Abstract
Study Objectives:
Sleep breathing disorders may trigger paroxysmal events during sleep such as parasomnias and may exacerbate preexisting seizures. We verified the hypothesis that the amount of EEG paroxysmal activity (PA) may be high in children with obstructive sleep apnea syndrome (OSAS).
Design:
Prospective study
Settings:
Sleep unit of an academic center.
Participants:
Polysomnographic studies were performed in a population of children recruited prospectively, for suspected OSAS, from January to December 2007, with no previous history of epileptic seizures or any other medical conditions. All sleep studies included ≥ 8 EEG channels, including centrotemporal leads. We collected data about clinical and respiratory parameters of children with OSAS and with primary snoring, then we performed sleep microstructure analysis in 2 OSAS subgroups, matched for age and sex, with and without paroxysmal activity.
Measurements and Results:
We found 40 children who met the criteria for primary snoring, none of them showed PA, while 127 children met the criteria for OSAS and 18 of them (14.2%) showed PA. Children with PA were older, had a predominance of boys, a longer duration of OSAS, and a lower percentage of adenotonsillar hypertrophy than children without PA. Moreover, PA occurred over the centrotemporal regions in 9 cases, over temporal-occipital regions in 5, and over frontocentral regions in 4. Children with PA showed a lower percentage of REM sleep, a lower CAP rate and lower A1 index during slow wave sleep, and lower total A2 and arousal index than children without EEG abnormalities.
Conclusions:
We found a higher percentage of paroxysmal activity in children with OSAS, compared to children with primary snoring, who did not exhibit EEG abnormalities. The children with paroxysmal activity have peculiar clinical and sleep microstructure characteristics that may have implications in the neurocognitive outcome of OSAS.
Citation:
Miano S; Paolino MC; Peraita-Adrados R; Montesano M; Barberi S; Villa MP. Prevalence of EEG paroxysmal activity in a population of children with obstructive sleep apnea syndrome. SLEEP 2009;32(4):522-529.
Keywords: Paroxysmal activity, obstructive sleep apnea syndrome, interictal discharges, cyclic alternating pattern, children
SLEEP BREATHING DISORDERS (SBD) MAY TRIGGER PAROXYSMAL EVENTS DURING SLEEP SUCH AS PARASOMNIAS AND MAY EXACERBATE PREEXISTING seizures. Treatment of SBD can eliminate or reduce these events in adults and children.1–4
In a series of adolescents and adults the improvement of SBD after ventilatory therapy was associated to a reduction in interictal epileptic discharges (IEDs); even if the mechanism is still unclear, sleep stability seems to play an important role.1 SBD is commonly reported in children with epilepsy,5 and treatment of SBD may reduce seizure frequency and improve daytime sleepiness.4,6–12 These studies have focused on the role of sleep respiratory events as trigger factors for seizure or interictal discharges in subjects with epilepsy. There are no reports of IEDs or seizures activated by SBD in subjects without a history of epileptic seizures. Recently we reported IEDs in 5 children clinically diagnosed with OSA. Video-polysomnographic (video-PSG) recordings revealed partial motor nocturnal epileptic seizures associated with UARS.13
IEDs are usually activated during NREM sleep and suppressed during REM sleep.14–18 The activating properties of NREM sleep depend on the synchronizing functions of the thalamocortical oscillations.19,20 The strong association between NREM sleep and IEDs may be one mechanism explaining the impact of IEDs on learning and memory and on daytime behavior, although this area needs further investigation.21 Recently, PSG recordings revealed an epileptiform activity during sleep in 1.45% of otherwise healthy children. Most of epileptiform patterns were found in the centrotemporal regions and were associated with suboptimal cognitive and behavioral function.22
Many studies have provided data on the relationship between IEDs during sleep (particularly centrotemporal or rolandic spikes) and neuropsychological dysfunction in children with language disorders, autism, and ADHD.16,23–26 Furthermore, the neuropsychological assessment in children affected by benign epilepsy of childhood with centrotemporal or rolandic spikes (BECRS) and IEDs during sleep revealed disorders in visuospatial short-term memory, attention span, cognitive flexibility, verbal fluency, phonological awareness, visuoperceptual skills,27–30 and academic performance,30 with a significant improvement after the remission of IEDs.28
Many children with SBD show neurobehavioral complications. Intermittent hypoxemia and subtle changes in sleep architecture might be implicated in the pathogenesis of neurocognitive deficits in pediatric OSAS.31 The neurocognitive profile of children with OSAS shows some similarities with that found in children with IEDs with or without epileptic seizures. Since sleep respiratory events could trigger IEDs during sleep, we expected to find a high prevalence of EEG paroxysmal activity (PA) in children with OSAS, disclosed by means of PSG (with extended EEG leads). Thus, the aims of our study were: (1) to assess the presence of PA in a group of children undergoing a diagnostic assessment for OSAS, without a history of epilepsy, and (2) to compare children with PA with children who had OSAS and normal EEG patterns. We hypothesized there would be differences in clinical and polysomnographic features between these 2 groups.
