Abstract
Drugs that have narrow absorption window in the gastrointestinal tract (GIT) will have poor absorption. For these drugs, gastroretentive drug delivery systems offer the advantage in prolonging the gastric emptying time. Swellable, floating, and sustained release tablets are developed by using a combination of hydrophilic polymer (hydroxypropyl methylcellulose), swelling agents (crospovidone, sodium starch glycolate, and croscarmelose sodium) and effervescent substance (sodium bicarbonate). Formulations are evaluated for percentage swelling, in vitro drug release, floating lag time, total duration of floating, and mean residence time (MRT) in the stomach. The drug release of optimized formulation follows the Higuchi kinetic model, and the mechanism is found to be non-Fickian/anomalous according to Krosmeyer–Peppas (n value is 0.68). The similarity factor (f2) is found to be 26.17 for the optimized formulation, which the release is not similar to that of marketed produced (CIFRAN OD®). In vivo nature of the tablet at different time intervals is observed in the radiographic pictures of the healthy volunteers and MRT in the stomach is found to be 320 ± 48.99 min (n = 6). A combination of HPMC K100M, crospovidone, and sodium carbonate shows the good swelling, drug release, and floating characters than the CIFRAN OD®.
Key words: ciprofloxacin, floating, gastroretentive, in vivo studies, swellable
INTRODUCTION
Oral sustained release dosage forms deliver the drug for longer period and helps in producing the therapeutic effect for 24 h for those drugs which are having low plasma half life. Drugs that have narrow absorption window in the gastro intestinal tract (GIT) will have poor absorption (1,2). For these drugs, gastroretentive drug delivery systems (GRDDSs) have been developed. Oral sustained release dosage form with prolonged residence time in the stomach helps in absorption of the drugs which are less soluble or unstable in the alkaline pH and those which are absorbed from the upper gastrointestinal tract (3). GRDDSs help in maintenance of constant therapeutic levels for prolonged periods and produce therapeutic efficacy and thereby reduce the total dose of administration.
Recently several gastroretentive approaches like swelling devices (4,5), floating systems (2,6), bioadhesive systems (7), low density systems (8), high density systems (9), expandable systems (10), superporous, biodegradable hydrogels (2,11,12), and magnetic systems (13) have been developed. To increase the gastric retention time (GRT), one should have a thorough knowledge about the physiology of GIT, and all the limitations should be well understood. To justify the in vitro studies, in vivo studies must be conducted.
The excellent floating system is effective only in the presence of sufficient fluid in the stomach; otherwise, buoyancy of the tablet may be hindered. This limitation can be overcome by using a combination of a floating system with other gastroretentive approaches (14). GRDDSs are formulated as floating microparticles, tablets, pellets, capsules, etc. among which the multiparticulate systems are more effective than the single unit dosage forms (15,16).
Srinatha and Pandit J.K developed ciprofloxacin loaded multi-unit floating alginate beads and studied in vitro release in simulated gastric fluid and effect of alginate when blended with gellan, hydroxypropyl methyl cellulose (HPMC), starch, and chitosan (17). Garg et al., suggested there will be a decrease in bioavailability of conventional oral controlled drug delivery system of narrow absorption window in gastrointestinal tract and developed floating microspheres of ciprofloxacin. Sa Hoo et al., developed floating microspheres of ciprofloxacin hydrochloride by using a polymer mixture of sodium alginate and HPMC (18). Varshosaz et al., formulated ciprofloxacin floating and bioadhesive release tablets by using sodium carboxy methyl cellulose, HPMC, polyacrylic acid, polymetacrylic acid, citric acid, and sodium bicarbonate (19).
Ciprofloxacin hydrochloride, a broad-spectrum fluoroquinolone antibacterial agent is more absorbed from the stomach and the proximal part of the small intestine (19). Oral bioavailability is about 70% and reaches the peak plasma concentration to 2.5 μg/ml in 1 to 2 h after administration of 500 mg. As the tablet passes down the GIT, the decrease absorption is the draw back with sustained release dosage form of ciprofloxacin hydrochloride. The extended release formulation of ciprofloxacin HCl (Cipro XR and Proquin XR) is used for complicated and uncomplicated urinary tract infections (UTIs) (20,21). Ciprofloxacin HCl extended release (500 mg once daily) shows higher plasma concentration than the immediate release (200 mg twice daily) formulation. The bioavailability is lower if Proquin XR tablets are given while fasting (21).
The objective of this research work is to obtain better delivery of ciprofloxacin HCl to the stomach and the proximal parts of the small intestine by increasing the mean residence time (MRT) in the stomach. For this, gastroretentive floating and swellable tablets are prepared to prolong the gastric emptying that provides maximum drug at the site of absorption.
