INTRODUCTION
Nanoemulsions are isotropic, thermodynamically stable transparent (or translucent) systems of oil, water, and surfactants with a droplet size usually in the range of 10–100 nm (1,2). Their long-term stability, ease of preparation (spontaneous emulsification), and high solubilization of drug molecules make them promising as a drug delivery tool. They have found wide applications in oral drug delivery to enhance the solubility and bioavailability of the lipophilic drugs (3–5). Recently, there has been a surge in the exploration of nanoemulsions for transdermal delivery (6–8). They are also being investigated ardently for potential applications in ocular (9,10), pulmonary (11), nasal (12,13), vaginal (14,15), and parenteral drug delivery (16–18).
The use of nanoemulsions in drug delivery has been reviewed, and it was noted that most studies have not been very systematic with regard to selection of surfactants and cosurfactants. The main objective of this study was to provide an efficient screening approach for the proper selection of oils, surfactants, and cosurfactants for the nanoemulsion formulation development. Ropinirole was selected as a model lipophilic drug for this purpose (Log P = 3.32). These systems often require high surfactant concentration, and this may lead to toxicity and irritancy problems. Therefore, judicious selection of surfactants along with their optimum concentration is required, which has been discussed in this report. Determination of the influence of the surfactant-to-cosurfactant mass ratio (Smix) on the nanoemulsion formation region also formed an important aspect of the study. Optimum selection would aid in better formulation with desirable attributes.
MATERIALS AND METHODS
Components
Ropinirole was a gift sample from USV (Bombay, India). Propylene glycol monocaprylate (Capryol 90) and caprylocaproyl macrogol-8-glyceride (Labrasol) (Gattefosse, Gennevilliers, France) were gift samples from Colorcon Asia (Mumbai, India), while propylene glycol monocaprylic ester (Sefsol 218) was a courtesy from Nikko Chemicals (Tokyo, Japan). Diethylene monoglycol ether (Carbitol) and polyoxy-35-castor oil (Cremophor EL) were purchased from Merck Schuchardt (Hohenbrunn, Germany) and Sigma Aldrich (St. Louis, MO), respectively. Isopropyl myristate, glycerol triacetate (Triacetin), castor oil, high-performance liquid chromatography (HPLC)-grade methanol, and ammonium acetate were purchased from E-Merck (Mumbai, India). Polyoxyethylene sorbitan monolaurate (Tween 20), polyoxyethylene sorbitan monostearate (Tween 60), polyoxyethylene sorbitan monooleate (Tween 80), ethanol, isopropyl alcohol, n-butanol, PEG 400, and propylene glycol were procured from S.D Fine Chemicals (Mumbai, India). Water was obtained from Milli Q water purification system (Millipore, MA). All other chemicals and solvents were of analytical grade.
Screening of Oil
The solubility of ropinirole in various oils was determined by adding an excess amount of drug in 2 mL of the oils (Capryol 90, Sefsol-218, triacetin, isopropyl myristate, castor oil, olive oil) separately in 5-mL-capacity stopper vials, and mixed using a vortex mixer. The mixture vials were then kept at 25 ± 1.0°C in an isothermal shaker (Nirmal International, Delhi, India) for 72 h to reach equilibrium. The equilibrated samples were removed from the shaker and centrifuged at 3,000 rpm for 15 min. The supernatant was taken and filtered through a 0.22-μm membrane filter. The concentration of ropinirole was determined in oils using a HPLC method (see below).
Screening of Surfactants
Five types of surfactants were screened for nanoemulsion formulation, which included Labrasol, Cremophor EL, Tween 20, Tween 60, and Tween 80. In water, 2.5 mL of 15 wt.% surfactant solution was prepared, and 4 μL of oil was added with vigorous vortexing. If a one-phase clear solution was obtained, the addition of the oil was repeated until the solution became cloudy.
Screening of Cosurfactants
Tween 20 was combined with six types of solubilizers as cosurfactants, namely, ethanol, isopropyl alcohol, n-butanol, PEG 400, Carbitol, and propylene glycol. At a fixed Smix ratio of 1:1, the pseudoternary phase diagrams were constructed. Twelve different combinations in different weight ratios of oil and Smix, 1:9, 1:8, 1:7, 1:6, 1:5, 1:4, 1:3, 1:2, 1:1, 6:4 (1:0.7), 7:3 (1:0.43), and 9:1, were taken so that maximum ratios were covered to delineate the boundaries of phases precisely formed in the phase diagrams.
Effect of Surfactant and Cosurfactant Mass Ratio on Nanoemulsion Formation
Surfactant was blended with cosurfactant in the weight ratios of 3:1, 2:1, 1:1, 1:0, 1:2, and 1:3. These Smix ratios were chosen in decreasing concentration of surfactant with respect to cosurfactant and increasing concentration of cosurfactant with respect to surfactant for detailed study of the phase diagrams. Aqueous titration method was used for the construction of the pseudoternary phase diagrams, which involves stepwise addition of water to each weight ratio of oil and surfactants, and then mixing the components with the help of vortex mixer at 25°C (19). The nanoemulsion phase was identified as the region in the phase diagram where clear, easily flowable, and transparent formulations were obtained based on the visual observation. Twelve different combinations in different weight ratios of oil and Smix, 1:9, 1:8, 1:7, 1:6, 1:5, 1:4, 1:3, 1:2, 1:1, 6:4 (1:0.7), 7:3 (1:0.43), and 9:1, were taken. One axis of the pseudo-three-component phase diagram represented the aqueous phase, the other represented the oil phase, and the third represented a mixture of surfactant and cosurfactant at a fixed weight ratio (Smix).