MATERIALS AND METHODS
We enrolled consecutive children undergoing a diagnostic assessment for OSAS for the first time in our Pediatric Sleep Centre (Rome, Italy). The diagnosis of OSAS was confirmed by laboratory PSG showing obstructive apnea/hypopnea index > 1, following the criteria of the American Academy of Sleep Medicine.32 Primary snoring was diagnosed in children with habitual snoring and apnea/hypopnea index < 1. Children were enrolled between January 2007 and December 2007. Patients with a history of epilepsy or with a history of previous treatment of OSAS (including tonsillectomy and adenoidectomy), acute or chronic cardiorespiratory or neuromuscular diseases, dysmorphism, major craniofacial abnormalities, or associated chromosomal syndromes, were excluded.
Subjects
A detailed personal and family history was obtained for all participants, together with a general clinical examination. Parents of participants completed the Brouillette questionnaire on symptoms of OSAS.33 Questions included those about sleep, breathing during sleep, medical diagnoses, and previous surgery, including adenotonsillectomy. Likelihood ratios for OSAS scores less than −1 predicted no OSAS, from −1 to 3.5 were considered inconclusive, and > 3.5 predicted the presence of OSAS.
All children underwent ENT examination to grade tonsillar hypertrophy according to a standardized scale ranging from 0 to 4.34 Adenoid hypertrophy was graded according to Greenfeld et al.35 All children underwent orthodontic assessment to detect possible jaw deviation from normal occlusion—deep bite, retrusive bite, and cross-bite.
The children underwent PSG in our sleep center after one adaptation night. The local ethics committee approved the study protocol and all children's parents gave their informed consent to the procedures.
Polysomnography and Sleep Stage Scoring
Standard overnight PSG recordings were obtained with a Grass Heritage polygraph. Variables recorded included at least an 8-channel electroencephalogram (EEG) (bilateral frontal, central, temporal and occipital, bipolar and monopolar montages referred to the contralateral mastoid; see figures); electrooculogram (electrodes placed 1 cm above the right outer canthus and 1 cm below the left outer canthus and referred to A1), submental electromyogram, and electrocardiogram (ECG) (1 derivation).
Sleep was subdivided into 30-s epochs, and sleep stages were scored according to standard criteria by Rechtschaffen and Kales.36 All the following sleep architecture parameters were evaluated: time in bed (TIB); total sleep time (TST = time from sleep onset to the end of the final sleep epoch minus time awake); sleep period time (SPT = time from sleep onset to sleep end); sleep efficiency (defined as the percentage ratio between TST and TIB), sleep onset latency (time from lights out to sleep onset, which was further defined as the first of 2 consecutive epochs of stage 1 sleep or 1 epoch of any other stage, in minutes); REM latency (time from sleep onset to the first epoch of REM sleep), wakefulness after sleep onset (time spent awake between sleep onset and end of sleep); total duration and percentage of stage 1, stage 2, stage 3, stage 4 NREM sleep, and REM sleep; and amount of movement time per hour.
Sleep scoring was performed by one of the investigators (SM) blinded to the children's group, age, and sex.
CAP Scoring
CAP was scored following the criteria by Terzano et al.37 CAP is a periodic EEG activity in NREM sleep characterized by repeated spontaneous sequences of transient events (phase A) which clearly break away from the background rhythm of ongoing sleep stage, with an abrupt frequency/amplitude variation, recurring at intervals up to 1 min long. The return to the background activity identifies the interval that separates the repetitive elements (phase B). CAP A phases have been subdivided into 3 subtypes:
A1: A phases with synchronized EEG patterns (intermittent alpha rhythm in stage 1; sequences of K complexes or delta bursts in the other NREM stages), associated with mild or trivial polygraphic variations;
A2: A phases with desynchronized EEG patterns preceded by or mixed with slow high-voltage waves (K complexes with alpha and beta activities, K-alpha, arousals with slow wave synchronization), linked with a moderate increase of muscle tone and/or cardiorespiratory rate;
A3: A phases with desynchronized EEG patterns alone (transient activation phases or arousals) or exceeding two-thirds of the phase A length, and coupled with a remarkable enhancement of muscle tone and/or cardiorespiratory rate.
The following CAP parameters were measured: CAP time (sum of the length of all CAP sequences, in minutes) in NREM sleep; CAP rate (percentage of total NREM sleep time occupied by CAP sequences); number and mean duration of CAP cycles (phase A + phase B); number and mean duration of CAP sequences; number, mean duration and percentage of A phases (including the phase A subtypes); A1, A2, and A3 index (number of phases A1, A2, or A3 per hour of NREM sleep, and per hour of sleep stages 1 and 2 NREM and of slow wave sleep, respectively); and number and mean duration of B phases. We defined as A1 phases as bursts of paroxysmal sharp waves and spike-and-wave complexes on EEG.
All these variables were visually detected and their parameters measured by means of the Hypnolab 1.0 sleep software analysis (SWS Soft, Italy). All recordings were visually scored by one of the investigators (SM), blinded to the subject's group, age, and sex; the CAP parameters derived were tabulated for subsequent statistical analysis.
