Abstract
This study aims to formulate and evaluate bioavailability of a self-nanoemulsified drug delivery system (SNEDDS) of a poorly water-soluble herbal active component oleanolic acid (OA) for oral delivery. Solubility of OA under different systems was determined for excipient selection purpose. Four formulations, where OA was fixed at the concentration of 20 mg/g, were prepared utilizing Sefsol 218 as oil phase, Cremophor EL and Labrasol as primary surfactants, and Transcutol P as cosurfactant. Pseudo-ternary phase diagrams were constructed to identify self-emulsification regions for the rational design of SNEDDS formulations. Sefsol 218 was found to provide the highest solubility among all medium-chained oils screened. Efficient self-emulsification was observed for the systems composing of Cremophor EL and Labrasol. The surfactant to cosurfactant ratio greatly affected the droplet size of the nanoemulsion. Based on the outcomes in dissolution profiles, stability data, and particle size profiles, three optimized formulations were selected: Sefsol 218/Cremophor EL/Labrasol (50:25:25, w/w), Sefsol 218/Cremophor EL/Labrasol/Transcutol P (50:20:20:10, w/w), and Sefsol 218/Cremophor EL/Labrasol/Transcutol P (50:17.5:17.5:15, w/w). Based on the conventional dissolution method, a remarkable increase in dissolution was observed for the SNEDDS when compared with the commercial tablet. The oral absorption of OA from SNEDDS showed a 2.4-fold increase in relative bioavailability compared with that of the tablet (p < 0.05), and an increased mean retention time of OA in rat plasma was also observed compared with that of the tablet (p < 0.01). These results suggest the potential use of SNEDDS to improve dissolution and oral bioavailability for poorly water-soluble triterpenoids such as OA.
Key words: bioavailability, dissolution, oleanolic acid, self-nanoemulsified drug delivery system (SNEDDS), triterpenoid
INTRODUCTION
Oleanolic acid (OA) belongs to the group of pentacyclic triterpenes, which is widely distributed in many traditional Chinese medicines (e.g., Fructus ligustri lucidi, Fructus forsythiae, and Radix ginseng), and used as the main bioactive component (1). In China, oleanolic acid is used as an OTC drug for oral delivery to treat human liver diseases, such as acute and chronic hepatitis (2). However, being hydrophobic (logP = 6.32, pKa = 5.11) (3), oleanolic acid exhibits poor aqueous solubility. During in vitro dissolution study, less than 1 μg/ml oleanolic acid was dissolved from raw solid form into an aqueous buffer (pH 1 or 7) after 2 h (4). In a human pharmacokinetic study, Tmax was reported to be 5.2 h after oral administration of oleanolic acid capsule, indicating that it had delayed in vivo absorption (5). In addition, absolute oral bioavailability of oleanolic acid was only 0.7% for oral doses of 25 and 50 mg/kg in rat, and it has been suggested that the poor oral bioavailability of oleanolic acid is due to poor solubility/dissolution and extensive metabolic clearance (6). Various formulation approaches have been utilized to improve the oral bioavailability of poorly water-soluble oleanolic acid, e.g., preparation of chemically modified derivatives, cyclodextrin inclusion complex, nanosuspension, and solid dispersions (7–10). In our previous work, different crystalline forms of oleanolic acid have been prepared by solvent recrystallization, but results showed that the dissolution of oleanolic acid could not be increased through solid-state manipulation (4). In recent years, much attention has been given to use lipid-based formulations to improve the oral bioavailability of poorly water-soluble pharmaceuticals. One of the attractive approaches is the application of self-nanoemulsified drug delivery system (SNEDDS). Self-nanoemulsified drug delivery systems are isotropic mixtures of oil, surfactant, cosurfactant, and drug that form fine oil-in-water (o/w) nanoemulsion when introduced into aqueous phases under gentle agitation (11). The nanoemulsions usually have a droplet size less than 100 nm and are thermodynamically stable, transparent dispersions of oil and water stabilized by an interfacial film of surfactant molecules. SNEDDS provides ultra low interfacial tensions and large o/w interfacial areas. Therefore, SNEDDS have the advantages in possessing higher solubilization capacity than simple micellar solutions, leading to the incorporation of poor water-soluble pharmaceutical inside the oil phase. Unlike the thermodynamically unstable dispersions systems, such as emulsions and suspensions, SNEDDS’ thermodynamic stability enables both easy manufacturability with little energy input (heat or mechanical mixing) and long shelf life (12). Furthermore, SNEDDS have been reported to result in the more reproducible plasma concentration profiles and oral bioavailability of pharmaceuticals (13–15).
In view of the potential benefits of SNEDDS systems, the objectives of the present study were, firstly, to develop and characterize SNEDDS of oleanolic acid; and secondly, to investigate the effects of composition on the physicochemical properties of each nanoemulsion system for formula optimization. Particularly, the effects of different types and weight ratios of surfactant to cosurfactant on the characteristics (e.g., droplet size, drug release, stability etc.) of nanoemulsions were investigated. Finally, in vivo oral bioavailability of the developed SNEDDS was evaluated in rats and was compared to the commercially available OA tablet.
MATERIALS AND METHODS
Materials
Oleanolic acid raw material (pharmaceutical grade, purity ≥95%) and standard (minimum purity 97%) were purchased from International Laboratory (San Bruno, USA) and Sigma-Aldrich (St. Louis, USA), respectively. Glycyrrhetinic acid standard was obtained from the China’s National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Miglyol 840, Neobee M5, and Captex 200P were gift samples from Sasol (Witten, Germany), Bronson & Jacobs (Hong Kong, China), and Abitex Corp. (Northampton, UK), respectively. Peanut oil, ethyl oleate, and Tween 80 were purchased from Sigma-Aldrich (St. Louis). Propylene glycol mono caprylic ester (Sefsol 218) was a gift sample from Nikkol Chemicals (Tokyo, Japan). Labrasol, Lauroglycol FCC, and Transcutol P were gift samples from Gattefosse (Saint Priest, Cedex France). Cremophor EL was a gift sample from BASF Corp (Ludwigshafen, Germany). All chemicals were used as received without further purification. All the solvents used in this study, including methanol and acetonitrile, were of high performance liquid chromatography (HPLC) grade. Water was obtained from the Milli-Q water purification system of Millipore Corporation (Bedford, USA).
High Performance Liquid Chromatography Analysis
The HPLC analysis of oleanolic acid followed the protocol reported by Chen et al. (16) with slight modifications. The analysis employed an Agilent 1100 series HPLC system, a 250 × 4.6 mm Agilent 5 μm Zorbax SB-C18 column, and a photodiode array detector. Injection volume was 20 μl. The analyte was eluted isocratically at a flow rate of 1.0 ml·min−1 with the mobile phase of acetonitrile and 0.5% phosphoric acid in water (85:15, v/v). Based on the UV spectrum of OA, the wavelength for oleanolic acid quantification was set at 204 nm. Glycyrrhetinic acid, with a chemical structure similar to oleanolic acid, was used as an internal standard at the concentration of 32 μg/ml. A calibration curve based on the area ratio between oleanolic acid and glycyrrhetinic acid had excellent linearity (R2 > 0.999) in the range between 2 and 64 μg/ml. The detection limit for oleanolic acid using this HPLC protocol was 1 μg/ml. The RSD of intra- and inter-day precision and accuracy for the oleanolic acid at three concentrations (4, 16, and 64 μg/ml) were less than 5% (n = 5).
