Abstract
Study Objectives:
Several lines of evidence suggest immune system derangement in obstructive sleep apnea syndrome (OSAS) patients. However, no data exist on the long-term effect of continuous positive airway pressure (CPAP) treatment on systemic immunity. Hence, we sought to evaluate this effect on various immunological parameters in OSAS patients.
Design:
Prospective case series.
Setting:
Sleep unit of a general hospital.
Patients:
Newly-diagnosed, nonsmoking, otherwise healthy OSAS male patients (n = 52) were evaluated on diagnosis and 6 months after CPAP treatment. According to compliance to CPAP use at 6-month follow-up, they were divided into 2 groups: group A (n = 32): good compliance (mean CPAP use ≥ 4 h/night); and group B (n = 20): poor compliance (mean CPAP use < 4 h/night).
Interventions:
N/A
Measurements and Results:
Blood samples were obtained at baseline and at the 6-month follow-up. Percentage and absolute count of lymphocyte subsets (by flow cytometry), serum TNF-α, IL-6, and uric acid levels were measured. No differences were recorded regarding the baseline anthropometric or sleep characteristics of the 2 groups. In group A, a significant decrease in the absolute count of total lymphocytes (P = 0.003), and of CD4+ cells (P = 0.001), and a decrease in TNF-α levels (P = 0.001) and uric acid levels (P < 0.001) was observed after CPAP application. On the contrary, no alterations occurred in any of the tested parameters in group B patients.
Conclusions:
The selective reduction of soluble and cellular immune response factors only in those OSAS patients who exhibited good compliance to CPAP therapy provides further evidence for an ongoing systemic immune process in OSAS.
Citation:
Steiropoulos P; Kotsianidis I; Nena E; Tsara V; Gounari E; Hatzizisi O; Kyriazis G; Christaki P; Froudarakis M; Bouros D. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. SLEEP 2009;32(4):537-543.
Keywords: Continuous positive airway pressure (CPAP), compliance, obstructive sleep apnea syndrome (OSAS), immune response, lymphocytes, uric acid, tumor necrosis factor-α (TNF-α), interleukin-6
OBSTRUCTIVE SLEEP APNEA SYNDROME (OSAS) HAS BEEN ASSOCIATED WITH A VARIETY OF AUTOIMMUNE DISEASES SUCH AS HASHIMOTO THYROIDITIS,1 adult onset diabetes,2 and myasthenia gravis.3 Although the pathogenetic mechanism underlying this association remains largely unknown, amelioration of OSAS has been found to improve the glycemic control of type 2 diabetes mellitus (DM), implying a direct role for OSAS in the immune deregulation that accompanies type 2 DM.4 Furthermore, the effect of OSAS in the immune system appears to be systemic, encompassing both its soluble and cellular elements. Increased plasma levels of tumor necrosis factor-α (TNF-α), C-reactive protein, and its major regulator, interleukin-6 (IL-6), which is secreted by monocytes in response to hypoxia, have been demonstrated in OSAS patients.5–7 A significant decrease in the levels of these factors has been reported after application of continuous positive airway pressure (CPAP).8–10 Also, the Th1 cytokine pattern in OSAS is further accompanied by functional and numerical alterations of peripheral blood lymphocytes. An activated phenotype is exhibited in CD8+ cells of OSAS patients, which is characterized by overexpression of perforin and TNF-α and excessive cytotoxicity against various cell lines, all of which decrease after CPAP therapy.11,12
Application of CPAP is the optimum therapy for OSAS patients, but its sleep-time use exhibits significant variations between patients.13,14 There is no consensus regarding which cut-off point is effective in terms of ameliorating not only OSAS symptoms, but also its systemic consequences. Weaver et al. in a recent study reached to the conclusion that effectiveness of CPAP treatment rises with increased hours of use. Four hours of CPAP use per night were proven effective for impaired OSAS patients in order to achieve normal scores in Epworth Sleepiness Scale (ESS), while more hours were necessary for improvement in multiple sleep latency test and Functional Outcomes associated with Sleepiness Questionnaire.15
To our knowledge, there is only one published study addressing the effect of CPAP therapy on the peripheral blood lymphocyte subsets of a very small number of OSAS patients.16 However, this study focused on the short-term effects of CPAP therapy, and the observation interval was only 6 days. As alterations in systemic immunity generally require longer periods to occur and there is also evidence that CPAP therapy may ameliorate immune activation, we sought to evaluate the long-term effect of CPAP on various immunologic parameters in otherwise healthy, nonsmoking, OSAS patients. To address this, we studied the changes in peripheral blood lymphocyte subsets of 52 OSAS patients before and after 6 months of CPAP application in accordance to their compliance with CPAP therapy. At the same time, serum TNF-α and IL-6 levels, salient mediators of immune response and inflammation, were also evaluated.