Arousal Analysis
Arousals were visually detected following the criteria reported in the recent manual for the scoring of sleep and associated events by the American Academy of Sleep Medicine.38
Paroxysmal Activity
The EEG was reviewed by one of the authors (SM), who was blinded to clinical information about the cases at 10 mm/s of screen resolution. The presence of spikes (transient, clearly distinguishable from background activity lasting 20–70 ms) and sharp waves (same as spike, but lasting 70–200 ms), either alone or accompanied by slow waves (the slow wave being of higher amplitude than the spike or the sharp wave), occurring isolated or in bursts, was considered to represent PA, according to definitions of the International Federation of Societies for Clinical Neurophysiology.39 To consider a subject as having PA, ≥ 10 spikes and/or sharp waves had to be present; however, all the subjects included in the group with PA had significantly higher numbers of PA in this study.
Sleep Respiration Analysis
Central, obstructive, and mixed apnea events were counted according to the criteria established by the American Academy of Sleep Medicine.38 An obstructive apnea was scored when there was > 90% drop in the signal amplitude of airflow for > 90% of the event, compared with the pre-event baseline amplitude, with continued chest wall and abdominal movement, for a duration ≥ 2 breaths. A central apnea was defined as the absence of airflow, with the cessation of respiratory effort, lasting > 20 sec or ≥ 2 missed breaths (or the duration of 2 baseline breaths) and is associated with an arousal, an awakening, or > 3% desaturation; central apnea occurring after gross body movements or after sighs was not considered as a pathologic finding. A mixed apnea was defined as an apnea that usually began as central and ended in obstruction, according to changes in the chest, abdominal, and flow traces. An event was scored as a hypopnea if there was > 50% drop in airflow signal amplitude compared with the pre-event baseline amplitude for ≥ 90% of the event; the event must last ≥ 2 missed breaths and should be associated with an arousal, awakening, or > 3% desaturation. Chest and abdominal movements were measured by strain gauges. Oronasal airflow was recorded with a thermocouple (or nasal pressure monitor when children tolerated a nasal cannula). Arterial oxygen saturation was monitored with a pulse oximeter. The apnea-hypopnea index (AHI) was defined as the average number of apneas and hypopneas per hour of sleep.
Leg movements (LMs) during sleep were defined as an activation of the tibialis anterior muscles lasting between 0.5 and 5 s, with an amplitude > 25% of the EMG amplitude at maximal flexion of the foot, recorded during pre-sleep test period. PLMs were identified as sequences of ≥ 4 or more LMs, separated by ≥ 5 s and ≤ 90 s, according to the American Academy of Sleep Medicine criteria.38 A PLM index (number of PLMs per hour of sleep) > 5 was considered as clinically significant.40
All recordings started at the patients' usual bedtime and continued until spontaneous awakening.
Study Design
On the basis of the presence of OSAS, primary snoring, and PA, we defined 3 subject groups. Group 0 was formed by subjects with primary snoring without PA (no children with primary snoring were found with PA). Group 1 comprised children with OSAS without PA, and Group 2 included children with OSAS and PA. These groups were then compared for their anthropometric and clinical characteristics and respiratory sleep parameters.
Because sleep scoring and evaluation are time-consuming and the groups were heterogeneous in age and sex distribution, only sleep architecture and microstructure (CAP and arousals) obtained in 2 age- and sex-matched subgroups selected from Groups 1 and 2 (Group A and Group B, respectively) were compared. There were fewer patients in these subgroups, thus allowing comprehensive analysis of patients with PA.
Statistical Analysis
Data are expressed as means ± SD. Analysis of variance (ANOVA) followed by Bonferroni post hoc comparisons, Mann-Whitney U, or χ2 test were chosen, when appropriate, to compare data; P values less than 0.05 were considered as statistically significant.
RESULTS
Study Population
In total, we found 40/167 (24%) children who met the criteria for primary snoring (Group 0) and 127/167 (76%) children who met the criteria for OSAS. Among those with primary snoring, none showed PA. Among children with a diagnosis of OSAS, 109/127 (85.8%) (Group 1) did not show PA, whereas 18/127 (Group 2) (14.2%) showed PA. The frequency of PA in primary snoring and in children with OSAS was significantly different (χ2 = 4.9, P < 0.05). Table 1 shows the clinical and anthropometric parameters of all groups, and Table 2 shows respiratory features. The nasal cannula was well tolerated in children older than 8 years; a thermocouple was used in children who did not tolerate the nasal cannula. No subject with PA reported family history of epilepsy.
Table 1.