MATERIALS AND METHODS
Materials
Ciprofloxacin HCl (Smruthi Organics, India), CIFRAN OD® 500 mg (Ranbaxy, India) hydroxypropyl methylcellulose (HPMC K4M and HPMC K100M), (Cadila Pharma, India), crospovidone (CP) (Danmed Pharma Pvt, India), sodium starch glycolate (SSG) (Amish Drugs & Chemicals, India), croscarmellose sodium (CCS) (Amish Drugs & Chemicals), Starch 1500 (STR) (Merck, Germany), poly vinyl pyrolidone k-30 (PVP K30) (S.D. Fine Chemical Pvt, India), sodium bicarbonate (S.D. Fine Chemical Pvt), magnesium stearate (S.D. Fine Chemical Pvt), and talc (S.D. Fine Chemical Pvt) are used.
Methods
Preparation of Tablets
Ciprofloxacin HCl tablets are prepared by wet granulation method using hydrophilic polymer (different grades of HPMC), gas generating agent, and swelling agents in each formulation. The swelling agents are used as half intra-granularly and other half as extra-granularly. The composition different excipients in formulations are listed in Table I. All the ingredients are passed through sieve no. 60 and mixed in a polybag and granulated by using PVP K30 (5% w/v in isopropyl alcohol). The wet mass is passed through sieve no. 14 and dried at 50°C. Dried granules are passed through sieve no. 16 and mixed with remaining portion of swelling agent, magnesium stearate, and talc. Granules are compressed by using caplet-shaped punches on cadmach 16-station rotary tablet press.
Table I.
Ingredients of Gastroretentive Tablets of Ciprofloxacin Hydrochloride (CFH)
| Formulae Code | Ingredients (mg) | ||||||
|---|---|---|---|---|---|---|---|
| CFH | HPMC K100M | CP | SSG | CCS | STR | SBC | |
| F1 | 580 | 145 | 232 | – | – | – | 116 |
| F2 | 580 | 145 | – | 232 | – | – | 116 |
| F3 | 580 | 145 | – | – | 232 | – | 116 |
| F4 | 580 | 145 | – | – | – | 232 | 116 |
| F5 | 580 | 116 | 232 | – | – | – | 116 |
| F6 | 580 | 101.5 | 232 | – | – | – | 116 |
| F7 | 580 | 87 | 232 | – | – | – | 116 |
| F8 | 580 | 87 | 116 | – | – | – | 116 |
| F9 | 580 | 87 | 174 | – | – | – | 116 |
| F10 | 580 | 87 | – | 174 | – | – | 116 |
| F11 | 580 | 87 | – | – | 174 | – | 116 |
| F12 | 580 | 87 | 203 | – | – | – | 116 |
| F13 | 580 | 87 | – | 203 | – | – | 116 |
| F14 | 580 | 87 | – | – | 203 | – | 116 |
All tablets contain 2% w/w magnesium stearate and 1% w/w talc that of tablet
CFH ciprofloxacin hydrochloride, HPMC K100M hydroxypropyl methylcellulose K100M, CP crospovidone, SSG sodium starch glycolate, CCS cross-carmellose sodium, STR Starch 1500, SBC sodium bicarbonate
Evaluation of Tablets
The prepared tablets are evaluated for hardness (Monsanto hardness tester), friability, weight variation, thickness, length, water uptake, in vitro drug release, in vitro floating lag time, and total floating time and in vivo buoyancy studies on healthy human volunteers.
Water Uptake Studies
The swelling behavior of dosage unit can be measured either by studying its dimensional changes, weight gain, or water uptake (22,23). Water uptake study of the dosage form is conducted by using USP dissolution apparatus-II in 900 ml of distilled water which is maintained at 37 ± 0.5°C, rotated at 50 rpm. At selected regular intervals, the tablet is withdrawn and weighed. Percentage swelling of the tablet is expressed as percentage water uptake (%WU) (24,25).
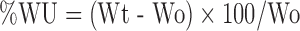 |
1 |
Where Wt is the weight of the swollen tablet, and Wo is the initial weight of the tablet.
In Vitro Buoyancy Determination
Floating behavior of the tablet is determined by using USP dissolution apparatus-II in 500 ml of 0.1 N HCl which is maintained at 37 ± 0.5°C, rotated at 50 rpm. The floating lag time as well as total floating time is observed.
In Vitro Dissolution Studies
In vitro dissolution studies are studied by using USP dissolution apparatus-II, in 900 ml of 0.1 N HCl (pH 1.2) at 50 rpm, and temperature is maintained at 37 ± 0.5°C. Five milliliters of sample is with drawn at predetermined time intervals 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, and 12 h. Every time, the dissolution medium is replaced with fresh 0.1 N HCl. Samples are filtered and diluted with fresh buffer and are analyzed in Elico UV/Vis spectrophotometer at 276 nm.