METHODS
Subjects
Fifty-five patients with newly polysomnographically confirmed OSAS (apnea hypopnea index [AHI] ≥ 5/h + daytime symptoms) were initially recruited. All of them had been referred to the Sleep Unit of ‘George Papanikolaou’ General Hospital between January and December 2005, with symptoms suggesting sleep related breathing disorders and had never been previously diagnosed or treated for OSAS. The study was approved by the ethics committee, and all participants gave their written informed consent after being fully informed of the study goals and procedures.
Exclusion criteria at baseline were infection, injury, surgical operation; known collagen, hematological, allergic, cardiovascular, respiratory, or malignant disease; and any medication, currently or during the month before the baseline examination. In order to exclude possible sexual difference in hormone secretion that can affect immunity, only male subjects were enrolled.17
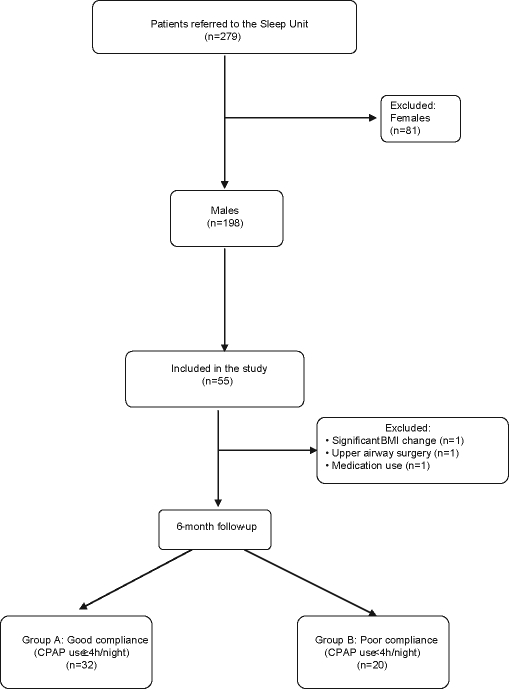
Additional exclusion criteria during the course of the study were change in body mass index (BMI) > 5% compared to baseline measurement, a newly diagnosed chronic disease, and any systemic medication use or surgery during the course of the study. Figure 1 summarizes in a flow chart the patients' inclusion procedure.
Figure 1.
Flow chart of the study protocol.
Study Design
Initial Assessment
At baseline, medical history was recorded and a physical examination was performed. Anthropometric data (age, BMI, neck, waist and hip circumferences) along with daytime habits, such as smoking or exercise, were recorded. During examination, participants were lightly clothed without shoes. Weight and height were measured to the nearest kilogram and centimeter, respectively, and BMI was calculated (BMI = weight [kg]/height2 [m2]). BMI > 30 was classified as obesity. Neck circumference was measured at the cricothyroid level, waist circumference in the middle between the 12th rib and the iliac crest, and hip circumference at the level of great trochanter by a measuring tape. Sleepiness was evaluated by the Greek version of ESS.18 To exclude any respiratory or cardiovascular disease, spirometric evaluation, arterial blood gas analysis while the patient breathed room air, chest radiograph, and resting 12-lead electrocardiogram were conducted. A patient was excluded for hypertension if the systolic and/or diastolic blood pressure exceeded 140 mm Hg and 90 mm Hg, respectively, on 3 different measurements.
