Abstract
Uncontrolled cell proliferation is a major feature of cancer. Experimental cellular models have implicated some members of the Rho GTPase family in this process. However, direct evidence for active Rho GTPases in tumors or cancer cell lines has never been provided. In this paper, we show that endogenous, hyperactive Rac3 is present in highly proliferative human breast cancer-derived cell lines and tumor tissues. Rac3 activity results from both its distinct subcellular localization at the membrane and altered regulatory factors affecting the guanine nucleotide state of Rac3. Associated with active Rac3 was deregulated, persistent kinase activity of two isoforms of the Rac effector p21-activated kinase (Pak) and of c-Jun N-terminal kinase (JNK). Introducing dominant-negative Rac3 and Pak1 fragments into a breast cancer cell line revealed that active Rac3 drives Pak and JNK kinase activities by two separate pathways. Only the Rac3–Pak pathway was critical for DNA synthesis, independently of JNK. These findings identify Rac3 as a consistently active Rho GTPase in human cancer cells and suggest an important role for Rac3 and Pak in tumor growth.
Rac proteins are members of the Rho GTPase family and act as molecular switches in regulating a variety of biological response pathways, including cell motility, gene transcription, cell transformation, and cell-cycle progression (1). The Rac family includes Rac1, the myeloid-lineage-specific Rac2, and the recently cloned Rac3 proteins (2). Rac3 differs from Rac1 and Rac2 in two domains, the insert region and the C terminus, which influence transformation (3, 4), interaction with guanine nucleotide exchange factors (GEFs) (5, 6), and subcellular localization (7, 8). Small GTPases, including Rac, cycle between an inactive GDP-bound state and an active GTP-bound state. Two classes of regulatory factors, GTPase-activating proteins (GAPs) and GEFs, determine by their opposing effects the ratio of GDP versus GTP, which is bound to the GTPase (1). GAP proteins increase the intrinsic rate of GTP hydrolysis, rendering the GTPase inactive, whereas GEFs enhance the exchange of bound GDP for GTP, thereby activating the protein. Active Rac regulates distinct downstream signaling pathways by interacting with specific effector proteins, including a family of serine-threonine protein kinases termed Paks (p21-activated kinases) (9–11).
Apart from its well documented role in cytoskeletal rearrangements in growth factor-stimulated cells (12), Rac1 is required for Ras-induced malignant transformation and is involved in transcription and growth control (1, 13, 14). Recently, the importance of the Rac effector Pak in cell transformation has been highlighted by inhibiting RasV12- and Rac1V12-induced transformation of Rat-1 fibroblasts with a catalytically inactive form of Pak (15, 16). The involvement of Rac1 in driving cell-cycle progression through the G1 phase and stimulating DNA synthesis has been shown by introducing dominant-active and -negative Rac1 mutants into fibroblasts (17, 18). However, the signaling pathways used by Rac to control mitogenesis and proliferation still remain poorly understood. Overexpression of constitutively active Rac-effector-domain mutants in fibroblasts indicated that although Rac1 mediated cyclin D1 transcription by Pak in NIH 3T3 cells (19), Pak was not involved in the DNA synthesis of Swiss 3T3 cells (20). Accumulating evidence, however, suggests higher complexity where Pak-binding proteins, such as the GEF Pix, contribute to the Rac–Pak interaction in vivo and influence subsequent cellular functions (21–23).
All biological functions listed above have been attributed to Rac1 in experimental cell systems using overexpression or microinjection of mutant forms. Endogenously active Rho GTPases, including Rac, have not yet been observed. In this paper, we describe a consistently active Rac3 GTPase leading to hyperactivity of its effector protein kinase, Pak, in human breast cancer-derived epithelial cell lines. Analysis of growth properties and DNA synthesis revealed that both proteins are required to convey the highly proliferative phenotype displayed by these cells.
Materials and Methods
Cell Culture.
Breast cancer cell lines were obtained from the American Type Culture Collection (ATCC) and were grown in medium containing 10% heat-inactivated FBS/1 mM glutamine/10 mM Hepes/100 units/ml penicillin-streptomycin at 37°C in a humidified atmosphere of 5% CO2. Cell lines MDA-MD 231 and MDA-MB 435 were grown in Leibovitch's L15 medium, T47D and Hs578T in RPMI medium 1640, and MCF 7 in Eagle's minimal essential medium, as recommended by the ATCC. Normal breast epithelial cell line HMEC 184 A1 was cultured as described in http://www.lbl.gov/∼mrgs.
Cell Lysates, Pak–p21-Binding Domain (PBD) Assay, and Immunoblot Analysis.
Cells were lysed after 18- to 24-h serum starvation in 25 mM Tris⋅HCl, pH 7.5/1 mM EDTA/5 mM MgCl2/1 mM DTT/0.1 mM EGTA/100 mM NaCl/1% Nonidet P-40/1 mM phenylmethylsulfonyl fluoride/1 μg/ml leupeptin/1 μg/ml aprotinin. Breast cancer cell lysates (300 μg), tissue lysates (450 μg), or transfected cell lysates (100 μg) (HeLa, MDA-MB 231, and MDA-MB 435) were incubated with 10 μg of recombinant glutathione S-transferase (GST)-PBD (amino acids 69 through 150 of human Pak1) for 1 h at 4°C and washed as reported (24). Immunoblotting was performed with anti-Rac1 (Upstate Biotechnology, Lake Placid, NY), anti-Cdc42 (Santa Cruz Biotechnology), anti-Rac3 (2) or 9E10 anti-Myc (Babco, Richmond, CA) antibodies and visualized by incubation with horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (Pierce). The specificity of antibodies directed against Rho GTPases was tested with recombinant proteins produced in Escherichia coli and in HeLa cells.