Drug Release Kinetics
The drug release kinetics are studied by plotting the data obtained from the in vitro drug release in various kinetic models like zero-order, first-order, Higuchi’s, and Hixson–Crowell model.
Zero-order (Eq. 2) data is plotted as cumulative percentage drug released versus time.
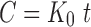 |
2 |
Where C is the concentration, Ko is the zero-order rate constant expressed as concentration/time, and t is time in hours.
First order (Eq. 3) is obtained by plotting log cumulative percentage drug released versus time.
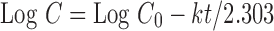 |
3 |
Where C0 is the initial concentration of the drug, k is the first-order rate constant, and t is the time.
As per Higuchi’s (Eq. 4) data is plotted as cumulative percentage drug released versus square root of the time.
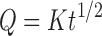 |
4 |
Where K is the constant of the system, and t is the time.
The mechanism of drug release is evaluated by plotting the percentage of drug released versus log time according to Krosmeyer–Peppas equation (equation). Exponent n indicates the mechanism of drug release calculated through the slope of the straight line.
 |
5 |
Where 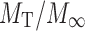 is the fractional solute release, t is the release time; K is a constant characteristic of the drug/polymer system. If the exponent n = 0.45, then the drug release follows the Fickian diffusion, and if
is the fractional solute release, t is the release time; K is a constant characteristic of the drug/polymer system. If the exponent n = 0.45, then the drug release follows the Fickian diffusion, and if 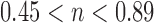 , then it is said to be non-Fickian or anomalous release.
, then it is said to be non-Fickian or anomalous release.
In Vivo Confirmation of Buoyancy by Using Radiographic Studies
For this study, the tablets are prepared by replacing half the amount of drug with barium sulfate. After overnight fasting of six healthy volunteers, they are fed with low calorie food and allowed to take water after these tablets are administered orally. Radiographs are obtained at 1, 2, 3 h, and up to 6 h over these periods, volunteers are allowed to take water (26,27).
RESULTS
The data of physical parameters like thickness, content uniformity, weight variation, length of the tablet, floating lag time, and total duration of floating of all the formulations is enclosed in Table II. As concentration of sodium bicarbonate is changed from 15%, 20%, and 25% w/w of drug, the floating lag time decreased, but the difference in the lag of formulation containing sodium carbonate as 20% and 25% w/w of drug is very narrow. So the sodium bicarbonate concentration is optimized as 20% w/w of drug. The floating lag time of marketed product is found to be 185 s, which is more compared to F12. All the parameters lie within the limits. The hardness is maintained as 9 ± 0.50 kg/cm2 in all the formulation. The friability of all the formulations falls in the acceptable limits.
Table II.
Physical Parameters of Gastroretentive Tablets of Ciprofloxacin Hydrochloride
| Formulae code | Physical parameters | ||||
|---|---|---|---|---|---|
| Weight variation (mg) (avg ± %SD) | Length (mm) (mean ± SD) | Assay (%) | Lag time (s) | Total floating time (h) | |
| F1 | 1,112.0 ± 2.58 | 13.43 ± 0.044 | 98.2 ± 2.9 | 45 | >12 |
| F2 | 1,120.0 ± 4.51 | 13.38 ± 0.021 | 101.6 ± 2.6 | 80 | Not stable |
| F3 | 1,095.0 ± 3.89 | 13.31 ± 0.012 | 94.8 ± 2.5 | 70 | >12 |
| F4 | 1,099.0 ± 2.56 | 13.31 ± 0.021 | 96.6 ± 2.7 | 137 | Not stable |
| F5 | 1,107.7 ± 3.57 | 13.43 ± 0.021 | 98.4 ± 3.1 | 30 | >12 |
| F6 | 1,048.0 ± 4.91 | 13.44 ± 0.025 | 98.4 ± 2.8 | 4 | >12 |
| F7 | 1,049.7 ± 1.14 | 13.45 ± 0.015 | 103 ± 2.5 | 17 | >12 |
| F8 | 865.0 ± 0.82 | 13.67 ± 0.021 | 101.8 ± 3.1 | 20 | >12 |
| F9 | 977.7 ± 2.40 | 13.68 ± 0.021 | 96.4 ± 2.4 | 43 | >12 |
| F10 | 956.6 ± 0.77 | 13.67 ± 0.015 | 92.8 ± 2.6 | 153 | Not stable |
| F11 | 986.3 ± 2.80 | 13.68 ± 0.015 | 104.8 ± 1.9 | 108 | >12 |
| F12 | 1,003.7 ± 1.82 | 13.33 ± 0.010 | 95 ± 1.5 | 15 | >12 |
| F13 | 994.8 ± 1.29 | 13.37 ± 0.029 | 98 ± 2.6 | 308 | 3 |
| F14 | 1,004.3 ± 1.66 | 13.32 ± 0.006 | 98 ± 2.9 | 215 | 5 |
The percentage swelling obtained from the water uptake studies of all the formulations is shown in Fig. 1a. Formulations with HPMC K100LV and HPMC K15M showed poor swelling and tablet integrity. The change in sodium bicarbonate concentration did not show any effect on swelling of the tablet. Formulation F3 containing high percentage of polymer (25% w/w that of drug) and swelling agent croscarmellose sodium shows the maximum swelling compared to that of the formulations F1, F2, and F4 containing other swelling agent crospovidone, sodium starch glycolate, and starch-500. But the formulation containing low polymer concentration with equal concentration of Croscarmellose sodium shows poor swelling and tablet integrity compared to that of formulations containing equal concentration of other swelling agent like crospovidone and sodium starch glycolate. The percentage swelling profile of formulation containing optimized concentration of polymer, swelling agent, and sodium bicarbonate is shown in Fig. 1b. The difference in size between dry and wet tablet is shown in Fig. 2
Fig. 1.
