Abstract
The aim of this work was to develop indinavir pediatric anti-HIV/AIDS formulations enabling convenient dose adjustment, ease of oral administration, and improved organoleptic properties by means of the generation of drug-loaded microparticles made of a polymer that is insoluble under intake conditions and dissolves fast in the stomach in order to completely release the active agent. Indinavir-loaded microparticles made of a pH-dependent polymeric excipient soluble at pH < 5, Eudragit E100, were prepared using a double emulsion solvent diffusion technique and the in vitro release profiles characterized. Finally, taste masking properties were evaluated in blind randomized sensory experiments by ten healthy human volunteers. The use of a w/o/o emulsion system resulted in indinavir loads around 90%. Thermal analysis of the microparticles by differential scanning calorimetry revealed that indinavir appeared mainly dispersed at the molecular level. Concentrations of residual organic solvents as determined by gas chromatography were below the upper limits specified by the European Pharmacopeia for pharmaceutical oral formulations. Then, the behavior of drug-containing microparticles in aqueous media at different pH values was assessed. While they selectively dissolved in gastric-like medium, in tap water (intake conditions), the matrix remained almost unchanged and efficiently prevented drug dissolution. Finally, sensoring taste tests performed by volunteers indicated that systems with indinavir loads ∼15% displayed acceptable taste. This work explored the production of indinavir-containing microparticles based on a common pharmaceutical excipient as a means for the improvement of medicines of drugs involved in the treatment of HIV/AIDS. For systems containing about 15% drug, taste studies confirmed the acceptability of the formulation. In pediatric regimes, this composition would require an acceptable amount of formulation (0.7–1.5 g).
Key words: bitterness masking, extemporaneous pediatric liquid formulations, indinavir-loaded microparticles, patient compliance and treatment adherence, pediatric HIV/AIDS
INTRODUCTION
The ability to swallow solid pharmaceutical formulations is restricted in children under the age of 7 years (1). Dose adjustment to children’s body weight is a common challenge faced when solid forms need to be employed in pediatric treatments. In addition, both the number of clinically approved drugs and the availability of liquid formulations are scarce for pediatric patients. The preparation of extemporaneous liquid formulations by processing capsules or tablets is a common strategy to deal with these problems, but it has raised significant safety, efficacy, and quality concerns (1). Also, organoleptic drawbacks (e.g., bitterness) are usually neglected, regardless of the impact these issues have on patient compliance and adherence (2). The last World Health Assembly stressed the importance of recognizing the children’s right to access safe, effective, and proven medicines (3).
Indinavir, a protease inhibitor used in HIV/AIDS pharmacotherapy, is available in capsules and presents pharmacokinetic profiles that depend on pharmacogenetic patterns (4). The palatability of experimental indinavir liquid preparations is unacceptable for most of the patients, thus affecting patient compliance (5). To attain therapeutic success, adherence levels to anti-HIV treatments need to be above 95% (no more than two doses missed monthly in a twice-a-day management; 6). Improving indinavir pediatric formulations is relevant because this drug plays a central role in second-line anti-HIV regimes (7) and in resource-constrained settings (8). In fact, demand forecasts indicated that indinavir still shows a sharp proportional increase (9).
The goal of the present preliminary developmental study was to explore a technologically cost-effective composition for the taste masking of the highly bitter indinavir. Indinavir-loaded Eudragit E-100 microparticles were produced and characterized by different techniques. The drug-loaded polymer matrix was poorly soluble under intake conditions (tap water) and it dissolved fast in stomach-mimicking medium allowing the complete drug solubilization.
MATERIALS AND METHODS
Indinavir sulfate monoethanolate and Eudragit E 100 were gifts of Richmond Laboratories and Degussa, respectively. All other reagents were of analytical grade.
