Abstract
Compacts containing selected bioadhesive polymers, fillers, and binders were investigated for their potential as a bioadhesive gastroretentive delivery system to deliver water soluble and water insoluble compounds in the stomach. Compacts with 90:10, 75:25, and 60:40 of polyvinylpyrrolidone (PVP) and polyethylene oxide (PEO) were evaluated for swelling, dissolution, bioadhesion, and in vitro gastric retention. Compacts containing higher PEO showed higher swelling (111.13%) and bioadhesion (0.62 ± 0.03 N/cm2), and retained their integrity and adherence onto gastric mucosa for about 9 h under in vitro conditions. In vivo gastroretentive property of compacts were evaluated in Yorkshire cross swines. Compacts containing 58% PVP, 40% PEO and 2% of water soluble or water insoluble marker compounds showed gastroadhesive and retentive properties in vivo. It is concluded that PEO in combination with PVP yields a non disintegrating type bioadhesive dosage form which is suitable for gastroretentive applications.
Key words: bioadhesion, compacts, gastroretentive, polyethylene oxide, polyvinylpyrrolidone
INTRODUCTION
Drugs such as riboflavin, levodopa, and metformin show preferential absorption only in the upper part of the gastrointestinal tract, especially the proximal part of small intestine, due to the presence of loose junctions and active transporters (1–7). Although the duodenum and jejunum are ideal sites for absorption, their relatively short residence time can prevent drugs from complete absorption, which may lead to poor oral bioavailability. For such drugs, enhancement of gastric residence is considered to be an approach for improving their oral bioavailability (8–11). However, the success of gastroretentive systems vary due to various biological factors such as mucus turnover, gastric emptying, age, posture, bed rest, exercise, psychological status, pathophysiology of gastrointestinal (GI) transit, food effects, and formulation factors of dosage forms such as time of dosing, type of formulation (solid versus liquids), density of formulation, dosage form size, GI transit, etc. To achieve consistent gastroretentive results, several formulations have been designed, including altered density systems (12–17), expandable swelling systems (18–20), and bioadhesive systems (21–24).
Bioadhesive systems explore the adhesive properties of some polymers on the mucus linings of various biological tissues for increasing the residence time of delivery devices in a specific biological location. Such increase in residence time prompts to enhance the bioavailability of drugs (8,22,25). A bioadhesive formulation that can swell and expand in size also increases retention in the stomach for a longer duration via retardation of premature passage of the dosage unit through the pyloric sphincter. In addition to bioadhesion and swelling, bioadhesive system should also possess sufficient mechanical strength in order to withstand the mechanical forces which are created by digestive activities of the stomach. Delivery systems in the forms of gels and films may not have sufficient mechanical strength. Although a nondisintegrating and swelling type tablet formulation made with a bioadhesive polymer can overcome mechanical issues, a concern with such systems is the fate of the formulation matrix after drug release. Very slow erosion or dissolution may lead to expulsion and other gastrointestinal safety issues. Undigested materials in the stomach will either be expelled out to the mouth for further grinding by reflux activities or the house keeper wave of stomach will push it through the relaxed and open pylorus to the intestine. Expulsion into the mouth or ingestion into the intestine happens with solid dosages that are designed for prolonged release in the stomach, which will affect the performance of the delivery system. Complete dissolution or erosion of the formulation matrix in a timely manner is, therefore, important for gastroretentive dosage forms.
Earlier studies performed by Bhaskara, R.J., et al. have shown that polymeric compacts made of hydroxypropylmethyl cellulose (HPMC), polyacrylic acid (PAA), polyethylene oxide (PEO) and Carbopol 974 showed regional variations in adhesion with porcine buccal, gastric and intestinal mucosa (26). In all three regions, PEO showed relatively higher adhesion than HPMC and PAA. The swelling characteristics of polymers were found to contribute to mucoadhesion. The swelling ratio of PEO was approximately twice as that of HPMC in acidic and neutral testing media. The swelling behavior of PEO hydrogels was not influenced by the pH or ionic strength of medium and, therefore, showed a similar bioadhesive trend in acidic and neutral pH environments. Carbopol, in contrast, showed pH dependant swelling pattern, which resulted in variations in its bioadhesion at different locations. Although Carbopol exhibited a comparable swelling ratio to that of PEO in acidic pH, a threefold higher swelling was observed in phosphate buffer at pH 6.8 due to ionization of carboxylic groups. The pH independent swelling and bioadhesion behavior of PEO makes it a reliable polymer for bioadhesion in stomach.