Thermodynamic Stability Studies
Selected formulations were subjected to different thermodynamic stability tests to assess their physical stability.
Heating–cooling cycle: Six cycles between refrigerator temperature (4°C) and 45°C with storage at each temperature of not less than 48 h were conducted, and the formulations were examined for stability at these temperatures.
Centrifugation test: Formulations were centrifuged at 3,500 rpm for 30 min, and we looked for phase separation.
Freeze–thaw cycle: Three freeze–thaw cycles between −21°C and +25°C, with formulation storage at each temperature for not less than 48 h, were performed.
Globule Size Analysis
The droplet size of the nanoemulsions was determined by photon correlation spectroscopy, which analyses the fluctuations in light scattering due to Brownian motion of the particles (20) using a Zetasizer 1000 HS (Malvern Instruments, Worcestershire, UK). Light scattering was monitored at 25°C at a 90° angle.
Viscosity
The viscosity of the nanoemulsions was determined by using Brookfield R/S plus rheometer (Brookfield Engineering, Middleboro, MA) using a C50-1 spindle in triplicate at 25°C.
Refractive Index
The refractive index of the system was measured by an Abbe refractometer (Bausch and Lomb Optical Company, Rochester, NY) by placing one drop of the formulation on the slide in triplicate at 25°C.
pH Measurements
The apparent pH of the formulations was measured by a pH meter (Mettler Toledo MP 220, Greifensee, Switzerland) in triplicate at 25°C.
Transmission Electron Microscopy (TEM)
Morphology and structure of the nanoemulsion were studied using Morgagni 268D electron microscope (Fei Company, Netherlands) operating at 70 kV capable of point-to-point resolution. Combination of bright field imaging at increasing magnification and of diffraction modes was used to reveal the form and size of the nanoemulsion. In order to perform transmission electron microscopy (TEM) observations, a drop of the nanoemulsion was suitably diluted with water and applied on a carbon-coated grid, then treated with a drop of 2% phosphotungstic acid and left for 30 s. The coated grid was dried and then taken on a slide and covered with a cover slip and observed under the microscope.
HPLC Analysis
Quantitative determination of ropinirole was performed by a validated HPLC method developed in our laboratory (21). A Shimadzu-model HPLC equipped with quaternary LC-10A VP pump, variable wavelength programmable UV/VIS detector, SPD-10AVP column oven (Shimadzu), SCL 10AVP system controller (Shimadzu), Rheodyne injector fitted with a 20-μl loop was used and the data were recorded and evaluated using Class-VP 5.032 software. Chromatographic separation was achieved on a reversed-phase C-18 column, LiChrospher®100 (5 μm, 250 × 4.6 mm inner diameter) using a mobile phase consisting of methanol and 0.05 M ammonium acetate buffer pH 7 (80:20 v/v) at a flow rate of 1 ml/min with UV detection at 250 nm. The mobile phase was filtered through 0.22-μm nylon filter prior to use.
Statistical Analysis
The differences in the results of size and viscosity of nanoemulsion formulations were evaluated using one-way analysis of variance, followed by Tukey’s multiple comparison post test. The data were considered to be significant at p < 0.05.
RESULTS AND DISCUSSION
The most important criteria for selection of all the nanoemulsion components is that all the excipients should be pharmaceutically acceptable for oral administration or topical application, etc., depending upon the requirement and falling under the generally-regarded-as-safe category.
Screening Criteria for Oil Selection
Lipophilic drugs are preferably solubilized in o/w nanoemulsions, whereas w/o systems seem to be a better choice for hydrophilic drugs. Drug loading per formulation is a very critical design factor in the development of nanoemulsion systems for poorly soluble drugs, which is dependent on the drug solubility in various formulation components. The volume of the formulation should be minimized as much as possible to deliver the therapeutic dose of the drug in an encapsulated form. Solubility of the drug in the oil phase is an important criterion for the selection of oils. This is particularly important in the case of oral formulation development, as the ability of nanoemulsion to maintain the drug in solubilized form is greatly influenced by the solubility of the drug in the oil phase. If the surfactant or cosurfactant is contributing to drug solubilization, there could be a risk of precipitation, as dilution of nanoemulsion in the gastrointestinal tract will lead to lowering of the solvent capacity of the surfactant or cosurfactant (22,23). Thus, an understanding of factors influencing drug loading capacity while maintaining the capability of the system to undergo monophasic dilution with water and minimizing the tendency for drug precipitation or crystallization in diluted systems is essential to the design of stable and appropriately low-volume nanoemulsion systems for drug delivery applications. As of late, novel semisynthetic medium chain derivatives, which can be defined as amphiphilic compounds with surfactant properties, are being preferred.
The solubility of ropinirole in different oils was determined (Table I). The solubility of ropinirole was found to be highest in Capryol 90 (183.12 ± 3.74 mg/ml) as compared to other oils. This may be attributed to the polarity of the poorly soluble drugs that favor their solubilization in small/medium molar volume oils, such as medium-chain triglycerides or mono- or diglycerides (22). Edible oils are not frequently useful due to their poor ability to dissolve large amounts of lipophilic drugs. Moreover, formulation of nanoemulsion with oil of low drug solubility would require incorporation of more oil to incorporate the target drug dose, which in turn would require higher surfactant concentration to achieve oil solubilization, which might increase the toxicity of the system. Novel semisynthetic medium chain derivatives having surfactant properties are progressively and effectively replacing the regular medium chain triglyceride oils (24,25). Thus, Capryol 90 was selected as the oil phase for the development of nanoemulsion formulation.