Polysomnography (PSG)
All subjects underwent an attended overnight polysomnography (Somnologica 3.1; Flaga; Reykjavik, Iceland) using a standard montage of electroencephalogram (EEG), electrooculogram, electromyogram (EMG), and electrocardiogram (ECG) signals, together with pulse oximetry and airflow (detected using combined oronasal thermistors). Thoracic cage and abdominal motion were recorded by inductive plethysmography. EEG recordings were manually scored according to standard criteria.19 Apnea was defined as complete cessation of airflow ≥ 10 sec; hypopnea as a 50% reduction in airflow ≥ 10 sec, accompanied by ≥ 4% desaturation or by an EEG recorded arousal. AHI was defined as the total number of apneas and hypopneas per hour of electroencephalographic sleep, and oxygen desaturation index (ODI) as the total number of oxygen desaturation ( ≥ 3%) events per hour of encephalographic sleep. PSG was performed from 22:00-06:00. Patients with pure or mainly central apneas were excluded from the study. Blood samples were obtained the morning after polysomnography, between 08:00 and 09:00 following an overnight fast.
Patients with OSAS who initially accepted CPAP treatment underwent a second sleep study for CPAP titration. Optimum CPAP pressure was defined as the pressure value that abolished all the respiratory events, arousals, and desaturation episodes.
Follow-Up
Follow-up examination was performed 1, 3, and 6 months after initiation of CPAP. At those visits, patients had physical examinations, and CPAP compliance was estimated by dividing total recorded hours in the device timer by the number of nights of use between treatment initiation and follow-up examination. All patients were strongly encouraged to comply with prescribed therapy. At the 6-month follow-up examination, 3 patients were excluded from the study; one had a significant change in his BMI (−13% from baseline), the second had undergone upper airway surgery; and the third was taking medication for an acute coronary syndrome. The remaining patients were classified into 2 groups according to their CPAP compliance—Group A: good compliance ( ≥ 4 h CPAP use/night); group B: poor compliance: ( < 4 h CPAP use/night). Blood samples from OSAS patients were collected at the baseline examination and also following an overnight fast between 08:00 and 09:00, to avoid differences due to circadian rhythm.
Biochemical Analysis of Blood Samples
After blood collection, venous blood samples were drawn directly into sterile tubes containing EDTA-K3 (0.17 mol/L) as anticoagulant. Blood cell enumeration and white blood cell differential counts were performed in an automated hematology analyzer (Sysmex K-4500, Roche Diagnostics, Kobe, Japan). Values are expressed as absolute count (cells per microliter [cells/μL]). All samples were studied within 1-2 hours following blood collection without storage. The absolute cell number of each cell subset (cells/μL) was calculated from the respective proportion of positive cells and the number of lymphocytes per microliter of blood.
Immunofluorescence Staining and Flow Cytometric Analysis
The percentage and absolute count of lymphocyte subsets were assessed by 2-color flow cytometry. Briefly, whole blood samples were stained with the following FITC- or PE-conjugated mouse anti-human monoclonal antibodies after erythrocyte lysis with ammonium chloride: anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CD3/CD16+56, anti-CD25, anti-HLA-DR and the appropriate isotype controls, all from BD Pharmingen. Data acquisition and analysis were performed on a FACSCalibur flow cytometer (BD Biosciences, California, USA), equipped with CellQuest software. At least 5×103 lymphocytes, identified via their forward and light-scatter characteristics, were acquired in each case.
Cytokine and Uric Acid Detection
TNF-α and IL-6 levels were detected with quantitative sandwich enzyme immunoassay technique (R&D Systems, Minneapolis, USA). Minimum detectable doses of TNF-α and IL-6 were 1.6 pg/mL and 0.7 pg/mL respectively. Uric acid was measured by enzymatic method (Olympus AU640; Hamburg, Germany).
Statistical Analysis
Results for continuous variables are expressed as mean ± SD. Student's t test was applied in order to examine differences between good and poor CPAP compliance. Paired samples t test was used for testing the difference of means before and after CPAP treatment. The reported P-values are 2-tailed, and a P-value < 0.05 was considered statistically significant. All analyses were performed using SPSS 15.0 software (SPSS Inc., Chicago, IL).
RESULTS
Characteristics of OSAS Patients
Fifty-two of 279 individuals examined at the sleep laboratory with polysomnography fulfilled inclusion criteria at baseline or during the 6-month follow up period. Anthropometric features and results of the sleep studies of these patients are shown in Table 1. Patients were middle-aged, obese, with normal respiratory function while awake. AHI ranged significantly between 9.1/h and 123.3/h (mean 57.1 ± 27.7/h). Mean values of lymphocyte subsets (absolute counts and percentages), TNF-α, IL-6, and uric acid levels at baseline are displayed in Table 2.