Determination of Oleanolic Acid Solubility in Different Excipients
The solubility of oleanolic acid in various oils, surfactants, and cosurfactants were determined. An excess amount of oleanolic acid was added into 3 ml of selected oils, surfactants, or cosurfactants. The mixtures were first vortexed to facilitate proper mixing of oleanolic acid with the vehicles, and the mixtures were then allowed to reach equilibrium at 40 ± 0.5°C in a shaking water bath (Schwahach FRG, Germany) under light protection. After 72 h, the mixtures were centrifuged at 3,000 rpm for 15 min, followed by filtration through a 0.45-μm membrane filter. The filtrates were diluted with methanol and OA solubility was subsequently quantified by HPLC. All measurements were in triplicate from three independent samples.
Screening of Oils and Surfactants
To evaluate the capability of oils and surfactants to form an emulsion spontaneously, oil was continually added into 1 ml surfactant solution (20%, w/w) with vigorous vortex. If a uniform clear solution was visually obtained, the addition of the oil will be continued until the solution became cloudy and then the total amount of oil added was recorded. Selected emulsions were subject to the physical stability test and all of them were confirmed to be stable for 3 weeks.
Screening of Cosurfactants
The cosurfactant is added to get more efficient self-nanoemulsion systems. The screening of the cosurfactant was conducted as follows. After mixing 4 g surfactant with 10 g oil phase, the surfactant/oil mixture was diluted to 20 ml by distilled water. The aforementioned resultant solution (1 ml) was titrated with increasing amount of cosurfactants until the system turned clear and the amount of cosurfactant used was recorded as the minimum amount. Then, more cosurfactants were added until the transparent solutions changed cloudy again, and the amount of cosurfactant used was recorded as the maximum amount.
Construction of Pseudo-ternary Phase Diagram
From the results of solubility studies and screening of excipients, Sefsol 218 was selected as the oil phase. Labrasol/Cremophor EL (1:1, w/w) was used as mixed surfactants and Transcutol P as the cosurfactant. Distilled water was used as an aqueous phase for construction of phase diagrams. Pseudo-ternary phase diagrams of mixed surfactant and cosurfactant (Smix), oil, and water but without drug incorporation were plotted, and each of them represents a side of the triangle. Ternary mixtures with varying compositions of surfactant, cosurfactant, and oil were prepared. Surfactant and cosurfactant were mixed in various ratios from 10:0 to 1:9 (Smix, w/w). For each phase diagram, oil and specific Smix ratio was mixed thoroughly in different weight ratios from 1:9 to 9:1 in different glass vials. Nine different combinations of oil and Smix, 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1, were made so that maximum ratios were covered for the study to delineate the boundaries of phase precisely formed in the phase diagrams. Pseudo-ternary phase diagrams were developed using the aqueous titration method. Slow titration with aqueous phase was done to each weight ratio of oil and Smix. The formation of the nanoemulsion was visually observed as transparent and easily flowable o/w nanoemulsion and marked on the pseudo-three-component phase diagram.
Optimization of Formula
From each diagram, different formulations were optimized based upon the following criteria:
-
A.
Select appropriate Smix which could construct a larger nanoemulsion area in the phase diagrams.
-
B.
Select appropriate amount of oil which could solubilize more OA in the formulation.
-
C.
Select the least amount of surfactant and cosurfactant to form SNEDDS.
-
D.
Check if there was any drug precipitation after storing each diluted formulation for 24 h.
Visual evaluation is the primary means of self-emulsification assessment. Each formulation (1 g) containing the prescribed amount of oleanolic acid (20 mg) was introduced into 1,000 ml of water in a glass flask at room temperature and was gently stirred manually. The performance of the formulations was visually assessed using the following grading systems (13):
-
Grade A:
Nanoemulsion with a clear or bluish appearance was rapidly formed within 1 min.
-
Grade B:
Less clear emulsion with a bluish white appearance was rapidly formed.
-
Grade C:
Fine milky emulsion was formed within 2 min.
-
Grade D:
Dull, grayish white emulsion with slightly oily appearance was slowly formed (longer than 2 min).
-
Grade E:
Poor or minimal emulsification with large oil globules was present on the surface.
Emulsion Droplet Size Analysis
Droplet size measurement and polydispersity index were carried out utilizing a Brookhaven 90 plus Nanoparticle Size Analyzer (Brookhaven Instruments Corporation, USA) at 25°C and using water as the solvent for dilution at 1:1,000. The analytical range of the analyzer is 1 nm–6 μm. All measurements were in triplicate from three independent samples. Data were represented as effective diameter (mean ± SD).
Transmission Electron Microscopy
To determine the size and shape of microemulsion drops, transmission electron microscopy (TEM) method was employed. After being diluted 1,000-fold with water, the OA self-emulsifying sample was negatively stained with 2% (w/v) phosphotungstic solution. The photograph of drops was obtained using a transmission electron microscope (JEM-100 CX II, Japan) under a high tension electricity of 80 kV.
In Vitro Drug Release Study
In vitro drug release from SNEDDS was performed by both the conventional method according to the standard USP 30-NF 25 (2007) and dialysis method using a standard dissolution tester (DT 706 1000LH, Erweka, Germany). In the conventional method, a hard gelatin capsule filled with pre-concentrate formulation or a commercial tablet containing 20 mg OA was put into 500 ml water or simulated gastric fluid at 37 ± 0.5°C with 100 rpm rotating speed. Simulated gastric fluid was prepared according to the USP 30-NF25 (2007). Samples (5 ml) were withdrawn at regular time intervals (5, 10, 20, 30, 45, 60, 90, and 120 min) and filtered using a 0.45-μm filter. An equal volume of the dissolution medium was added to maintain the volume constant. In the dialysis method, OA SNEDDS containing 20 mg OA (diluted by water or 1% sodium dodecyl sulfate (SDS) for 50 or 100 times) were put into the dialysis bag (MWCO 12,000, Biodee Biotechnology Co., Ltd., Beijing, China). The dialysis bag was sealed and place into 1% SDS solution as the dissolution medium at 37 ± 0.5°C with 100 rpm rotating speed. Sample was withdrawn at 1, 2, 3, 4, 6, 8, 12, and 16 h. The drug content in the samples was assayed using HPLC. All measurements were in triplicate from three independent samples.