Table I.
Solubility of Ropinirole in Different Oils at 25°C (mean ± SD, n = 3)
| S.No | Oil | Solubility (mg/ml) |
|---|---|---|
| 1 | Capryol 90 | 183.12 ± 3.74 |
| 2 | Sefsol-218 | 174.73 ± 1.98 |
| 3 | Isopropyl myristate | 135.28 ± 2.53 |
| 4 | Triacetin | 147.36 ± 2.06 |
| 5 | Castor oil | 43.27 ± 0.78 |
| 6 | Olive oil | 31.62 ± 0.66 |
Screening Criteria for Surfactants
The most critical problem related to the nanoemulsion-based systems is the toxicity of the components. Large amounts of surfactants may cause gastrointestinal and skin irritation when administered orally and topically, respectively. Therefore, the proper selection of surfactants becomes necessary. It is, therefore, important to determine the surfactant concentration properly and use the minimum concentration in the formulation. Nonionic surfactants are relatively less toxic than their ionic counterparts and typically have lower CMCs. Also, o/w nanoemulsion dosage forms for oral or parenteral use based on nonionic surfactants are likely to offer in vivo stability (26). Therefore, proper selection of surfactants becomes a crucial factor. Another important criterion is the selection of surfactant with proper HLB value. Hydrophilic surfactant and cosurfactant are considered to prefer the interface and to lower the necessary energy to form the nanoemulsions, consequently improving the stability. For example, the required HLB value to form o/w nanoemulsion is greater than 10 (27). The right blend of low and high HLB surfactants leads to the formation of a stable nanoemulsion upon dilution with water.
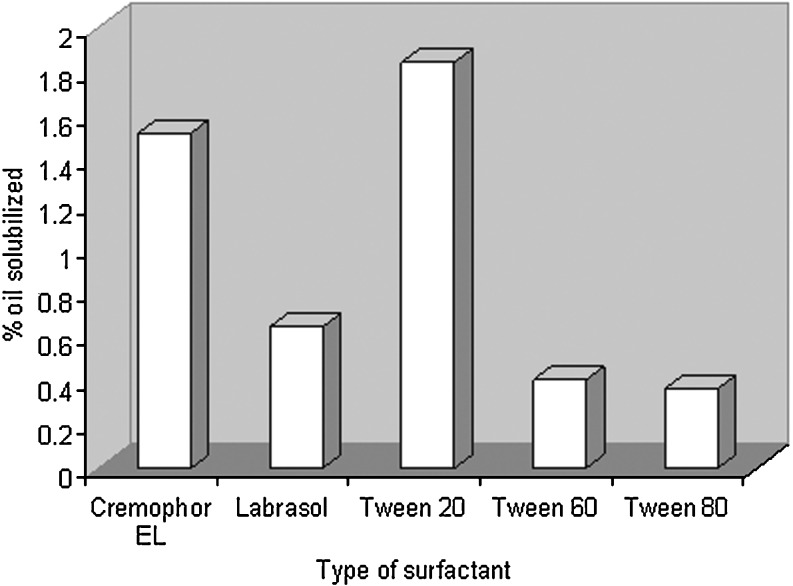
After selection of capryol 90 as the oil phase, the goal was to identify the surfactant that has the highest solubilization capacity for the oil. In the present study, five nonionic surfactants, namely, Labrasol, Cremophor EL, Tween 20, Tween 60, and Tween 80, were chosen for screening. Nonionic surfactants were selected since they are known to be less affected by pH and changes in ionic strength, are generally regarded as safe, and are biocompatible. Ionic surfactants were excluded from the study due to toxicological concerns. Although some authors had selected surfactants on the basis of drug solubility, we suggest that solubilization of oil with the surfactant is also an important factor. It is not necessary that the same surfactant that has good solubilizing power for drugs would have equally good affinity for the oil phase. Here, we have selected the surfactant giving the maximum nanoemulsion area alone, i.e., without the addition of the cosurfactant. The greater the nanoemulsion area is, the greater the nanoemulsification capacity of the surfactant is. As Tween 20 solubilized the maximum amount of Capryol 90, i.e., 1.84 wt.%, it was chosen as the surfactant for the nanoemulsion development. Surfactant–oil miscibility can thus give an initial indication on the possibility of nanoemulsion formation with this system. Figure 1 shows the solubilization behavior of the employed oil into five types of surfactant solutions.
Fig. 1.
Oil solubilized (wt.%) by different surfactants
Screening of Cosurfactants
Cosurfactants are added to obtain nanoemulsion systems at low surfactant concentration (28). Short- to medium-chain-length alcohols (C3–C8) are commonly added as cosurfactants, which further reduce the interfacial tension and increase the fluidity of the interface (20,29). They also increase the mobility of the hydrocarbon tail and allow greater penetration of the oil into this region. Alcohols may also increase the miscibility of the aqueous and oily phases due to its partitioning between these phases. Therefore, ethanol, isopropyl alcohol, 1-butanol, and propylene glycol were selected as cosurfactants. PEG 400 and Carbitol were also selected, as they also show increased permeation when incorporated into formulations and are relatively tolerable. All the cosurfactants studied are the pharmaceutically acceptable ingredients.
