Abstract
The abbreviated impactor measurement concept is a potential improvement to the labor-intensive full-resolution cascade impactor methodology for inhaler aerosol aerodynamic particle size distribution (APSD) measurement by virtue of being simpler and therefore quicker to execute. At the same time, improved measurement precision should be possible by eliminating stages upon which little or no drug mass is collected. Although several designs of abbreviated impactor systems have been developed in recent years, experimental work is lacking to validate the technique with aerosols produced by currently available inhalers. In part 1 of this two-part article that focuses on aerosols produced by pressurized metered dose inhalers (pMDIs), the evaluation of two abbreviated impactor systems (Copley fast screening Andersen impactor and Trudell fast screening Andersen impactor), based on the full-resolution eight-stage Andersen nonviable cascade impactor (ACI) operating principle, is reported with a formulation producing dry particles. The purpose was to investigate the potential for non-ideal collection behavior associated with particle bounce in relation to internal losses to surfaces from which particles containing active pharmaceutical ingredient are not normally recovered. Both abbreviated impactors were found to be substantially equivalent to the full-resolution ACI in terms of extra-fine and fine particle and coarse mass fractions used as metrics to characterize the APSD of these pMDI-produced aerosols when sampled at 28.3 L/min, provided that precautions are taken to coat collection plates to minimize bounce and entrainment.
Key words: cascade impactor, inhaler testing, particle bounce, particle re-entrainment, particle size distribution
INTRODUCTION
The full-resolution multi-stage cascade impactor (CI) is regarded in the compendial literature as the “gold standard” for the assessment of the aerodynamic particle size distribution (APSD) of oral inhaled aerosols containing medication (1, 2). Typically, such instruments size-fractionate incoming aerosol into seven or eight discrete components by size, with as many as five stages encompassing the range from 0.5- to 5.0-μm aerodynamic diameter (3), where most information is required in connection with respiratory deposition to receptors located in the airways beyond the oropharynx (4,5). These instruments provide direct measures of both mass of active pharmaceutical ingredient (API) and aerodynamic particle size (6). However, they require mastery of a complex technique before consistent results can be achieved (7), with the result that measurements are time-consuming, typically taking more than an hour to complete (8). There is, therefore, an interest in the development of more rapid (and by implication simpler) techniques that might have particular application for routine product quality testing as well as in product development applications in which many similar impactor measurements are often necessary (9,10).
In recent years, there has been an increasing interest in understanding the sources of variability in CI-based measurements. Apart from operator-related issues, an important contributor to method imprecision is known to be the presence of collection surfaces where the mass of API to be assayed is near to the limit of quantitation of a given assay method (11). In a well-designed measurement method developed to assess most inhaler-produced aerosols, these surfaces are typically associated with size-fractionation stages located at the beginning (largest particles) and/or end (finest particles) of the flow path through the impactor. The bulk of the particles typically collect at the middle size-fractionation stages where the technique has optimum size resolution. However, a further contributor to imprecision can be large differences associated in dilutions of recovered particulate in preparation for assay of API from stages collecting the maximum and minimum mass deposited per measurement (11).
It has recently been shown that the relationships between aerodynamic particle size and regional deposition in the respiratory tract are not as size sensitive as the fractionating capability of a typical multi-stage CI (12). Such instruments are not in vitro analogs of the human respiratory tract (12), and, when used correctly, their function is primarily to determine APSD with sufficient precision and accuracy to enable such predictions to be made using appropriate in vitro–in vivo models (6). On this basis, it has been postulated that measures of coarse, fine, and extra-fine particle fractions obtained by abbreviated impactors rather than full-resolution APSD data may be adequate to describe the likely behavior of the aerosol once inhaled (13). The abbreviated impactor measurement (AIM) concept has been developed out of this thinking with the objective of providing these APSD-related metrics in ways that are relatively simple to execute compared with full-resolution CI measurements (13,14). AIM-based abbreviated systems may also offer the opportunity to improve both method precision and productivity by eliminating operating stages. However, it has been recognized from the outset that such changes to the internal geometry of the aerosol measurement system may affect non-ideal behavior including particle bounce and re-entrainment (13). Furthermore, removal of stages from the full-resolution system from which API is not normally recovered following compendial procedures may affect the distribution of particle losses to internal surfaces in abbreviated impactors to the point at which changes in APSD metrics become observable.
This article describes the outcome from experimental studies in which two abbreviated cascade impactors based on the Andersen Mark-II nonviable cascade impactor (referred to from now onward as the Andersen cascade impactor (ACI)) operating principle (6) have been compared with the full-resolution eight-stage instrument. The purpose of these investigations was to explore specifically particle bounce and re-entrainment using a commercially available pressurized metered dose inhaler (pMDI) product that generates aerosols comprising “dry” particles following flash evaporation of the hydrofluoroalkane propellant. The underlying purpose was to validate the AIM concept from the standpoint of having equipment available that is known to be capable of making accurate and precise measurements of the metrics identified as representing inhaler aerosol APSD.
MATERIALS AND METHODS
Abbreviated Impactors
The stainless steel fast screening Andersen impactor (C-FSA; Copley Scientific Ltd., Nottingham, UK) has recently been developed to fulfill the role of an AIM-based abbreviated measurement system (Fig. 1). The C-FSA comprises two inertial size-fractionation stages followed by a backup filter and can therefore be used to determine extra-fine, fine, and coarse particle fractions of the sampled aerosol. The cut-point size (aerodynamic diameter at which 50% of the incoming particles are collected) of the uppermost stage of the commercially available instrument is 5.0-μm aerodynamic diameter, in conformance with the upper size limit for fine particles specified in the European Pharmacopeia, when operated at the standard flow rate of 28.3 L/min for pMDI assessments (1). However, in the version evaluated in this study, this cut point was reduced slightly to 4.7-μm aerodynamic diameter to conform to the cut point of stage “2” of the complete eight-stage ACI (15). This upper stage is identified as “2A” to indicate its relation to the corresponding stage of the ACI, but it should be noted that its upper exterior surface profile was modified so that it accepted the inlet cone directly, as is the case for the uppermost stage “0” for the ACI. The cut point for the lower stage is 1.0-μm aerodynamic diameter, slightly smaller than 1.1 μm for stage “5” of the ACI. This stage is identified by “1.0 μm at 28.3 L/min.”