Stability Study
Short-term stability assessments were performed under the following conditions for 15 days, e.g., 60°C, 4–25°C cold–heat cycles (each cycle was 24 h by storing the sample at 4°C for 12 h and followed by storing sample at 25°C for 12 h). Long-term stability was assessed by keeping the ready-to-use SNEDDS formulations into the sealed amber glass vial at room temperature and 4°C. The physical stability of the ready-to-use SNEDDS formulations was evaluated by monitoring the time-dependent change in the physical characteristics (e.g., drug precipitation). The chemical stability of the oleanolic acid in the SNEDDS formulations was analyzed by HPLC. All measurements were repeated for five independent samples.
Bioavailability Study of Oleanolic Acid in Rats
The animal experiment was approved by the Animal Ethics Committee at the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences (Beijing, China). The animals used were male Sprague–Dawley rats (210 ± 10 g), which were supplied by Vital River Experimental Animal Co. Ltd (Beijing, China). The rats were housed under standard conditions of temperature, humidity, and light, and had free access to standard rodent diet and water before the experiment. On the day before the experiment, the jugular vein of rat was cannulated by a polyethylene catheter (0.50 mm I.D., 1.00 mm O.D., Portex Ltd., Hythe, Kent, England) for blood sampling. After surgery, the rat was placed individually and allowed to recover for 24 h and fasted overnight prior to drug dosing.
Two formulations, including water suspension of commercial OA tablet triturate and OA SNEDDS formulation I, were given orally to SD rat (n = 5) at a dose of 50 mg/kg by gastric gavages. The OA tablet was ground into small particles and sufficiently mixed with water to give a suspension at OA concentration of 10 mg/ml predetermined by HPLC. A certain volume (about 1.0 ml for each rat) of the suspension was withdrawn for dosing after thoroughly vortexing. The blood samples (0.2 ml) were collected via the catheter before and at 5, 15, 30, 60, 90, 120, 180, 240, 360, 480, and 720 min post dosing, and immediately placed into heparinized tubes. After centrifugation at 4,000 rpm for 5 min, the plasma (100 μl) were obtained and stored at −20°C until assay. After each blood collection, 0.2 ml of normal saline containing 20 I.U. of heparin was immediately injected back into the catheter to flush the catheter and prevent coagulation.
Liquid Chromatography/Mass Spectroscopy/Mass Spectroscopy Analysis of OA in Rat Plasma
Plasma concentration of OA was determined by a published liquid chromatography/mass spectroscopy/mass Spectroscopy (LC/MS/MS) method (5) with slight modifications. Glycyrrhetinic acid was used as an internal standard. Plasma sample (100 μl) was mixed with 20 μl of 500 ng/ml glycyrrhetinic acid in 80% methanol and 3 ml ether. After vortexing for 3 min with subsequent standing for more 30 min, the clear supernatant ether was dried by nitrogen at 45°C. The residue was then reconstituted in 150 μl of 80% methanol and above solution (50 μl) was injected into LC/MS/MS system for assay.
LC/MS/MS assay was performed on a HPLC system (Agilent-1100, USA) connecting to a mass spectrometry system (Applied Biosystem 3200 Q-TRAP, USA). The LC separation was conducted by using a C18 column (100 × 2.1 mm, 3 μm, Alltima HP, USA) kept at 35°C. Mobile phases consisting of acetonitrile with 0.2% formic acid (A) and water with 0.2% formic acid (B) were eluted with the following linear gradients at 0.3 ml/min: 0–3 min (70% A, 30% B), 5–11 min (100% A, 0% B), and 12–15 min (70% A, 30% B). Mass spectrometry adopted ESI in the positive mode, with ion spray voltage at −4,500.0 V, ion source temperature at 300°C, entrance potential at −7.5 V, declustering potential at −110.0 V, curtain gas pressure at 10.0 psi, and nebulizer gas and auxiliary gas both at 50.0 psi. Quantification was performed using selective ion monitoring of m/z 455.9 and 469.3 for oleanolic acid and glycyrrhetinic acid, respectively.
The method showed good linearity (r2 > 0.994) between the concentration ranges of 1.3–25 and 25–400 ng/ml in rat plasma. The mean recoveries of spiked oleanolic acid at 50 and 150 ng/ml are 99.84 ± 3.86 (n = 5) and 100.44 ± 2.96 (n = 5), respectively. Intra-day and inter-day variations at the above two concentrations were lower than 12.2%. The limit of detection of oleanolic acid in this protocol was 0.4 ng/ml.
Pharmacokinetic and Student’s t Test Analysis
The plasma concentrations versus time profiles were analyzed using WinNonlin software (Pharsight Corporation, Mountain View, CA, USA, Version 2.1). The non-compartmental model was employed to estimate the following pharmacokinetic parameters for individual rat in each group, peak plasma concentration (Cmax), the time to reach Cmax (Tmax), area under the plasma concentration versus time curve from zero to last sampling time 12 h (AUC0→12 h), and mean retention time (MRT). The pharmacokinetic data between the two formulations were compared for statistical significance by Student’s t test. Values are reported as mean ± SD and the data were considered as statistically significant as p < 0.05.
RESULTS
Solubility Study
Solubility of oleanolic acid in various oils, surfactants, and cosurfactants was presented in Table I. It is clearly shown that the highest solubility of oleanolic acid was found in the oil phase of Sefsol 218, the surfactant of Labrasol, and cosurfactant of Transcutol P.
Table I.
