Abstract
Gliclazide is a second generation of hypoglycemic sulfonylurea and acts selectively on pancreatic β cell to control diabetes mellitus. The objective of this study was to produce a controlled release system of gliclazide using chitosan beads. Chitosan beads were produced by dispersion technique using tripolyphosphate (TPP) as gelating agent. The effects of process variables including chitosan molecular weight, concentration of chitosan and TPP, pH of TPP, and cross-linking time after addition of chitosan were evaluated by Taguchi design on the rate of drug release, mean release time (MRT), release efficiency (RE8%), and particle size of the beads. The blood glucose lowering effect of the beads was studied in normal and streptozotocin-diabetic rats. The optimized formulation CL2T5P2t10 with about 31% drug loading, 2.4 h MRT, and 69.16% RE8% decreased blood glucose level in normal rats for 24 h compared to pure powder of gliclazide that lasted for just 10 h.
Key words: blood glucose, chitosan beads, controlled drug delivery, gliclazide, streptozotocin-diabetic rats
INTRODUCTION
Multiple-unit solid dosage forms such as microspheres or beads have gained in popularity as oral drug delivery systems because of more uniform distribution of the drug in the gastrointestinal tract, more uniform drug absorption, reduced local irritation, and elimination of unwanted intestinal retention of polymeric material when compared to non-disintegrating single-unit dosage forms (1–3).
Chitosan is a natural polysaccharide prepared by deacetylation of chitin, the principal component in shells of crustaceans (4–6). However, chitosan is a basic polymer and dissolves in acidic environment (7). Therefore, processing techniques have been extensively developed since the 1980s to prepare stable microspheres of chitosan. Four main approaches for the preparation of chitosan microspheres are: ionotropic gelation with oppositely charged polyelectrolytes such as tripolyphosphate (TPP) or alginate (8–12), simple or complex coacervation (13), spray drying (14,15), and emulsification–solvent evaporation (16,17). TPP is a nontoxic and multivalent anion. It can form gel by ionic interaction between positively charged amino groups of chitosan and negatively charged counterion of TPP. This interaction could be controlled by the charge density of TPP and chitosan, which is dependent on the pH of solution (18,19).
Statistically experimental design methods provide a systematic and efficient plan for experimentation to achieve certain goals so that many control factors can be simultaneously studied. The simplex method (20,21), evolutionary operation (22,23), response surface methodology (24–26), and the Taguchi method (27–29) are frequently applied experimental design methods. The Taguchi method has the advantages of optimization of many more factors simultaneously and extraction of much quantitative information by only a few experimental trials (30). Optimization of the process implies the use of a designed experiment in order to: (1) identify factors affecting the procedure, (2) estimate the factor levels yielding an optimum response, and (3) decrease the process variability without controlling or eliminating causes of variation. Thus, optimizing process parameters by the Taguchi method is an attempt not only to bring the average quality near to the target value but also to simultaneously minimize the variation in quality (31).
Gliclazide, 1-(3-azabicyclo(3.3.0)oct-3-yl)-3-p-tolylsulphonylurea is an oral hypoglycemic agent used in the treatment of non-insulin-dependent diabetes mellitus. Its plasma half-life is 6–14 h (32). After a single oral dose of 80 mg to 23 subjects, peak plasma concentrations of 0.7–4.9 mg L−1 were attained in about 4 h (33). Sulfonylureas have been used successfully for the treatment of type 2 diabetes and useful for islet β cell function in whom dietary and lifestyle modifications have been proven to be insufficient (34). Gliclazide modified release is a new once-daily formulation of the sulfonylurea gliclazide (35). The bioavailability after administration of a single dose of 30 mg is almost complete (97%) (36). The release of gliclazide over a 24-h period has been shown to parallel the circadian glycemic profile of type 2 diabetics (36,37). In spite of the good clinical results of this sustained release formulation, the binding of gliclazide to sulfonylurea receptors on pancreatic β cells is very rapidly reversible (38,39). This property may explain the low rate of hypoglycemia and pancreatic exhaustion and failure of modified release gliclazide (35,40).
The objective of this study was to design a sustained release formulation of chitosan microparticles containing gliclazide to increase the duration of its blood glucose-lowering effect. For this purpose, the chitosan beads were prepared by TPP. Considering that there were many variables interfering in the preparation process, to evaluate the effect of the these variables on the drug release behavior, various conditions such as concentration and molecular weight (MW) of wall material (chitosan), pH, and concentration of cross-linker (TPP) solution and curing time were evaluated by a Taguchi design.
MATERIALS AND METHODS
Materials
Chitosan with low MW (1.5 × 105) and medium MW (4 × 105; the degree of deacetylation was 86.8, 86.4%, respectively) were obtained from Fluka (Switzerland). Gliclazide was obtained from Synteco Chemical Ltd. (Italy). Sodium TPP (Sigma, USA), streptozotocin (Sigma, USA), glacial acetic acid, HCl, and other reagents were all analytical reagents grade from Merck Chemical Company (Germany).
Experimental Design and Analysis
Table I displays the five control factors selected in the optimization study. A standard orthogonal array L8 (41) was used to examine this five-factor system. L and subscript 8 denote the Latin square and the number of the experimental runs, respectively. The run involved the corresponding combination of levels to which the factors in the experiment was set. All studied factors had two levels and all experiments were performed in triplicate. The mean release time (MRT) and release efficiency (RE8%) of the release of gliclazide from chitosan beads were considered to be the responses. The optimum conditions were determined by the Taguchi’s two-step optimization method (42) to yield a heightened performance with the lowest possible effect of the noise factor. The first step was to select the factor/level combination to maximize the response. The second step was to find the condition for attaining optimal desirability (31).