a Maximum percentage swelling of all the formulations. b Effect of different swelling agent of percentage swelling
Fig. 2.

Picture of tablet in dry and wetted state
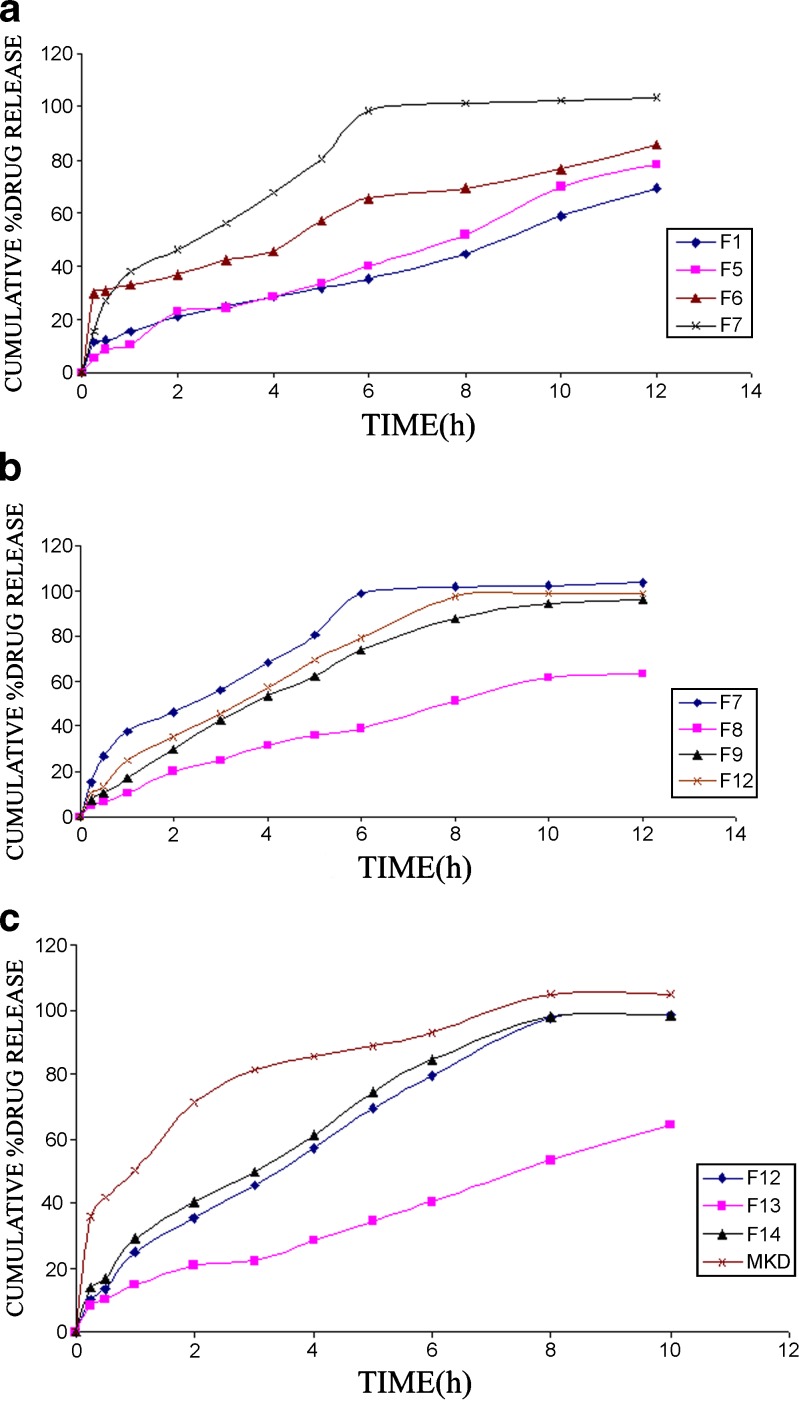
The formulations containing HPMC k100LV and K15 M showed early release. As the concentration of these polymers increased, the drug release is retarded, but percentage swelling is not satisfactory. The drug release profiles from the formulations F1, F5, F6, and F7 which contain different concentrations of polymer is shown in Fig. 3a. The effect of crospovidone concentration on the drug release of formulations F7, F8, F9, and F12 is shown in Fig. 3b. The comparison of drug release of F12, F13, F14, which contained optimized .polymer, swelling agent, and sodium bicarbonate concentration and that of marketed product is shown in Fig. 3c. Higher concentration of sodium bicarbonate facilitates the drug release.