Table 1.
Baseline Characteristics in All OSAS Patients
| Age (years) | 46 ± 10.34 |
| BMI (kg/m2) | 34.18 ± 7.50 |
| Neck circumference (cm) | 43.28 ± 3.49 |
| Waist circumference (cm) | 115.09 ± 14.04 |
| Hip circumference (cm) | 119.93 ± 21.52 |
| WHR | 0.97 ± 0.094 |
| FEV1 (% pred.) | 92.04 ± 10.86 |
| FVC (% pred.) | 91.04 ± 10.68 |
| pH | 7.41 ± 0.023 |
| PaO2 (mm Hg) | 83.99 ± 7.843 |
| PaCO2 (mm Hg) | 38.21 ± 2.94 |
| ESS | 11 ± 5.67 |
| AHI (/hour) | 57.11 ± 27.73 |
| ODI (/hour) | 58.09 ± 27.63 |
| avSpO2 (%) | 90.3 ± 4.72 |
| minSpO2 (%) | 72.4 ± 10.13 |
| t < 90 (%TST) | 31.51 ± 27.29 |
| Sleep efficiency (%TST) | 88.56 ± 10.44 |
| S1 (%TST) | 12.84 ± 7.73 |
| S2 (%TST) | 52.38 ± 14.47 |
| S3+4 (%TST) | 13.59 ± 13.22 |
| REM (%TST) | 10.1 ± 6.46 |
Values are expressed as mean ± SD
BMI, body mass index; WHR, waist to hip ratio; FEV1, forced expiratory volume (1st second); FVC, forced vital capacity; ESS, Epworth Sleepiness Scale; AHI, apnea hypopnea index; ODI, oxygen desaturation index; avSpO2, average saturation in pulse oximetry; minSpO2, minimum, saturation in pulse oximetry; t < 90, percentage of total sleep time with SpO2 < 90%; TST, total sleep time; S1, stage 1 of sleep; S2, stage 2 of sleep; S3+4, stage 3 and stage 4 of sleep; REM, rapid eye movement
Table 2.
Baseline Levels of Immunological Factors in All OSAS Patients
| WBC (cells/μL) | 7343.53 ± 2047.95 |
| Neutrophils (cells/μL) | 4187.76 ± 1517.33 |
| Neutrophils (%) | 55.34 ± 9.66 |
| Lymphocytes (cells/μL) | 2620.58 ± 854.52 |
| Lymphocytes (%) | 36.14 ± 8.77 |
| CD4+ (cells/μL) | 1209.19 ± 308.07 |
| CD4+ (%) | 48.62 ± 8.263 |
| CD3+ (cells/μL) | 1773.6 ± 388.49 |
| CD3+ (%) | 71.12 ± 7.36 |
| B cells (CD19+) (cells/μL) | 376.15 ± 269.98 |
| B cells (CD19+) (%) | 14.4 ± 6.61 |
| N/K cells (CD3-16+56+) (cells/μL) | 358.83 ± 157.42 |
| N/K cells (CD3-16+56+) (%) | 14.52 ± 6.01 |
| CD3+16+56+ (cells/μL) | 141.04 ± 146.28 |
| CD3+16+56+ (%) | 5.48 ± 5.26 |
| CD4+CD25+ (cells/μL) | 609 ± 228.15 |
| CD4+CD25+ (%) | 24.54 ± 8.7 |
| CD4+HLA-DR+ (cells/μL) | 222.96 ± 74.62 |
| CD4+HLA-DR+ (%) | 8.98 ± 2.71 |
| CD8+ (cells/μL) | 826.42 ± 251.41 |
| CD8+ (%) | 33.12 ± 8.28 |
| CD3+8+ (cells/μL) | 826.42 ± 251.41 |
| CD3+8+ (%) | 25.08 ± 8 |
| CD4+/8+ | 1.66 ± 0.8 |
| TNF-α (pg/mL) | 7.98 ± 5.01 |
| IL-6 (pg/mL) | 2.6 ± 1.147 |
| Uric acid (mg/dL) | 6.72 ± 1.45 |
Values are expressed as mean ± SD
Abbreviations: WBC, white blood cells; NK, natural killer cells; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6
According to compliance to CPAP use, which was evaluated at the 6-month follow up examination, OSAS patients were divided into 2 groups: Group A: (n = 32) with good compliance and group B: (n = 20) with poor compliance. The 2 groups did not differ significantly in terms of anthropometric or sleep characteristics at baseline (Table 3).