Fig. 1.

Copley fast screening Andersen impactor (C-FSA)
The second abbreviated system to be evaluated (for convenience referred to as the “Trudell fast screening Andersen impactor,” T-FSA) was similar to the design of the C-FSA but made up entirely from existing ACI components (Fig. 2). It was created so that components from the ACI could be used without the need for additional stage mensuration, which is an important requirement to validate continued CI measurement accuracy (16). The T-FSA comprised stage “0” from the ACI located immediately after the inlet, but this stage was rendered nonfunctional as a size fractionator by omitting its collection plate. The purpose of including this stage was to augment the internal dead volume in the upper part of the impactor to make it closer to that for the ACI (see part 2 of this investigation). The inclusion of stage “0” also provided a direct connection to the inlet cone without having to modify the profile of the stage exterior as was done for stage “2A” of the C-FSA. The upper size-fractionating stage of the T-FSA was stage “2” from an ACI with the same cut-point size (4.7 μm) as that of stage “2A” for the C-FSA. Stage “5” from an ACI was utilized to form the lower size-fractionating stage in the T-FSA. In addition to the avoidance of extra stage mensuration, this change from the C-FSA configuration avoided the need to machine a special stage having jet diameters with slightly reduced size from those of ACI stage “5” to achieve a cut-point size of exactly 1.0-μm aerodynamic diameter.
Fig. 2.

“Trudell” fast screening Andersen impactor (T-FSA)
Evaluation Procedures
The investigation was undertaken using HFA Flovent®-110 (110 μg per actuation fluticasone propionate (FP) ex actuator mouthpiece; GSK Inc., Zebulon, NC, USA) to provide substantially dry particles with mass median aerodynamic diameter close to 2.4 μm after flash evaporation of the HFA 134 propellant (17). The APSD of this pMDI-delivered aerosol is sufficiently disperse that appreciable mass of particles are collected on all but the uppermost stage and backup filter of a full-resolution ACI when operated at 28.3 L/min (17). In all the situations that were investigated, recovery of collected particulate (together with surfactant when collection plate coating took place) and subsequent assay for FP were undertaken by high-performance liquid chromatography UV spectrophotometry using a validated procedure.
All stages of the stainless steel ACI that was used to provide the reference data were mensurated before use to determine that all stages conformed to the pharmacopeial specification (1). Similarly, the upper and lower impaction stages of the C-FSA were mensurated to ensure that the jet sizes conformed to the manufacturer’s specifications. The stages used to create the T-FSA were taken from the previously mensurated ACI. All systems were used with stainless steel collection plates that were visually inspected before use to ensure that they were flat and without appreciable distortion.
In the first part of the study, benchmark APSD data (n = 5 replicates) were obtained with the ACI equipped with Ph.Eur/USP induction port and operated at 28.3 L/min (±5%) in conformance with the method provided in the European and US Pharmacopeias (1,2). Five actuations of Flovent®-110 were delivered at 30-s intervals from primed inhalers to the induction port, conforming to a validated procedure that ensures adequate API is collected for recovery and assay from all stages. The collection plates were each coated with a thin layer of surfactant (3 g Brij-35 (polyoxyethylene 23 lauryl ether; Sigma-Aldrich, Canada), in 5 g glycerol) to minimize the risk of particle bounce and re-entrainment that had been reported several years ago by Graham et al. (18) for ACI-based measurements with pMDI-generated aerosols containing salbutamol. Coating was undertaken by painting the upper collection surface of each plate evenly using a fine-haired laboratory brush. The use of a sticky coating to modify the coefficient of restitution associated with particle impact is based on previous work by Kamiya et al. (19). This group demonstrated that coating the collection surfaces of multi-stage impactors is a necessary prerequisite for the most accurate work even with pMDI-generated aerosols that are typically sampled at lower flow rates than dry powder inhaler (DPI) aerosols (1,2).
The second part of this study focused on the C-FSA. This system was initially evaluated at 28.3 L/min (±5%) without coating the collection plates, on the basis that, although stage coating is widely undertaken for DPI assessments, it is less frequently encountered with pMDI-based measurements (20). Five replicate determinations were made after one actuation of medication into the impactor from a primed inhaler. After recovery of API and subsequent cleaning of the system, the process was repeated after delivering two, five, and ten actuations per measurement to accumulate more collected mass per stage. Finally, the entire procedure was repeated, this time, after coating the stages with Brij-35 on each occasion by the same procedure that had been used with the ACI. Internal losses were quantified at each component of the abbreviated impactor based on 10 actuations per determination to optimize sensitivity, by recovering FP that had deposited on interior surfaces other than the collection plates.
The final part of the investigation was concerned with establishing the performance of the T-FSA in comparison with the other two systems. The measurements with this abbreviated impactor were not undertaken with uncoated plates on the basis that equivalent behavior to that observed with the C-FSA would be expected, given the near-similar geometry of the two systems. Instead, the collection plates of the T-FSA were always prepared using the Brij-35 coating technique already described for measurements in which the number of actuations per determination was increased progressively from one to ten. Internal losses were quantified as described for the C-FSA.
RESULTS
Individual values of total mass recovery of FP (mass balance) with both the ACI and the abbreviated impactors were within ±10% of label claim per actuation (110 μg), therefore comfortably within ±15% label claim limit in guidance from one regulatory agency (21). In addition, the magnitude of the mass recovery did not appear to be substantially different for any of the impactor systems.