Preparation of Indinavir-Loaded Microparticles
Indinavir-loaded microparticles were prepared by means of a double emulsion/solvent evaporation method (10). Firstly, a w/o/w emulsion system was intended; indinavir sulfate monoethanolate water solution (200 mg/mL, 1 mL) was added to the polymer solution in dichloromethane (80 mg/mL, 10 mL) and vortexed (1 min) until the formation of the primary w/o emulsion. Then, the emulsion was poured into an aqueous solution of polyvinyl alcohol (2.5 mg/mL, 200 mL) and mechanically stirred using a three-blade propeller (90 min, 450 rpm) in order to allow evaporation of the organic solvent. Microparticles were isolated by centrifugation, washed with distilled water (3 × 50 mL), and dried in a diseccator until constant weight. Relatively low experimental indinavir loads (∼67% of the theoretical load) were obtained. To study the effect of the external phase composition on drug loading, Eudragit E100 solution (10% w/v, 0.4–0.8 g) in dicholoromethane/ethanol (1:1) and indinavir sulfate monoethanolate aqueous solution (200–600 mg/mL in distilled water, containing 0.25% Tween 80, 1 mL) were vortexed until the formation of a stable emulsion. The w/o emulsion was then poured into corn oil (0.05% Span 80, 100 mL) and stirred (4 h, 450 rpm) to eliminate the dichloromethane. The hardened microcapsules were centrifuged (1 min, 1,500 rpm) and washed with n-hexane (50 mL) to remove the remaining oil. Microcapsules were finally filtered and dried under vacuum. Indinavir load was estimated by high-performance liquid chromatography (HPLC; see below). Table I presents the composition of different drug-containing systems produced in different batches. Theoretical indinavir sulfate monoethanolate contents were between 20% and 60%. A correction factor was applied to express values as indinavir free base. Drug-loaded microparticles (MPs) were denominated MP20, MP40, and MP60 for 20%, 40%, and 60% theoretical drug loadings. The values of loading and loading % are expressed as mean ± standard deviation (SD; n = 5). From the data presented, it is concluded that the protocols are highly reproducible. To establish the stability of the drug in the formulation, samples were stored in vials at room temperature and titrated after 24 months. Stability levels are informed as % of the initial indinavir load.
Table I.
Composition of Different Microparticular Systems Containing Indinavir Sulfate Obtained by a Double Emulsion/Solvent Evaporation Method
| Formulation | Type of emulsion | Theoretical indinavir sulfate monoethanolate loading (wt.%) | Theoretical indinavir free base loading (wt.%) | Experimental indinavir free base loading (wt.%) ± SD | % f load ± SD |
|---|---|---|---|---|---|
| MP20Aa | w/o/w | 20 | 16.2 | 10.9 ± 0.4 | 67.3 ± 2.5 |
| MP20 | w/o/o | 20 | 16.2 | 15.2 ± 0.8 | 91.3 ± 5.2 |
| MP40 | w/o/o | 40 | 32.4 | 30.1 ± 1.2 | 92.8 ± 1.2 |
| MP60 | w/o/o | 60 | 48.6 | 43.6 ± 1.5 | 89.8 ± 3.1 |
MP microparticular
aMP20A denotes preliminary microparticles prepared by a w/o/w technique. The procedure was modified in order to improve the levels of % f load
Characterization of Drug-Loaded Microparticles
Particle size range and size distribution of the different batches was determined by means of analytical sieving (ASTM Sieves, Zonytest, Sieves #20 (840 μm), #40 (420 μm), #70 (210 μm), #140 (105 μm), and #240 (53 μm)). Size ranges were expressed as % of the total sample weight (mean ± SD, n = 5). The morphology of the particles was analyzed by optical microscopy.
Thermal Analysis of the Indinavir-Containing Microparticles
The thermal properties of the drug within the microparticles were analyzed by differential scanning calorimetry (DSC; Shimadzu DSC-50 differential scanning calorimeter, single heating ramp 25–250°C, 5°C/min) under dry N2 atmosphere. Values of enthalpy of the drug were normalized to the indinavir free base content in the sample. ΔHm of pure indinavir free base (132 J/g) was used to calculate the amount of crystalline drug in each microparticulate system.