Solubility of Oleanolic Acid in Various Vehicles (n = 3)
| Vehicle | Composition | Solubility of OA (mg/ml) |
|---|---|---|
| Oils | ||
| Miglyol 840 | Propylene glycol dicaprylate/dicaprate | 2.54 ± 0.05 |
| Neobee M5 | Caprylic/capric triglycerides | 2.74 ± 0.01 |
| Captex 200P | Diesters of caprylic/capric acids on propylene glycol | 2.46 ± 0.01 |
| Sefsol 218 | Propylene glycol caprylate | 27.38 ± 0.38 |
| Surfactants/cosurfactants | ||
| Labrasol | Saturated polyglycolyzed C8–C10 glycerides (HLB = 14) | 21.85 ± 1.09 |
| Cremophor EL | Polyoxyl 35 castor oil (HLB = 13.5) | 16.04 ± 0.55 |
| Tween 80 | Polyethylene glycol sorbitan monooleate (HLB = 15) | 7.44 ± 0.70 |
| Lauroglycol FCC | Propylene glycol laurate (HLB = 4) | 8.28 ± 0.24 |
| Transcutol P | Diethylene glycol monoethyl ether | 40.34 ± 1.42 |
OA oleanolic acid, HLB hydrophilic–lipophilic balance
Screening of Oils and Surfactants
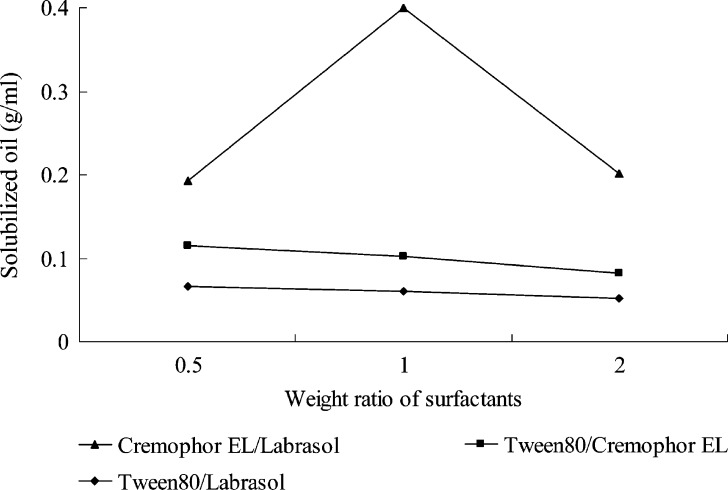
In the investigation of the solubilization behaviors of the employed oils with selected surfactants, only Sefsol 218 formed spontaneous nanoemulsion with selected surfactants (Labrasol, Cremophor EL, and Tween 80). Figure 1 showed solubilization capability of Sefsol 218 with various mixed surfactants. When Labrasol and Tween 80 were used alone, good nanoemulsions were not generated, though good flowability was observed in the preparations. Cremophor EL could form a transparent and uniform nanoemulsion with Sefsol 218 upon dilution with water, but it has a long self-emulsification time due to its high viscosity (data not shown). Therefore, the combined use of the surfactant mixture was considered for further studies. When Tween 80 was combined with Labrasol or Cremophor EL, more amount of oil could be solubilized into the surfactant solutions (Fig. 1). It is also demonstrated that the combined use of Cremophor EL and Labrasol at 1:1 weight ratio had excellent emulsification capability for Sefsol 218, followed by their 2:1 and 1:2 mixtures (Fig. 1). Li et al. (17) had also reported the advantages of combined use of surfactants (Tween 20/Cremophor EL, 1:1 w/w) in pre-concentrate, which could enlarge self-emulsifying area, increase drug loading, and improve its physical stability.
Fig. 1.
Solubilized oil (Sefsol 218) in different weight ratios of the two mixed surfactants
Screening of Cosurfactant
Lauroglycol FCC could not form a clear solution with selected oil and mixed surfactants (data not shown). A large amount of Transcutol P could be incorporated in the oil and surfactant, indicating that it had good compatibility with Cremophor EL/Labrasol (1:1) and Sefsol 218. Furthermore, as a high performance solubilizer, Transcutol P could dissolve oleanolic acid efficiently and was selected as the cosurfactant in the following formulations.
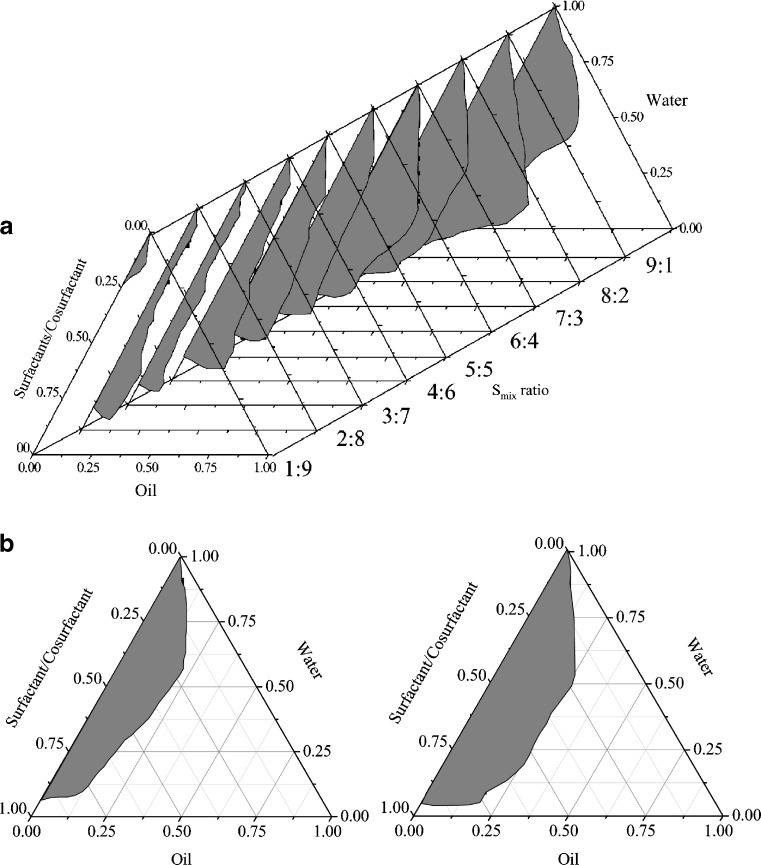
Pseudo-ternary Phase Diagram
As shown in Fig. 2a, the self-emulsification region in Sefsol 218–Cremophor EL/Labrasol (1:1, w/w)–Transcutol P ternary systems became smaller in area when the Smix ratio decreased from 9:1 to 1:9. The preparations obtained within the small self-emulsification area at Smix of 4:6, 3:7, 2:8, and 1:9 were thermodynamically unstable, showing phase separation after 24 h.
Fig. 2.
a Pseudo-ternary phase diagrams of Sefsol 218–Cremophor EL/Labrasol (1:1, w/w)–Transcutol P formulations. Regions in gray color indicate the o/w self-emulsion region at different S mix (w/w, surfactant/cosurfactant) ratios. b Comparison of phase diagram of Sefsol 218–Cremophor EL/Labrasol–Transcutol P formulations with (left) and without oleanolic acid (right) at S mix ratio of 8:2
Comparing phase diagrams with and without drug incorporated in the same formulation (Fig. 2b), it was evident that self-emulsion regions were smaller if oleanolic acid (20 mg/g) was added into the formulation. Similar trends were observed when Smix changed from 10:0 to 7:3 (data not shown).
Formulations that passed the dispersibility test with grade A and B results were taken for further study, as grade A and B formulations will remain as nanoemulsions when dispersed in GIT. All the formulations that fell in grades C, D, and E of dispersibility tests were not considered for further study. Based on the pseudo-ternary phase diagram, nanoemulsions composed of Sefsol 218 as oil (50%, w/w), Cremophor EL/Labrasol (1:1, w/w) as surfactant, and Transcutol P as cosurfactant with different weight ratios of Smix (10:0, 9:1, 8:2) were selected for further characterization and optimization in droplet size, emulsification time, in vitro drug release, and physicochemical stability.