The ACI measurements expressed as cumulative mass percent are summarized in Table I. The use of normalized metrics rather than the absolute mass of each size fraction was chosen for data presentation and for most of the analysis because this procedure corrects for minor variations in mass output ex pMDI from one determination to another. It therefore enables direct comparisons between systems to be made with the APSD data presented on a cumulative percentage by mass-weighted basis (12). The following aerodynamic diameter-related metrics were defined to characterize these data:
Coarse particle fraction >4.7-μm aerodynamic diameter (CPF>4.7 μm) for all systems
Fine particle fraction <4.7-μm aerodynamic diameter (FPF<4.7 μm) for all systems
Extra-fine particle fraction <1.1-μm aerodynamic diameter (EPF<1.1 μm) for the ACI and T-FSA or EPF <1.0-μm aerodynamic diameter (EPF<1.0 μm) for the C-FSA
Table I.
Cumulative Mass-Weighted Data for Flovent®-110 Measured by Full-Resolution ACI
| Location in CI | Size rangea (μm) | Upper size limit (μm) | Size fraction | Cumulative mass % < stated upper size limit (mean ± SD) |
|---|---|---|---|---|
| Induction port | >9 | Undefined | CPF>4.7 μm | 100.0 |
| Stage 0 | >9 | Undefined | 49.5 ± 1.9 | |
| Stage 1 | 5.8–9.0 | 9.0 | 47.4 ± 1.9 | |
| Stage 2 | 4.7–5.8 | 5.8 | 45.3 ± 2.0 | |
| Stage 3 | 3.3–4.7 | 4.7 | FPF<4.7 μm | 42.3 ± 2.2 |
| Stage 4 | 2.1–3.3 | 3.3 | 30.6 ± 2.1 | |
| Stage 5 | 1.1–2.1 | 2.1 | 10.8 ± 1.3 | |
| Stage 6 | 0.7–1.1 | 1.1 | EPF<1.1 μm | 1.2 ± 0.2 |
| Stage 7 | 0.4–0.7 | 0.7 | 0.3 ± 0.1 | |
| Backup filter | < 0.4 | 0.4 | 0.1 ± 0.1 |
n = 5 replicates
aBased on manufacturer’s nominal calibration data at 28.3 L/min
These metrics were selected to characterize in vitro inhaler performance based on recommendations in a Canadian standard for evaluating pMDI performance with add-on devices (22,23). By definition, CPF>4.7 μm = [100 − FPF<4.7 μm], if all fractions are expressed as percentages of the mass of aerosol entering the induction port of each system. Since increases in FPF<4.7 μm therefore resulted in equal and corresponding decreases in CPF>4.7 μm, values of CPF>4.7 μm are included in the tables only for the sake of completeness, as well as to indicate that this additional information is available from the abbreviated systems.
All calculations of cumulative mass percent < stated upper size limit in this first part of the overall investigation were based on the total mass of aerosol that entered the induction port. This method of data analysis was chosen in preference to considering only the mass that entered the impactor (so-called impactor sized mass (24)) because it was important to be able to assess the behavior of the induction port and upper two stages of the ACI in relation to previously published data for internal losses associated with stages “0” and “1” of this impactor, in which an induction port had not been used (25–27).
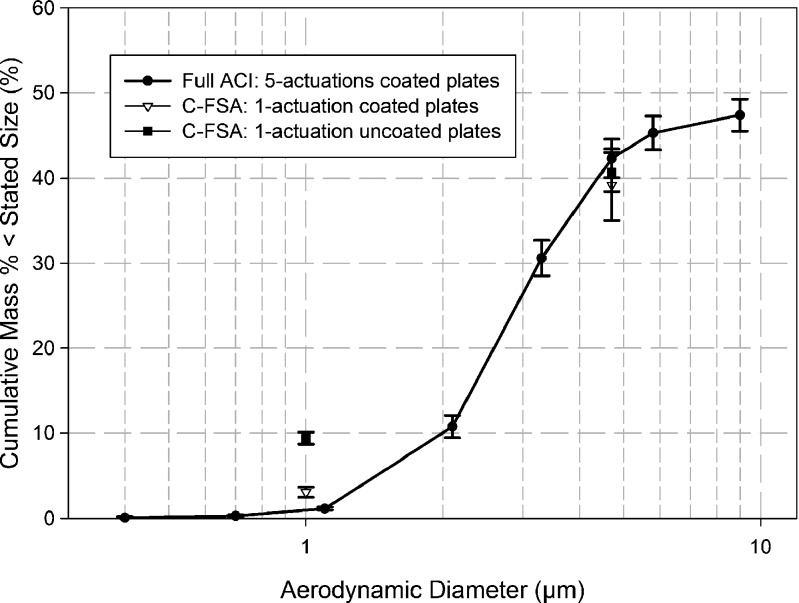
Equivalent results for the C-FSA without and with coated collection plates are summarized in Tables II and III, respectively. Single-actuation data for the C-FSA with collection plates at both conditions are compared against the cumulative mass-weighted APSD determined by the full ACI in Fig. 3 to indicate the outcome when the minimum mass of API was collected by the abbreviated system. Despite the small amount of API present for assay, these single-actuation data were reproducible, with the coefficients of variation for FPF<4.7 μm from the C-FSA being 5.7% (uncoated plates) and 10.7% (coated plates). Equivalent coefficients of variation for EPF<1.0 μm via the C-FSA were 7.5% (uncoated plates) and 19.3% (coated plates). The noticeably larger coefficient of variation associated with EPF<1.0 μm with the use of coated plates is most likely the result of the very small mass (3.3 ± 0.8 μg) that was collected at this condition. This value can be compared directly with 9.3 ± 1.0 μg that was obtained for one actuation per measurement using uncoated plates. Much of this additional mass was likely associated with the accumulation of re-entrained particles that had bounced off the plates and was collected quantitatively on the filter.