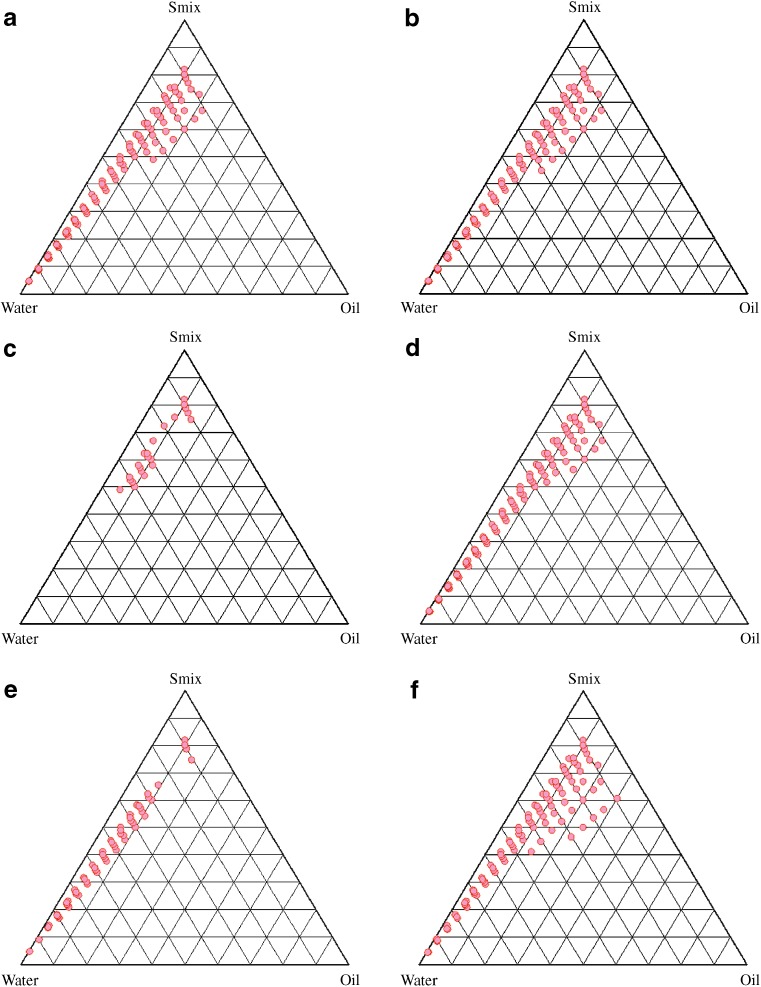
Nanoemulsion area was used as the assessment criteria for the evaluation of cosurfactants. The size of the nanoemulsion region in the phase diagrams were compared at a fixed Smix (1:1), keeping the surfactant the same but replacing the cosurfactant (Fig. 2). The larger the size of the nanoemulsion field is, the greater the nanoemulsification efficiency of the system is. It was found that, when the chain length was increased from ethanol (Fig. 2a) to isopropyl alcohol (Fig. 2b), it increased the area of the existence of the nanoemulsion. However, with n-butanol (Fig. 2c), a considerable decrease in the area was observed. Also, increasing the number of hydroxyl groups as we move from isopropyl alcohol to propylene glycol (Fig. 2d) reduced the nanoemulsion area. A limited nanoemulsion formation zone was obtained with PEG 400 (Fig. 2e). Carbitol gave the maximum nanoemulsion region as compared with the other tested cosurfactants (Fig. 2f). The presence of the cosurfactant/secondary surfactant and its type can thus affect the phase behavior of the nanoemulsion. Based on the results, Carbitol was found to be an efficient cosurfactant for its maximum nanoemulsion area, and hence, it was selected as the cosurfactant for the nanoemulsion formulation development.
Fig. 2.
Pseudoternary phase diagrams of nanoemulsion composed of Capryol 90, Tween 20, water and different cosurfactants a ethanol; b isopropyl alcohol; c butanol; d propylene glycol; e PEG 400; f carbitol at S mix 1:1
Effect of Surfactant and Cosurfactant Mass Ratio on Nanoemulsion Formation
Nanoemulsion formation is a function of composition of the system. The existence of nanoemulsion formation zone can be illustrated with the help of the pseudoternary phase diagram. The order of mixing of various components is not expected to influence the formation of nanoemulsion if the system is indeed thermodynamically stable (path-independent). Phase diagrams were constructed using Capryol 90 as the oil phase and Tween 20 and Carbitol as the surfactant and cosurfactant, respectively. No distinct conversion from w/o to o/w nanoemulsions was observed. The rest of the region on the phase diagram represents the turbid and conventional emulsions. Formulations were carefully observed so that the metastable systems were not selected, although the free energy required to form a nanoemulsion is very low and the formation is thermodynamically spontaneous (30).