Determination of Residual Organic Solvents
Different batches were assayed for residual organic solvents by gas chromatography (GC; Hewlett Packard 6890, column DB-1301, length 30 m, i.d. 530 μm). Microspheres (∼250 mg) were dissolved in a standard solution (10 mg of n-heptane in 10 mL of dimethylformamide). Temperatures were 40°C, 140°C, and 250°C for oven, injector, and detector, respectively. Each batch of microparticles was analyzed by duplicate.
Titration of Indinavir
The indinavir content in every batch was determined by HPLC (Waters 515 HPLC pump; reverse-phase C18 column Phenomenex, 5 μm, 5 × 150 mm; flow rate 1.2 mL/min at room temperature; UV–visible detector at λ = 210 nm); samples were dissolved in HCl 0.1 N. The mobile phase was a mixture of acetonitrile/0.85% sodium orthophosphate/triethylamine (75:25:0.2) adjusted to pH 3. The retention time of indinavir was 5 min. Percent load factors (% fload) were calculated according to % fload = Eload/Tload.100, where Eload and Tload are the experimental and the theoretical indinavir free base loads in the manufactured formulation, respectively. Results are expressed as mean ± SD (n = 5).
In Vitro Release Studies
The release of indinavir under saliva (phosphate buffered saline, pH 6.8; 10) and gastric-mimicking (HCl, pH 1.5) conditions was assessed by dispersing the different MP20 and MP40 batches (100 mg) in the test medium (500 mL) using the United States Pharmacopeia (USP) dissolution apparatus 2, at 25°C and 37°C. The paddles rotation was set at 100 rpm. Results are expressed as mean ± SD (n = 5).
Release Under Intake Conditions
Release profiles following the extemporaneous reconstitution of the drug-containing powder were assayed by dispersing the different specimens (amount equivalent to 400 mg indinavir) in tap water (125 mL) at 25°C. Supernatant solutions were removed and analyzed for indinavir content at 1, 5, and 15 min. Finally, samples were acidified to pH < 3 (HCl 30%, 50 μL) to completely dissolve the microparticles and the total drug released was titrated. Results are expressed as mean ± SD (n = 5).
Taste Masking Evaluation
Taste masking properties were evaluated in blind randomized sensory experiments by ten healthy human volunteers. Aqueous solutions containing indinavir free base concentrations similar to those released by the microparticles in tap water (see above, 1 mL) at 1 and 5 min were held in the center of the tongue (20 s) and then spat out. Volunteers rinsed their mouths with plenty of water before the test of the next solution. Samples were scored using a numerical scale between 0 and 4, where 0, 1, 2, 3, and 4 were no detectable, threshold, slight, moderated, and strong taste, respectively. A final score of bitterness was calculated by averaging the values recorded for each sample. Means <2 (bitterness lower than slight) were considered of acceptable taste, values >2 were defined as unacceptable. Commercially available capsules and tap water were included in the set of samples. The assay was performed following the Declaration of Helsinki guidelines and the local ethical regulations for human studies. Participants were previously informed and they expressed their consent prior to the test.
RESULTS
Preparation of Indinavir-Loaded Microparticles
First, a w/o/w double emulsion solvent evaporation methodology was employed. Spherical microparticles displaying sizes mainly in the 210 to 420 μm range were obtained (not shown). Indinavir load levels were between 60% and 70% of the optimal ones, i.e., MP20A contained about 11 wt.% drug instead of the expected 16.2 wt.% (Table I, entry 1). In formulations produced using a w/o/o emulsion, indinavir % fload values sharply increased to about 90%, regardless of the final indinavir content (Table I, entries 2–4).