Table II.
Cumulative Mass-Weighted Data for Flovent®-110 Measured by C-FSA Without Coating on Collection Plates
| Location in C-FSA | Size rangea (μm) | Upper size limit (μm) | Size fraction | Number of actuations per determination | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | ||||
| Cumulative mass % < stated upper size limit (mean ± SD) | |||||||
| Induction port | >9 | Undefined | CPF>4.7 μm | 59.3 ± 2.3 | 61.7 ± 2.6 | 60.5 ± 1.7 | 61.1 ± 3.4 |
| Stage 2Ab | >4.7 | Undefined | |||||
| Stage 5b | 1.0–4.7 | 4.7 | FPF< 4.7 μm | 40.7 ± 2.3 | 38.3 ± 2.6 | 39.5 ± 1.7 | 37.8 ± 4.0 |
| Backup filter | < 1.0 | 1.0 | EPF<1.0 μm | 9.4 ± 0.7 | 7.5 ± 0.6 | 6.4 ± 0.4 | 5.3 ± 0.4 |
n = 5 replicates
aBased on manufacturer’s nominal calibration data at 28.3 L/min
bBased on numbering in full-resolution ACI
Table III.
Cumulative Mass-Weighted Data for Flovent®-110 Measured by C-FSA with Coating on Collection Plates
| Location in C-FSA | Size rangea (μm) | Upper size limit (μm) | Size fraction | Number of actuations per determination | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | ||||
| Cumulative mass % < stated upper size limit (mean ± SD) | |||||||
| Induction port | >9 | Undefined | CPF>4.7 μm | 60.8 ± 4.2 | 60.8 ± 3.3 | 60.1 ± 1.2 | 61.4 ± 2.5 |
| Stage 2Ab | >4.7 | Undefined | |||||
| Stage 5b | 1.0–4.7 | 4.7 | FPF< 4.7 μm | 39.2 ± 4.2 | 39.2 ± 3.3 | 39.9 ± 1.2 | 37.9 ± 3.0 |
| Backup filter | < 1.0 | 1.0 | EPF<1.0 μm | 3.1 ± 0.6 | 3.5 ± 0.3 | 3.5 ± 0.4 | 3.3 ± 0.2 |
n = 5 replicates
aBased on manufacturer’s nominal calibration data at 28.3 L/min
bBased on numbering in full-resolution ACI
Fig. 3.
Cumulative mass-weighted data for one actuation per measurement of Flovent®-110 into C-FSA compared with full-resolution ACI
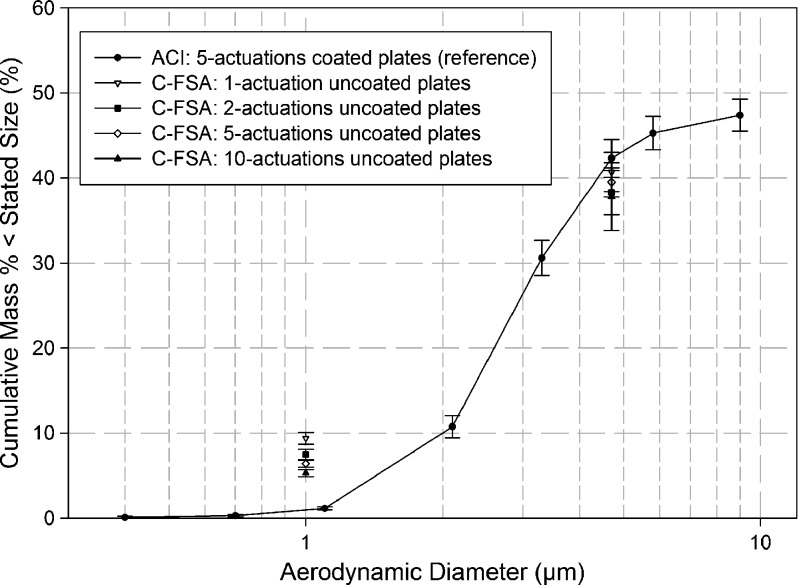
Figure 4 illustrates the effect of number of actuations per determination with the C-FSA compared with the reference ACI data when the collection plates of this abbreviated impactor were uncoated. EPF<1.0 μm decreased systematically with increasing number of actuations, being 9.4 ± 0.7% (one actuation), 7.5 ± 0.6% (two actuations), 6.4 ± 0.4% (five actuations), and 5.3 ± 0.4% (ten actuations). However, EPF<1.0 μm was never as low as the equivalent values that were obtained when coated collection plates were used (Fig. 5). In contrast with the EPF data, FPF<4.7 μm and CPF>4.7 μm from the C-FSA were quite similar, irrespective of the number of inhaler actuations per measurement. Thus, values of FPF<4.7 μm ranged from 37.8% to 40.7% with associated coefficients of variation close to or less than 10%.
Fig. 4.
Effect of number of actuations of Flovent®-110 on C-FSA measured data when used with uncoated collection plates
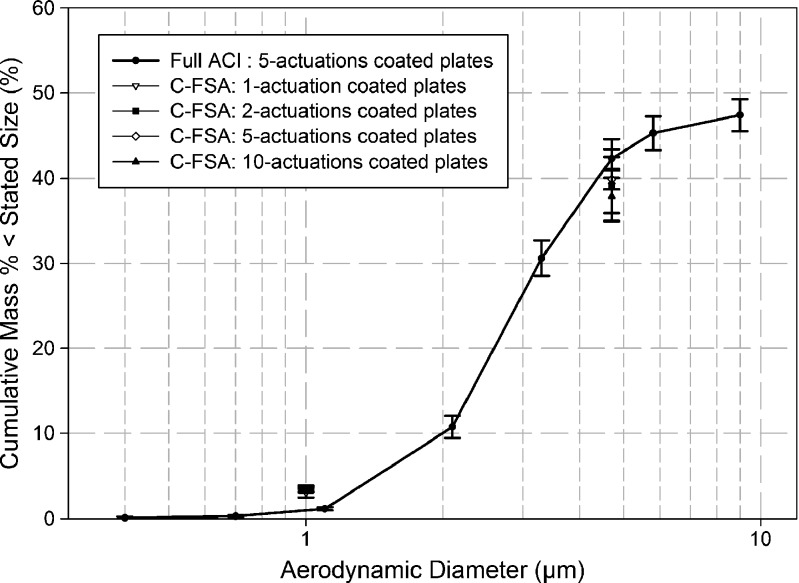
Fig. 5.