Fig. 3.
Drug release profiles. a Effect of polymer concentration on drug release. b Effect of crospovidone concentration on drug release. c effect of swelling agent on the drug release. Standard deviation of all the data was found less than 3%
The regression coefficient (R2) of release data of all formulation obtained by curve fitting method on various kinetic models is reported in Table III. The data of release exponent “n” according to Krosmeyer–Peppas is also represented in Table III which indicates the mechanism of drug release. The n value of all formulation lies in between 0.45–0.85.
Table III.
Regression Coefficient (R 2) Values of Drug Release Data Obtained from Various Kinetic Models and n Value According to Krosmeyer–Peppas
| Formulae code | Zero-order R 2 | Higuchi model R 2 | Peppas model | First-order R 2 | |
|---|---|---|---|---|---|
| R 2 | n | ||||
| F1 | 0.9759 | 0.9396 | 0.9357 | 0.46 | 0.9541 |
| F2 | 0.9737 | 0.9561 | 0.9692 | 0.48 | 0.9653 |
| F3 | 0.8916 | 0.9543 | 0.9375 | 0.29 | 0.9462 |
| F4 | 0.9831 | 0.9695 | 0.9860 | 0.75 | 0.9980 |
| F5 | 0.8818 | 0.9502 | 0.8777 | 0.28 | 0.9613 |
| F6 | 0.9877 | 0.9376 | 0.9777 | 0.67 | 0.9509 |
| F7 | 0.9530 | 0.9769 | 0.9783 | 0.52 | 0.7561 |
| F8 | 0.9709 | 0.9790 | 0.9957 | 0.70 | 0.9919 |
| F9 | 0.9354 | 0.9792 | 0.9918 | 0.71 | 0.9791 |
| F10 | 0.9651 | 0.9802 | 0.9850 | 0.63 | 0.9837 |
| F11 | 0.9351 | 0.9447 | 0.9735 | 0.87 | 0.8684 |
| F12 | 0.9542 | 0.9806 | 0.9923 | 0.66 | 0.911 |
| F13 | 0.9888 | 0.9492 | 0.9661 | 0.57 | 0.9754 |
| F14 | 0.9691 | 0.9864 | 0.9900 | 0.58 | 0.8783 |
| CIFRAN OD | 0.7871 | 0.9496 | 0.9834 | 0.32 | 0.9739 |
All formulations are compared with the marketed product of ciprofloxacin hydrochloride CIPFRAN OD® 500 mg drug. The f2 value of formulation F12, F13, and F14 is found to be 26.714, 47.235, and 29.227, respectively.