Anthropometric and Clinical Characteristics in Children with Primary Snoring (Group 0), Children with OSAS without Paroxysmal Activity (Group 1), and Children with OSAS and Paroxysmal Activity (Group 2)
| Group 0 (n 40) | Group 1 (n 109) | Group 2 (n 18) | ANOVA, P < | Bonferroni, P < |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | 0 vs 1 | 0 vs 2 | 1 vs 2 | ||
| Age (years) | 6.7 | 3.4 | 5.0 | 2.5 | 8.4 | 3.8 | 0.001 | 0.005 | NS | 0.000 |
| BMI (kg/m2) | 19.4 | 5.5 | 17.2 | 3.0 | 18.7 | 3.6 | 0.006 | 0.007 | NS | NS |
| Duration of disease (years) | 3.1 | 2.6 | 2.6 | 1.7 | 4.1 | 3.3 | 0.03 | NS | NS | 0.03 |
| Brouillette score | −0.9 | 1.8 | −0.4 | 1.6 | −0.8 | 1.9 | NS | |||
| χ2(P<) | ||||||||||
| n | % | n | % | N | % | 0 vs 1 | 0 vs 2 | 1 vs 2 | ||
| Sex (male) | 22 | 55 | 68 | 62.4 | 15 | 83.3 | NS | NS | NS | |
| Tonsillar hypertrophy | 6 | 15 | 69 | 63.3 | 3 | 16.7 | 25.4 (0.001) | NS | 11.8 (0.001) | |
| Narrow palate | 23 | 57.5 | 61 | 56 | 9 | 50 | NS | NS | NS | |
| Crossbite | 8 | 20 | 19 | 17.4 | 5 | 27.8 | NS | NS | NS | |
| Open bite | 7 | 17.5 | 20 | 18.3 | 4 | 22.2 | NS | NS | NS | |
| Angle Class II-III | 14 | 35 | 43 | 39.6 | 6 | 33.4 | NS | NS | NS | |
| Allergic test positivity | 8 | 20 | 29 | 26.6 | 5 | 27.8 | NS | NS | NS | |
| Respiratory allergy | 8 | 20 | 28 | 25.7 | 5 | 27.8 | NS | NS | NS | |
| Food allergy | - | - | 3 | 2.8 | - | - | NS | NS | NS | |
| Perinatal injuries | 6 | 15 | 12 | 11 | 5 | 27.8 | NS | NS | NS | |
| Born preterm | 4 | 10 | 9 | 8.3 | 3 | 16.7 | NS | NS | NS | |
| Psychomotor delay | 2 | 5 | 10 | 9.2 | 2 | 11.1 | NS | NS | NS | |
| Language disorders | 2 | 5 | 6 | 5.5 | - | - | NS | NS | NS | |
Table 2.
Respiratory Parameters in Children with Primary Snoring (Group 0), Children with OSAS without Paroxysmal Activity (Group 1), and Children with OSAS and Paroxysmal Activity (Group 2)
| Group 0 (n 40) |
Group 1 (n 109) |
Group2 (n 18) |
ANOVA | Bonferroni, P < |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P < | 0 vs 1 | 0 vs 2 | 1 vs 2 | |
| AHI (events/h) | 0.3 | 0.3 | 7.3 | 6.5 | 8.7 | 14.0 | 0.001 | 0.001 | 0.001 | NS |
| SpO2 (%) | 97.6 | 1.4 | 97.2 | 1.3 | 96.7 | 1.8 | NS | |||
| SpO2 desaturation (%) | 94.2 | 2.4 | 92.6 | 1.9 | 92.3 | 2.3 | 0.01 | 0.01 | NS | NS |
| SpO2 nadir (%) | 92.9 | 3.2 | 88.6 | 5.0 | 87.2 | 5.6 | 0.003 | 0.003 | NS | NS |
AHI, apnea-hypopnea index; SpO2 oxygen saturation by pulse oximetry
There were many differences between Groups 1 and 2: children belonging to Group 2 were older and had a longer OSAS duration than those of Group 1. A higher percentage of adenotonsillar hypertrophy was found in Group 1 than in the other 2 groups.
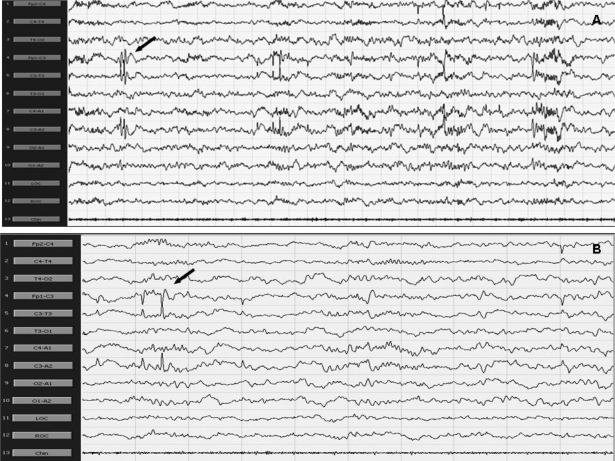
PA was more prominent during NREM sleep and occurred rarely during REM sleep. PA was mostly represented by runs of sharp waves. These appeared over the occipital areas in the youngest children and involved the centrotemporal regions with increasing age; they were most often evident over the frontocentral regions in the oldest children in our group. In particular, PA occurred mostly over the centrotemporal regions in 9 children (Figure 1), over the temporal-occipital regions in 6 children, and in 4 over the frontocentral regions.
Figure 1.
Polysomnographic recording showing the presence of sharp waves over the left centrotemporal and rolandic regions; 30-s epochs (A) and 10-s epochs (B) 300 μV. Arrows point to examples of paroxysmal activity.