Effect of number of actuations of Flovent®-110 on C-FSA measured data when used with coated collection plates
When the C-FSA was equipped with coated collection plates, the values of both EPF<1.0 μm and FPF<4.7 μm were almost identical (Fig. 5). This agreement is especially noticeable with the measurements of EPF<1.0 μm, where only 3.1% to 3.5% of the mass of API collected. Their associated coefficients of variation were close to or slightly less than 10%, except for the single-actuation data (Fig. 3) where the coefficient of variation was close to 20%. Similarly, values of FPF<4.7 μm and CPF> 4.7 μm were both similar and reproducible, with FPF<4.7 μm ranging from 37.9% to 39.9% and associated coefficients of variation close to or less than 10%. The lack of sensitivity in these metrics to mass loading of coated collection plates is in agreement with earlier pMDI-based experimental work with full-resolution CIs (both ACI and next-generation pharmaceutical impactor) by Kamiya et al. (19).
Values of the same size fractions made with five actuations of Flovent®-110 into the slightly larger T-FSA (Table IV), equipped with coated collection plates, were substantially equivalent to those obtained with either the C-FSA or ACI. This outcome indicates that the addition of the nonoperative stage “0” had not introduced a discernable bias compared with either of the other systems at this condition.
Table IV.
Key Size Fraction Metrics Determined for Five Actuations of Flovent®-110 into the T-FSA: Comparison with Equivalent Data from the C-FSA and ACI
| Location | Size rangea (μm) | Upper size limit (μm) | Size fraction | Cumulative mass % < stated upper size limit (mean ± SD) | ||
|---|---|---|---|---|---|---|
| T-FSA | C-FSA | ACI | ||||
| Induction port | >9 | Undefined | CPF>4.7 μm | 57.6 ± 3.5 | 60.1 ± 1.2 | 57.7 ± 2.2 |
| Stage 2Ab; C-FSA 2b; T-FSA | >4.7 | Undefined | ||||
| Stage 5b | 1.0–4.7: C-FSA; 1.1 – 4.7: T-FSA, ACI | 4.7 | FPF<4.7 μm | 42.4 ± 3.5 | 39.9 ± 1.2 | 42.3 ± 2.2 |
| Backup filter | <1.0: C-FSA; <1.1: T-FSA, ACI | 1.0: C-FSA; 1.1: T-FSA, ACI | EPF<1.0 μm; EFF<1.1 μm | 3.8 ± 0.5 | 3.5 ± 0.4 | 1.2 ± 0.2 |
n=5
aBased on manufacturer’s nominal calibration data at 28.3 L/min
bBased on numbering in full-resolution ACI
Internal losses for the C-FSA were close to 4.3 μg per actuation of FP (3.9% of mass ex inhaler mouthpiece), of which 2.8 μg was recovered from the metalwork associated with the inlet cone and stage “2A” before size fractionation into coarse and fine components takes place. Similar internal losses of 4.0 μg per actuation (3.6% of mass of FP ex inhaler mouthpiece) were evident in the T-FSA, of which 3.3 μg was recovered from the stage metalwork associated with the inlet cone, inoperative stage “0” and stage “2.” Slightly more than one-half of this lost API (1.7 μg per actuation) was recovered from the inoperative stage 0 associated with this impactor.
DISCUSSION
The measurements undertaken in this proof-of-concept study confirm that for the C-FSA, the mass of FP per actuation was adequate for acceptable assays of recovered particulate to be undertaken even for measurements in which only one actuation was delivered from the inhaler to the abbreviated system. Given that the behavior of the slightly larger T-FSA was substantially comparable with the C-FSA in terms of both precision and accuracy based on five actuations per measurement (Table IV), it would be reasonable to assume that this abbreviated design could also be used to assess inhaler performance based on as little as a single actuation per determination. The ability to make measurements of key size mass fractions (EPF, FPF, and CPF) in experiments in which the clinical dose (often only one or two actuations) is delivered to the particle sizing equipment is attractive, given the tendency for regulatory agencies to request that the minimum number of actuations be delivered for reliable assay (21).
It is anticipated that the precision of metrics determined directly from AIM-based systems will be improved in relation to equivalent measures derived by full-resolution CI. The rationale for this expectation arises because stages fractionating particles at either end of the APSD are eliminated. Such stages typically collect amounts of API that are close to the limit of detection and, therefore, contribute significantly to the overall variability of the method. In the present study, the error bars (±1 SD) for the individual measurements by full-resolution ACI (Figs. 2, 3, and 4) were noticeably larger than the equivalent variability observed with either abbreviated impactor. Looking more closely at the derived metrics, the coefficients of variation for EPF<1.1 μm, FPF<4.7 μm, and CPF>4.7 μm by full ACI based on five inhaler actuations per measurement were 16.7%, 8.9%, and 3.8%, respectively. If the equivalent abbreviated impactor data with coated stages are compared (EPF<1.1 μm = 11.4% (C-FSA), 13.2% (T-FSA); FPF<4.7 μm = 3.0% (C-FSA), 8.3% (T-FSA); CPF>4.7 μm = 2.0% (C-FSA), 6.1% (T-FSA)), there is slight evidence to support an improvement in precision particularly for the C-FSA. However, a study designed to investigate method precision by substantially increasing the number of replicate measurements at each condition would be needed to substantiate this finding.