Emulsion Droplet Size Analysis
As large amounts of surfactants/cosurfactant would cause irritation to the gastrointestinal tract (GI) tract, it is therefore essential to optimize the amount and weight ratio of surfactant/cosurfactant in the formula. In Table II, in all four formulations tested, the droplet size increased upon decreasing weight ratio of Smix. All the polydispersity values were below 0.3, suggesting good uniformity in the droplet size distribution after diluted with water. It is believed that droplet size distribution is one of the most important characteristics of emulsion for stability evaluation and in vivo absorption (18). Therefore, detailed studies were carried out to investigate the effect of every formulation variable, i.e., dilution times, amount, and weight ratio of surfactant and cosurfactant.
Table II.
Droplet Size and Polydispersity Values of Three Oleanolic Acid Formulations (n = 3)
| Formulation | SNEDDS composition (Sefsol 218–Cremophor EL/Labrasol (1:1)–Transcutol P, w/w) | Effective diameter (nm) | Polydispersity |
|---|---|---|---|
| Mean ± SD | |||
| I | 50:50:0 | 38.4 ± 0.2 | 0.055 |
| II | 50:45:5 | 46.4 ± 0.5 | 0.120 |
| III | 50:40:10 | 75.3 ± 0.3 | 0.238 |
| IV | 50:35:15 | 110.4 ± 0.6 | 0.258 |
SNEDDS self-nanoemulsified drug delivery system
Effect of Dilution Times
Distilled water was used as a dispersion medium in the present study. No significant difference is observed when the nanoemulsions prepared by nonionic surfactants are dispersed in water, or simulated gastric and intestinal fluids (18,19).
The droplet size of the nanoemulsion increased when the dilution times of the aqueous phase increased from 10 to 50. The droplet size maintained stable at around 120 nm upon dilution with 100–2,000 times of water (data not shown). This result demonstrates that the preparation could be administered as an oleanolic acid containing pre-concentrate inside suitable delivery system, such as soft gelatin capsule, and the pre-concentrate would readily form nanoemulsion inside the gastro/intestinal tract.
Effect of Surfactant Concentration
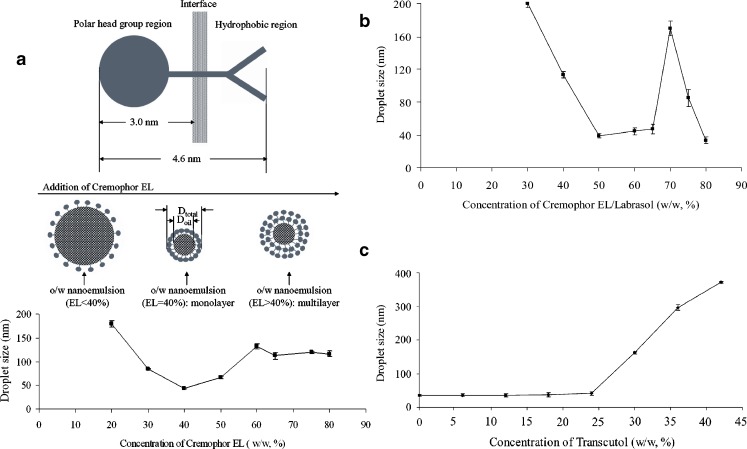
The effects of the surfactant concentration on the droplet size distribution in various SNEDDS are presented in Fig. 3a, b. In the case of SNEDDS containing Cremophor EL and Sefsol 218 (Fig. 3a), it was observed that the droplet size decreased significantly if the concentration of Cremophor EL increased from 20% to 40% (w/w), and gradually increased from 40% afterwards. The minimum droplet size (43.5 ± 1.3 nm) was found at 40% content of Cremophor EL.
Fig. 3.
a Effect of concentration of Cremophor EL in a mixture of Sefsol 218–Cremorphor EL on the mean droplet size (n = 3). b Effect of concentration of Cremophor EL/Labrasol (1:1) (surfactant) in a mixture of Sefsol 218–Cremophor EL/Labrasol (1:1) on the mean droplet size (n = 3). c Effect of Transcutol P (cosurfactant) concentration in mixture of Sefsol 218–Cremophor EL/Labrasol (1:1)–Transcutol P on mean droplet size (n = 3)
A similar trend of droplet size changes was also observed where the minimum droplet size (38.9 ± 2.7 nm) was found at 50% content of mixed surfactants (Cremophor EL/Labrasol, 1:1, w/w; Fig. 3b). A few other studies have also reported similar trends in droplet size for various self-emulsified systems (14).
Effect of Cosurfactant Concentration
The effects of the cosurfactant (Transcutol P) concentration on the droplet size in the system containing Sefsol 218, Cremophor EL, and Labrasol were also investigated. Stable droplet size at around 35 nm was observed with the cosurfactant concentration below 24% (w/w), and the droplet size of emulsion was continually increased beyond 24% (w/w) up to ∼400 nm (42%, w/w; Fig. 3c). A similar observation was found when the surfactant mixture was replaced by Cremophor EL alone, where the smaller droplet size (<100 nm) was observed with the cosurfactant concentration below 30% (w/w), and increased up to 600 nm with the increased concentration of surfactant up to 50% (w/w; data not shown)
Effect of Weight Ratio of Surfactant to Cosurfactant
The effect of weight ratio of surfactant to cosurfactant is conducted by increasing their weight ratio in the system containing Sefsol 218, Cremophor EL, Labrasol, and Transcutol P. The droplet size of nanoemulsion dramatically decreased from 370 to 35 nm when the weight ratio increased from 0.4 to 1.5 and became constant at above 1.5 (data not shown). Gao et al. (18) have reported similar observations with the microemulsion containing Captex-355, Cremophor EL, Transcutol P, and saline, where the droplet size decreased with increasing surfactant to cosurfactant ratio and then became constant at a ratio above 2. A similar result was also observed in the system containing Cremophor EL, Capmul MCM-C8, and coenzyme Q10/essential oil, where the droplet size was relatively constant (<100 nm) at ratios greater than 0.5 (19). The aforementioned result maybe explained by the fact that the addition of surfactants to the microemulsion systems causes the interfacial film to stabilize and condense, while the addition of cosurfactant causes the film to expand (20).
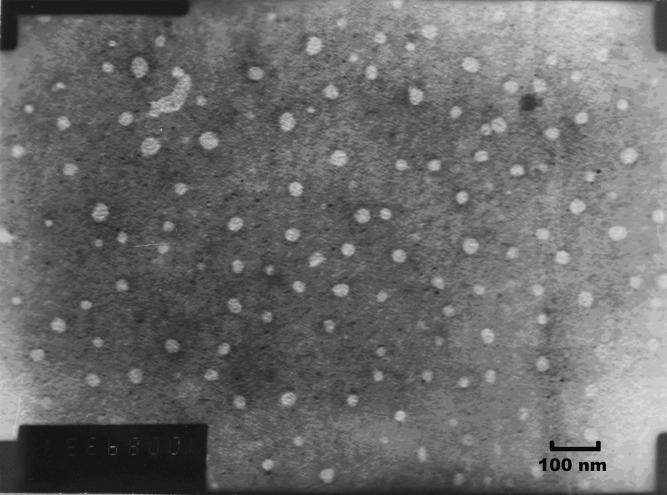
Transmission Electron Microscopy
The photograph of the transmission electron microscopy (TEM) was shown in Fig. 4. The nanoemulsion generated from formulation IV appeared as bright spots on a dark background. The emulsion droplets generated were spherical and uniform in size with a large population of smaller droplets in the size range between 30 and 50 nm, which was a bit smaller than the droplet size measurement results (Table II).