Comparison Between Groups A and B
Group B comprised 14 subjects. Four children from Group 2 were excluded from this analysis because of EEG artifact in their recordings that might have interfered with CAP analysis; another subject was excluded because he was 17.6 years old and no one had a comparable age in the other group. Subsequently, 14 children with OSAS and without PA (Group A) were age- and sex-matched with Group B.
Sleep architecture, respiratory, and limb parameters showed only a lower percentage of REM sleep in Group B compared to Group A, as shown in Table 3. However, sleep microstructure analysis (Table 4) disclosed many differences between these 2 Groups: Group B showed a lower CAP percentage during SWS, lower A1 index during SWS, lower A2 index during total NREM sleep, and finally a lower arousal index than Group A.
Table 3.
Polysomnographic Parameters in OSAS Children without Paroxysmal Activity (Group A) Compared to OSAS Children with Paroxysmal Activity (Group B) Matched for Age and Sex
| Group A (n = 14, 11 M) |
Group B (n = 14, 11 M) |
Mann-Whitney | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | P < | |
| Age (years) | 7.7 | 3.0 | 8.4 | 3.3 | NS |
| BMI (kg/m2) | 20.3 | 6.5 | 19.3 | 4.3 | NS |
| Macrostructure parameters | |||||
| TIB, min | 476.28 | 39.3 | 426.50 | 112.0 | NS |
| SPT, min | 444.39 | 35.8 | 402.70 | 109.3 | NS |
| TST, min | 422.82 | 44.5 | 379.32 | 65.8 | NS |
| SOL, min | 23.39 | 20.5 | 24.07 | 5.5 | NS |
| FRL, min | 109.32 | 32.5 | 111.80 | 79.0 | NS |
| SS/h | 8.08 | 2.4 | 13.66 | 3.3 | NS |
| AWN/ h | 1.16 | 0.9 | 1.15 | 0.7 | NS |
| MT/h | 0.70 | 0.6 | 0.58 | 0.4 | NS |
| SE % | 89.1 | 9.2 | 88.1 | 6.1 | NS |
| REM n/h | 7.64 | 2.2 | 6.14 | 2.3 | NS |
| WASO % | 4.99 | 4.2 | 6.45 | 6.1 | NS |
| S1 % | 2.47 | 1.9 | 2.07 | 1.6 | NS |
| S2 % | 41.97 | 6.2 | 44.75 | 4.4 | NS |
| SWS % | 31.32 | 6.6 | 31.57 | 7.1 | NS |
| REM % | 19.21 | 3.6 | 15.14 | 5.6 | 0.03 |
| Respiratory and LM parameters | |||||
| AHI (h) | 2.9 | 4.7 | 8.9 | 16.0 | NS |
| SpO2 (%) | 97.3 | 1.2 | 96.7 | 2.0 | NS |
| SpO2 desaturation (%) | 93.3 | 2.8 | 90.3 | 2.9 | NS |
| SpO2 nadir (%) | 91.4 | 4.2 | 87.1 | 4.6 | NS |
| PLM index | 5.63 | 3.9 | 12.81 | 14.6 | NS |
Abbreviations: AHI, apnea-hypopnea index; TIB, time in bed; SPT, sleep period time; TST, total sleep time; SOL, sleep onset latency; FRL, first REM sleep latency; SS/h = stage shifts per hour; AWN/h, awakenings per hour; MT/h, movement time per hour; WASO, wakefulness after sleep onset; SE, sleep efficiency; S1, S2, sleep stages 1 and 2; SWS, slow-wave sleep; SpO2 = oxygen saturation by pulse oximetry; PLM: periodic limb movements.
Table 4.
Microstructure Parameters in OSAS Children without Paroxysmal Activity (Group A) Compared to OSAS Children with Paroxysmal Activity (Group B) Matched for Age and Sex
| Group A (n = 14) |
Group B (n = 14) |
Mann-Whitney | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | P < | |
| CAP Rate % total NREM | 56.63 | 14.12 | 48.53 | 19.1 | NS |
| CAP rate % S1 | 23.77 | 16.6 | 16.62 | 16.2 | NS |
| CAP rate % S2 | 41.17 | 15.3 | 36.78 | 23.8 | NS |
| CAP rate % SWS | 81.80 | 13.2 | 68.45 | 14.7 | 0.02 |
| A1 % | 69.02 | 7.8 | 73.56 | 10.4 | NS |
| A2 % | 23.35 | 6.9 | 18.13 | 7.2 | NS |
| A3 % | 7.60 | 2.9 | 8.28 | 3.9 | NS |
| A1 index (h) | 62.75 | 22.5 | 53.20 | 2.4 | NS |
| A1 index, S1 | 6.63 | 13.0 | 2.75 | 4.3 | NS |
| A1 index, S2 | 33.11 | 13.9 | 34.88 | 21.0 | NS |
| A1 index, SWS | 116.15 | 34.0 | 93.04 | 22.7 | 0.04 |
| A2 index (h) | 20.72 | 9.2 | 12.35 | 7.4 | 0.01 |
| A2 index, S1 | 22.40 | 15.9 | 13.10 | 12.4 | NS |
| A2 index, S2 | 26.73 | 12.9 | 16.87 | 9.9 | 0.03 |
| A2 index, SWS | 17.99 | 10.5 | 10.16 | 5.4 | 0.02 |
| A3 index (h) | 5.41 | 1.8 | 4.67 | 3.4 | NS |
| A3 index, S1 | 13.99 | 14.8 | 15.98 | 18.9 | NS |
| A3 index, S2 | 9.63 | 3.5 | 8.62 | 5.5 | NS |
| A3 index, SWS | 2.36 | 1.3 | 2.01 | 0.7 | NS |
| A mean duration, s | 8.41 | 1.1 | 8.28 | 1.7 | NS |
| B mean duration, s | 17.11 | 3.4 | 19.33 | 2.9 | NS |
| CAP S1 duration, s | 26.52 | 12.7 | 18.48 | 21.1 | NS |
| CAP S2 duration, s | 28.99 | 2.9 | 29.58 | 2.6 | NS |
| CAP SWS duration, s | 23.84 | 3.8 | 26.39 | 2.7 | 0.05 |
| Sequence mean duration, s | 385.84 | 178.0 | 371.09 | 361.13 | NS |
| Number of sequences | 32.78 | 8.7 | 32.0 | 10.4 | NS |
| Cycle sequence | 17.07 | 9.6 | 14.66 | 13.3 | NS |
| Arousal index (h) | 25.29 | 8.1 | 18.04 | 8.6 | 0.03 |
DISCUSSION