Table 3.
Comparison of CPAP use and baseline characteristics between the 2 groups, according to CPAP compliance
| Good compliance | Poor compliance | P | |
|---|---|---|---|
| CPAP use (h/night) | 4.68 ± 0.56 | 1.41 ± 1.47 | < 0.001 |
| Age (years) | 45.63 ± 10.73 | 46.6 ± 9.91 | 0.744 |
| BMI (kg/m2) | 34.58 ± 8.92 | 33.55 ± 4.51 | 0.632 |
| Neck circumference (cm) | 43.58 ± 3.68 | 42.82 ± 3.25 | 0.496 |
| Waist circumference (cm) | 115.46 ± 16.11 | 114.53 ± 10.56 | 0.834 |
| Hip circumference (cm) | 120.77 ± 23.88 | 118.56 ± 17.69 | 0.751 |
| FEV1 (% pred) | 91.07 ± 10.53 | 93.65 ± 11.51 | 0.431 |
| FVC (% pred) | 90.67 ± 10.9 | 91.66 ± 10.58 | 0.758 |
| pH | 7.41 ± 0.02 | 7.41 ± 0.03 | 0.998 |
| PaO2 (mm Hg) | 83.99 ± 7.68 | 83.98 ± 8.39 | 0.997 |
| PaCO2 (mm Hg) | 38.4 ± 2.66 | 37.88 ± 3.46 | 0.572 |
| ESS | 11.91 ± 5.59 | 9.55 ± 5.62 | 0.146 |
| AHI (/h) | 61.12 ± 28.29 | 50.71 ± 26.23 | 0.191 |
| ODI (/h) | 63.53 ± 28.47 | 49.38 ± 24.44 | 0.072 |
| avSpO2 (%) | 89.83 ± 4.78 | 91.06 ± 4.64 | 0.368 |
| minSpO2 (%) | 71 ± 9.49 | 74.65 ± 10.94 | 0.209 |
| t < 90 (%TST) | 35.09 ± 27.48 | 25.49 ± 26.59 | 0.228 |
#Values are given as mean ± SD
Abbreviations: CPAP, continuous positive airway pressure; BMI, body mass index; FEV1, forced expiratory volume (1st second); FVC, forced vital capacity; ESS, Epworth Sleepiness Scale; AHI, apnea hypopnea index; ODI, oxygen desaturation index; avSpO2, average saturation in pulse oximetry; minSpO2, minimum saturation in pulse oximetry; t < 90, percentage of total sleep time with SpO2 < 90%; TST, total sleep time.
Group A and group B patients were also comparable regarding absolute counts of lymphocyte subsets, and serum levels of TNF-α, IL-6, and uric acid at baseline. Respectively, total lymphocytes: 2528.1 ± 578.2 vs 2475 ± 595.5 cells/μL (P = 0.751); CD4+: 1262.7 ± 329.4 vs 1123.7 ± 255.4 cells/μL (P = 0.114); CD8+: 812.69 ± 252.07 vs 848.4 ± 254.32 cells/ μL (P = 0.623); CD19+: 362.59 ± 167.05 vs 397.85 ± 386.41 cells/μL (P = 0.651); CD3+16+56+: 136.25 ± 162.65 vs 148.7 ± 119.05 cells/ μL (P = 0.769); CD4+CD25+: 635.88 ± 249.26 vs 566 ± 187.57 cells/μL (P = 0.287); CD4+HLA-DR+: 217.16 ± 80.76 vs 232.25 ± 64.46 cells/μL (P = 0.483); TNF-α: 8.4 ± 5.7 vs 6.8 ± 2.4 pg/mL (P = 0.440); IL-6: 2.7 ± 1.3 vs 2.3 ± 0.7 pg/mL (P = 0.421) and uric acid: 6.79 ± 1.48 vs 6.62 ± 1.46 mg/dL (P = 0.693).
