google Scholar]
- 7.Bhat RY, Hannam S, Pressler R, Rafferty GF, Peacock JL, Greenough A. Effect of prone and supine position on sleep, apnoeas and arousal in preterm infants. Pediatrics. 2006;118:101–7. doi: 10.1542/peds.2005-1873. [DOI] [PubMed] [Google Scholar]
- 8.Galland BC, Reeves G, Taylor BJ, Bolton DPG. Sleep position, autonomic function, and arousal. Arch Dis Child. 1998;78:189–94. doi: 10.1136/fn.78.3.f189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horne RSC, Ferens D, Watts A-M, et al. Effects of maternal tobacco smoking, sleeping position and sleep state on arousal in healthy term infants. Arch Dis Child Fetal Neonatal Ed. 2002;87:F100–F5. doi: 10.1136/fn.87.2.F100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco P, Groswasser J, Hassid S, Lanquart J, Scaillet S, Kahn A. Prenatal exposure to cigarette smoking is associated with a decrease in arousal in infants. J Pediatr. 1999;135:34–8. doi: 10.1016/s0022-3476(99)70324-0. [DOI] [PubMed] [Google Scholar]
- 11.Chang AB, Wilson SJ, Masters IB, et al. Altered arousal response in infants exposed to cigarette smoke. Arch Dis Child. 2003;88:30–3. doi: 10.1136/adc.88.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lijowska AS, Reed NW, Mertins Chiodini BA, Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J Appl Physiol. 1997;83:219–28. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- 13.McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;83:219–28. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- 14.McNamara F, Lijowska AS, Thach BT. Spontaneous arousal activity in infants during NREM and REM sleep. J Physiol (Lond) 2002;538:263–9. doi: 10.1113/jphysiol.2001.012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Paediatric Work Group on Arousals. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months) J. Sleep Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 16.Kato I, Franco P, Groswasser J, et al. Incomplete Arousal Processes in Infants Who Were Victims of Sudden Death. Am J Respir Crit Care Med. 2003;168:1298–303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 17.Byard RW, Krous HF. Sudden Infant Death Syndrome: Overview and update. Pediatr Dev Pathol. 2003;6:112–27. doi: 10.1007/s10024-002-0205-8. [DOI] [PubMed] [Google Scholar]
- 18.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the Sudden Infant Death Syndrome. Biol Neonate. 1994;65:194–7. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 19.Richardson HL, Walker AM, Horne RSC. Sleep position alters arousal processes maximally at the high-risk age for sudden infant death syndrome. J Sleep Res. 2009;17:450–7. doi: 10.1111/j.1365-2869.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- 20.Anders T, Emde R, Parmelee A. Los Angeles, CA: BRI Publications; 1971. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. [Google Scholar]
- 21.Bland JM, Altman DG. Statistics Notes: The odds ratio. BMJ. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–23. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell EA, Milerad J. Smoking and the sudden infant death syndrome. Rev Environ Health. 2006;21:81–103. doi: 10.1515/reveh.2006.21.2.81. [DOI] [PubMed] [Google Scholar]
- 24.Matturri L, Ottaviani G, Lavezzi AM. Maternal smoking and sudden infant death syndrome: epidemiological study related to pathology. Virchows Arch. 2006;449:697–706. doi: 10.1007/s00428-006-0308-0. [DOI] [PubMed] [Google Scholar]
- 25.Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr. 1995;127:691–9. doi: 10.1016/s0022-3476(95)70155-9. [DOI] [PubMed] [Google Scholar]
- 26.Harper RM. Impaired arousals and sudden infant death syndrome. Am J Respir Crit Care Med. 2003;168:1262–3. doi: 10.1164/rccm.2309010. [DOI] [PubMed] [Google Scholar]
- 27.Duncan JR, Randall LL, Belliveau RA, et al. The effect of maternal smoking and drinking during pregnancy upon 3H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain Pathol. 2008;18:21–31. doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachmanoff DB, Panigrahy A, Filiano JJ, et al. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1998;57:1018–25. doi: 10.1097/00005072-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Duncan JR, Paterson DS, Kinney HC. The development of nicotinic receptors in the human medulla oblongata: Inter-relationship with the serotonergic system. Auton Neurosci Basic Clin. 2008 doi: 10.1016/j.autneu.2008.09.006. doi: 10.1016/j.autneu.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricceri L, Minghetti L, Moles A, et al. Cognitive and neurological deficits induced by early and prolonged basal forebrain cholinergic hypofunction in rats. Exp Neurol. 2004;189:162–72. doi: 10.1016/j.expneurol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Falk L, Nordberg A, Seiger A, Kjaeldgaard A, Hellstrom-Lindahl E. Smoking during early pregnancy affects the expression pattern of both nicotinic and muscarinic acetylcholine receptors in human first trimester brainstem and cerebellum. Neuroscience. 2005;132:389–97. doi: 10.1016/j.neuroscience.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 32.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–45. [PubMed] [Google Scholar]
- 33.Lavezzi AM, Ottaviani G, Mingrone R, Matturri L. Analysis of the human locus coeruleus in perinatal and infant sudden unexplained deaths. Possible role of the cigarette smoking in the development of this nucleus. Dev Brain Res. 2005;154:71–80. doi: 10.1016/j.devbrainres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Kinney HC, O'Donnell TJ, Kriger P, Frost White W. Early developmental changes in [3H]nicotine binding in the human brainstem. Neuroscience. 1993;55:1127–38. doi: 10.1016/0306-4522(93)90326-b. [DOI] [PubMed] [Google Scholar]
- 35.Cohen G, Roux J, Malcolm G, Changeux J. Perinatal exposure to nicotine causes deficits associated with a loss of nicotinic receptor function. Proc Natl Acad Sci USA. 2005;102:3817–21. doi: 10.1073/pnas.0409782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell EA, Ford RPK, Stewart AW, et al. Smoking and the sudden infant death syndrome. Pediatrics. 1993;91:893–6. [PubMed] [Google Scholar]
- 37.Storm H, Nylander G, Saugstad OL. The amount of brainstem gliosis in sudden infant death syndrome (SIDS) victims correlates with maternal cigarette smoking during pregnancy. Acta Paediatr. 1999;88:13–8. [PubMed] [Google Scholar]
- 38.Stephan E, Cardot V, Leke A, et al. Sleep structure modifications in premature neonates exposed to maternal smoking in utero. Sleep Biol Rhythms. 2007;5:A73. [Google Scholar]
- 39.Andres RL, Day M. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5:231–41. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]