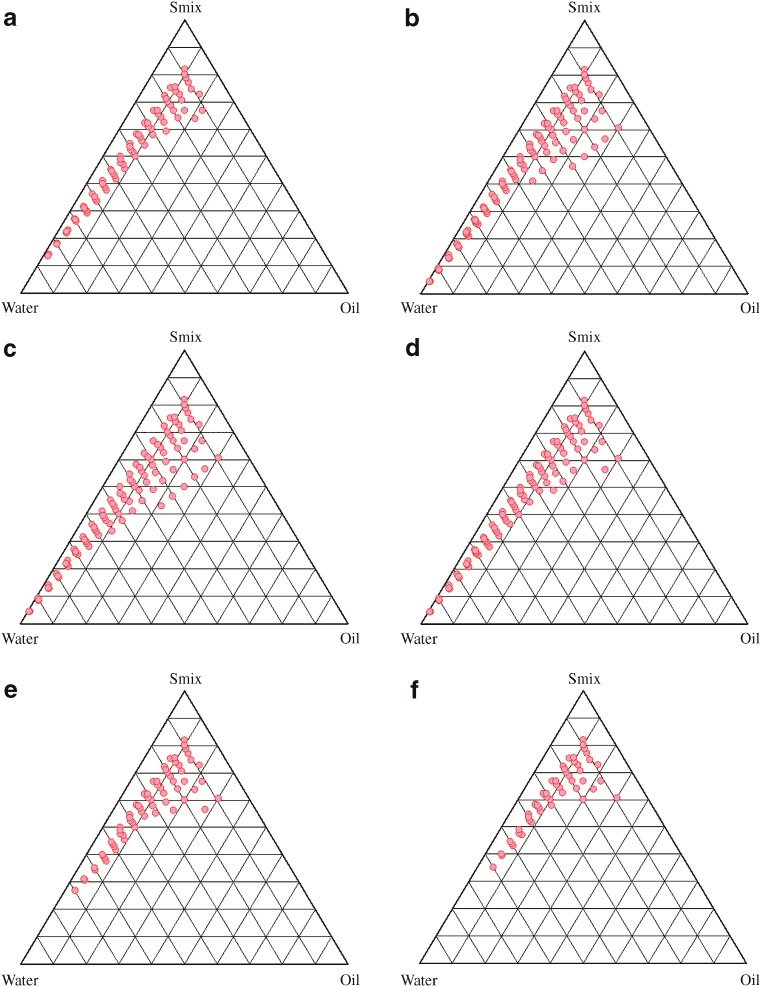
Effect of surfactant and cosurfactant mass ratio on nanoemulsion formation was evaluated for the further optimization of the system. In Fig. 3, a low-nanoemulsion area was observed when Tween 20 was used alone without cosurfactant, i.e., at the Smix ratio 1:0 (Fig. 3a). Probably, when the cosurfactant is absent or present at lower concentrations, the surfactant is not able to sufficiently reduce the o/w interfacial tension. An o/w nanoemulsion region was found towards the water-rich apex of the phase diagram. The maximum concentration of oil that could be solubilized, as can be seen in the phase diagram, was only 24% weight/weight (wt/wt) at 66% wt/wt of Smix. When cosurfactant was added with surfactant in equal amounts, a higher nanoemulsion region was observed, perhaps because of the further reduction of the interfacial tension and increased fluidity of the interface at Smix 1:1 (Fig. 3b). The maximum concentration of oil that could be solubilized was 31% wt/wt at 60% wt/wt of Smix. On further increasing the surfactant concentration, i.e., at Smix 2:1 (Fig. 3c), the nanoemulsion region increased in size as compared to the region in Smix 1:0 and Smix 1:1. When the surfactant concentration is further increased in the Smix ratio of 3:1 (Fig. 3d), a decrease in the nanoemulsion region was observed when compared with Smix 2:1, although the maximum amount of oil that could be solubilized by this ratio of Smix was 31% wt/wt with 60% wt/wt of Smix. It can be said that, when surfactant concentration was increased in comparison to cosurfactant, the nanoemulsion region increased up to the 2:1 Smix ratio, but in the 3:1 ratio, it was decreased, indicating that the optimum emulsification has been achieved. Therefore, there was no need to attempt an Smix ratio of 4:1. Thus, the areas of one phase nanoemulsion zones are dependent on surfactant composition (31). When the cosurfactant concentration with respect to surfactant was increased to the Smix 1:2, it was observed that the nanoemulsion area decreased as compared to Smix 1:1. When cosurfactant concentration was further increased to make Smix 1:3, a further decrease in the area was obtained. In case of Smix 1:2, for 5%wt/wt oil solubilization, the amount of Smix required was 32%wt/wt, while it was 35%wt/wt with the Smix 1:3. In contrast, only 20%wt/wt Smix was required in the case of the Smix 2:1 ratio. However, at higher Smix concentrations (60%wt/wt), the difference was less pronounced. A narrower nanoemulsion field at Smix 1:2 and Smix 1:3 was most likely due to a decrease in surfactant concentration by the increased presence of carbitol. It could be observed that the formulations prepared from phase diagrams in which the nanoemulsion area was extended towards an aqueous-rich apex could be diluted to a larger extent.
Fig. 3.
Pseudoternary phase diagrams indicating o/w nanoemulsion region of Capryol 90 (oil), water, Tween 20 (surfactant), and Carbitol (cosurfactant) at different S mix ratios indicated in a (S mix 1:0), b (S mix 1:1), c (S mix 2:1), d (S mix 3:1), e (S mix 1:2), and f (S mix 1:3)
The surfactant and cosurfactant mass ratio had been found to be a key factor influencing the phase properties, i.e., size and position of nanoemulsion region (32,33). The kind and concentration of oil employed also plays a role (34,35). Smix 2:1 showed the maximum area as compared to the other ratios. Such an effect was attributed to differences in the packing of surfactant and cosurfactant at the o/w interface. Attwood et al (36) also showed how size and location of nanoemulsion was changed on changing the mass ratio of polysorbate 40/sorbitol from 1:1 to 1:3.5 (36). Similar studies using polysorbate 80 (37) and polysorbate 60 (38) had shown a change in the optimum polysorbate/sorbitol mass ratio (i.e., that producing the largest nanoemulsion region) from 1:2.5 for polysorbate 80 to 1:2 for polysorbate 60 to 1:1.5 for polysorbate 40. It was also observed that decreasing the oil level led to an increase in the area of nanoemulsion formation. This fact suggested that the oil constitutes the inner phase of the nanoemulsion droplets, which is consistent with a direct o/w-type structure (39).