Table I.
Definition and Trial Levels of Factors in Taguchi’s L8 Orthogonal Array Experiment in Production of Chitosan Beads Loaded with Gliclazide
| Studied variables | Levels | |
|---|---|---|
| I | II | |
| Chitosan MW | Low (L) | Medium (M) |
| Chitosan concentration (%, w/w) | 1 | 2 |
| pH of TPP solution | 2 | 9.6 |
| TPP concentration (%, w/v) | 2 | 5 |
| Cross-linking time (min) | 10 | 20 |
Chitosan beads containing gliclazide were prepared by a dispersion technique (43). Briefly, chitosan (100 or 200 mg) was dissolved in 10 ml of 2% (v/v) acetic acid containing 2% Tween 20 to form a homogeneous solution. The solution was filtered through glass wool and degassed overnight. Gliclazide powder (200 mg) was added to the chitosan gel and was homogenized thoroughly for 20 min. The curing solution used was a 2 or 5 wt.% TPP aqueous solutions (pH 2 or 9.6). The beads were formed by injecting 5 mL of bubble-free chitosan solution dropwise using a disposable plastic syringe with a 22-gauge needle and a push–pull syringe pump into 10 mL of the curing solution under gentle agitation. The dropping rate was 30 beads/min. The falling distance was 5 cm. The beads formed instantaneously and were kept for 10 or 20 min to cure. The solidified beads were extensively rinsed with deionized water and then dried at 37°C for 24 h (43). The final products were stored in a desiccator for future characterization and analysis.
Five studying variables (Table I) were investigated for optimization of beads properties by Taguchi’s design and eight formulations were prepared according to an orthogonal L8 array as shown in Table II. Calculations and statistical analysis of the results by analysis of variance were carried out to determine which factors had statistically significant effect on the response parameters by Qualitek4 software)Nutek, Inc., USA.).
Table II.
Formulations of Chitosan Beads Produced by a Taguchi Design in a Standard Orthogonal Array L8
| Formulation code | Chitosan MW | Chitosan concentration (%, w/w) | TPP concentration (%, w/v) | pH of TPP solution | Cross-linking time (min) |
|---|---|---|---|---|---|
| CL1T2P2t10 | Low | 1 | 2 | 2 | 10 |
| CM2T2P2t20 | Medium | 2 | 2 | 2 | 20 |
| CL2T5P2t20 | Low | 2 | 5 | 2 | 20 |
| CM1T5P2t10 | Medium | 1 | 5 | 2 | 10 |
| CL1T2P9.6t20 | Low | 1 | 2 | 9.6 | 20 |
| CM2T2P9.6t10 | Medium | 2 | 2 | 9.6 | 10 |
| CL2T5P9.6t10 | Low | 2 | 5 | 9.6 | 10 |
| CM1T5P9.6t20 | Medium | 1 | 5 | 9.6 | 20 |
Particle Size Analysis
The loaded beads were first overwhelmed for 1 h in water and then 625 particles were measured microscopically (Central Scientific Co., USA).
Drug Content of Beads
Encapsulation efficiency was studied by triturating 10 mg of the beads in 500 mL of phosphate buffer solution (pH 7.4) for 24 h. Tween 20 (0.5 mL) was added into the solution to enhance the drug solubility. The amount of drug loaded was determined by spectrophotometer at 227.8 nm. All the experiments were carried out in triplicate and drug free beads were used to prepare the blank.
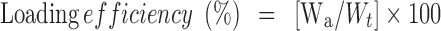 |
1 |
where Wa is the actual gliclazide content and Wt is theoretical gliclazide content (44).
In Vitro Release Studies
Beads (0.01 g) were suspended in 500 ml of phosphate-buffered solution (pH 7.4) containing Tween 20 (0.5 mL) in a 900-mL dissolution flask of USP apparatus II and maintained at 37°C. The release medium was stirred by USP paddle method at 50 rpm. Samples (3 mL) were periodically removed until 8 h and the volume of each sample was replaced by the same volume of fresh medium. The amount of released gliclazide was analyzed with a spectrophotometer at 227.8 nm. The in vitro release studies were performed in triplicate for each of the samples and drug free beads were used as the blank.
Analysis of Release Data
RE (45) after 8 h of release test was used to compare the results of release tests of different formulations:
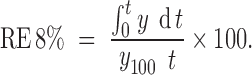 |
2 |
The other release parameter used for comparing the different formulations was MRT, which is calculated from the amount of drug released to the total cumulative drug. MRT is a measure of the rate of the release process: the higher the MRT, the slower the release rate. The following equation was used to calculate the MRT from the mean release data:
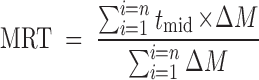 |
3 |
where i is the release sample number, n is the number of release sample time, tmid is the time at the midpoint between i and i − 1, and ΔM is the additional amount of drug released between i and i − 1 (46).
Experimental Animals
The animal experiments were conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations per the spirit of Association for Assessment and Accreditation of Laboratory Animal Care International’s expectations for animal care and use/ethics committees. Male albino Wistar rats of body weight 180–250 g were selected for all the experiments. Animals were kept in the animal house at an ambient temperature of 25–30°C and 45–55% relative humidity with 12 h each of dark and light cycle. Animals were fed pellet diet and water ad libitum. They were kept fasted 12–14 h before blood sampling.