Lymphocyte subsets Before and After CPAP Application on OSAS Patients
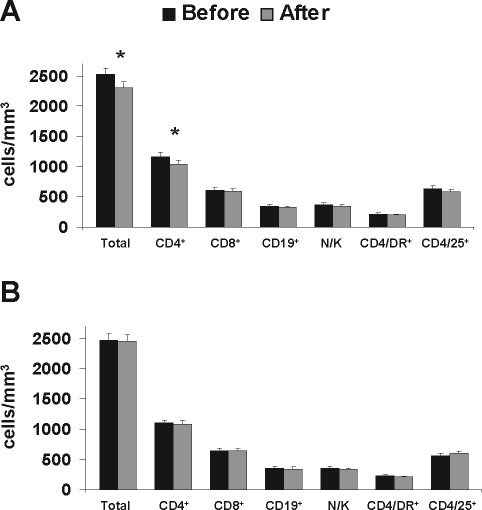
In the good compliance group (group A), a statistically significant reduction was observed in the total lymphocyte count (2528.13 ± 578.23 vs 2318.75 ± 549.16 cells/μL, P = 0.003), and in the total count of CD4+ cells (1262.66 ± 328.38 vs 1129.69 ± 297.76 cells/μL, P = 0.001). No significant alteration was observed in any of the rest examined parameters, namely: absolute count of CD8+ cells 812.69 ± 252.67 vs 762.28 ± 276 cells/μL, (P = 0.095); CD19+ cells: 362.59 ± 167.05 vs 335.83 ± 158.46 cells/μl (P = 0.096); N/K cells (CD3−/16+56+): 354.16 ± 157.03 vs 319.16 ± 135.72 cells/μl (P = 0.147); and CD4+HLA-DR+ cells: 217.16 ± 80.76 vs 209.44 ± 69.48 cells/μl (P = 0.494). Interestingly, the absolute count of CD4+CD25+ subset, which represents activated lymphocytes, was slightly decreased only in group A patients (635.88 ± 249.26 vs 585.69 ± 202.37 cells/μl, P = 0.182), in contrast to group B, where CD4+CD25+ cells exhibited an increase, although not statistically significant (566 ± 187.57 vs 601.6 ± 205.55 cells/μl, P = 0.415). No significant alteration was observed in the examined parameters in the poor compliance group (Figure 2).
Figure 2.
Changes in peripheral blood lymphocyte subsets according to CPAP compliance. A: Absolute counts of lymphocyte subsets before (black bars) and after (grey bars) 6 consecutive months of CPAP application in good compliance patients (n = 32). B: Absolute counts of lymphocyte subsets before (black bars) and after (grey bars) 6 consecutive months of CPAP application in poor compliance patients (n = 20). Error bars represent SEM.
Alterations of Soluble Immune Mediators According to CPAP Compliance
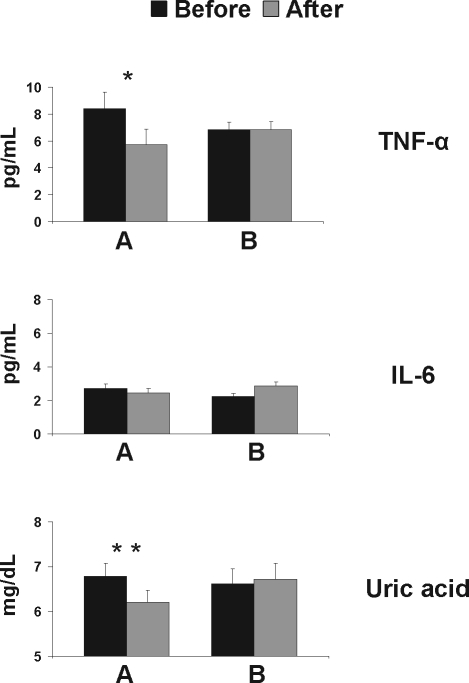
Changes in serum levels of TNF-α, IL-6, and uric acid were recorded prior and after CPAP therapy. TNF-α levels decreased significantly after 6 months of good compliance to CPAP treatment (8.41 ± 5.7 vs 5.72 ± 4.91 pg/mL, P = 0.001). These levels remained unaffected in the poor compliance group (6.86 ± 2.4 vs 6.87 ± 2.43 pg/mL, P = 0.985). Likewise, uric acid levels declined significantly in CPAP compliant patients (8.79 ± 1.48 vs 6.2 ± 1.37 mg/dL, P < 0.001). This result was not observed to those OSAS patients who complied poorly (6.62 ± 1.46 vs 6.71 ± 1.53 mg/dL, P = 0.223). In contrast, IL-6 levels showed no significant change in either group (Group A: 2.71 ± 1.27 vs 2.46 ± 1.08 pg/mL [P = 0.266]; group B: 2.32 ± 0.72 vs 3.86 ± 3.03 pg/mL (P = 0.173)] (Figure 3).