This study represents, to the best of our knowledge, the first report of the presence of paroxysmal activity in PSG recordings of children with OSAS. Even if we did not compare our data with those of a group of healthy children, we found a higher percentage of PA in children with OSAS (14.2%) than in those with primary snoring (0%). Recently, it has been reported that 1.43% of healthy children with epileptiform activity can be found by PSG;22 we might assume that the frequency of PA in our OSAS children is high also as compared to that expected in healthy children.
Interestingly, children with PA and OSAS showed peculiar clinical features compared to those without PA: they were older, with a longer duration of the disease, and showed a lower percentage of adenotonsillar hypertrophy. Benign focal spikes in childhood are a well-known age-related phenomenon and we cannot exclude that some of the younger children with OSAS or primary snoring will develop PA in the future. A longitudinal study would be able to clarify this point. Despite the lack of statistical significance, subjects with PA had a higher occurrence of perinatal injuries (see Table 1). These findings suggest the possibility that a primary brain insult may predispose to both OSAS and the paroxysmal EEG activity in at least some of these subjects. Moreover, even if the number of EEG channels included in our study can be viewed as high from a standard PSG point of view, it is certainly low from an epileptological point of view and, taken into account the often focal nature of PA at this age, we can expect an even higher frequency of these abnormalities if a higher number of leads is used. In our study, PA mostly occurred over the centrotemporal regions, suggesting some similarities with the IEDs of the benign epilepsy with central-temporal spikes (BECTS).
The occurrence of PA during sleep in children might be associated with cognitive and behavioral dysfunction in children with IEDs during sleep (BECTS, ADHD, autism, and language disorders).16,24–27 Recently, it has been questioned whether benign rolandic epilepsy is indeed “benign” or whether long-term cognitive outcome may be adversely affected,41 taking into account that IEDs may disrupt cognitive abilities and impair learning and memory.21 Our findings may represent a new possibility to explain the neurocognitive dysfunction in children with OSAS; this will require further evaluation in future studies.
A model of prefrontal cortical process disruption has been proposed to clarify as OSAS causes impairment in daytime cognitive and behavioral functioning: the prefrontal regions of the brain cortex are the last areas of the brain to mature and the most susceptible to OSAS injury. The same PFC regions generate the slow oscillations during NREM sleep, mostly represented by the A subtypes of CAP, particularly the A1 events.42–45
The interictal epileptiform activity is produced by the synchronizing action of the thalamocortical oscillations.19,20 The high prevalence of PA in children with OSAS might represent another sign of prefrontal cortical dysfunction: PA could represent a sort of shift of brain activity during sleep, in particular during NREM sleep, with a predominance of thalamocortical over prefrontal activity. This hypothesis is also corroborated by our CAP analysis findings: a lower CAP rate and A1 index during SWS, and a lower A2 index during total NREM sleep, found in children with PA with respect to children without PA.
These children may have a dysfunction of the arousal system, since they show also a lower arousal index. At this time, we are not able to explain this phenomenon; it may be related to longer duration of disease, with a sort of habituation of the arousal system to the respiratory events.
We found similar CAP features in children with ADHD46 and in another group of children with OSAS,47 but in the latter study we did not check for the presence of sleep EEG abnormalities.
Parrino et al.48 found that, in primary generalized epilepsy and in lesional epilepsies with frontotemporal focus, a clear activation of interictal discharges occurred during CAP, particularly during phase A, with the strongest inhibition during phase B. Conversely, benign rolandic spikes are not modulated by the activity of A1 subtypes of CAP. Since PA found in our group was mostly represented over the centrotemporal regions and temporal regions, the relatively low CAP rate we found indirectly confirmed the results by Parrino et al.48 and confirmed that the PA of our population might be similar to that observed in benign partial epilepsy.