The abbreviated impactors investigated in this study represent two ways by which the AIM concept might be realized without resorting to a novel geometry but rather making use of existing and well-proven technology associated with the ACI design. This approach is, therefore, consistent with the proposals of Van Oort and Roberts (9) and Lundbäck and Wiktorsson (10). However, given the prevalence of non-ideal collection behavior known to be present within impaction systems used for pharmaceutical inhaler characterizations (6,19,28), similar validation studies should be undertaken each time a new abbreviated apparatus is introduced or if an existing abbreviated system is used with a new inhaler class (i.e., for DPI rather than just pMDI assessments).
These measurements were purposefully undertaken with aerosols comprising dry particles without surfactant added as excipient. Such particles, having comparatively higher coefficients of restitution, are more likely to be subject to bounce and re-entrainment from a given impaction surface than liquid droplets or partly dry solid particles containing low volatile solvents such as water or ethanol (29). The absence of low volatile excipients also avoided the possibility of size-related bias in the abbreviated systems as the result of changes in evaporation behavior taking place within the smaller dead space of the C-FSA or T-FSA compared with that in the ACI. This important potential cause of size-related bias is the topic of part 2 of the investigation.
The reduction of particle bounce with increasing the number of actuations per measurement when the C-FSA was evaluated with uncoated collection plates (Fig. 4) was anticipated from the outcomes of previous studies based on full-resolution CIs (18,19,28). Significantly lower values of EPF<1.0 μm were observed for the C-FSA when coated collection plates were used compared with the situation that existed when the plates were uncoated. This behavior was most evident with the data for one actuation per measurement (unpaired t test, p < 0.001), where collection surface modification by previously deposited particles from prior actuations could not have taken place. The trend towards lower values of EPF<1.0 μm as the number of actuations per determination to uncoated plates was increased is evidence of progressive surface modification by particles arriving from previous inhaler actuations. The metric EPF should be more diagnostic of particle bounce and re-entrainment than either FPF or CPF in a CI since once particles have bounced from a particular stage, their high retained kinetic energy will most likely carry them through to the filter at the base of the impactor (6). This explanation is consistent with the increased sensitivity to the effect of particle bounce and re-entrainment observed by Nasr et al. (30) with data from the bottom-most stages and after filter of ACI and Marple-Miller five-stage impactor in an investigation of particle collection from pMDI-generated albuterol aerosols. Two possible mechanisms may be operating to cause such behavior. Firstly, deposited particles from initial actuations may create a modified surface that is more absorptive of the incoming energy of particles from the later actuations. This process would result in progressively reduced bounce with a corresponding decrease in mass transfer of API to the backup filter (28). Secondly, the modified surface may at the same time become more rugged, increasing the effectiveness of capture of incoming particles by interception (18). In summary, the findings from the present study with the C-FSA reinforce previous observations (18,19,28), of the importance of coating impaction surfaces for the most accurate work when sampling aerosols comprising “dry” particles emitted from pMDIs.
It is notable that even though EPF<1.0 μm for the C-FSA with coated collection plates (3.5 ± 0.4% for five actuations per determination) was close to the corresponding value of EPF<1.1 μm for the ACI (1.4 ± 0.3%; Table IV), the difference (approximately 2%) was significant (Mann–Whitney rank-sum test, p = 0.008). However, although statistically different, its magnitude was sufficiently small that it could likely be neglected. Its cause can, however, be understood in terms of mass transfer of the small amount of API associated with nonrecovered particles that are deposited on internal surfaces of stages within the full-resolution ACI that are missing in the abbreviated system. The US pharmacopeial monograph permits an upper limit for such internal losses of 5% of the delivered API mass per actuation throughout a CI system (2), and this limit appears to be a reasonable reflection of what is achievable for full-resolution instruments when sampling polydisperse aerosols, albeit not from an inhaler-based source (31). The ACI calibration data of Mitchell et al., using monodisperse particles, show that the magnitude of inter-stage losses as a proportion of the sampled mass becomes progressively greater as a function of increasing particle size. Furthermore, these losses tend to distribute such that they are at their maximum at stages associated with the collection of the bulk of the incoming aerosol (25,26). This trend is supported by the monodisperse particle calibration data of Vaughan, who was also able to show, using a fluorescent tracer, that deposits to internal surfaces other than the collection plates are concentrated near to the jet entrances of stages that capture most particles (27). He attributed the cause of such deposition to the overshoot of particles near each jet entry. In the present study, close to 32 μg per actuation of FP that penetrated beyond the induction port (50 μg) was collected by stages “3” and “4” in the measurements using the full ACI (Table I). Both stages are missing in either the C-FSA or T-FSA. The acquisition of the extra 1.3–1.6 μg FP observed on the lower stage of these abbreviated systems compared with the corresponding ACI data (Table IV) represents 4–5% of the mass that was collected by these missing stages. This mechanism is, therefore, believed to be feasible, given that the total internal loss for the ACI could be as large as 5% and still comply with the compendial requirement (2). Unfortunately, it is not yet a practical proposition to conduct a complete internal loss distribution assessment with the ACI utilizing inhaler-produced aerosols so as to quantify the magnitude of this potential mass transfer process. This limitation arises primarily because of the imprecision introduced in assigning losses on a stage-by-stage basis throughout a multi-stage CI comprising as many as ten to 12 components (excluding collection plates), when total losses are such a small portion of the sampled mass of API. Under these circumstances, many of the components will typically collect less than the lower limit of API for detection, even when as many as ten inhaler actuations per measurement are employed.