Figure 3.
Changes in TNF-α, IL-6, and uric acid levels according to CPAP compliance. A: TNF-α, IL-6, uric acid levels before (black bars) and after (hatched bars) 6 consecutive months of CPAP application in good compliance patients (n = 32). B: TNF-α, IL-6, uric acid levels before (black bars) and after (hatched bars) 6 consecutive months of CPAP application in poor compliance patients (n = 20). Error bars represent SEM.
DISCUSSION
Our study demonstrates that a significant reduction of total lymphocyte count occurred exclusively in the peripheral blood of OSAS patients who used CPAP therapy > 4 h per night for 6 consecutive months. This reduction was the result of a selective decrease in CD4+ lymphocytes, as the other subsets (CD8+, B, and N/K cells) did not display numerical alterations after CPAP therapy, independent of compliance. Similarly, a significant decrease in serum TNF-α and uric acid levels, indicative of recession of a preexistent immune activation status was evident only in the good compliance group.
Nakamura et al. demonstrated that the absolute count of CD4+ and CD4+HLA-DR+ cells in OSAS patients declined significantly 24 hours after the application of CPAP. As this reduction was transient and CD4+ cells were restored to their initial count after one week, it was attributed to the sleep disturbance and hypoxia that accompanies OSAS, and the authors postulated that long-term CPAP use may have no influence on immunity.16
In contrast to the above report, our study showed that the significant decrease in total and CD4+ lymphocyte count in OSAS patients was still evident after 6 months of adherent CPAP use, whereas OSAS patients who complied poorly with CPAP displayed no numerical alterations of CD4+ lymphocytes. Although we cannot exclude possible fluctuations of the CD4+ cell count, since we did not perform serial measurements during the follow-up period, our findings suggest that the effect of CPAP application on immunity is constant. Notably, in order to avoid differences due to the circadian rhythm of the CD4+ cell count,20 blood was always drawn at the same time in the morning.
Immune system activation in OSAS patients is corroborated by a plethora of clinical and experimental evidence.21 A potential reason for the above association may be the obesity that represents a major risk factor and usually accompanies OSAS. Increased levels of proinflammatory cytokines have been observed in obese patients, while leptin is considered to possess immunomodulatory properties.22 Nevertheless, the anthropometric characteristics were identical in both groups of our patients, and their BMI remained stable, thus excluding a role for obesity in the alterations of immune parameters in the good compliance group.
Catecholamine and other hormonal changes induced by sleep deprivation can provide another explanation for immune derangement in OSAS, as there is substantial evidence for a mutual association between sleep and immune system. Sustained wakefulness has been shown to induce expansion of most cell subpopulations in peripheral blood that acutely resolves after sleep restoration.20 The mechanism underlying this phenomenon might involve migration of cells to extravascular sites during sleep, possibly due to attenuation of acute antigen challenge. However, our OSAS patients displayed selective decrease of only CD4+ cells, while no differences were present in all other lymphocyte subsets.
Another possible interpretation for the CD4+ cell count reduction is that the OSAS-induced inflammatory assault prompted the expansion of a particular CD4+ cell subset that returned to normal after the cessation of the immune process due to CPAP therapy. It has recently been hypothesized that sleep apnea might be a triggering factor for the development of autoimmune phenomena.21 Hypoxia-induced repeated cell injury, leading to hyperuricemia, results in the precipitation of monosodium urate, which in turn, affects dendritic cell maturation and antigen presentation,23 allowing for the loss of the immune tolerance. In support of this mechanism, the serum levels of both TNF-α and uric acid were significantly reduced after 6 months of therapy with CPAP, whereas they remained unchanged in poorly treated patients.