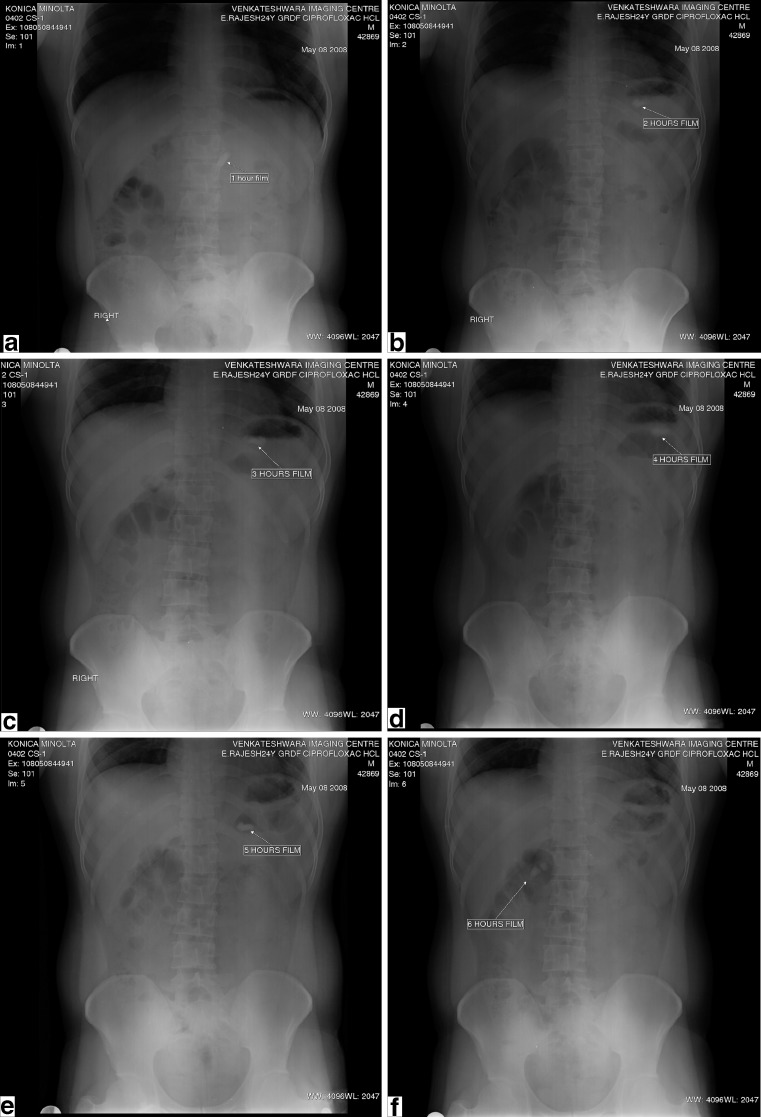
In vivo studies conducted on six healthy, adult, male volunteers using barium sulfate-supplemented tablets show a mean gastric residence time of 320 ± 48.99 min. In vivo tablet behavior is shown in radiographic picture Fig. 4.
Fig. 4.
Radiographic pictures of tablets at 1-, 2-, 3-, 4-, 5-, and 6-h interval
DISCUSSION
Formulations with HPMC K100LV and HPMC K15M grades show poor swelling and sustained action due to the low viscosity. These polymer grades are unable to withhold swelling produced by a swelling agent in the tablets due to the poor gelling/viscosity. The HPMC K100M showed good tablet integrity, swelling, and sustained release of ciprofloxacin HCl.
Swelling agents used in this study are super disintegrating agents. The immediate tablet disintegration is due to the swelling nature of these agents. This character is utilized in the preparation of floating, swellable, and extended release dosage form of ciprofloxacin HCl (24).
Sodium bicarbonate in the acidic environment reacts with the acid and produces carbon dioxide. The evolved gas will get entrapped in the matrix leading to floating of the tablet. The floating lag time decreased as the concentration of the sodium bicarbonate increased. The formulations (F12, F13, and F14) containing different swelling agents with optimized concentration of polymer, swelling agent and sodium bicarbonate show different lag time. This may be due to variation in the mechanism of action of different swelling agents. CS and CCS produced its action by both swelling and wicking in the presence of water, whereas SSG shows only swelling. Wicking is caused due to the porosity and capillary action because of which the density of dosage form is reduced. The total duration of floating is also changed with change in swelling agent. Formulations F2, F10, and F13 show high lag time and fluctuations in floating.
The percentage swelling of the tablet is high, and it is obtained within 3-h interval in formulations F1, F5, F6, F7, F8, F9, and F12, which contain CP than the formulations with CCS, SSG, and STR. This is due to the swelling and wicking nature of CS. Swelling is low in case of formulations (F4, F11, and F14) containing CS as swelling agent which is due to highest swelling nature because of which tablet erodes. At the highest polymer concentration, the CCS shows good swelling than the tablet containing equal amounts of CCS and low concentrations of polymer F3 and F14 with polymer concentration as 25% and 15% w/w of that of drug, respectively. At a low concentration, the CCS (F11) shows good integrity, but the maximum swelling is less (15% swelling) which may not satisfy the conditions required for gastric retention.
The formulations containing SSG (F2 and F13) show the good swelling nature, but the maximum swelling will occur at later hours, i.e., in formulation F2, swelling 233.36% is obtained in 24 h interval, and for F13, the maximum swelling 178.73 is obtained in 5-h interval. The swelling at later hours may be due to gelling of SSG with water. STR (F4) shows poor swelling nature.