Due to the mucoadhesive capability of the dosage form, the tablet may adhere to the esophagus during the administration. A coated solid dosage form which is made of a swelling type bioadhesive polymer with improved dissolution properties may avoid adherence in the mouth or esophagus, improve gastroadhesion, avoid premature expulsion from the stomach, and assure the safety and efficacy of the delivery system. Such systems are expected to reside in the stomach for relatively longer duration than solution dosages, disintegrating type solid formulations, and other conventional formulations, improving the absorption of drugs that show preferential absorption in the stomach or upper part of intestine. The objective of the present study is to formulate a slow dissolving bioadhesive matrix using PEO in combination with different hydrophilic diluents and evaluate the dissolution, swelling, in vitro and in vivo gastric retention properties of polymeric compacts for the delivery of water soluble and water insoluble compounds in the stomach.
MATERIALS
Three different grades of polyethylene oxides were obtained from DOW Chemical Company. The molecular weight of Polyox WSR 301, polyox WSR coagulant, and polyox WSR 303 used in this study have molecular weights of 4,000,000, 5,000,000, and 7,000,000, respectively (27). PVP K-30 USP and PVP K-90 USP were obtained from BASF Corporation. Compressible sugar (Domino Specialty Ingredients), mannitol (Roquette), starch (Spectrum Chemicals), lactose (Hilmar Ingredients), microcrystalline cellulose (FMC Biopolymer), and dicalcium phosphate (Innophos) were of pharmacopeial grades. Methylene blue and phenylazoaniline obtained from Sigma–Aldrich were use as water soluble and water insoluble model compounds, respectively.
METHODS
Preparation and Characterization of Compacts
Bioadhesive matrices were formulated using PEO in combination with different hydrophilic diluents/binders. Circular shaped-flat surfaced compacts of 6.35-mm internal diameter (65 mG weight) were directly compressed after mixing different components as listed in Table I. All of the components were passed through a US standard sieve 45 prior to weighing. Weighed quantities of components were mixed by geometric dilution and compressed into compacts using a Stokes 16-Station tablet machine at 1.5 ton compression force. All operations were performed at room temperature (23–25°C). The batch size of each composition was 10 G. Compacts were evaluated for disintegration, swelling, gastroadhesion, in vitro dissolution, in vitro gastroretention, and in vivo gastroretention.
Table I.
Composition of Compacts
| Formulation code | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| A1 | 100% PEO 301 | – | – |
| A2 | 100% PEO Coagulant | – | – |
| A3 | 100% PEO 303 | – | – |
| B1 | 100% Compressible sugar | – | – |
| B2 | 100% Mannitol | – | – |
| B3 | 100% Starch | – | – |
| B4 | 100% Lactose | – | – |
| B5 | 100% Microcrystalline cellulose | – | – |
| B6 | 100% Dicalcium phosphate | – | – |
| B7 | 100% PVP K-30 | – | – |
| B8 | 100% PVP K-90 | – | – |
| C1 | 90% PVP K-90 | 10% PEO 303 | – |
| C2 | 75% PVP K-90 | 25% PEO 303 | – |
| C3 | 60% PVP K-90 | 40% PEO 303 | – |
| D1 | 58% PVP K-90 | 40% PEO 303 | 2% MB |
| D2 | 58% PVP K-90 | 40% PEO 303 | 2% PAA |
PEO Polyethylene oxide, PVP polyvinylpyrrolidone, MB Methylene blue, PAA Phenylazoaniline
The disintegration of compacts was determined in simulated gastric fluid (SGF). Compacts were immersed in 10 mL of SGF in glass vials and agitated at 100 rpm using a water bath shaker at 37 ± 0.5°C. Each test vial was carefully observed for disintegration or deformation of the compacts. The time required for retaining at least 25% of the original dimension of compacts in SGF was recorded and used an indicative parameter to compare different formulations. The study was carried out up to 6 h.
Swelling of compacts was determined by immersion method. Compacts were immersed in glass Petri-dish containing 5 mL of SGF at room temperature. The radius of each compact was measured at 1, 3 and 6 h without disturbing Petri dish. The diameter of the compacts was determined and compared with that of the original diameter to calculate the percent of swelling. The following equation was used for calculating the swelling of compacts.