Experimental Design
Gliclazide powder and the optimized beads of gliclazide were suspended in 1% (w/v) carboxymethylcellulose (CMC) suspension in normal saline and administered orally to the rats as much as 10 mg/kg of drug alone using a gastric tube. Animals were randomly divided into the following ten experimental groups, each group including six animals: groups I to V included non-diabetic animals and groups VI to X were streptozocin-induced diabetic rats. Group I were non-diabetic control animals and treated orally with CMC 1% suspension, groups II to IV included non-diabetic animals which were administrated orally suspension of placebo chitosan beads in CMC 1%, suspension of gliclazide beads in CMC 1%, suspension of gliclazide powder in CMC 1% respectively, and group V which were non-diabetic animals received 2.5 IU/kg of NPH insulin subcutaneously. Groups VI to X were exactly similar to groups I to V except that included diabetic control animals. The animals were carefully monitored everyday (47).
Induction of Diabetes in Rats
Diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (STZ) at 60 mg/kg body weight in freshly prepared normal saline solution (80 mg/4 mL) injected in a volume of about 0.5 mL. Control rats received only CMC 1% suspension in normal saline. The diabetic status of rats was assessed by measuring the fasting blood glucose (48).
Blood Collection and Biochemical Analysis
After 3 days of injection the STZ when the blood glucose level of rats reached to 300 mg/dL, rats were fasted 12–14 h and 0.5 mL of blood was withdrawn after 0, 1, 2, 3, 4, 8, 12, and 24 h through the retro-orbital plexus using a glass capillary and collected in Eppendorf tubes containing 20 mL of 10% EDTA. Collected blood was centrifuged for 10 min at 3000 rpm. The plasma thus obtained was used for glucose measuring. Blood glucose concentrations (mg/100 mL) were determined using a commercial kit based on the glucose oxidase method (49).
RESULTS AND DISCUSSION
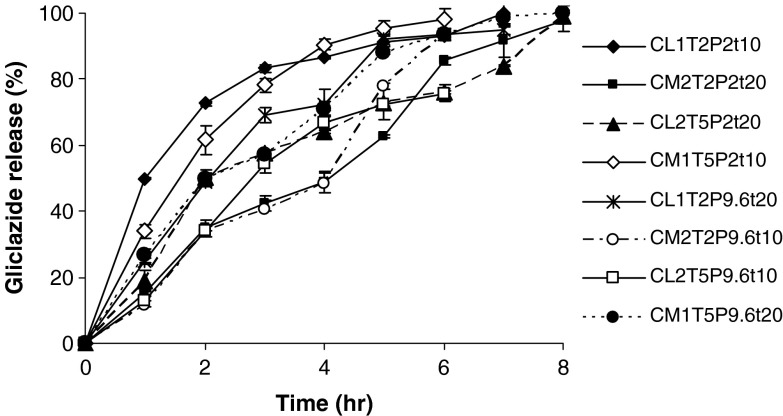
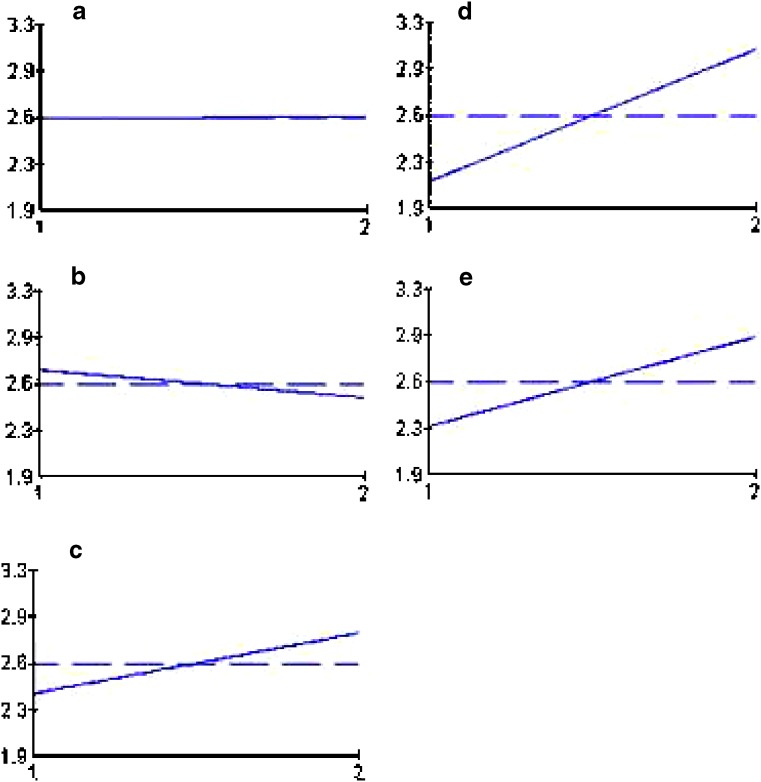
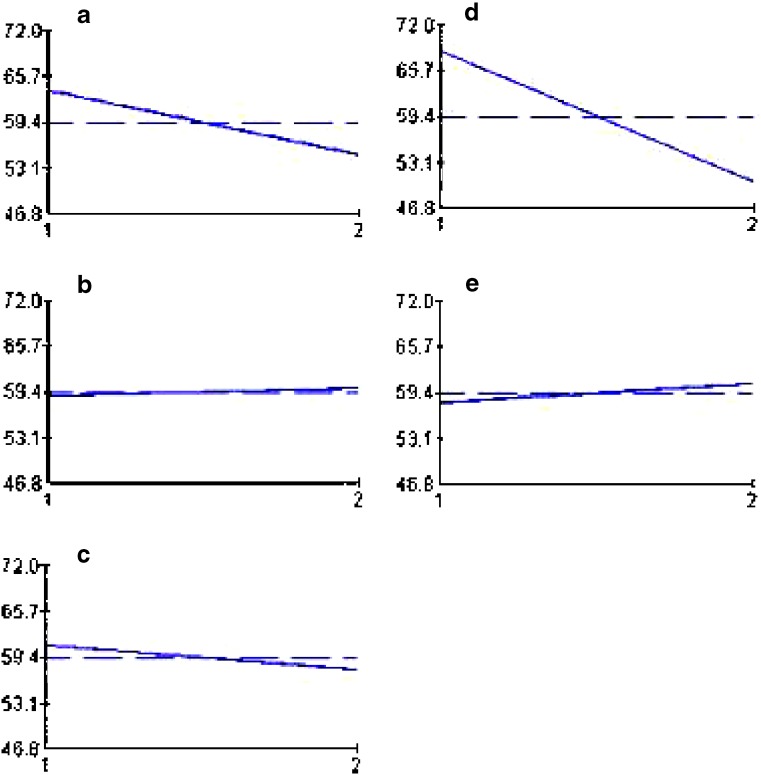
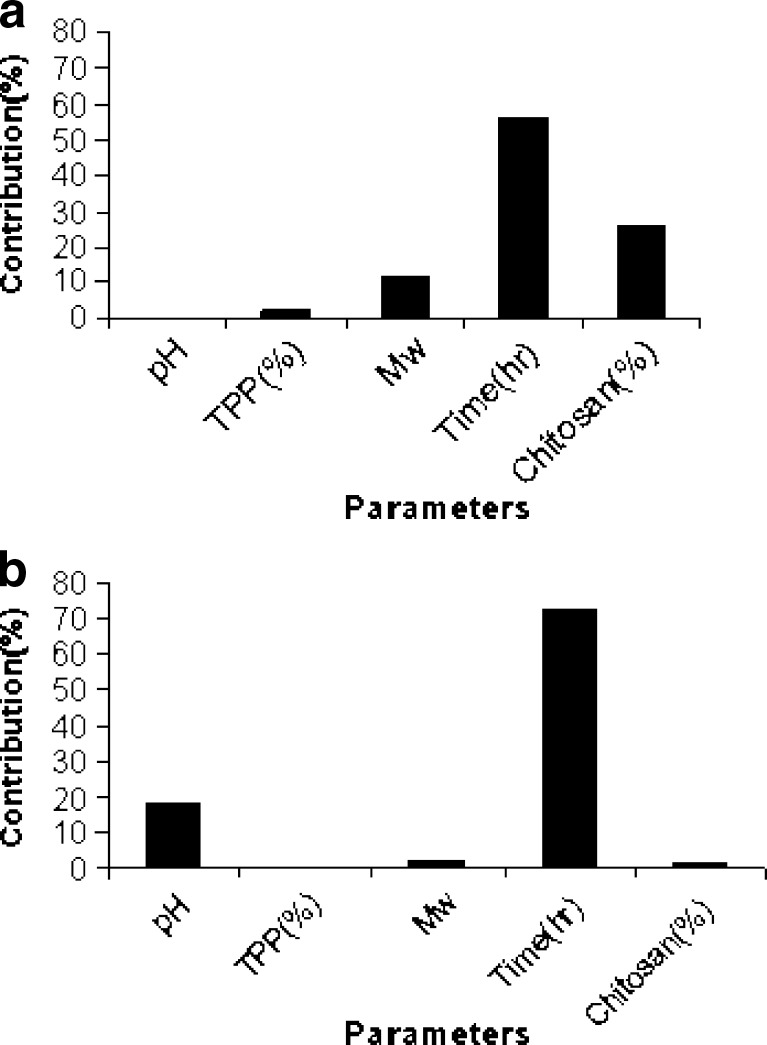