Characterization of Drug-Loaded Microparticles
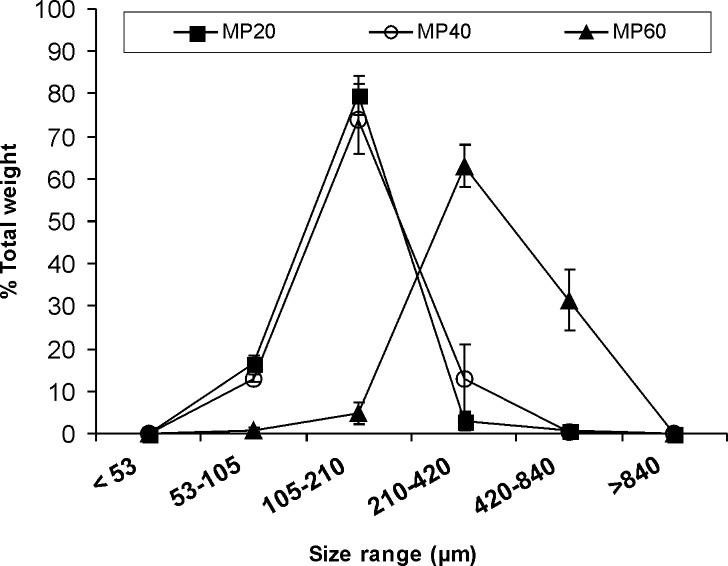
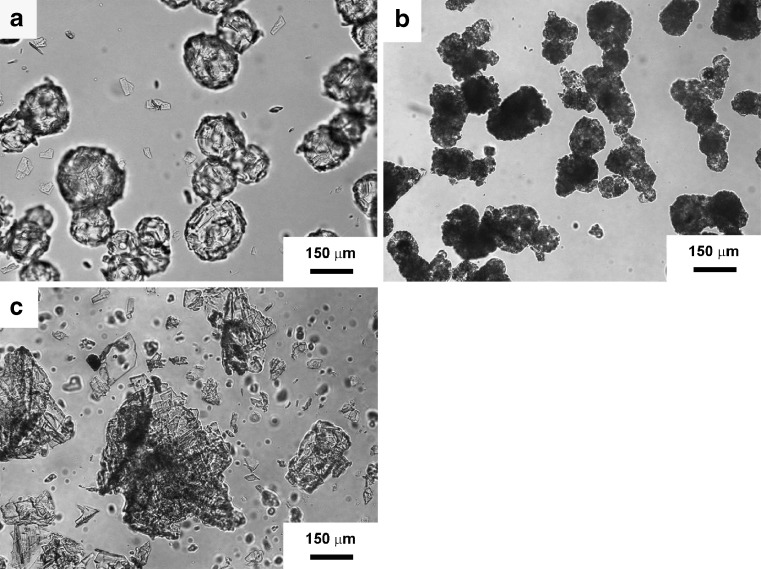
Size analysis indicated that for MP20 and MP40, 80 wt.% of the samples displayed sizes between 105 and 210 μm (Fig. 1). As compared to MP20, with only a 3% of the particles being larger than 420 µm, the fraction between 210 and 420 μm in MP40 increased to 13%. MP60 showed a slight increase in the size range means (210–420 μm) and a wider distribution. Under the microscope, MP20 samples displayed more rounded shapes than those containing higher indinavir concentrations (Fig. 2). As the amount of loaded indinavir increased, microparticles displayed more irregular shapes. In order to establish the stability of the drug in the microparticles, the indinavir content was assayed in different samples 2 years after production (n = 2). For MP20, initial indinavir free base % fload remained unchanged (15.2%). In contrast, both MP40 and MP60 showed titer losses in the 20–25% of the initial level.
Fig. 1.
Size range and distribution of MP20, MP40, and MP60 as determined by analytical sieving. Values of the amount retained in each sieve are expressed as % of the total sample weight
Fig. 2.