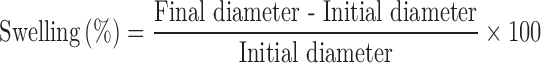 |
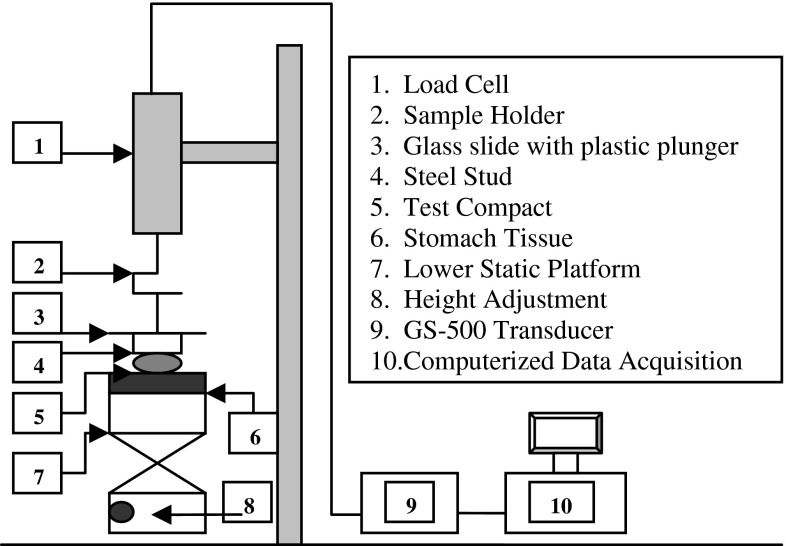
Adhesive strength of the compacts to porcine stomach mucosa was evaluated using a system consisting of a precision load cell (GS-500 Transducer techniques, Temecula, CA) with a sample holder and a computerized data acquisition system (Model 500 A, Keithley Metrabyte, Taunton, MA and an IBM computer). Data were analyzed using EasyLx software (Keithley Metrabyte). As shown in Fig. 1, a hanging platform consisting of a glass slide attached with plastic plunger (8 cm) on the top and a circular-steel stud (0.5 cm) with flat surface on the bottom was attached to the load cell. A flat surfaced steel block having 3-cm diameter was used as a lower static platform. The mucosa was mounted onto the lower platform using a screw-clamp. The compacts were attached to the bottom of the hanging platform using a synthetic adhesive. The hanging platform with the compact was brought down and placed over the surface of the mucosa with an applied force of 20 G for 1 min, and the force required to detach the compacts from the mucosal surface was determined. Between each measurement, the mucosal surface was rinsed with 4 mL of purified water. The excess water was wiped with a soft tissue paper and the mucosa was wetted with 25 μl of SGF. The force of detachment between the solid body of the hanging platform (without compacts) and the mucosal surface without any applied force was considered blank. A calibration curve was developed using known weights attached onto the hanging platform. The detachment force in mV/cm2 was converted into G/cm2 from the calibration curve and transformed into N/cm2 by using a conversion factor (1 G = 0.009806 N). The test was performed at room temperature (23–25°C), and the mean of six measurements was used as the mucoadhesive strength of the compacts. The thickness of stomach mucosa employed in experiments ranged from 1.3 to 2.5 mm.
Fig. 1.
Bioadhesion testing setup
Dissolution time of compacts in SGF was determined using a USP dissolution apparatus 2 with paddle attachment. Prior to dissolution of compacts, about 250 mL of SGF was transferred into dissolution jars and heated to 37 ± 0.5°C. Compacts were added into SGF containing jars and stirred at 100 rpm. The dissolution times of the compacts were measured by visual observation every 15 min for their complete dissolution in the medium. All studies were conducted in triplicate.