The results of drug loading efficiency and particle size of the beads are shown in Table III. Among the studied parameters, only TPP concentration had a statistically significant effect on the particle size of the beads. Figure 1 depicts the release profiles of different studied formulations of the gliclazide beads. The release parameters MRT and RE8% are also shown in Table III. According to the statistical analysis, the results show that changing the pH of the TPP solution from 2 to 9.6 does not have a significant effect on the MRT (Fig. 2a), while increasing its concentration from level 1 to 2 has a reducing effect on the MRT (Fig. 2b). Changing the MW of chitosan, curing time, and chitosan concentration from level 1 to 2 increased the MRT significantly (Fig. 2c–e). Changing the pH of the TPP solution and curing time from level 1 to 2 decreased the RE8% (Fig. 3a, d), while changing TPP concentration, MW, and concentration of chitosan from level 1 to 2 did not change the RE8% significantly (Fig. 3b, c, e). Figure 4a shows that MRT is more affected by the curing time of the beads in the TPP solution and the chitosan concentration. These parameters affect their cross-linking density and the wall thickness of the beads, respectively. Curing time and also the pH of the TPP solution are the most effective variables on RE8% (Fig. 4b).
Table III.
Drug Loading, Particle Size, Release Efficiency up to 8 h of Release Test (RE8%), and MRT of Chitosan Beads Loaded with Gliclazide (n = 3)
| Formulation code | Mean particle size (mm) | Drug loading (%) | RE8 (%) | MRT (h) |
|---|---|---|---|---|
| CL1T2P2t10 | 1.1 ± 0.05 | 41.8 ± 0.57 | 75.03 ± 0.05 | 1.73 ± 0.01 |
| CM2T2P2t20 | 1.45 ± 0.04 | 24.86 ± 0.45 | 53.30 ± 1.52 | 3.60 ± 0.09 |
| CL2T5P2t20 | 1.30 ± 0.04 | 19.0 ± 0.25 | 59.00 ± 2.50 | 3.21 ± 0.15 |
| CM1T5P2t10 | 1.22 ± 0.05 | 38.0 ± 0.52 | 67.93 ± 0.90 | 1.83 ± 0.04 |
| CL1T2P9.6t20 | 1.19 ± 0.02 | 34.6 ± 0.66 | 64.00 ± 2.60 | 2.26 ± 0.07 |
| CM2T2P9.6t10 | 1.58 ± 0.03 | 20.0 ± 1.02 | 43.00 ± 2.00 | 3.22 ± 0.05 |
| CL2T5P9.6t10 | 1.38 ± 0.04 | 23.8 ± 1.00 | 46.30 ± 0.55 | 2.31 ± 0.12 |
| CM1T5P9.6t20 | 1.28 ± 0.03 | 33.0 ± 1.12 | 66.50 ± 0.75 | 2.65 ± 0.05 |
Fig. 1.