The usual preference is to select formulations with the lowest surfactant concentration for oral administration. However, for transdermal delivery, where enhanced skin permeation is the aim, it is not purposeful to select the lowest surfactant concentration. The surfactant concentration should be chosen so that it gives the maximum flux, which is an important criterion. This is usually not obtained with formulations that contain the highest amount of surfactant since high surfactant concentration decreases the thermodynamic activity of the drug in the vehicle, and the affinity of the drug to the vehicle becomes greater (40). Therefore, formulations should be optimized judiciously. As it could be seen from the phase diagrams, the surfactant or Smix that is able to increase the dispersion entropy, reduce the interfacial tension and increase the interfacial area, and thus, lower the free energy of the nanoemulsion system to a very low value with the minimum concentration, and that is thermodynamically stable, is a prospective candidate for efficient drug delivery.
Thermodynamic Stability Tests
In order to exclude the possibility of metastable formulations, stress testing is required. Some representative formulations were taken from the o/w nanoemulsion region of the phase diagram constructed at Smix 2:1, as it showed the maximum nanoemulsion area, and were subjected to the thermodynamic stability tests such as heating–cooling cycle, freeze–thaw cycle, and centrifugation. No phase separation, turbidity, creaming, or cracking was observed. All of them were found to be stable (data not shown). Thermodynamic stability confers long shelf life to the nanoemulsion as compared to ordinary emulsions. It differentiates them from emulsions that have kinetic stability and will eventually phase-separate (23,40). Table II shows the composition of these formulations.
Table II.
Composition of Selected Nanoemulsion Formulations
| Formulation Code | Oil (wt.%) | S mix (wt.%) | Water (wt.%) |
|---|---|---|---|
| NE T1 | 5 | 40 | 55 |
| NE T2 | 10 | 40 | 50 |
| NE T3 | 15 | 40 | 45 |
| NE T4 | 20 | 40 | 40 |
Characterization of the Selected Nanoemulsions
The nanoemulsions were selected so that all the formulations contain increasing concentrations of oil at a fixed Smix (40 wt.%) (Table II). Table III depicts the characteristics of these formulations.
Table III.
The Characteristics/Evaluation of the Nanoemulsion Formulations
| Formulation Code | Mean Globule Size (nm) | Polydispersity | Viscosity (mPas) | pH | Refractive Index |
|---|---|---|---|---|---|
| NET1 | 29.66 | 0.187 | 38.85 ± 1.69 | 5.48 ± 0.03 | 1.461 ± 0.018 |
| NET2 | 39.99 | 0.194 | 42.53 ± 1.38 | 5.54 ± 0.05 | 1.463 ± 0.015 |
| NET3 | 53.25 | 0.201 | 46.39 ± 1.55 | 5.57 ± 0.04 | 1.465 ± 0.023 |
| NET4 | 76.74 | 0.216 | 50.85 ± 1.46 | 5.62 ± 0.05 | 1.468 ± 0.025 |
Value represents as mean ± SD (n = 3)
The droplet size increased with increase in the concentration of the oil in the formulations (Table III). However, the droplet size of all the formulations was in the nano range. The low polydispersibility values observed for all the formulations indicated uniformity of droplet size within each formulation.
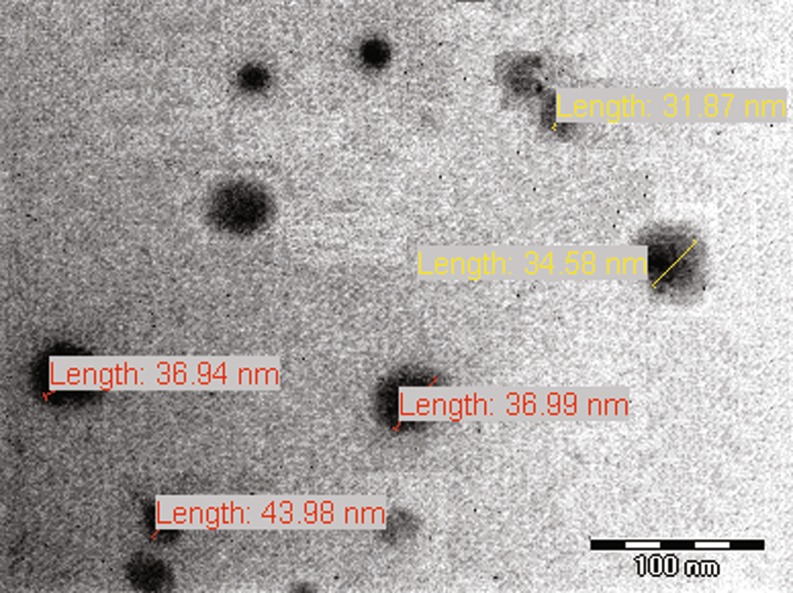
The droplets in the nanoemulsion appear dark, and the surroundings are bright; a “positive” image was seen using TEM (Fig. 4). Some droplet sizes were measured using TEM, as it is capable of point-to-point resolution.
Fig. 4.