Optical microscope photographs of indinavir-containing microparticles. a MP20, b MP40, and c MP60. Scale bar = 150 μm
Thermal Analysis of Indinavir-Containing Microparticles
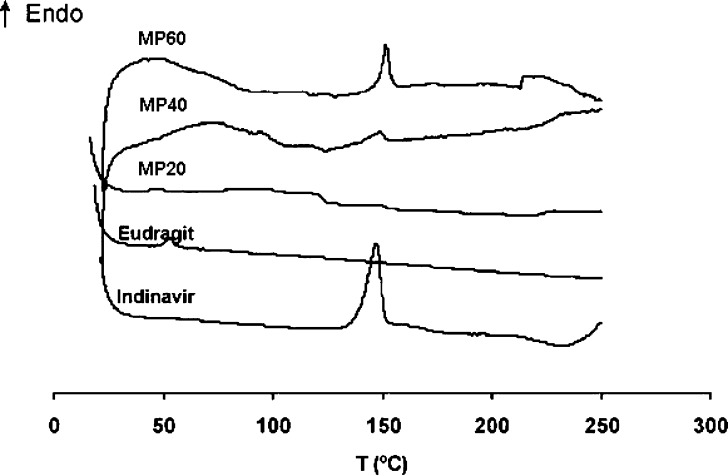
Indinavir could theoretically be present in crystalline form or dispersed at the molecular level within the polymer matrix. Representative thermograms are presented in Fig. 3. First, the polymer and the drug were investigated separately. The fully amorphous Eudragit E100 displayed only a typical Tg of polymethacrylate derivatives ∼60°C and indinavir sulfate monoethanolate showed a melting endotherm at ∼150°C. The ΔHm of indinavir (normalized to indinavir free base) involved in this transition (132 J/g) was considered a 100% degree of crystallinity. In drug-containing specimens, the indinavir melting endotherm appeared at the same temperature. Normalization of enthalpy values to drug content showed a gradual increase of the crystallization extent from 21.8 to 57.2 J/gindinavir for MP20 and MP60, respectively, and represented a degree of crystallinity of 16.5% and 43.3%. MP40 displayed an intermediate value (30.9 J/g, 23.4% crystallinity). Thermal analysis of MP20 stored 2 years coincided with the original pattern and indicated that this system was stable over this period of time.
Fig. 3.
DSC thermograms of Eudragit E100, indinavir sulfate monoethanolate, and MP20, MP40, and MP60 drug-loaded microparticles
Determination of Residual Organic Solvents
The presence of residual solvents used in the production of the microparticles constitutes a parameter of concern toward acceptability of the formulations for in vivo testing. GC analysis indicated values below the maximal levels specified by the European Pharmacopeia for pharmaceutical oral formulations (600 and 290 ppm for dichloromethane and n-hexane, respectively).
In Vitro Release Studies
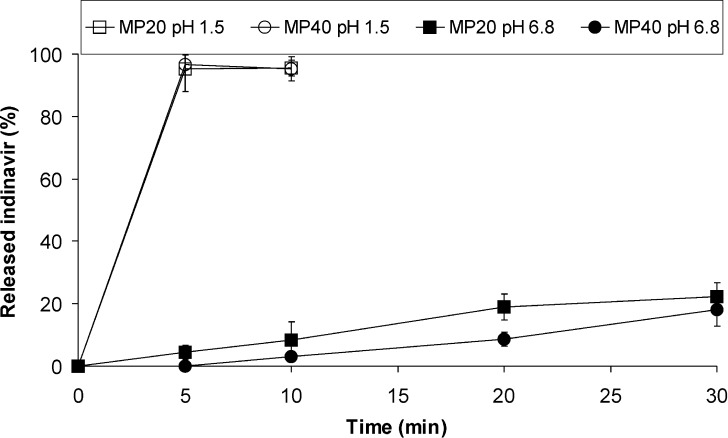
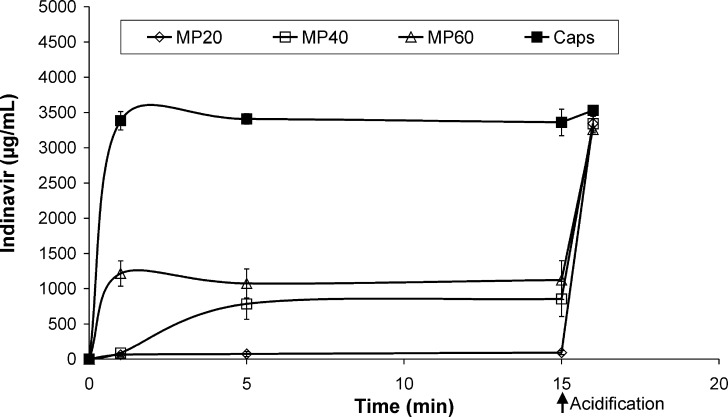