Release profiles of gliclazide from different studied formulations of the chitosan beads (n = 3)
Fig. 2.
Effect of different levels of studied variables. a pH of TPP solution, b TPP concentration, c MW of chitosan, d curing time, and e chitosan concentration on MRT of gliclazide release from chitosan beads. The vertical axis shows the mean MRT (h) and horizontal one shows two levels of the studied variables
Fig. 3.
Effect of different levels of studied variables. a pH of TPP solution, b TPP concentration, c MW of chitosan, d curing time, and e chitosan concentration on RE8% of gliclazide release from chitosan beads. The vertical axis shows the mean RE8 (%) and horizontal one shows two levels of the studied variables
Fig. 4.
Contribution effect of studied variables on a MRT and b RE8% of gliclazide release from chitosan beads
With respect to the objective properties considered in this study, both responses (MRT and RE8%) are the-larger-the-better, as the higher MRT shows a more prolonged release and the higher RE shows the greater amount of the loaded drug in the beads that can be released. Accordingly, Taguchi’s design predicted the optimized situation of the gliclazide bead formulations (Table IV). These formulations were prepared and their MRT and RE8% were determined experimentally as shown in Table IV. The results show that the actual and predicted values of the optimized formulations according to both responses are near and the Taguchi design can successfully predict the best situation of preparing the chitosan beads for sustained delivery of gliclazide.
Table IV.
Comparison of the Predicted MRT and RE8% of Optimized Formulations of Chitosan Beads Designed by Qualitek4 Software and the Real Values of These Parameters
| Optimized formulation | MRT (h) | RE8(%) | ||
|---|---|---|---|---|
| Predicted | Actual | Predicted | Actual | |
| CM2T2P9.6t20 | 3.75 | 3.75 | 49.5 | 45.1 |
| CL2T5P2t10 | 2.5 | 2.4 | 76.4 | 69.16 |
Considering the higher RE8% values obtained by the optimized formulation CL2T5P2t10, this formulation was chosen for in vivo studies.
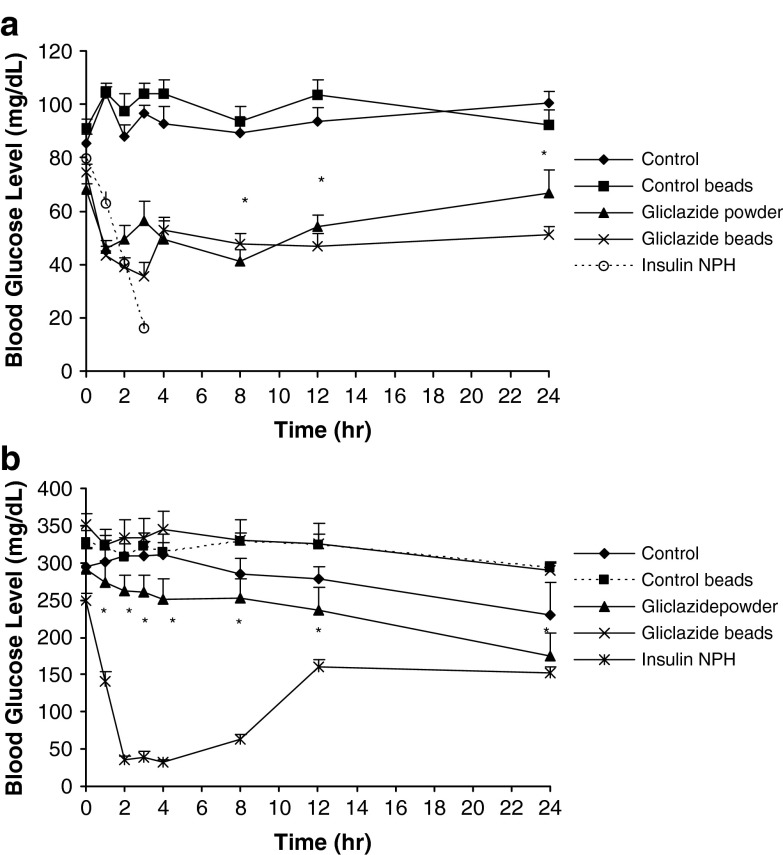
Figure 5 shows the effect of gliclazide powder or beads on the blood glucose levels in normal rats (Fig. 5a) and STZ-diabetic rats (Fig. 5b). As Fig. 5a indicates, gliclazide beads have reduced more significantly (p < 0.05) blood glucose levels at 2, 3, and 24 h after administration in the normal rats compared with gliclazide powder. This indicates the sustained increased duration of action of gliclazide beads in lowering blood glucose levels. These groups both showed significantly lower (p < 0.05) blood glucose levels from the control groups administered 1% CMC suspension or blank beads. However, in diabetic rats, there is not any significant difference between the control groups and treated groups administered gliclazide powder or beads form (p > 0.05). In other words, not only the gliclazide beads but also its powder have not been able to reduce the blood glucose level in diabetic rats. This may be related to the utilized dose of STZ that has damaged completely the pancreas of the rats. There are different and controversial reports about the effect of gliclazide on different doses of STZ used for induction of diabetes in rats: Pulido et al. (50) examined whether the treatment of STZ-diabetic rats with gliclazide in 5 mg/kg body weight twice daily orally increases muscle glucose uptake. They concluded that gliclazide has a glucose-lowering effect in STZ-diabetic rats that could be attributed to an increase in muscle glucose clearance by a post-insulin receptor mechanism, probably related to a normalization of GLUT4 content (50). However, Qiang et al. (51) used doses of gliclazide administered (28.2 to 100.7 mg kg−1 day−1) that were extremely higher than clinical doses for humans (approximately 10 to 40 times as much as those for humans), but equivalent to the rat experiments previously reported (52,53), while serum levels of gliclazide (2.5 to 10.8 μg/mL) were comparable to those in both rats (53) and humans (52), which effectively lowered blood glucose levels in the short-time experiment. In their study, although the blood glucose levels of diabetic rats were more than 300 mg/dL 24 h after the STZ injection, the hyperglycemia remained stable throughout the entire experiment. There was no difference in blood glucose levels between non-treated rats and gliclazide- or glibenclamide-treated rats both in diabetic and nondiabetic rats, i.e., administration of gliclazide or glibenclamide had no effect on blood glucose levels in this experiment. Gliclazide or glibenclamide administration did not affect non-fasting serum insulin or glucose levels in either non-diabetic and diabetic rats, although the exact reason was unclear (51). The authors concluded that a possible reason was that the rats were chronically administered sulfonylureas with foods, which might induce secondary failure to sulfonylureas (54).