Despite the limitations of our study, we believe that we were able to add clues to the understanding of the neurobehavioral dysfunction of this syndrome, because the presence of PA in OSAS has never been demonstrated before. Recently we reported IEDs in 5 children clinically diagnosed as having OSA and their video-PSG recordings revealed the presence of nocturnal epileptic seizures associated with UARS.13 If confirmed, our results justify the use of a higher number of EEG channels for the detection of paroxysmal activity during routine PSG in children with sleep breathing disorders.
At this time we are not able to confirm if some of our children with OSAS and PA will develop seizures and if the treatment of OSAS will directly influence the rate of PA, thus improving prognosis. All of these issues need to be clarified in future studies.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Oliveira AJ, Zamagni M, Dolso P, Bassetti MA, Gigli GL. Respiratory disorders during sleep in patients with epilepsy: effect of ventilatory therapy on EEG interictal epileptiform discharges. Clin Neurophysiol. 2000;111:141–5. doi: 10.1016/s1388-2457(00)00415-6. [DOI] [PubMed] [Google Scholar]
- 2.Guilleminault C, Palombini L, Pelayo R, Chervin RD. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003;111:17–25. doi: 10.1542/peds.111.1.e17. [DOI] [PubMed] [Google Scholar]
- 3.Guilleminault C, Kirisoglu C, Bao G, Arias V, Chan A, Li KK. Adult chronic sleepwalking and its treatment based on polysomnography. Brain. 2005;128:1062–9. doi: 10.1093/brain/awh481. [DOI] [PubMed] [Google Scholar]
- 4.Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4:509–15. doi: 10.1016/j.sleep.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Becker DA, Fennell EB, Carney PR. Sleep disturbance in children with epilepsy. Epilepsy Behav. 2003;4:651–8. doi: 10.1016/j.yebeh.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Whiler A, Weymuller EA. Epilepsy complicated by sleep apnea. Ann Neurol. 1981;9:403–4. doi: 10.1002/ana.410090415. [DOI] [PubMed] [Google Scholar]
- 7.Tirosh E, Tal Y, Jaffe M. CPAP treatment of obstructive sleep apnea and neurodevelopmental deficits. Acta Paediatr. 1995;84:791–4. doi: 10.1111/j.1651-2227.1995.tb13758.x. [DOI] [PubMed] [Google Scholar]
- 8.Ezpeleta D, García Peña A, Peraita-Adrados R. Epilepsy and sleep apnea syndrome. Rev Neurol. 1998;26:389–92. [PubMed] [Google Scholar]
- 9.Kohl S, Ward SL, Lin M, Chen LS. Sleep apnea treatment improves seizure control in children with neurodevelopmental disorders. Pediatr Neurol. 2000;22:36–9. doi: 10.1016/s0887-8994(99)00114-9. [DOI] [PubMed] [Google Scholar]
- 10.Bazil C. Sleep and epilepsy: something else we did not know. Epilepsy Curr. 2003;3:48–49. doi: 10.1046/j.1535-7597.2003.03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughn BV, D'Cruz OF. Sleep and epilepsy. Semin Neurol. 2004;24:301–13. doi: 10.1055/s-2004-835068. [DOI] [PubMed] [Google Scholar]
- 12.Malow BA. The interaction between sleep and epilepsy. Epilepsia. 2007;48(Suppl 9):36–8. doi: 10.1111/j.1528-1167.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 13.Peraita-Adrados R, Bachiller C, Gutierrez M, Salcedo A, Miano S. Central-temporal and rolandic spikes associated with OSA/UARS in children: a clinical overlap of diurnal and nocturnal symptoms. J Sleep Res. 2008;17:P169. [Google Scholar]
- 14.Dalla Bernardina B, Colamaria V, Capovilla G, Bondavalli S. Epilepsy, sleep and sleep deprivation. Amsterdam: Elsevier; 1984. Sleep and benign partial epilepsies of childhood; pp. 119–33. [Google Scholar]
- 15.Ferri R, Bergonzi P, Elia M, Ferri P, Musumeci SA. Modulation of the interictal epileptiform EEG activity during sleep: from oscillations to complex dynamics. Neurophysiol Clin. 1991;21:1–14. doi: 10.1016/s0987-7053(05)80349-1. [DOI] [PubMed] [Google Scholar]
- 16.Malow BA. Sleep disorders, epilepsy, and autism. Ment Retard Dev Disabil Res Rev. 2004;10:122–5. doi: 10.1002/mrdd.20023. [DOI] [PubMed] [Google Scholar]
- 17.Tassinari CA, Rubboli G. Cognition and paroxysmal EEG activities: from a single spike to electrical status epilepticus during sleep. Epilepsia. 2006;47:40–3. doi: 10.1111/j.1528-1167.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 18.Kotagal P, Yardi N. The relationship between sleep and epilepsy. Semin Pediatr Neurol. 2008;15:42–9. doi: 10.1016/j.spen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Steriade M, Contreras D, Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 20.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–22. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binnie CD. Cognitive impairment during epileptiform discharges: Is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–30. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- 22.Sans Capdevila O, Dayyat E, Kheirandish-Gozal L, Gozal D. Prevalence of epileptiform activity in healthy children during sleep. Sleep Med. 2008;9:303–309. doi: 10.1016/j.sleep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Ballaban-Gil K, Tuchman R. Epilepsy and epileptiform EEG: association with autism and language disorders. Mental retardation and developmental disabilities research reviews. 