The formulations (F1 to F4) that contain polymer concentration 25% w/w that of the drug show more retarded release, i.e., 69.19 ± 3.1, 60.85 ± 2.6, 75.11 ± 2.9, and 60.01 ± 3.2% release in 12 h of the formulations F1, F2, F3, and F4, respectively. The polymer HPMC K100M concentration is optimized to 15% w/w that of drug. Further, the change in swelling agent concentration changes the drug release in formulation. The decrease in swelling agent concentration retards the drug release. The drug release is also dependent upon the type of swelling agent in the F12, F13, and F14, which releases 97.33 ± 2.9, 43.48 ± 1.9, and 97.85 ± 3.3%, respectively, in 8 h interval is shown in Fig. 3c. Increase in sodium bicarbonate concentration increases release rate.
The regression coefficient (R2) values of release data of all formulations obtained by curve fitting method for zero-order, first-order, and Higuchi model are reported in Table III. Most of the formulations follow the zero order and Higuchi model. For the optimized formulation F12, the R2 value of Higuchi 0.9806 (nearer to 1) is dominant than the other models which indicates that the drug release depended on the square root of the time (Eq. 4).
The mechanism of drug release is predicted by using Eq. 5 according to Krosmeyer–Peppas. The n value of optimized formulation F12 is 0.66 and that of all formulations is between “0.45 to 0.85”. This indicates that the drug release depends on swelling, erosion, and diffusion. All formulations follow the non-Fickian/anomalous type of diffusion.
The similarity factor f2 value of optimized formulation F12 is found to be 26.714, which indicates lack of similarity with marketed product (CIFRAN OD® 500 mg). The 92.8 ± 11.5% drug release within 5 h interval is observed for marketed product.
The in vivo nature of the tablet is observed in the radiographic pictures at different time intervals. Initially, the tablet appeared very clear, but later on, the tablet appeared dull, due to swelling of the tablet. The gastric retention is due to floating in the first few hours, and later, it is due to obstruction of the tablet at duodenum as seen in Fig. 4. The radiographic pictures of healthy volunteers confirm the in vivo buoyancy in the stomach for 320 ± 48.99 min (n = 6). This gastric retention occurred due to swelling and floating characters of the dosage form.
CONCLUSION
We conclude that HPMC K100M grade is better than other grades of HPMC in formulating swellable and floating extended release tablets. The optimized formulation F12 follows the Higuchi kinetic model, and the mechanism of drug release is found to be non-Fickian/anomalous. Swelling studies and in vitro buoyancy studies indicate that the formulation is suitable for gastroretention. The similarity factor (f2) of formulation F12 shows that it is not similar to marketed product. In vivo studies conducted on healthy volunteers supported prolonging of the gastric residence time. The mean gastric retention is found to be 320 ± 48.99 min (n = 6). This result indicates increase in MRT of the ciprofloxacin HCl in the stomach.
Acknowledgements
The authors would like to thank the St. Peter’s Institute of Pharmaceutical Sciences, India for providing the facilities.
References
- 1.Baumgartner S., Kristl J., Vrecer F., Vodopivec P., Zorko B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000;195:125–135. doi: 10.1016/S0378-5173(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 2.Singh B. N., Kim K. H. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J. Control. Rel. 2000;63:235–259. doi: 10.1016/S0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 3.Streubel A., Siepmann J., Bodmeier R. Floating matrix tablets based on low-density foam powder: effects of formulation and processing parameters on drug release. Eur. J. Pharm. Sci. 2003;18(1):37–45. doi: 10.1016/S0928-0987(02)00223-3. [DOI] [PubMed] [Google Scholar]
- 4.R. C. Mamajek, and E. S. Moyer, Drug-dispensing device and method. US Patent 4, 207, 890, 1980.
- 5.J. Urquhart, and F. Theeuwes, Drug delivery system comprising a reservoir containing a plurality of tiny pills. U.S. patent 4, 434, 153, February 28, 1984.