Fig. 5.
Blood glucose levels in a normal rats and b streptozocin-diabetic rats (n = 6). Control group were treated orally with CMC 1% suspension, control beads groups were administrated orally suspension of placebo chitosan beads in CMC 1%, gliclazide powder, and gliclazide beads groups were administered as much as 10 mg/kg of pure drug and the loaded chitosan beads containing the same amount of pure gliclazide suspended in 1% (w/v) carboxymethylcellulose (CMC) suspension in normal saline, respectively, and NPH insulin group received 2.5 IU/kg of NPH insulin subcutaneously. *p < 0.05
These reports can explain our results on the glucose-lowering effect of gliclazide sustained release beads in normal rats, but not in the STZ-diabetic ones.
CONCLUSIONS
It may be concluded that the optimized beads prepared by 2% low-molecular-weight chitosan that were cross-linked by 5% TPP at pH 2 with a curing time of 10 min could decrease blood glucose level in normal rats for 24 h compared to powder of gliclazide that lasted for just 10 h. These sustained release beads may be considered for further human evaluation as a promising controlled release dosage form for gliclazide.
Acknowledgments
The authors wish to acknowledge the Vice Chancellor of Research of Isfahan University of Medical Sciences for the financial support of this project.
References
- 1.Lauwo J. A. K., Agrawal D. K., Emenike I. V. Some pharmaceutical studies on sustained release coprecipitates of ampicillin-trihydrate with acrylic resin (Eudragit®-RS) Drug Dev. Ind. Pharm. 1990;16:1375–1389. doi: 10.3109/03639049009115967. [DOI] [Google Scholar]
- 2.Bodmeier R., Chen H., Tyle P., Jarosz P. Pseudophedrine HCL microspheres formulated into an oral suspension dosage form. J. Control. Release. 1991;15:65–77. doi: 10.1016/0168-3659(91)90104-L. [DOI] [Google Scholar]
- 3.Anal A. K., Stevens W. F., Remuñán-López C. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 2006;312(1–2):166–173. doi: 10.1016/j.ijpharm.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 4.van der Lubben I. M., Verhoef J. C., Borchard G., Junginger H. E. Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 2001;52:139–144. doi: 10.1016/S0169-409X(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 5.Martino A. D., Sittinger M., Risbud M. V. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Wang L. Y., Gu Y. H., Zhou Q. Z., Ma G. H., Wan Y. H., Su Z. G. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf., B: Biointerfaces. 2006;50:126–135. doi: 10.1016/j.colsurfb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Wei W., Wang L. Y., Su Z. G., Ma G. H. Preparation of uniform-sized pH-sensitive quaternized chitosan microsphere by combining membrane emulsification technique and thermal-gelation method. Colloids Surf., B: Biointerfaces. 2008;63(2):164–175. doi: 10.1016/j.colsurfb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Bodmeier R., Oh K. H., Pramar Y. Preparation and evaluation of drug-containing chitosan beads. Drug Dev. Ind. Pharm. 1989;15:1475–1494. doi: 10.3109/03639048909062758. [DOI] [Google Scholar]
- 9.Sezer A. D., Akbûga J. Release characteristics of chitosan treated alginate beads. I. Sustained release of a macromolecular drug from chitosan treated alginate beads. J. Microencap. 1999;16(2):195–203. doi: 10.1080/026520499289176. [DOI] [PubMed] [Google Scholar]
- 10.Anal A. K., Bhopatkar D., Tokura S., Tamura H., Stevens W. F. Chitosan-alginate multilayer beads for gastric passage and controlled intestinal release of protein. Drug Dev. Ind. Pharm. 2003;29:713–724. doi: 10.1081/DDC-120021320. [DOI] [PubMed] [Google Scholar]
- 11.Anal A. K., Stevens W. F. Chitosan–alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005;290:45–54. doi: 10.1016/j.ijpharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Bhopatkar D., Anal A. K., Stevens W. F. Ionotropic alginate beads for controlled intestinal protein delivery: Effect of chitosan and barium counterions on entrapment and release. J. Microencap. 2005;22:91–100. doi: 10.1080/02652040400026434. [DOI] [PubMed] [Google Scholar]
- 13.Remuñán-López C., Bodmeier R. Effect of formulation and process variables on the formation of chitosan–gelatin coacervates. Int. J. Pharm. 1996;135:63–72. doi: 10.1016/0378-5173(95)04347-0. [DOI] [Google Scholar]
- 14.Williams R. O., III, Barron M. K., Alonso M. J., Remuñán-López C. Investigation of a pMDI formulation containing chitosan microspheres. Int. J. Pharm. 1998;174:209–222. doi: 10.1016/S0378-5173(98)00266-X. [DOI] [Google Scholar]