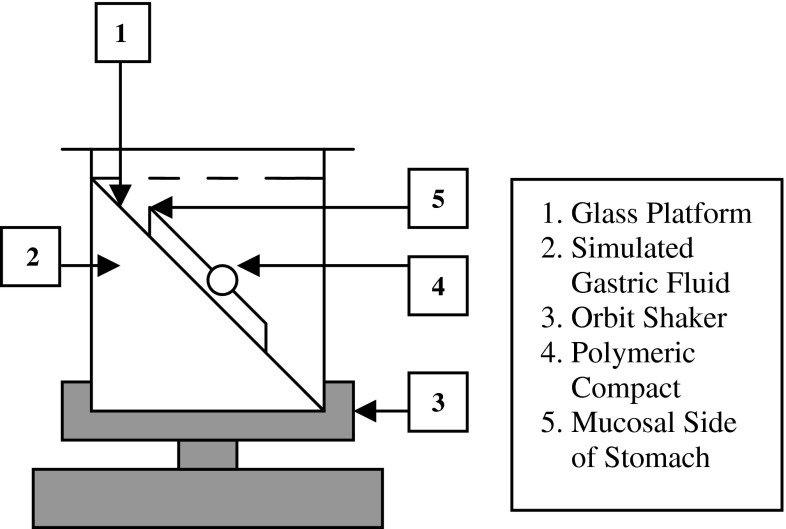
The in vitro gastric retention of compacts was studied using porcine stomach tissue immersed in SGF. The stomach of freshly slaughtered pig was obtained from a local slaughter house. The stomach was preserved in McIlvaine buffer at 4–8°C during transportation. Prior to the experiment, the tissue was allowed to equilibrate to room temperature and incised with a surgical scissor. The gastric contents on the mucosal surface were gently rinsed with about 250 ml of 0.1 N hydrochloric acid. About 4 cm2 tissue piece from the body region of the stomach was removed and fixed on to a solid platform. It was then immersed into 250 ml of SGF and allowed to equilibrate to 37 ± 0.5°C for 30 min. The tissue platform was gently pulled out of SGF and a test polymeric compact was gently placed on the wet mucosal surface. After a minute of contact, it was immersed back into the SGF. The platform with test compact was positioned at a 60° angle within the medium, and agitated at 100 rpm using an orbital shaker. The entire set up was maintained at 37 ± 0.5°C throughout the study duration (Fig. 2). The attachment of compacts with the gastric mucosa surface was visually inspected. The time required for the compact to detach from the mucosa was recorded as the in vitro retention time. Studies were conducted in triplicate.
Fig. 2.
In vitro gastroretention study setup
In vivo studies
A composition that showed higher bioadhesion and longer in vitro retention on the gastric mucosa was selected and its in vivo gastroretention was evaluated in Yorkshire cross swines. Methylene blue (MB) or phenylazoaniline (PAA; 2%) were incorporated into the compacts as markers to identify their presence in the GI tract. MB is a water soluble blue colored dye and PAA is a water insoluble yellow dye. Compacts were coated with 3–4% of HPMC to avoid adhesion in the mouth or esophagus during administration or swallowing. The test products were administered to healthy swines of either sex, aged 9–10 weeks, with body weights ranging from 47 to 53 lbs after overnight fasting. Compacts were administered using orogastric tubes with 75 mL of water. All animals had free access to water. Animals were observed for any signs of nausea, vomiting or spitting of tested compacts for about 1 h after administration, and observed for their normal behavior and/or any possible adverse effects throughout the study. Animals were sacrificed at different time intervals for gastroretentive examination. The esophagus, cardiac, body, and pylorus regions of stomach, duodenum, and intestine were carefully examined for the presence of compacts or signs of blue or yellow coloration of the marker compounds. Animal studies were performed per IACUC protocols of the University of the Pacific.
RESULTS AND DISCUSSIONS
The disintegration and bioadhesion of test compacts prepared with different excipients are shown in Table II. Compacts made of different grades of polyox were physically stable for more than 6 h in simulated gastric fluid at 37°C, and exhibited higher bioadhesion on the porcine gastric mucosa compared to all other excipients. Although more than 25% of the initial dimension of all PEO compacts were retained for about 6 h in SGF, the extent of dissolution of compacts containing polyox WSR 301 and WSR coagulant grades of PEO were higher than that of polyox WSR 303. In addition, polyox WSR 303 showed highest adhesive strength (1.13 N/cm2) compared to other grades of PEO and all other excipients. Better retention and bioadhesion of compacts containing WSR 303 grade of PEO could be attributed to its higher molecular weight (27,28). During the process of bioadhesion, bioadhesive polymers undergo wetting, swelling, and interdiffusion or interpenetration into the mucus or epithelial surface. In this process, polymers with optimum molecular weights are believed to make strong entanglements and reside in the application site for prolonged period of time (22).
Table II.