Transmission electron micrograph of nanoemulsion NET1 showing the size of some oil droplets
Viscosity tends to increase with the oil content. As the oil content was increased from 5%wt/wt to 20%wt/wt, an increase in the viscosity of the formulations was observed (Table III). The viscosity of formulation NET1 was significantly lower than that of the other formulations (p < 0.05), which might be due to its lower oil content. Overall, very low viscosity of the formulations was observed, which is expected for nanoemulsions.
Refractive index is the net value of the components of nanoemulsion and indicates the isotropic nature of the formulation. The mean value of the refractive index for all the formulations was relatively similar. However, a slight increase in the refractive index was seen from formulations NT1 to NT4 (Table III). This might be attributed to a decrease in the water content, as water has a comparatively lower refractive index (the refractive index of water is 1.334).
Summary and Conclusion
Proper selection of components is critical to an efficient nanoemulsion formulation. Low-molar-volume oils are preferable instead of high-molar-volume oils, as they usually show better solubilization of the drug. As of late, novel semisynthetic medium chain derivatives, which can be defined as amphiphilic compounds with surfactant properties, are being preferred. Attention should be paid with regard to the tolerability of the constituting excipients. Recent efforts have, therefore, been focused on how to decrease or eliminate the toxicity or irritation of the nanoemulsion formulations. The study clearly illustrated the impact of the surfactant/cosurfactant weight ratio in the formulation of nanoemulsion systems. It is possible to achieve desirable properties by appropriately varying the level of oil, surfactants, and secondary surfactants.
Acknowledgements
The authors are thankful to the University Grants Commission, Govt. of India, for funding the project work. Adnan Azeem thanks the Colocon Asia Pvt. for providing gift samples of surfactants and oils (Gattefosse, France), Nikko Chemicals (Tokyo, Japan) for Sefsol-218, and USV (Mumbai, India) for the gift sample of ropinirole.
References
- 1.Shafiq S., Faiyaz S., Sushma T., Ahmad F.J., Khar R.K., Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Mei Z., Chen H., Weng T., Yang Y. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56:189–196. doi: 10.1016/S0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 3.Pineyro-Lopez A., Pineyro-Garza E., Torres-Alanis O., Reyes-Araiza R., Gomez-Silva M., Waksman N., Salazar-Leal M.E., Lujan-Rangel R. Evaluation of the bioequivalence of single 100-mg doses of two oral formulations of cyclosporin a microemulsion: A randomized, open-label, two-period crossover study in healthy adult male mexican volunteers. Clin Therapeutics. 2007;29:2049–2054. doi: 10.1016/j.clinthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 4.P.K. Ghosh, R.J. Majithiya, M.L. Umrethia and R.S.R. Murthy. Design and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS PharmSciTech. 2006; 7: Article 77. [DOI] [PMC free article] [PubMed]
- 5.Karasulu H.Y., Karabulut B., Goker E., Guneri T., Gabor F. Controlled release of methotrexate from w/o microemulsion and its in vitro antitumor activity. Drug Deliv. 2007;14:225–33. doi: 10.1080/10717540601067760. [DOI] [PubMed] [Google Scholar]
- 6.Ambade K.W., Jadhav S.L., Gambhire M.N., Kurmi S.D., Kadam V.J., Jadhav K.R. Formulation and evaluation of flurbiprofen microemulsion. Curr Drug Deliv. 2008;5:32–41. doi: 10.2174/156720108783331032. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J.S., Ansari M., Samaan M., Acosta E.J. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm. 2008;349:130–143. doi: 10.1016/j.ijpharm.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Shakeel F., Baboota S., Ahuja A., Ali J., Aqil M., Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech. 2007;8:E104. doi: 10.1208/pt0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fialho S.L., da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Expt Opthal. 2004;32:626–632. doi: 10.1111/j.1442-9071.2004.00914.x. [DOI] [PubMed] [Google Scholar]
- 10.Lv F-F., Li N., Zheng L-Q., Tung S-H. Studies on the stability of the chloramphenicol in the microemulsion free of alcohols. Eur J Pharm Biopharm. 2006;62:288–294. doi: 10.1016/j.ejpb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Sommerville M.L., Cain J.B., Johnson C.S., Jr., Hickey A.J. Lecithin inverse microemulsions for the pulmonary delivery of polar compounds utilizing dimethylether and propane as propellants. Pharm Dev Technol. 2000;5:219–230. doi: 10.1081/PDT-100100537. [DOI] [PubMed] [Google Scholar]
- 12.Lianly I.L., Nandi I., Kim K.H. Development of an ethyl laurate based microemulsion for rapid onset of intranasal delivery of diazepam. Int J Pharm. 2002;237:77–85. doi: 10.1016/S0378-5173(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 13.Vyas T.K., Babbar A.K., Sharma R.K., Singh S., Misra A. Intranasal mucoadhesive microemulsions of clonazepam: Preliminary studies on brain targeting. J Pharm Sci. 2005;95:570–580. doi: 10.1002/jps.20480. [DOI] [PubMed] [Google Scholar]