To investigate the release of the drug from the microparticles, two standardized tests were performed. At pH 1.5 (37°C), microparticles dissolved within 5 min releasing 100% of the loaded indinavir. In contrast, a moderated release was observed at pH 6.8 (Fig. 4). Systems investigated at 25°C showed very similar profiles (not shown). Then, an assay mimicking clinical intake conditions was conducted. Different samples were dispersed in tap water and the indinavir concentration monitored at 25°C (Fig. 5). Due to the basicity of the polymer and a dispersion medium lacking buffering ability, pH values slightly increase to about 7.5 in the dispersion medium. After 1 min, MP20 and MP40 systems released concentrations of 60 and 90 μg/mL. After 5 min, MP20 attained values ∼75 μg/mL. Contrary to this, MP40 displayed a sharp increase to 785 μg/mL. MP60 released notably faster (1215 μg/mL after 1 min). These results were compared to the powder within 400 mg commercial capsules that, as expected, dissolved completely after 1 min, releasing 100% of the drug. Acidification to attain a gastric-like pH (<3) showed the pronounced release of more than 95% of the indinavir after 5 min in all the samples.
Fig. 4.
Released indinavir (expressed as %) versus time for MP20 and MP40 systems. Microparticles (100 mg) were dispersed in the test medium (500 mL, pH values 1.5 and 6.8) and assayed in a dissolution test using the USP dissolution apparatus 2, at 37°C
Fig. 5.
Released indinavir versus time for MP20, MP40, and MP60 microparticles (amount equivalent to 400-mg drug) under intake conditions (tap water, 125 mL), at 25°C. At time point 15 min, test samples were acidified to pH < 3 with HCl 30% in order to mimic the gastric environment
Taste Masking Evaluation
The ultimate goal of the encapsulation process was to mask the bitterness of an indinavir-based liquid formulation by constraining the drug dissolution. In order to evaluate the tolerability of the developed indinavir-loaded microparticles, solutions containing concentrations similar to those released by MP20, MP40, and MP60 in tap water (after 1 and 5 min) were tested by volunteers and scored accordingly. Tap water and commercial capsules were used as blank and control. MP20 solutions displayed scores of 1.00 and 1.50 at times 1 and 5 min, respectively (Table II). MP40 and MP60 showed higher scores. For example, MP40 displayed 2.20 and 3.10 for the same time points. As expected, for MP60, scores were even higher with 3.50 and 3.80, respectively. All the scores were lower than those determined for the commercial product.
Table II.