Disintegration and Bioadhesion of Compacts
| Formulation | Disintegration (Min) | Bioadhesion (N/cm2) |
|---|---|---|
| PEO 301 | >360 | 0.44 ± 0.05 |
| PEO coagulant | >360 | 0.69 ± 0.03 |
| PEO 303 | >360 | 1.13 ± 0.09 |
| Compressible sugar | 4.33 ± 1.53 | 0.02 ± 0.02 |
| Mannitol | 3.67 ± 0.58 | 0.08 ± 0.03 |
| Starch | 1.33 ± 0.58 | 0.12 ± 0.03 |
| Lactose | 3.00 ± 1.00 | 0.06 ± 0.01 |
| Microcrystalline cellulose | 15.33 ± 1.53 | 0.11 ± 0.05 |
| Dicalcium phosphate | 4.67 ± 2.08 | 0.08 ± 0.01 |
| PVP K-30 | 47.00 ± 5.29 | 0.16 ± 0.03 |
| PVP K-90 | 176.33 ± 8.02 | 0.26 ± 0.02 |
PEO Polyethylene oxide, PVP polyvinylpyrrolidone
Among the other excipients screened, only PVP compacts retained their structure for more than 45 min. Excipients such as compressible sugar, mannitol, starch, lactose, dicalcium phosphate, and microcrystalline cellulose either dissolved completely or disintegrated in SGF quickly. None of the excipients showed any significant level of bioadhesive interaction with the gastric mucosa. Incorporation of such rapidly dissolving or disintegrating and poorly bioadhesive excipients could substantially reduce the retentive and adhesive properties of gastroretentive formulations. PVP is a nonirritant material and was not absorbed from the GI tract (29,30). It is extensively used as a tablet binder and also as a thickening, suspending, and stabilizing agent in oral formulations (29). Among two different molecular grades of PVP screened, K-90 showed about threefold slower disintegration and twofold higher bioadhesive forces. The dissolution rate of various grades of PVP is generally controlled by the viscosity of the resulting solution, which is primarily controlled by the molecular weight. High molecular weight grades of PVP offer higher binding capacity. The molecular weight of PVP K-90 is approximately 20-fold higher than PVP K-30. This obviously resulted in slow dissolution of PVP K-90 compacts. Binding and sticking properties of PVP also contribute to the bioadhesion (30). Additionally, PVP’s high plastic deformation property makes it suitable as a binder-filler for direct compression processing.
Physically stable structures are generally unsuitable for gastroretentive studies due to the reflux or house keeping activities and possible premature expulsion of dosage form from the stomach (8,9,31). Although PEO compacts showed highest bioadhesion in this study, they did not dissolve in SGF for about 6 h. In contrary, PVP K-90 showed relatively lower bioadhesion than PEO compacts (0.26 ± 0.02 N/cm2), and complete dissolution within 3 h time. Although it may not provide additional bioadhesion to compacts, PVP serves as a modulator for dissolution of the PEO matrix and avoids the issues of excessive residence in the stomach. As both dissolution and adhesive properties are required for an ideal gastroretentive formulation, combination of PEO and PVP would be complimentary to each other in providing a bioadhesive and soluble dosage form.
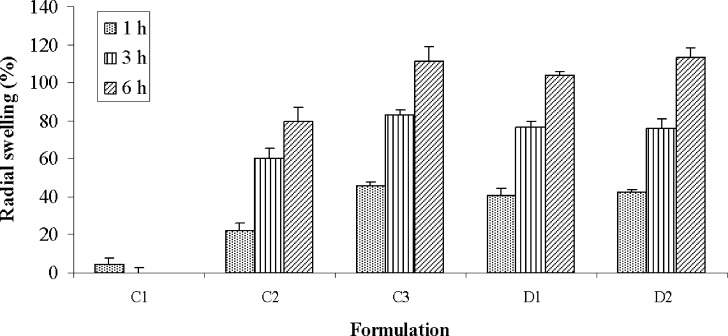
Compacts made with PEO showed gradual swelling in SGF, whereas PVP compacts did not show any swelling due to its dissolution characteristics. The extent of radial swelling shown by PEO compacts after 1, 3, and 6 h was 48%, 87%, and 136%, respectively. The swelling of compacts that were formulated with three different proportions of PEO and PVP are shown in Fig. 3. Incorporation of 10% PEO into PVP matrix did not improve the swelling property or delay the dissolution of compacts. Increasing the content of PEO above 25% however showed marked changes in swelling or dissolution properties. The swelling of compacts containing 25% and 40% of PEO after 6 h were 80% and 111%, respectively, and their dissolution times were increased to 6.33 ± 0.38 and 8.92 ± 0.38 h. Incorporation of 2% water soluble and insoluble marker dyes did not alter the swelling property of compacts.
Fig. 3.