A possible limitation of our study is that it was not a randomized controlled trial. However, such a study is difficult to perform, since it would be unethical to leave patients with confirmed OSAS untreated in order to record potential changes in immunological parameters.
In conclusion, the significant decrease of the CD4+ cell count along with the parallel decline of serum levels of TNF-α and uric acid in OSAS patients who complied with long-term CPAP use, argue for a direct effect of OSAS on systemic immunity, potentially independent from the effect of obesity and sleep disturbance that accompany OSAS. Moreover, the above effect appears to be specific to OSAS, since OSAS represents a unique model of intermittent hypoxemia, and, to our knowledge, there are no studies reporting an analogous reduction of the CD4+ subpopulation after CPAP treatment in any other sleep related hypoxemic syndrome. In addition, no alterations were observed in other major lymphocyte subsets studied, including B-cells, suggesting that humoral immunity might be unaffected in patients with OSAS, as reported previously.24
Our results are in line with the concept of an ongoing inflammatory process in OSAS patients, thus rendering CPAP an important tool not only for the therapeutic manipulation of respiratory events in OSAS, but also for its inflammatory sequelae.
ACKNOWLEDGMENTS
Institution: Sleep Unit, 2nd Chest Department ‘George Papanikolaou’ General Hospital, Thessaloniki, Greece
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Erden S, Cagatay T, Buyukozturk S, Kiyan E, Cuhadaroglu C. Hashimoto thyroiditis and obstructive sleep apnea syndrome: is there any relation between them? Eur J Med Res. 2004;9:570–2. [PubMed] [Google Scholar]
- 2.Brown LK. A waist is a terrible thing to mind: central obesity, the metabolic syndrome, and sleep apnea hypopnea syndrome. Chest. 2002;122:774–8. doi: 10.1378/chest.122.3.774. [DOI] [PubMed] [Google Scholar]
- 3.Quera-Salva MA, Guilleminault C, Chevret S, et al. Breathing disorders during sleep in myasthenia gravis. Ann Neurol. 1992;31:86–92. doi: 10.1002/ana.410310116. [DOI] [PubMed] [Google Scholar]
- 4.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 5.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 6.Alberti A, Sarchielli P, Gallinella E, Floridi A, Mazzotta G, Gallai V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–11. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 7.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 8.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 9.Steiropoulos P, Tsara V, Nena E, et al. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2007;132:843–51. doi: 10.1378/chest.07-0074. [DOI] [PubMed] [Google Scholar]
- 10.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 11.Dyugovskaya L, Lavie P, Hirsh M, Lavie L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur Respir J. 2005;25:820–8. doi: 10.1183/09031936.05.00103204. [DOI] [PubMed] [Google Scholar]
- 12.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–50. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 13.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130:1772–8. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T, Chin K, Shimizu K, Kita H, Mishima M, Ohi M. Acute effect of nasal continuous positive airway pressure therapy on the systemic immunity of patients with obstructive sleep apnea syndrome. Sleep. 2001;24:545–53. doi: 10.1093/sleep/24.5.545. [DOI] [PubMed] [Google Scholar]
- 17.Khansari DN, Murgo AJ, Faith RE. Effects of stress on the immune system. Immunol Today. 1990;11:170–5. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 18.Tsara V, Serasli E, Amfilochiou A, Constantinidis T, Christaki P. Greek version of the Epworth Sleepiness Scale. Sleep Breath. 2004;8:91–5. doi: 10.1007/s11325-004-0091-6. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Washington, DC: National Institute of Health; 1968. A manual of standardized terminology techniques and scoring system for sleep stages of human subjects. [DOI] [PubMed] [Google Scholar]
- 20.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 21.Abrams B. Long-term sleep apnea as a pathogenic factor for cell-mediated autoimmune disease. Med Hypotheses. 2005;65:1024–7. doi: 10.1016/j.mehy.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 22.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 24.Dopp JM, Wiegert NA, Moran JJ, et al. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy. 2007;27:1483–9. doi: 10.1592/phco.27.11.1483. [DOI] [PubMed] [Google Scholar]