Sensoring Blind Test on Healthy Volunteers (n = 10)
| Bitterness score | ||
|---|---|---|
| Sample (min) | 1 | 5 |
| MP20 | 1.00 | 1.50 |
| MP40 | 2.20 | 3.10 |
| MP60 | 3.50 | 3.80 |
| Capsules | 4.00a | – |
| Tap water | 0.20 | – |
Samples were scored using a numerical scale from 0 to 4. Scores of 0, 1, 2, 3, and 4 corresponded to no detectable, threshold, slight, moderated, and strong bitterness, respectively. Score for each sample are expressed as means. Means <2 were considered of acceptable taste. In contrast, values >2 were defined as unacceptable
aCapsules disintegrated and the drug completely dissolved in tap water after ∼1 min
DISCUSSION
The palatability-related unsatisfactory pediatric patient compliance with indinavir regimes motivated us to explore the development of a taste-masked formulation based on the production of indinavir-loaded microparticles made of a pH-dependent pharmaceutical excipient, Eudragit E100. Our results support the feasibility of the proposed system to mask the bitterness of the drug, in contrast to the direct extemporaneous dispersion of the content of indinavir capsules that results in drug solutions of unacceptable taste (5).
Simple and highly reproducible double emulsion techniques were used for the production of drug-loaded microparticles in a laboratory scale (technology transfer to an industrial setting was out of the scope of the present studies). The initial w/o/w double emulsion process resulted in migration of indinavir across the relatively nonviscous organic phase and dissolution in the external aqueous medium. To improve the loading extents, a w/o/o system was implemented; the high hydrophobicity of the external corn oil phase and the poor solubility of indinavir prevented the passage of the drug from the internal (aqueous) to the external (oily) phase, increasing the % fload from 67% to about 90% (Table I). Size and morphology characterization showed a gradual loss in the regular spherical shape as drug content increased. This observation probably stemmed from a higher tendency of the drug to crystallize within the polymeric matrix. Also, a higher concentration of indinavir crystals on the surface of the microparticle led to the surface roughening (Fig. 2). The total amorphousness of the polymer matrix enabled to unequivocally identify the drug melting peak in DSC thermograms, even in systems with relatively low drug loads (MP20). Findings confirmed that even if the drug was mainly in its noncrystalline form, molecularly dispersed and crystalline forms coexisted.
In order to confirm the selective disintegration of the particles in the gastric environment, microparticles were assayed under pH conditions below and above the pKa of the polymer, 6.3. As expected, at pH 1.5, the amine-side chain groups of the polymer underwent protonation and microparticles fully disintegrated (Fig. 4). In contrast, in saliva-like pH medium, the polymer remained unprotonated and due to the limited water solubility of the carrier, only a very limited amount of indinavir was found in solution. It is apparent from the data presented that MP20 released faster than MP40, though values are expressed in % of drug released.
Clinical intake of powder formulations usually comprises the use of a liquid medium lacking buffering properties. Accordingly, microparticles were dispersed in tap water and the concentration of indinavir monitored over time (Fig. 5). Initially, both MP20 and MP40 maintained relatively low drug levels. In contrast, MP60 rapidly released the drug. Previous studies showed threshold taste detection limits for indinavir around 20 to 45 μg/mL (5). After 1 min, MP20 and MP40 displayed drug levels comparable to these values. In contrast, MP60 displayed concentrations associated with high bitterness. At 5 min, only MP20 maintained drug levels suitable for a formulation displaying an acceptable taste. The low concentration of indinavir at the microparticle/aqueous medium interface in MP20 prevented dissolution and formation of pores across the matrix that would lead to additional and enhanced dissolution. The smooth surface observed under the microscope supported the full entrapment of the drug by the polymer (Fig. 2a). Higher indinavir loads (MP40 and MP60) rendered systems where the polymer was unable to fully isolate the drug from the aqueous medium. This phenomenon resulted in the faster solubilization of outer indinavir clusters and generated an increasingly porous structure that favored water diffusion into the microparticle and further solubilization.
Remarkably, indinavir solutions with concentrations matching with the amounts released by MP20 systems displayed taste scores <2 in human volunteers, the upper limit value defined as acceptable in this study. This fact suggested the stability and acceptability at clinically relevant intake times. Even though after 1 min MP40 released in the same order of magnitude of MP20 (Fig. 5), a taste score slightly above 2 was obtained and the sample was defined as unacceptable. MP60 showed even higher scores.