Fig. 4.
Transmission electron micrograph of SNEDDS of OA (mag 100 K; dilution 1,000-fold with water)
In Vitro Drug Release
No detectable oleanolic acid (<1 μg/ml) could be dissolved from commercial available tablet. However, more than 75% of oleanolic acid could be rapidly released from its four developed nanoemulsions either in water or in simulated gastric fluid by conventional dissolution method (data not shown). As suggested by Zhang et al. (21), the drug in the SNEDDS system could be existed as free molecular form, or mixed in the micelles or in the microemulsion droplets when diluted into aqueous solution. Therefore, the dialysis method needs to be utilized to separate the different forms. Using the dialysis method, no OA could be detected after 16 h in the dissolution medium, which indicates that most of the OA exists in the nanoemulsion droplets or micelles but not in the free form.
Stability Test
Short-term stability studies showed that except for the formulation II where oleanolic acid was precipitated out, OA was physically and chemical stable in other three formulations when being stored at 4°C for 3 months, as well as in the cold and heat cycles for 15 days (data not shown). However, oleanolic acid was not chemically stable and decreased by nearly 10% in concentrations after 15 days when being stored at 60°C (data not shown), indicating that oleanolic acid should be stored at ambient or sub-ambient temperatures. In the long-term stability assessment, oleanolic acid in the optimized three formulations were stable for at least 4 months when being stored at ambient condition in amber glass bottles (data not shown).
Bioavailability Study
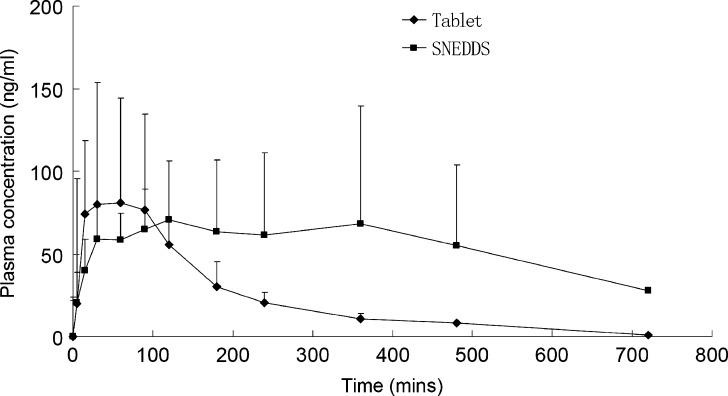
The plasma profiles of OA after oral administration of suspension of commercial tablet and developed SNEDDS formulation I were compared. As the droplet size and polydispersity of the formed nanoemulsion in formulation I were the smallest among the four developed formulations, this formula was selected for in vivo bioavailability study. The plasma concentrations versus time profiles are presented in Fig. 5 and the pharmacokinetic parameters are given in Table III. The absorption of OA from the SNEDDS formulation resulted in a 2.4-fold increase in bioavailability (as calculated by AUC), although the SNEDDS formulation did not significantly change the Cmax compared with the tablet formulation (p > 0.05). In addition, large variability of the plasma concentrations from both formulae were observed, which was consistent with previous pharmacokinetic studies in human and rat (5,6). It is expected that the poor oral absorption and individual variation may be the reason that lead to these large variability. However, a more detailed study is needed to provide an appropriate justification in the future.
Fig. 5.
Plasma concentration profiles of OA after oral administration of commercial tablet and SNEDDS of OA in rats (50 mg/kg, n = 5)
Table III.
Pharmacokinetic Parameters of OA Tablet and OA SNEDDS (Formulation I) After Oral Administration at 50 mg/kg in Rat (n = 5)
| Commercial OA tablet | OA SEDDS | |
|---|---|---|
| T max (min) | 48 ± 27 | 90 ± 73 |
| C max (ng ml−1) | 104.93 ± 61.79 | 99.81 ± 42.64 |
| AUC0→t (ng min ml−1) | 14,974.89 ± 10,906.19 | 36,041.38* ± 28,965.03 |
| MRT (min) | 138 ± 43 | 243** ± 105 |
| Rel. BAa (%) | – | 240.68 |
OA oleanolic acid, SNEDDS self-nanoemulsified drug delivery system
*p < 0.05 and **p < 0.01 when compared with tablet formulation using Student’s t test
aRelative bioavailability
The sustained release effect of the OA from SNEDDS formulation was clearly observed which are reflected by the significantly increased MRT value (p < 0.01) compared with the tablet. These results may suggest that although the nanoemulsion could be rapidly formed within minutes, the release rate of free drug from the fine emulsified oil droplets or micelles formed at high concentration of the surfactant is relative slow, if the absorption of nanoemulsion from the intestinal lymphatic system is not considered.
The sustained effects of SNEDDS have also been reported before (22), but no detailed explanation could be provided. One possible mechanism is that the surfactant (e.g., Cremophor EL) at high concentration may lead to sequestration of the drug into the surfactant micelles or emulsified oil droplet and delay/reduce the permeation of the drug across the GI tract supported by the result of using Caco-2 cell monolayer model (23). The fraction of OA dissolved in the fine oil droplets is slowly released in the GI tract (e.g., after digestion of lipid components in SNEDDS) and provides the sustained effect in vivo, which is consistent with in vitro drug release results by dialysis method.
DISCUSSION
Self-nanoemulsification is spontaneous and the resulting dispersion is thermodynamically stable (24). Free energy of nanoemulsion formation depends on the extent to which the surfactant lowers the surface tension of the oil–water interface and the change in dispersion entropy (13), and our results demonstrated that increasing surfactant proportion led to a more favorable formation of nanoemulsion. By ΔGf = γ ΔA − T ΔS, where ΔGf is the free energy of formation, γ is the interfacial tension of the oil–water interface, ΔA is the change in interfacial area on nanoemulsification, ΔS is the change in entropy of the system which is effectively the dispersion entropy, and T is the temperature, where a nanoemulsion can only be formed if γ ΔA is offset by the entropic component T ΔS, as γ is always positive, and ΔA is exceptionally large in nanoemulsion systems. The dominant favorable entropic contribution is the very large dispersion entropy arising from the mixing of one phase in the other in large numbers of nanosized droplets. In addition, favorable entropic contribution also arises from other dynamic processes such as surfactant diffusion in the interfacial layer and monomer–micelle surfactant exchange (24). As a result, surfactant composition, and their concentration in particular, play a crucial role in the spontaneous formation of nanoemulsion, as indicated in Fig. 3a.