It is interesting to note that estimates of FPF<4.7 μm (and by definition CPF>4.7 μm) should be less affected by mass transfer of internal losses associated with missing ACI stages because the induction port typically removes almost all of the coarser fraction of pMDI-generated aerosols before the remaining airborne particles enter the CI (32). In the present study, this behavior was more evident with the T-FSA (FPF<4.7 μm of 42.4 ± 3.5% compared with 42.3 ± 2.2% in the ACI) than the C-FSA (FPF<4.7 μm 39.9 ± 1.2%; Table IV). The divergence between the two abbreviated systems is sufficiently small to have arisen as the result of measurement variability. In summary, there may be an advantage in including an inoperative stage “0” as an addition to the C-FSA (essentially creating the T-FSA configuration) to obtain closer agreement in the volume of internal voids (dead space) to that within the ACI. Such an approach could be advantageous when formulations containing volatile species are being assessed (see part 2).
CONCLUSIONS
This experimental comparison of two abbreviated CIs (C-FSA and T-FSA) to the full-resolution ACI has demonstrated that substantially equivalent measures of extra-fine, fine, and coarse fractions of the mass of API ex inhaler can be obtained, as long as stage collection surfaces are coated with a thin layer of sticky substance compatible with API recovery and assay, thereby minimizing particle bounce and re-entrainment. When this precaution is taken, small differences that remain between the results from the two AIM-concept-based methods can likely be explained in terms of relocated particulate that would normally be lost internally to recovery in full-resolution ACI measurements. In practice, these differences are probably sufficiently small to make method transfers possible without further procedural changes. However, before implementing this type of simplified methodology for routine use in inhaler product characterization, it is recommended that the system suitability be evaluated on a product-by-product basis to establish substantial equivalency.
Acknowledgements
The authors acknowledge the support of Copley Scientific Ltd. for the C-FSA and the advice and support of Mark Copley and Daryl Roberts (MSP Corp., St. Paul, MN, USA) during the development and execution of this investigation.
Abbreviations
- ACI
Andersen cascade impactor
- AIM
abbreviated impactor measurement
- API
active pharmaceutical ingredient
- APSD
aerodynamic particle size distribution
- CI
cascade impactor
- C-FSA
Copley fast screening Andersen impactor
- CPF
coarse particle fraction (based on mass entering induction port)
- EPF
extra-fine particle fraction (based on mass entering induction port)
- FP
fluticasone propionate
- FPF
fine particle fraction (based on mass entering induction port)
- pMDI
pressurized metered dose inhaler
- T-FSA
Trudell fast screening Andersen impactor
References
- 1.European Pharmacopeia . Section 2.9.18—Preparations for Inhalation: Aerodynamic Assessment of Fine Particles. 5. Strasbourg: Council of Europe; 2005. pp. 2799–2811. [Google Scholar]
- 2.United States Pharmacopeia . USP 30-NF 25. Chapter 601—Physical Tests and Determinations: Aerosols. Rockville: United States Pharmacopeia; 2007. pp. 220–240. [Google Scholar]
- 3.Marple V. A., Roberts D. L., Romay F. J., Miller N. C., Truman K. G., Van Oort M., Olsson B., Holroyd M. J., Mitchell J. P., Hochrainer D. Next generation pharmaceutical impactor. Part 1: design. J. Aerosol Med. 2003;16:283–299. doi: 10.1089/089426803769017659. [DOI] [PubMed] [Google Scholar]
- 4.Heyder J., Svartengren M. U. Basic principles of particle behavior in the human respiratory tract. In: Bisgaard H., O’Callaghan C., Smaldone G.C., editors. Drug delivery to the lung. New York: Marcel Dekker; 2002. pp. 21–45. [Google Scholar]
- 5.Rudolph G., Kobrich R., Stahlhofen W. Modeling and algebraic formulation of regional aerosol deposition in man. J. Aerosol Sci. 1990;21(S1):S403–S406. doi: 10.1016/0021-8502(90)90266-Z. [DOI] [Google Scholar]
- 6.Mitchell J. P., Nagel M. W. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J. Aerosol Med. 2003;16:341–377. doi: 10.1089/089426803772455622. [DOI] [PubMed] [Google Scholar]
- 7.Christopher D., Curry P., Doub B., Furnkranz K., Lavery M., Lin K., Lyapustina S., Mitchell J., Rogers B., Strickland H., Tougas T., Tsong Y., Wyka B. Considerations for the development and practice of cascade impaction testing including a mass balance failure investigation tree. J. Aerosol Med. 2003;16:235–247. doi: 10.1089/089426803769017604. [DOI] [PubMed] [Google Scholar]
- 8.Nichols S., Russell-Graham D. Comparative Efficiency of the Use of the Next Generation Impactor Compared to the Andersen Cascade Impactor when Used with Dry Powder Inhalers. Drug Delivery to the Lungs-18. London: The Aerosol Society; 2007. pp. 120–123. [Google Scholar]
- 9.Van Oort M., Roberts W. Variable flow–variable stage–variable volume strategy for cascade impaction testing of inhalation aerosols. In: Dalby R. N., Byron P. R., Farr S. J., editors. Respiratory Drug Delivery-V. Buffalo Grove: Interpharm; 1996. pp. 418–420. [Google Scholar]
- 10.Lundbäck H., Wiktorsson B. High throughput inhaler testing I: Fine particle dose. In: Dalby R. N., Byron P. R., Peart J., Suman J. D., Farr S. J., editors. Respiratory Drug Delivery 2006. River Grove: Davis Healthcare; 2006. pp. 467–469. [Google Scholar]
- 11.Bonam M., Christopher D., Cipolla D., Donovan B., Goodwin D., Holmes S., Lyapustina S., Mitchell J., Nichols S., Pettersson G., Quale C., Rao N., Singh D., Tougas T., Van Oort M., Walther B., Wyka B. Minimizing variability of cascade impaction measurements in inhalers and nebulizers. AAPS PharmSciTech. 2008;9(2):404–413. doi: 10.1208/s12249-008-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell J. P., Dunbar C. Analysis of cascade impactor mass distributions. J. Aerosol Med. 2005;18(4):439–451. doi: 10.1089/jam.2005.18.439. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell J. P. The role of aerosol measurement for aerodynamic particle size distribution (APSD) in a quality-by-design (QbD) environment. In: Dalby R. N., Byron P. R., Peart J., Suman J. D., Farr S. J., Young P. M., editors. Respiratory Drug Delivery 2008. River Grove: Davis Healthcare; 2008. pp. 779–783. [Google Scholar]
- 14.J. P. Mitchell. The Abbreviated Impactor Measurement (AIM) concept for aerodynamic particle size distribution (APSD) in a quality-by-design (QbD) environment. In: Proc. Biennial IPAC-RS Conference, Bethesda, MD, USA. 2008. http://www.ipacrs.com/ipac2008.html. Accessed 5/10/2008.