- 15.He P., Davis S. S., Illum L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999;187:53–65. doi: 10.1016/S0378-5173(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 16.Remuñán-López C., Lorenzo-Lamosa M. L., Vila-Jato J. L., Alonso M. J. Development of new chitosan–cellulose multicore microparticles for controlled drug delivery. Eur. J. Pharma. Biopharm. 1998;45:49–56. doi: 10.1016/S0939-6411(97)00122-7. [DOI] [PubMed] [Google Scholar]
- 17.Mi F. L., Shyu S. S., Wong T., Jang S. F., Lee S. T., Lu K. T. Chitosan–polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. Effect of pH-dependent ionic cross-linking or interpolymer complex using tripolyphosphate or polyphosphate reagent. J. Appl. Polym. Sci. 1999;74:1093–1107. doi: 10.1002/(SICI)1097-4628(19991031)74:5<1093::AID-APP6>3.0.CO;2-C. [DOI] [Google Scholar]
- 18.Ko J. A., Park H. J., Hwang S. J., Park J. B., Lee J. S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002;249(1–2):165–174. doi: 10.1016/S0378-5173(02)00487-8. [DOI] [PubMed] [Google Scholar]
- 19.Mi F. L., Shyu S. S., Chen C. T., Schoung J. Y. Porous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: Preparation of antigen-adsorbed microsphere and in vitro release. Biomaterials. 1999;20(17):1603–1612. doi: 10.1016/S0142-9612(99)00064-2. [DOI] [PubMed] [Google Scholar]
- 20.Nakai S., Koide K., Eugster K. A new mapping super-simplex optimization for food product and process development. J. Food Sci. 1984;49:1143–1170. doi: 10.1111/j.1365-2621.1984.tb10414.x. [DOI] [Google Scholar]
- 21.Chen K. C., Lee T. C., Houng J. Y. Search method for the optimal medium for the production of lactase by Kluyveromyces fragilis. Enzyme Microb. Technol. 1992;14:659–664. doi: 10.1016/0141-0229(92)90043-N. [DOI] [Google Scholar]
- 22.Banerjii R., Bhattacharyya B. C. Evolutionary operation (EVOP) to optimize three-dimensional biological experiments. Biotechnol. Bioeng. 1993;41:67–71. doi: 10.1002/bit.260410109. [DOI] [PubMed] [Google Scholar]
- 23.Tunga R., Banerjii R., Bhattacharyya B. C. Optimization of n variable biological experiments by evolutionary operation-fractional design technique. J. Biosci. Bioeng. 1999;87(2):224–230. doi: 10.1016/S1389-1723(99)89017-3. [DOI] [PubMed] [Google Scholar]
- 24.Houng J. Y., Chen K. C., Hsu W. H. Optimization of cultivation medium composition for isoamylase production. Appl. Microb. Biotechnol. 1989;31:61–64. doi: 10.1007/BF00252528. [DOI] [Google Scholar]
- 25.Chopra S., Patil G. V., Motwani S. K. Release modulating hydrophilic matrix systems of losartan potassium: Optimization of formulation using statistical experimental design. Eur. J. Pharm. Biopharm. 2007;66:73–82. doi: 10.1016/j.ejpb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Sreekumar O., Chand N., Basappa A. C. Optimization and interaction of media components in ethanol production using Zymomonas mobilis by response surface methodology. J. Biosci. Bioeng. 1999;88(3):334–338. doi: 10.1016/S1389-1723(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 27.Jahanshahi M., Najafpour G., Rahimnejad M. Applying the Taguchi method for optimized fabrication of bovine serum albumin (BSA) nanoparticles as drug delivery vehicles. Afr. J. Biotechnol. 2008;7(4):362–367. [Google Scholar]
- 28.Cobb B. D., Clarkson J. M. A simple procedure for optimising the polymerase chain reaction (PCR) using modified Taguchi methods. Nucleic Acids Res. 1994;22(18):3801–3805. doi: 10.1093/nar/22.18.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone R. A., Veevers A. The Taguchi influence on designed experiments. J. Chemometr. 1994;8:103–110. doi: 10.1002/cem.1180080203. [DOI] [Google Scholar]
- 30.Bendell J. D., Disney J., Pridmore W. A. Taguchi Methods: Applications in World Industry. Bedford: IFS Publications; 1989. [Google Scholar]
- 31.Houng J. Y., Hsu H. F., Liu Y. H., Wu J. Y. Applying the Taguchi robust design to the optimization of the asymmetric reduction of ethyl 4-chloro acetoacetate by bakers’ yeast. J. Biotechnol. 2003;100(3):239–250. doi: 10.1016/S0168-1656(02)00179-7. [DOI] [PubMed] [Google Scholar]
- 32.Główka F. K., Hermann T. W., Zabel M. Bioavailability of gliclazide from some formulation tablets. Int. J. Pharm. 1998;172(1–2):71–77. [Google Scholar]
- 33.Cambell D. B., Lavielle R., Nathan C. The mode of action and clinical pharmacology of gliclazide: A review. Diabetes Res. 1991;14(Suppl):S21–S36. doi: 10.1016/0168-8227(91)90005-x. [DOI] [PubMed] [Google Scholar]