- 6.Saito N., Konishi K., Sato F., Kato M., Takeda H., Sugiyama T., Asaka M. Plural transformation-processes from spiral to coccoid Helicobacter pylori and its viability. J. Infect. 2003;46(1):49–55. doi: 10.1053/jinf.2002.1047. [DOI] [PubMed] [Google Scholar]
- 7.Park K., Robinson J. R. Bioadhesive polymers as platforms for oral-controlled drug delivery: method to study bioadhesion. Int. J. Pharm. 1984;19(2):107–127. doi: 10.1016/0378-5173(84)90154-6. [DOI] [Google Scholar]
- 8.Kawashima Y., Niwa T., Takeuchi H., Hino T., Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J. Pharm. Sci. 1992;81(2):135–140. doi: 10.1002/jps.2600810207. [DOI] [PubMed] [Google Scholar]
- 9.Clarke G. M., Newton J. M., Short M. D. Gastrointestinal transit of pellets of differing size and density. Int. J. Pharm. 1993;100(1–3):81–92. doi: 10.1016/0378-5173(93)90078-T. [DOI] [Google Scholar]
- 10.Klausner E. A., Lavy E., Friedman M., Hoffman A. Expandable gastroretentive dosage forms. J. Control. Release. 2003;90(2):143–162. doi: 10.1016/S0168-3659(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Blevins W. E., Park H., Park K. Gastric retention properties of superporous hydrogel composites. J. Control. Release. 2000;64(1–3):39–51. doi: 10.1016/S0168-3659(99)00139-X. [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Park K. Synthesis and characterization of superporous hydrogel composites. J. Control. Release. 2000;65(1–2):73–82. doi: 10.1016/S0168-3659(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 13.Ito R., Machida Y., Sannan T., Nagai T. Magnetic granules: a novel system for specific drug delivery to esophageal mucosa in oral administration. Int. J. Pharm. 1990;61(1–2):109–117. doi: 10.1016/0378-5173(90)90049-A. [DOI] [Google Scholar]
- 14.Chitnis V. S., Malshe V. S., Lalla J. K. Bioadhesive polymer synthesis, evaluation and application in controlled release tablets. Drug Dev. Ind. Pharm. 1991;17(6):879–892. doi: 10.3109/03639049109040824. [DOI] [Google Scholar]
- 15.Efentakis M., Koutlis A., Vlachou M. Development and evaluation of oral multiple-unit and single-unit hydrophilic controlled-release systems. AAPS PharmSciTech. 2000;1(4):34. doi: 10.1208/pt010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Kamel A. H., Sokar M. S., Al Gamal S. S., Naggar V. F. Preparation and evaluation of ketoprofen floating oral delivery system. Int. J. Pharm. 2001;220:13–21. doi: 10.1016/S0378-5173(01)00574-9. [DOI] [PubMed] [Google Scholar]
- 17.Srinath A., Pandit J. K. Multiple-unit floating alginate system: effect of additives on ciprofloxacin release. Drug Deliv. 2008;15(7):471–476. doi: 10.1080/10717540802329282. [DOI] [PubMed] [Google Scholar]
- 18.Sahoo S. K., Mohapatra S., K Dhal S., Behera B. C., Barik B. B. Formulation of floating microspheres of ciprofloxacin hydrochloride by crosslinking technique. Indian Pharm. 2007;58(6):65–68. [Google Scholar]
- 19.Varshosaz J., Tavakoli N., Roozbahani F. Formulation and in vitro characterization of ciprofloxacin floating and bioadhesive extended-release tablets. Drug Deliv. 2006;13:277–285. doi: 10.1080/10717540500395106. [DOI] [PubMed] [Google Scholar]
- 20.Bayer. Cipro® XR (Ciprofloxacin extended-release tablets) prescribing information. West Haven, CT, December 2005.
- 21.Esprit Pharma. ProQuin® XR (Ciprofloxacin hydrochloride) extended-release tablets prescribing information. East Brunswick, NJ, October 2005.
- 22.Agarwal V., Mishra B. Design, development and biopharmaceutical properties of buccoadhesive compacts of pentazocine. Drug Dev. Ind. Pharm. 1999;25(6):701–709. doi: 10.1081/DDC-100102229. [DOI] [PubMed] [Google Scholar]
- 23.Mohammed F. A., Khedr H. Preparation and in vitro/in vivo evaluation of the buccal bioadhesive properties of slow-release tablets containing miconazole nitrate. Drug Dev. Ind. Pharm. 2003;29(3):321–337. doi: 10.1081/DDC-120018206. [DOI] [PubMed] [Google Scholar]
- 24.Chanvanpatil M., Jain P., chaudhari S., Shear R., Vavia P. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006;316:86–92. doi: 10.1016/j.ijpharm.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Gerogiannis V. S., Rekkas D. M., Dallas P. P., Choulis N. H. Floating and swelling characteristics of various excipients used in controlled release technology. Drug Dev. Ind. Pharm. 1993;19(9):1061–1081. doi: 10.3109/03639049309063001. [DOI] [Google Scholar]
- 26.Machida Y., Inouyå K., Tokumura T., Iwata M., Nagai T. Preparation and evaluation of intragastric buoyant preparations. Drug Des. Deliv. 1989;4:155–161. [PubMed] [Google Scholar]
- 27.Iannucelli V., Coppi G., Sansone R., Ferolla G. Air compartment multiple-unit system for prolonged gastric residence. Part II. In vivo evaluation. Int. J. Pharm. 1998;174:55–62. doi: 10.1016/S0378-5173(98)00230-0. [DOI] [Google Scholar]