- 15.Marple V. A., Olson B. A., Miller N. C. The role of inertial particle collectors in evaluating pharmaceutical aerosol delivery systems. J. Aerosol Med. 1998;11(S1):S-139–S-153. [PubMed] [Google Scholar]
- 16.Roberts D. L., Romay F. J. Relation of stage mensuration data to the performance of new and used cascade impactors. J. Aerosol Med. 2005;18(4):396–413. doi: 10.1089/jam.2005.18.396. [DOI] [PubMed] [Google Scholar]
- 17.Cripps A., Riebe M., Schulze M., Woodhouse R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir. Med. 2000;94(SB):3–9. [PubMed] [Google Scholar]
- 18.Graham S. J., Lawrence R. C., Ormsby E. D., Pike R. K. Particle size distribution of single and multiple sprays of salbutamol metered-dose inhalers (MDIs) Pharm. Res. 1995;12(9):1380–1384. doi: 10.1023/A:1016294228280. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya A., Sakagami M., Hindle M., Byron P. Aerodynamic sizing of metered dose inhalers: an evaluation of the Andersen and Next Generation pharmaceutical impactors and their USP methods. J. Pharm. Sci. 2004;93(7):1828–1837. doi: 10.1002/jps.20091. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell J. P. Practices of Coating Collection Surfaces of Cascade Impactors: a Survey of Members of the European Pharmaceutical Aerosol Group. Drug Delivery to the Lungs-14. London: The Aerosol Society; 2003. pp. 75–78. [Google Scholar]
- 21.US Federal Drug Administration (FDA). Draft guidance: Metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products chemistry, manufacturing and controls documentation. Docket 98D-0997 (1998).
- 22.Canadian Standards Association . Spacers and Holding Chambers for Use with Metered-Dose Inhalers. Mississauga: Canadian Standards Association; 2008. [Google Scholar]
- 23.Dolovich M. B., Mitchell J. P. Canadian Standards Association (CSA) Standard Z264.1-02: a new voluntary standard for spacers and holding chambers used with pressurized metered-dose inhalers (pMDIs) Can. Respir. J. 2004;11(7):489–495. doi: 10.1155/2004/497946. [DOI] [PubMed] [Google Scholar]
- 24.Christopher D., Adams W., Amann A., Bertha C., Byron P. R., Doub W., Dunbar C., Hauck W., Lyapustina S., Mitchell J. P., Morgan B., Nichols S., Pan Z., Singh G. J. P., Tougas T., Tsong Y., Wolff R., Wyka B. Product Quality Research Institute evaluation of cascade impactor profiles of pharmaceutical aerosols: part 3—final report on a statistical procedure for determining equivalence. AAPS PharmSciTech. 2007;8(4):Article 90. doi: 10.1208/pt0804090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J. P. Mitchell, P. A. Costa, and S. Waters. The calibration of Andersen Mark-II cascade impactors. AEE Winfrith (UK) Report AEEW-R 2162, December 1986.
- 26.Mitchell J. P., Costa P. A., Waters S. An assessment of an Andersen Mark-II cascade impactor. J. Aerosol Sci. 1986;19(2):213–221. doi: 10.1016/0021-8502(88)90224-8. [DOI] [Google Scholar]
- 27.Vaughan N. P. The Andersen impactor: calibration, wall losses and numerical simulation. J. Aerosol Sci. 1989;20(1):67–90. doi: 10.1016/0021-8502(89)90032-3. [DOI] [Google Scholar]
- 28.Kamiya A., Sakagami M., Hindle M., Byron P. R. Particle sizing with the next generation impactor: a study of Vanceril™ metered dose inhaler. J. Aerosol Med. 2003;16(2):216. [Google Scholar]
- 29.Berg E., Lamb P., Ali A., Dennis J., Tservistas M., Mitchell J. Assessment of the need to coat particle collection cups of the NGI to mitigate droplet bounce when evaluating nebuliser-produced droplets. PharmEuropa Sci. Notes. 2008;2008(1):21–26. [PubMed] [Google Scholar]
- 30.Nasr M. N., Ross D. L., Miller N. C. Effect of drug load and plate coating on the particle size distribution of a commercial albuterol metered dose inhaler (MDI) determined using the Andersen and Marple–Miller cascade impactors. Pharm. Res. 1997;14(10):1437–1443. doi: 10.1023/A:1012180924063. [DOI] [PubMed] [Google Scholar]
- 31.Chan T. L., Lawson D. R. Characteristics of cascade impactors in size determination of diesel particles. Atmos. Environ. 1981;15(7):1273–1279. doi: 10.1016/0004-6981(81)90320-6. [DOI] [Google Scholar]
- 32.Stein S. W., Gabrio B. J. Understanding throat deposition during cascade impactor testing. In: Dalby R. N., Byron P. R., Farr S. J., Peart J., editors. Respiratory Drug Delivery-VII. Raleigh: Serentec; 2000. pp. 573–576. [Google Scholar]