- 14.D’Cruz, Yiv S.H., Uckun F.M. GM-144, a novel lipophilic vaginal contraceptive gel-microemulsion. AAPS Pharm. Sci. Tech. 2001;2:E5. doi: 10.1208/pt020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Cruz O.J., Uckun F.M. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception. 2001;64:113–123. doi: 10.1016/S0010-7824(01)00233-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Chen D., Gao P., Ding P., Li K. Synthesis of Ibuprofen eugenol ester and its microemulsion formulation for parenteral delivery. Chem. Pharm. Bull. 2005;53:1246–1250. doi: 10.1248/cpb.53.1246. [DOI] [PubMed] [Google Scholar]
- 17.Jumma M., Muller B.W. The effect of oil components and homogenization conditions on the physicochemical properties and stability of parenteral fat emulsions. Int J Pharm. 1998;163:81–89. doi: 10.1016/S0378-5173(97)00369-4. [DOI] [Google Scholar]
- 18.von Corswant C., Thoren P., Engstrom S. Triglyceride based microemulsion for intravenous administration of sparingly soluble substances. J Pharm Sci. 1998;87:200–208. doi: 10.1021/js970258w. [DOI] [PubMed] [Google Scholar]
- 19.Chen H., Chang X., Weng T., Zhao X., Gao Z., Yang Y., Xu H., Yang X. A study of microemulsion systems for transdermal delivery of triptolide. J Control Rel. 2004;98:427–436. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Attwood D. Microemulsions. In: Kreuer J., editor. Colloidal Drug Delivery Systems. New York: Marcel Dekker; 1994. pp. 31–71. [Google Scholar]
- 21.Azeem A., Iqbal Z., Ahmad F.J., Khar R.K., Talegaonkar S. Development and validation of a stability indicating method for determination of ropinirole in the bulk drug and in pharmaceutical dosage forms. Acta Chromatographica. 2008;20:95–107. doi: 10.1556/AChrom.20.2008.1.8. [DOI] [Google Scholar]
- 22.Lawrence M.J., Rees G.D. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 23.Narang A.S., Delmarre D., Gao D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 2007;345:9–25. doi: 10.1016/j.ijpharm.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 24.Constantinides P.P. Lipid microemulsions for improving drug dissolution and oral absorption and biopharmaceutical aspects. Pharm Res. 1995;12:1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 25.Karim A., Gokhale R., Cole M., Sherman J., Yeramian P., Bryant M., Franke H. HIV protease inhibitor SC-52151: a novel method of optimizing bioavailability profile via a microemulsion drug delivery system. Pharm Res. 1994;11:S368. [Google Scholar]
- 26.Kawakami K., Yoshikawa T., Moroto Y., Kanaoka E., Takahashi K., Nishihara Y., Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. II. In vivo study. J Control Rel. 2002;81:75–82. doi: 10.1016/S0168-3659(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 27.Kommuru T.R., Gurley B., Khan M.A., Reddy I.K. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212:233–246. doi: 10.1016/S0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 28.Kreilgaard M., Pedersen E.J., Jaroszewski J.W. NMR characterization and transdermal drug delivery potential of microemulsion systems. J Control Rel. 2000;69:421–433. doi: 10.1016/S0168-3659(00)00325-4. [DOI] [PubMed] [Google Scholar]
- 29.Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 1999;16:461–521. [PubMed] [Google Scholar]
- 30.Craig D.Q.M., Barker S.A., Banning D., Booth S.W. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm. 1995;114:103–110. doi: 10.1016/0378-5173(94)00222-Q. [DOI] [Google Scholar]
- 31.Ajith S., Rakshit A.K. Studies of mixed surfactant microemulsion systems: Brij 35 and Tween 20 and sodium dodecyl sulfate. J. Phys. Chem. 1995;99:14778–14783. doi: 10.1021/j100040a030. [DOI] [Google Scholar]
- 32.Kreilgaard M., Pedersen E.J., Jaroszewski J.W. NMR characterization and transdermal drug delivery potential of microemulsion systems. J Control Rel. 2000;69:421–433. doi: 10.1016/S0168-3659(00)00325-4. [DOI] [PubMed] [Google Scholar]
- 33.Hua L., Weisan P., Jiayu L., Ying Z. Preparation, evaluation and NMR characterization of vinpocetine microemulsion for transdermal delivery. Drug Dev Ind Pharm. 2004;30:657–666. doi: 10.1081/DDC-120039183. [DOI] [PubMed] [Google Scholar]
- 34.Malcolmson C., Satra C., Kantaria S., Sidhu A., Lawrence M.J. Effect of oil on the level of solubilization of testosterone propionate into nonionic oil-in-water microemulsions. J Pharm Sci. 1998;87:109–116. doi: 10.1021/js9700863. [DOI] [PubMed] [Google Scholar]
- 35.Yuan Y., Li S-M., Mo F-K., Zhong D-F. Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm. 2006;321:117–123. doi: 10.1016/j.ijpharm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Attwood D., Mallon C., Ktistis G., Taylor C.J. A study on factors influencing the droplet size in nonionic oil-in-water microemulsions. Int J Pharm. 1992;88:417–422. doi: 10.1016/0378-5173(92)90341-X. [DOI] [Google Scholar]
- 37.Ktistis G. A viscosity study on oil-in-water microemulsions. Int J Pharm. 1990;61:213–218. doi: 10.1016/0378-5173(90)90211-L. [DOI] [Google Scholar]
- 38.Attwood D., Ktistis G. A light scattering study on oil-in-water microemulsions. Int. J. Pharm. 1989;52:165–171. doi: 10.1016/0378-5173(89)90292-5. [DOI] [Google Scholar]
- 39.Rhee Y-S., Choi J-G., Park E-S., Chi S-C. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228:161–170. doi: 10.1016/S0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 40.Shinoda K., Lindman B. Organized surfactant systems: microemulsions. Langmuir. 1987;3:167–180. doi: 10.1021/la00074a001. [DOI] [Google Scholar]