When oleanolic acid was incorporated into the formulations, self-emulsion regions in the phase diagrams were decreased at about 6.0% (Fig. 2b). Since oleanolic acid could be regarded as an oil phase material, this fact further illustrated the concept that increasing amount of oil phase hindered the formation of nanoemulsion system and should be offset by a higher entropic contribution, i.e., increase of surfactant amount in the preparation.
While oleanolic acid nanoemulsion was diluted by water, it was evident that the droplet size at 10× dilution was much smaller than that at 50× dilution. Comparison of droplet size data with the visual observations shows that good emulsification property is reflected by the small globule size, except the formula at 10× dilution, where the formulation is less transparent. This reflects that the visual test is only a qualitative measurement of emulsification spontaneity, which is subject to only limited interpretation, rather than a quality measure of the formed emulsion (25). Generally, it was well correlated between visual observation and droplet size measurement. The SMEDDS formulations formed microemulsions (i.e., visual grading of A), where the droplet size was less than 50 nm, and the SNEDDS usually formed emulsions which were grade C and these had a droplet size range of approximately 100–200 nm. However, sometimes it was not possible to measure the droplet size of the emulsions formed, which emulsions were usually grade D or E and typically contained unemulsified oil which resulted in inaccurate droplet size measurements (26).
Minimum droplet size (43.5 nm) was obtained at 40% concentration of Cremophor EL in the SNEDDS containing Cremophor EL and Sefsol 218 (Fig. 3a), where the volume ratio between Cremophor EL to Sefsol 218 is calculated to be 0.57 since the density of Cremophor EL and Sefsol 218 is 1.055 and 0.906 g/ml, respectively. It is hypothesized that Cremophor EL (40%, w/w) forms a micellar monolayer structure with Sefsol 218 upon 1,000× dilution with water during droplet size analysis. Increasing the Cremophor EL concentration from 20% to 40% decreases the droplet size because the micelles are decreasingly swollen with Sefsol 218. In addition, smaller particles cannot be formed at lower concentrations of Cremophor EL because of the required total larger interfacial area for smaller particles. At 20%–40% Cremophor EL, the surfactant monolayer is maintained, though the distance between the surfactant molecules is decreased by including less oil droplets.
Meanwhile, increasing Cremophor EL from 40% afterwards provides additional surfactant molecules for possible condensation phenomenon and multi-layer formulation, and so, moderate increase of droplet size is observed. The hypothetical model is schematically summarized in Fig. 3a. However, in a thermodynamic sense, the addition of Cremophor EL cannot reduce the globular size forever. When a limiting value is reached, Cremophor EL may just condense onto the existing Cremophor EL layers as multiple layers. In principle, when there is sufficient Cremophor EL in the system, the surfactants will aggregate together and phase separation takes place, leading to smaller droplet size observed in Fig. 3a.
To support the above hypothesis, the critical micelle concentration (CMC) of Cremophor EL, which is reported at 0.039 mM, equivalent to around 0.0098% (w/v) in water at 25°C, should be considered (27). The concentration of Cremophor EL within the experimental range is therefore well above the CMC of the nanoemulsion upon 1,000 times dilution during droplet size analysis. Theoretically speaking, the characteristic nanomicellar structure should be retained until at least 4,000 times dilution. In sodium dodecyl sulfate (SDS), the surfactant with the most abundant information literature, it is reported that the lamella thickness of anhydrous SDS crystal is 1.943 nm (28). Although there is substantial difference between hydrogen bonding environments in crystal form and aqueous solution state, the captioned value suffices to yield a reasonable estimation in the molecular size of Cremophor EL. By comparison of the space filling models between SDS and Cremophor EL (27), the length of Cremophor EL is 4.6 nm approximately, and its polar head group region is about 3.0 nm (Fig. 3a). As the nanoemulsion droplet is spherically shaped, the volume ratio between the surfactant Cremophor EL to oil phase Sefsol 218 can be calculated. Assuming the formation of tightly packed outermost surfactant monolayer film and the hydrophobic region of Cremophor EL’s miscible with Sefsol 218, the polar head group region of Cremophor EL (3.0 nm) is further used for the calculation. With the consideration of Cremophor EL CMC in solution, at 40% content of Cremophor EL, the nanoemulsion droplet diameter (Dtotal) is 43.5 nm and droplet volume (Vtotal) is 43,099.02 nm3. The radius of oil phase is 18.75 nm, calculated by subtracting the polar head group region of Cremophor EL (3.0 nm) from the total measured nanoemulsion droplet diameter (21.75 nm). Using this value, the oil phase volume Voil is computed as 27,611.72 nm3. The surfactant volume (Vsur) is calculated by subtracting Voil from Vtotal, which is 15,487.30 nm3. The calculated volume ratio between Vsur to Voil is 0.56, a value consistent to a previously calculated value based on the droplet diameter of 43.5 nm when the weight ratio of Cremophor EL is at 40%, thereby supporting our hypothesis. A similar hypothesis could be used to explain the droplet size changes using the Cremophor EL and Labrasol as the mixed surfactants.
The droplet size analysis indicated that the nanoemulsion could be obtained with the system containing Sefsol 218, Cremophor EL/Labrasol, and Transcutol P (0–24%). Gao et al. (18) have reported similar observations with the microemulsion systems containing Captex-355, Cremophor EL, Transcutol P, and saline, where an increase in cosurfactant concentration (Transcutol P) increased the droplet size.
It is expected that the cosurfactant (Transcutol P) could form more stable interfacial film with the surfactants, which will further lower the interfacial tension between the oil and water phases, fluidize the hydrocarbon region of the interfacial film, and modify the film curvature (29). However, when an excess amount of the cosurfactant exists, it will not only stay into the interfacial film but also enter into the inner oil phase, leading to the expansion of interfacial film and increase of droplet size. As a result, the formed SNEDDS will become cloudy finally, i.e., turning into a macroemulsion (30).
CONCLUSIONS
The present study illustrated the potential use of SNEDDS for the delivery of oleanolic acid by the oral route. The surfactant to cosurfactant weight ratio greatly affected the effective self-emulsion region, droplet size, and emulsification rate of the resultant nanoemulsion systems. Compared with a single surfactant, the combined use of surfactants could improve the formed nanoemulsion generated upon dilution with water. The developed SNEDDS increased in vitro release of OA either in water or in simulated gastric fluid. Meanwhile, it also improved the oral bioavailability of OA by prolonging the retention time of OA in rat plasma. It is expected that the approach utilized in the present study may be helpful to formulate other lipophilic triterpenoid as well.
Acknowledgments
Financial support from the Research Council of the University of Macau (Research Grant RG 072/05-06S/07R for YZ) and the Macao Science and Technology Development Fund (FDCT Fund Project No: 005/2007/A1 for HHYT & 008/2007/A1 for YZ) is gratefully acknowledged. Valuable comments from Mr. Zhao Yi are highly appreciated.