Swelling of PEO-PVP compacts
The dissolution, bioadhesion and gastroretention of PEO-PVP compacts are shown in Table III. The bioadhesive strength of the PEO 303 compact was 1.13 ± 0.09 N/cm2, whereas PVP K-90 compacts yielded a bioadhesive strength of 0.26 ± 0.02 N/cm2. Incorporation of PEO into a PVP matrix gradually increased the bioadhesive strength of the compacts. Bioadhesion of PVP compacts increased linearly as PEO content was increased (r2 = 0.99). Such a linear relationship between the polymer content and bioadhesion provides a basis in designing systems with predictable bioadhesion and dissolution properties. The in vitro gastroretentive time of compacts increased significantly with an increase in PEO content, which was mainly due to the increase in the bioadhesive strength. PEO-PVP compacts did not detach from the gastric mucosa throughout the duration of the study and dissolved completely into the surrounding medium at different time intervals. The in vitro gastric residence of 10:90, 25:75 and 40:60 PEO-PVP compacts were1.58 ± 0.38, 5.58 ± 0.63 and 8.67 ± 0.63 h, respectively. Bioadhesion and in vitro gastroretention were not influence by the incorporating 2% of MB or PAA into the compact matrix. Dissolution rate decreased in the case of PAA containing compacts, while incorporation of MB did not change the dissolution rate.
Table III.
Dissolution, Bioadhesion, and In Vitro Gastroretention of PEO-PVP Compacts
| Code | Composition | Dissolution Time (h) | Bioadhesion (N/cm2) | In vitro GRT (h) |
|---|---|---|---|---|
| C1 | 90% PVP K-90 + 10% PEO | 1.83 ± 0.29 | 0.32 ± 0.03 | 1.58 ± 0.38 |
| C2 | 75% PVP K-90 + 25% PEO 303 | 6.33 ± 0.38 | 0.48 ± 0.03 | 5.58 ± 0.63 |
| C3 | 60% PVP K-90 + 40% PEO 303 | 8.92 ± 0.38 | 0.62 ± 0.03 | 8.67 ± 0.63 |
| D1 | 58% PVP K-90 + 40% PEO 303 + 2% MB | 8.75 ± 0.25 | 0.61 ± 0.02 | 8.58 ± 0.63 |
| D2 | 58% PVP K-90 + 40% PEO 303 + 2% PAA | 9.08 ± 0.14 | 0.59 ± 0.01 | 8.67 ± 0.88 |
PEO Polyethylene oxide, PVP polyvinylpyrrolidone, MB Methylene blue, PAA Phenylazoaniline
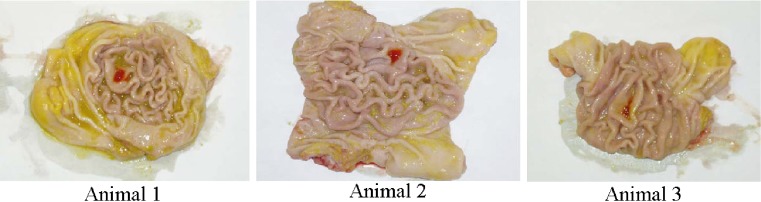
For the in vivo gastroretentive study conducted in Yorkshire cross swines, all animal showed normal behavior and no signs on nausea or vomiting upon administration of the test compacts. PEO–PVP compacts containing MB were hydrated with gastric fluid and were found to adhere to the body region of the stomach. The compacts were retained on the mucosal surface of stomach in all three animals after 1 h. The force of adhesion of the swollen matrix withstood manual flushing of water (Fig. 4). The MB compacts on the tissue were deformed with finger pressure. After 3 h, a mild blue stain was observed in the body region of stomach in only one animal. In the remaining two animals, neither blue stain nor compact fragments could be located in the stomach or intestine. PAA containing compacts were also found in the stomach region of all three animals after 1 h (Fig. 5). However, the force of adhesion of the hydrated compact matrix on the gastric mucosa was not as strong as those of methylene blue compacts. The swollen mass of compact could be easily removed without deformation. This could be due to its poor swelling nature as observed in in vitro studies. After 4 h, a mild red stain of PAA was observed in the stomach of two animals. No fragments of compact could be located in any other part of GI tract. In the third animal, neither red stain nor compact fragments could be located in stomach or intestine. Variations at 3–4 h may be due to dissolution of entire matrix under in vivo conditions. Although prolonged in vitro gastroretention time (8–9 h) shown by PEO–PVP compacts could not be reproduced in vivo, the preliminary results of in vivo studies provided valuable information for the design of such bioadhesive dosage form. In addition, the in vivo study can serve as a tool for screening the influences of various formulation and process variables on bioadhesion and gastroretention of dosage forms.