2000;6:300–8. doi: 10.1002/1098-2779(2000)6:4<300::AID-MRDD9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Chez MG, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A. Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav. 2006;8:267–71. doi: 10.1016/j.yebeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Holtmann M, Matei A, Hellmann U, Becker K, Poustka F, Schmidt MH. Rolandic spikes increase impulsivity in ADHD – A neuropsychological pilot study Brain Dev. 2006;28:633–40. doi: 10.1016/j.braindev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Silvestri R, Gagliano A, Calarese T, et al. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007;75:130–7. doi: 10.1016/j.eplepsyres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Baglietto MG, Battaglia FM, Nobili L, et al. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes Benign epilepsy. Dev Med Child Neurol. 2001;43:407–12. doi: 10.1017/s0012162201000755. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren A, Kihlgren M, Melin L, Croona C, Lundberg S, Eeg-Olofsson O. Development of cognitive functions in children with rolandic epilepsy. Epilepsy Behav. 2004;5:903–10. doi: 10.1016/j.yebeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Northcott E, Connolly AM, Berroya A, et al. Memory and phonological awareness in children with Benign Rolandic Epilepsy compared to a matched control group. Epilespy Res. 2007;75:57–62. doi: 10.1016/j.eplepsyres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Piccinelli P, Borgatti R, Aldini A, et al. Academic performance in children with rolandic epilepsy. Dev Med Child Neurol. 2008;50:353–6. doi: 10.1111/j.1469-8749.2007.02040.x. [DOI] [PubMed] [Google Scholar]
- 31.Gozal D, Sans Capdevila O, Crabtree VM, Serpero LD, Witcher LA, Kheirandish-Gozal L. Plasma IGF-1 levels and cognitive dysfunction in children with obstructive sleep apnea. Sleep Med. 2008 doi: 10.1016/j.sleep.2008.01.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.International Classification of Sleep Disorders-ICSD. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 33.Brouillette R, Hanson D, David R, et al. A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr. 1984;105:10–4. doi: 10.1016/s0022-3476(84)80348-0. [DOI] [PubMed] [Google Scholar]
- 34.Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope. 2004;114:454–9. doi: 10.1097/00005537-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Greenfeld M, Tauman R, DeRowe A, Sivan Y. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Othorhinolaryngol. 2003;67:1055–60. doi: 10.1016/s0165-5876(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 36.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. A manual of standardized terminology, techniques and scoring system for sleep stage of human subjects. [Google Scholar]
- 37.Terzano MG, Parrino L, Smerieri A, et al. Consensus Report. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2:537–53. doi: 10.1016/s1389-9457(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 38.Iber C, Ancoli-Israel S, Chesson A, Quan SF. for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 39.International Federation of Societies for Clinical Neurophysiology. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37:538–44. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree VM, Ivanenko A, O'Brien LM, Gozal D. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003;12:73–81. doi: 10.1046/j.1365-2869.2003.00332.x. [DOI] [PubMed] [Google Scholar]
- 41.Perkins FF, Jr, Breier J, McManis MH, et al. Benign rolandic epilepsy -- perhaps not so benign: use of magnetic source imaging as a predictor of outcome. Child Neurol. 2008;23:389–93. doi: 10.1177/0883073807309239. [DOI] [PubMed] [Google Scholar]
- 42.Beebe DW, Gozal D. Obstructive sleep apnea and the pre frontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 43.Ferri R, Bruni O, Miano S, Terzano MG. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP) Sleep Med. 2005;6:29–36. doi: 10.1016/j.sleep.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Ferri R, Huber R, Aricò D, et al. The slow-wave components of the cyclic alternating pattern (CAP) have a role in sleep-related learning processes. Neurosci Lett. 2008;432:228–31. doi: 10.1016/j.neulet.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 45.Bruni O, Ferri R. Neurocognitive deficits in pediatric obstructive sleep apnea: A multifaceted pathogenetic model. Sleep Med. 2008 doi: 10.1016/j.sleep.2008.02.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Miano S, Donfrancesco R, Bruni O, et al. NREM sleep instability is reduced in children with attention deficit/hyperactivity disorder. Sleep. 2006;29:797–803. [PubMed] [Google Scholar]
- 47.Kheirandish-Gozal L, Miano S, Bruni O, et al. Reduced NREM sleep instability in children with sleep disordered breathing. Sleep. 2007;30:450–7. doi: 10.1093/sleep/30.4.450. [DOI] [PubMed] [Google Scholar]
- 48.Parrino L, Smerieri A, Terzano MG. Combined influence of cyclic arousability and EEG synchrony on generalized interictal discharges within the sleep cycle. Epilepsy Res. 2001;44:7–18. doi: 10.1016/s0920-1211(00)00192-3. [DOI] [PubMed] [Google Scholar]