Contributor Information
Henry H. Y. Tong, Phone: +853-83998632, FAX: +853-28753159, Email: henrytong@ipm.edu.mo
Ying Zheng, Phone: +853-83974687, FAX: +853-28841358, Email: yzheng@umac.mo.
References
- 1.Tian L. T., Ma L., Du N. S. Survey of pharmacology of oleanolic acid. Chin. J. Chin. Mater. Med. 2002;27:884–886. [PubMed] [Google Scholar]
- 2.Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 3.Claude B., Morin P. h., Lafosse M., Andre P. Evaluation of apparent formation constants of pentacyclic triterpene acids complexes with derivatized β- and χ-cyclodextrins by reversed phase liquid chromatography. J. Chromatogr. A. 2004;1049:37–42. [PubMed] [Google Scholar]
- 4.Tong H. H., Wu H. B., Zheng Y., Xi J., Chow H. L., Chan C. K. Physical characterization of oleanolic acid nonsolvate and solvates prepared by solvent recrystallization. Int. J. Pharm. 2008;355:195–202. doi: 10.1016/j.ijpharm.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Song M., Hang T., Wang Y., Jiang L., Wu X., Zhang Z., Shen J., Zhang Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006;40:190–196. doi: 10.1016/j.jpba.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Jeong D. W., Kim Y. H., Kim H. H., Ji H. Y., Yoo S. D., Choi W. R., Lee S. M., Han C. K., Lee H. S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007;28:51–57. doi: 10.1002/bdd.530. [DOI] [PubMed] [Google Scholar]
- 7.Yan Y. D., Feng B., Huang X. J., Zhou Z. G. Study on β-cyclodextrin inclusion compound of oleanolic acid. Chin. Tradit. Patent Med. 1995;6:2–4. [Google Scholar]
- 8.Xiang D. X., Tao Y. F., Wang F., Li H. D. Studies on formation and solubilizing mechanism of oleanolic acid solid dispersion. Chin. Tradit. Herb Drugs. 2002;33:311–314. [Google Scholar]
- 9.Guo M. Q., Zhang S. Q., Song F. R., Wang D. W., Liu Z. Q., Liu S. Y. Studies on the non-covalent complexes between oleanolic acid and cyclodextrins using electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003;38:723–731. doi: 10.1002/jms.486. [DOI] [PubMed] [Google Scholar]
- 10.Neu Yen Thi V. T., Zhao H. R. Preparation of oleanolic acid solid dispersion systems and their dissolution in vitro. J. Chin. Pharm. Univ. 2003;34:236–239. [Google Scholar]
- 11.Date A. A., Nagarsenker M. S. Design and evaluation of self-nanoemulsified drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int. J. Pharm. 2007;329:166–172. doi: 10.1016/j.ijpharm.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Constantinides P. P. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 1995;12:1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 13.Shafiq S., Shakeel F., Talegaonkar S., Ahmad F. J., Khar R. K., Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007;66(2):227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Kommuru T. R., Gurley B., Khan M. A., Reddy I. K. Self-emulsified drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int. J. Pharm. 2001;212:233–246. doi: 10.1016/S0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami K., Yoshikawa Y., Moroto E., Kanaoka K., Takaashi Y., Nishihara K., Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs II. In vivo study. J. Control Release. 2002;81:75–82. doi: 10.1016/S0168-3659(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Liu J., Yang X., Zhao X., Xu H. Oleanolic acid nanosuspensions: preparation, in-vitro characterization and enhanced hepatoprotective effect. J. Pharm. Pharmacol. 2005;57:259–264. doi: 10.1211/0022357055407. [DOI] [PubMed] [Google Scholar]
- 17.Li P., Ghosh A., Wagner R. F., Krill S., Joshi Y. M., Serajuddin A. T. M. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int. J. Pharm. 2005;288:27–34. doi: 10.1016/j.ijpharm.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Gao Z. G., Choi H. G., Shin H. J., Park K. M., Lim S. J., Hwang K. J., Kim C. K. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosphorin A. Int. J. Pharm. 1998;161:75–86. doi: 10.1016/S0378-5173(97)00325-6. [DOI] [Google Scholar]
- 19.Nazzal S., Smalyukh I. I., Lavrentovich O. D., Khan M. A. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int. J. Pharm. 2002;235:247–265. doi: 10.1016/S0378-5173(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 20.Charman S. A., Charman W. N., Rogge M. C., Wilson T. D., Dutko F. J., Pouton C. W. Self-emulsified drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm. Res. 1992;9:87–93. doi: 10.1023/A:1018987928936. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P., Liu Y., Feng N. P., Xiu J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2008;355:269–276. doi: 10.1016/j.ijpharm.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Atef E., Belmonte A. A. Formulation and in vitro and in vivo characterization of a phenytoin self-emulsifying drug delivery system (SEDDS) Eur. J. Pharm. Sci. 2008;35:257–263. doi: 10.1016/j.ejps.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Chiu Y. Y., Higaki K., Neudeck B. L., Barnett J. L., Welage L. S., Amidon G. L. Human jejunal permeability of cyclosporin A: influence of surfactants on P-glycoprotein efflux in Caco-2 cells. Pharm. Res. 2003;20:749–756. doi: 10.1023/A:1023481418576. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence M. J., Ress G. D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 25.Craig D. Q. M., Barker S. A., Banning D., Booth S. W. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int. J. Pharm. 1995;114:103–110. doi: 10.1016/0378-5173(94)00222-Q. [DOI] [Google Scholar]
- 26.Khoo S. M., Humberstone A. J., Porter C. J. H., Edwards G. A., Charman W. N. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int. J. Pharm. 1998;167:155–164. doi: 10.1016/S0378-5173(98)00054-4. [DOI] [Google Scholar]
- 27.Priev A., Zalipsky S., Cohen R., Barenholz Y. Determination of critical micelle concentration of lipopolymers and other amphiphiles: comparison of sound velocity and fluorescent measurements. Langmuir. 2002;18:612–617. doi: 10.1021/la0110085. [DOI] [Google Scholar]
- 28.Smith L. A., Hammond R. B., Roberts K. J., Machin D., McLeod G. Determination of the crystal structure of anhydrous sodium dodecyl sulphate using a combination of synchrotron radiation powder diffraction and molecular modeling techniques. J. Mol. Struct. 2000;554:173–182. doi: 10.1016/S0022-2860(00)00666-9. [DOI] [Google Scholar]
- 29.Ghosh P. K., Murthy R. S. R. Microemulsions: a potential drug delivery system. Curr. Drug Deliv. 2006;3:167–180. doi: 10.2174/156720106776359168. [DOI] [PubMed] [Google Scholar]
- 30.Kale N. J., Allen L. V. Studies on microemulsions using Brij 96 as surfactant and glycerin, ethylene glycol and propylene glycol as cosurfactants. Int. J. Pharm. 1989;57:87–93. doi: 10.1016/0378-5173(89)90296-2. [DOI] [Google Scholar]