Finally, the analysis of the indinavir content in different batches after 2 years (the expiry period of commercialized capsules is 18 months) indicated that MP20 samples were highly stable. These findings were supported by the thermal analysis. Contrary to that, more concentrated formulations displayed titer losses around 20–25%.
CONCLUSIONS
This is the first reported attempt to design optimized taste-masked indinavir HIV/AIDS pediatric formulations. For systems containing about 15% drug, taste studies confirmed the acceptability of the formulation. In pediatric regimes where doses in the 100–200-mg range are demanded, this composition would require the intake of an acceptable amount of formulation (0.7–1.5 g). In the case of doses around 400 mg (usually administered to teenagers), a 3-g dose also appears as a reasonable amount of microparticles, comparable to powders for extemporaneous preparation already in the market. Efforts are being dedicated to the optimization of the encapsulation process. Moreover, this technology could also serve as a platform for the taste masking of other protease inhibitors displaying unbearable organoleptic properties (e.g., ritonavir). Added flavors could eventually render a further improvement toward the design of cost-effective and scalable formulations for administration in children that, on one hand, enable easy swallowing and dose adjustment and, on the other hand, display acceptable organoleptic properties.
Acknowledgments
Authors thank the University of Buenos Aires and CONICET for financial support. Dr Christian Höcht is gratefully acknowledged for the stability analysis of the samples.
Footnotes
Diego A. Chiappetta and Ángel M. Carcaboso contributed equally to this work.
References
- 1.Nunn T., Williams J. Formulation of medicines for children. Br. J. Clin. Pharmacol. 2005;59:674–676. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyke R.B., Lee S., Johnson G.M., Wiznia A., Mohan K., Stanley K., Morse E.V., Krogstad P.A., Nachman S. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:61–67. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
- 3.WHO/Make medicines child size, In http://www.who.int/childmedicines/en/ (accessed 01/07/08).
- 4.Aarnoutse R., Schapiro J.M., Bouchr C.A.B., Hkster Y.A., Burger D.M. Therapeutic drug monitoring. Drugs. 2003;63:741–753. doi: 10.2165/00003495-200363080-00002. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman S., Zervakis J., Heffron S., Heald A.S. Effect of protease inhibitors on the sense of taste. Nutrition. 1999;15:767–772. doi: 10.1016/S0899-9007(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 6.Andrews L., Friedland G. Progress in the HIV therapeutics and the challenges of adherence to antiretroviral therapy. Inf. Dis. Clin. N. Amer. 2000;14:1–26. doi: 10.1016/S0891-5520(05)70215-4. [DOI] [PubMed] [Google Scholar]
- 7.Boyd M.A., Cooper D.A. Second-line combination antiretroviral therapy in resource-limited settings: facing the challenges through clinical research. AIDS. 2007;21(Suppl 4):S55–S63. doi: 10.1097/01.aids.0000279707.01557.b2. [DOI] [PubMed] [Google Scholar]
- 8.Boyd M.A. Indinavir: the forgotten HIV-protease inhibitor. Does it still have a role? Exp. Op. Pharm. 2007;8:957–964. doi: 10.1517/14656566.8.7.957. [DOI] [PubMed] [Google Scholar]
- 9.Galárraga O., O’Brien M.E., Gutiérrez J.P., Renaud-Théry F., Nguimfack B.D., Beusenberg M., Waldman K., Soni A., Bertozzi S.M., Greener R. Forecast of demand for antiretroviral drugs in low and middle-income countries: 2007–2008. AIDS. 2007;21(Suppl 4):S97–S103. doi: 10.1097/01.aids.0000279712.32051.29. [DOI] [PubMed] [Google Scholar]
- 10.Kim B.K., Hwang S.J., Park J.B., Park H.J. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J. Microencap. 2002;19:811–822. doi: 10.1080/0265204021000022770. [DOI] [PubMed] [Google Scholar]