Fig. 4.
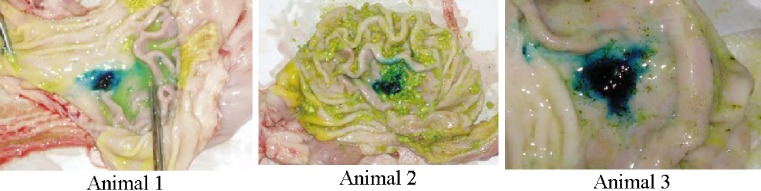
Adhesion of MB compact in the body region of stomach after 1 h
Fig. 5.
Adhesion of PAA compact in the body region of stomach after 1 h
CONCLUSIONS
Compared to liquids and disintegrating type products, non disintegrating-bioadhesive compacts which reside in the stomach for more than 1 h are likely to enhance bioavailability of drugs that have preferential absorption in stomach or duodenum. The preliminary results have shown the flexibility in altering the bioadhesive, swelling, and dissolution characteristics of PEO compacts by incorporation of PVP, and possibility of delivering varying quantities of water soluble as well as insoluble drugs without compromising their gastroretentive performance.
Acknowledgements
The authors acknowledge Long Ranch (Manteca) for providing isolated porcine stomach tissues and Pork Power Farm (Turlock) for providing animal research facility for conducting animal studies.
Dedication of the Manuscript: This manuscript is dedicated to the memory of Joseph Robinson, Ph.D.
References
- 1.Ali J., Hasan S., Ali M. Formulation and development of gastroretentive drug delivery system for ofloxacin. Methods Find. Exp. Clin. Pharmacol. 2006;28(7):433–439. doi: 10.1358/mf.2006.28.7.1003574. [DOI] [PubMed] [Google Scholar]
- 2.Ali J., et al. Development and evaluation of a gastroretentive drug delivery system for the low-absorption-window drug celecoxib. PDA J. Pharm. Sci. Technol. 2007;61(2):88–96. [PubMed] [Google Scholar]
- 3.Basak S. C., Rahman J., Ramalingam M. Design and in vitro testing of a floatable gastroretentive tablet of metformin hydrochloride. Pharmazie. 2007;62(2):145–148. [PubMed] [Google Scholar]
- 4.Christensen S. The biological fate of riboflavin in mammals. A survey of literature and own investigations. Acta Pharmacol. Toxicol. (Copenh). 1973;32:3–72. [PubMed] [Google Scholar]
- 5.Dave B. S., Amin A. F., Patel M. M. Gastroretentive drug delivery system of ranitidine hydrochloride: formulation and in vitro evaluation. AAPS PharmSciTech. 2004;5(2):e34. doi: 10.1208/pt050234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deleu D., Ebinger G., Michotte Y. Clinical and pharmacokinetic comparison of oral and duodenal delivery of levodopa/carbidopa in patients with Parkinson’s disease with a fluctuating response to levodopa. Eur. J. Clin. Pharmacol. 1991;41(5):453–458. doi: 10.1007/BF00626368. [DOI] [PubMed] [Google Scholar]
- 7.Marathe P. H., et al. Pharmacokinetics and bioavailability of a metformin/glyburide tablet administered alone and with food. J. Clin. Pharmacol. 2000;40(12 Pt 2):1494–1502. [PubMed] [Google Scholar]
- 8.Davis S. S. Formulation strategies for absorption windows. Drug Discov. Today. 2005;10(4):249–257. doi: 10.1016/S1359-6446(04)03351-3. [DOI] [PubMed] [Google Scholar]
- 9.Moes A. J. Gastroretentive dosage forms. Crit. Rev. Ther. Drug Carrier. Syst. 1993;10(2):143–195. [PubMed] [Google Scholar]
- 10.Streubel A., Siepmann J., Bodmeier R. Gastroretentive drug delivery systems. Expert. Opin. Drug Deliv. 2006;3(2):217–233. doi: 10.1517/17425247.3.2.217. [DOI] [PubMed] [Google Scholar]
- 11.Streubel A., Siepmann J., Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006;6(5):501–508. doi: 10.1016/j.coph.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Jaimini M., Rana A. C., Tanwar Y. S. Formulation and evaluation of famotidine floating tablets. Curr. Drug Deliv. 2007;4(1):51–55. doi: 10.2174/156720107779314730. [DOI] [PubMed] [Google Scholar]
- 13.Rouge N., et al. Comparative pharmacokinetic study of a floating multiple-unit capsule, a high-density multiple-unit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm. Acta Helv. 1998;73(2):81–87. doi: 10.1016/S0031-6865(97)00050-2. [DOI] [PubMed] [Google Scholar]