- 34.European Diabetes Policy Group A desktop guide to type 2 diabetes mellitus. Diabet. Med. 1999;16:716–730. doi: 10.1046/j.1464-5491.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 35.Harrower A. Gliclazide modified release: From once daily administration to 24-hour blood glucose control. Metabolism. 2000;49(suppl 2):7–11. doi: 10.1053/meta.2000.17823. [DOI] [PubMed] [Google Scholar]
- 36.Francillard M., Frey N., Paraire M. Pharmacokinetics of diamicron modified release (MR) in 1007 type 2 diabetic patients. J. Nutr. Health Aging. 2001;5:31. [Google Scholar]
- 37.Delrat P., Paraire M., Jochemsen R. Complete bioavailability and lack of food effect on pharmacokinetics of gliclazide 30 mg modified release in healthy volunteers. Biopharm. Drug Dispos. 2002;23:151–157. doi: 10.1002/bdd.303. [DOI] [PubMed] [Google Scholar]
- 38.Gribble F. M., Ashcroft F. M. Differential sensitivity of β-cell and extrapancreatic K ATP channels to gliclazide. Diabetologia. 1999;42:845–848. doi: 10.1007/s001250051236. [DOI] [PubMed] [Google Scholar]
- 39.Gribble F. M., Tucker S. J., Seino S. Tissue specificity of sulfonylureas: Studies on cloned cardiac and β cell KATP channels. Diabetes. 1998;47:1412–1418. doi: 10.2337/diabetes.47.9.1412. [DOI] [PubMed] [Google Scholar]
- 40.Schernthaner G. Gliclazide modified release: a critical review of pharmacodynamic, metabolic, and vasoprotective effects. Metabolism. 2003;52(Suppl 1):29–34. doi: 10.1016/S0026-0495(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 41.Taguchi G. System of Experimental Design. Engineering Methods to Optimize Quality and Minimize Costs. White Plains, New York: Kraus International; 1987. [Google Scholar]
- 42.Fowlkes W. Y., Creveling C. M. Engineering Methods for Robust Product Design. Using Taguchi Methods® in Technology and Product Development. New York: Addison-Wesley; 1995. [Google Scholar]
- 43.Lin W. C., Yu D. G., Yang M. C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Colloids Surf., B: Biointerfaces. 2005;44(2–3):143–151. doi: 10.1016/j.colsurfb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Ko J. A., Park H. J., Hwang S. J., Park J. B., Lee J. S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002;249(1–2):165–174. doi: 10.1016/S0378-5173(02)00487-8. [DOI] [PubMed] [Google Scholar]
- 45.Costa P., Manuel J., Lobo S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001;13:123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 46.Gohel M. C., Panchal M. K. Novel use of similarity factors f2 and Sd for development of diltiazem HCl modified-release tablets using a 32 factorial design. Drug Dev. Ind. Pharm. 2002;28(1):77–87. doi: 10.1081/DDC-120001488. [DOI] [PubMed] [Google Scholar]
- 47.Eidi A., Eidi M., Sokhteh M. Effect of fenugreek (Trigonella foenum-graecum L) seeds on serum parameters in normal and streptozotocin-induced diabetic rats. Nutr. Res. 2007;27(11):728–733. doi: 10.1016/j.nutres.2007.09.006. [DOI] [Google Scholar]
- 48.Suresh Kumar G., Shetty A. K., Sambaiah K., Salimath P. V. Antidiabetic property of fenugreek seed mucilage and spent turmeric in streptozotocin-induced diabetic rats. Nutr. Res. 2005;25(11):1021–1028. doi: 10.1016/j.nutres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Chandra A., Mahdi A. A., Ahmad S., Singh R. K. Indian herbs result in hypoglycemic responses in streptozotocin-induced diabetic rats. Nutr. Res. 2007;27(3):161–168. doi: 10.1016/j.nutres.2006.12.008. [DOI] [Google Scholar]
- 50.Pulido N., Suarez A., Casanova B., Romero R., Rodriguez E., Rovira A. Gliclazide treatment of streptozotocin diabetic rats restores GLUT4 protein content and basal glucose uptake in skeletal muscle. Metabolism. 1997;46(Suppl 1):10–13. doi: 10.1016/S0026-0495(97)90310-3. [DOI] [PubMed] [Google Scholar]
- 51.Qiang X., Satoh J., Sagara M., Fukuzawa M., Masuda T., Miyaguchi S., Takahashi K., Toyota T. Gliclazide inhibits diabetic neuropathy irrespective of blood glucose levels in streptozotocin-induced diabetic rats. Metabolism. 1998;47(8):977–981. doi: 10.1016/S0026-0495(98)90354-7. [DOI] [PubMed] [Google Scholar]
- 52.Palmer K. J., Brogden R. N. Gliclazide, an update of its pharmacological properties and therapeutic efficacy non-insulin-dependent diabetes mellitus. Drugs. 1993;46:92–125. doi: 10.2165/00003495-199346010-00007. [DOI] [PubMed] [Google Scholar]
- 53.Duhault J., Boulanger M., Tisserand F. The pharmacology of S 1702, a new highly effective oral antidiabetic drug with unusual properties. Arzneim Forsch. 1972;22:1682–1685. [PubMed] [Google Scholar]
- 54.Pontiroli A. E., Calderara A., Pozza G. Secondary failure of oral hypoglycaemic agents: Frequency, possible causes, and management. Diabetes Metab. Rev. 1994;10:31–43. doi: 10.1002/dmr.5610100104. [DOI] [PubMed] [Google Scholar]