- 14.Streubel A., Siepmann J., Bodmeier R. Multiple unit gastroretentive drug delivery systems: a new preparation method for low density microparticles. J. Microencapsul. 2003;20(3):329–347. doi: 10.1080/0265204021000058384. [DOI] [PubMed] [Google Scholar]
- 15.Streubel A., Siepmann J., Bodmeier R. Multiple unit gastroretentive drug delivery systems: a new preparation method for low density microparticles. J. Microencapsul. 2003;20(3):329–347. doi: 10.1080/0265204021000058384. [DOI] [PubMed] [Google Scholar]
- 16.Talukder R., Fassihi R. Gastroretentive delivery systems: hollow beads. Drug Dev. Ind. Pharm. 2004;30(4):405–412. doi: 10.1081/DDC-120030935. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead L., et al. Floating dosage forms: an in vivo study demonstrating prolonged gastric retention. J. Control Release. 1998;55(1):3–12. doi: 10.1016/S0168-3659(97)00266-6. [DOI] [PubMed] [Google Scholar]
- 18.Groning R., Cloer C., Muller R. S. Development and in vitro evaluation of expandable gastroretentive dosage forms based on compressed collagen sponges. Pharmazie. 2006;61(7):608–612. [PubMed] [Google Scholar]
- 19.Groning R., et al. Compressed collagen sponges as gastroretentive dosage forms: in vitro and in vivo studies. Eur. J. Pharm. Sci. 2007;30(1):1–6. doi: 10.1016/j.ejps.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Klausner E. A., et al. Expandable gastroretentive dosage forms. J. Control. Release. 2003;90(2):143–162. doi: 10.1016/S0168-3659(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 21.Duchene D., Ponchel G. Principle and investigation of the bioadhesion mechanism of solid dosage forms. Biomaterials. 1992;13(10):709–714. doi: 10.1016/0142-9612(92)90132-8. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y., et al. Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J. Control. Release. 2000;65(1–2):63–71. doi: 10.1016/S0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 23.Ponchel G., Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug. Deliv. Rev. 1998;34(2–3):191–219. doi: 10.1016/S0169-409X(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 24.Tao S. L., Desai T. A. Gastrointestinal patch systems for oral drug delivery. Drug Discov. Today. 2005;10(13):909–915. doi: 10.1016/S1359-6446(05)03489-6. [DOI] [PubMed] [Google Scholar]
- 25.Atuma C., et al. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280(5):G922–G929 . doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 26.R. J. Bhaskara, et al. Effect of Polymer Swelling on Mucoadhesion in Various GI Locations. Controlled Release Society symposium, Hawaii. (2005).
- 27.Schmitt R. L. Polyethylene oxide. In: Raymond C R., Paul J. S., Paul J. W., editors. Handbook of pharmaceutical excipients. 4. London: Pharmaceutical Press and American Pharmaceutical Association; 2003. pp. 460–461. [Google Scholar]
- 28.Betageri G. V., Deshmukh D.V., Gupta R. B. Oral sustained-release bioadhesive tablet formulation of didanosine. Drug Dev. Ind. Pharm. 2001;27(2):129–136. doi: 10.1081/DDC-100000479. [DOI] [PubMed] [Google Scholar]
- 29.Kibbe A. H. Povidone. In: Raymond C. R., Paul J. S., Paul J. W., editors. Handbook of pharmaceutical excipients. 4. London: Pharmaceutical Press and American Pharmaceutical Association; 2003. pp. 508–513. [Google Scholar]
- 30.Wessel W., Schoog M., Winkler E. Polyvinylpyrrolidone (PVP), its diagnostic, therapeutic and technical application and consequences thereof. Arzneimittelforschung. 1971;21(10):1468–1482. [PubMed] [Google Scholar]
- 31.Hou S. Y., Cowles V. E., Berner B. Gastric retentive dosage forms: a review. Crit. Rev. Ther. Drug Carrier Syst. 2003;20(6):459–597. doi: 10.1615/CritRevTherDrugCarrierSyst.v20.i6.30. [DOI] [PubMed] [Google Scholar]