Plasmids and Transfections.
Rac3 wild-type (wt) cDNA was cloned from HMEC 184 cells by reverse transcription–PCR using Rac3-specific primers (2) and subcloned into the Myc-tagged pRK5 mammalian expression vector. Mutagenesis was performed with the QuikChange site-directed mutagenesis kit (Stratagene). The cDNAs encoding Myc-tagged Rac3wt, dominant-negative Rac3N17, constitutively active Rac3V12, Pak1 PBD (amino acids 69 through 150), Pak1 PBD F107, and Pak1 Pak-inhibitory domain (PID, amino acids 83 through 149), were subcloned into the pSFV3 vector to produce recombinant viral Semliki Forest virus stocks as described (25). PCR fidelity and site-directed mutagenesis were confirmed by sequence analysis. Cells were infected with activated virus in serum-free medium for 2 h and allowed to express protein for 12–14 h in serum-free medium before being used in experiments. Cells were fixed and stained with rhodamine-phalloidin and with anti-Myc antibody, followed by FITC-conjugated anti-mouse antibody to visualize cells expressing Myc-tagged proteins. Viral transfection efficiency was between 70% (HMEC 184) and 95% (MDA-MB 435 and MDA-MB 231) for all recombinant virus.
Kinase Assays.
Cell lysates were prepared from 20- to 24-h serum-starved cells as described above. Pak activities (60 μg of protein) were measured after renaturation by using in-gel kinase assays with SDS/6.5% PAGE gels containing a peptide substrate that corresponds to amino acid residues 297 through 331 of p47phox. This technique was performed as described (26). Pak activities were also analyzed after immunoprecipitation with specific Pak antibodies followed by in vitro kinase assays (27) using histone H4 (Fluka) as a substrate. c-Jun N-terminal kinase (JNK) activity was determined after incubation of 300 μg of cell lysate with 2 μg of recombinant GST-c-Jun (amino acid residues 1 through 89), followed by in vitro kinase reaction on beads and detection with phospho-specific c-Jun antibody (New England Biolabs).
BrdUrd Incorporation.
Cells infected with Semliki Forest virus encoding β-galactosidase (LacZ), wild-type and mutated forms of Rac3, or Pak fragments were allowed to express protein for 12–14 h in serum-free medium containing 10–20 μM BrdUrd for the times indicated. Cells were fixed and stained with FITC-conjugated anti-BrdUrd antibody (Becton Dickinson) (18).
Results and Discussion
Highly Proliferating Cancer Cells Contain Hyperactive Rac3.
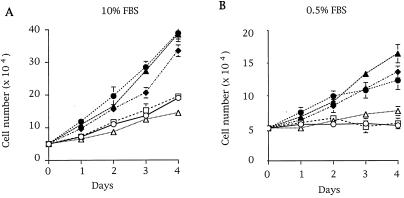
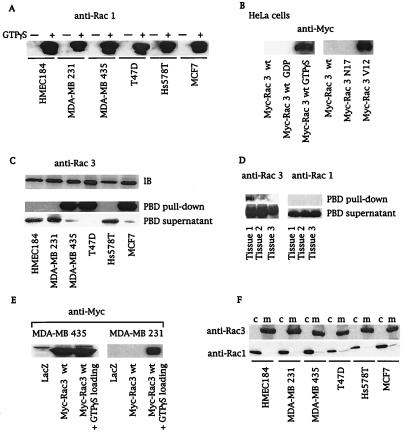
Comparison of growth rates among several breast cancer cell lines showed that three lines (MDA-MB 435, T47D, and MCF 7) grew faster under normal and low-serum conditions (Fig. 1). Interestingly, in contrast to MDA-MD 231 and Hs578T cells, these three highly proliferative cell lines do not possess mutated Ras (28, 29). To assess whether Rho GTPases drive this cellular phenotype, we determined whether these cell lines contained active GTP-bound Rac or Cdc42. We used a recently described assay, the PBD-pulldown assay (24), which is based on the specific binding of the GTP-bound forms of Rac and Cdc42 to the PBD of Pak (10). Neither active Rac1 (Fig. 2A) nor active Cdc42 (data not shown) could be detected in any of the cell lysates obtained from serum-starved cells. However, both proteins were detected if the PBD-pulldown assay was performed with in vitro guanosine 5′-[γ-thio]triphosphate (GTP[γS])-loaded cell lysates, confirming that Rac1 and Cdc42 were present in their inactive GDP-bound forms in these cells (Fig. 2A for Rac1). Next we wanted to determine whether active Rac3 was present in breast cancer cell lines. Because Rac3 effectors have not yet been characterized, we demonstrated by overlay binding and kinase assays that Rac3 bound to and activated Pak as efficiently as Rac1 (data not shown). We verified that the PBD-pulldown assay specifically detected the active GTP-bound form of Rac3 (GTP[γS]-loaded Rac3wt or Rac3V12, Fig. 2B) and not the inactive form. To probe for Rac3 protein in breast cell lysates, a Rac3-specific antibody was used. GST-PBD-pulldown experiments from cell lysates revealed the presence of hyperactive Rac3 in highly proliferative cell lines (MDA-MB 435, T47D, and MCF 7), but not in normal breast cell lines or in less proliferative breast cancer cells (Fig. 2C). Additionally, as indicated by the virtual absence of Rac3 in the supernatant of the PBD pulldown, all the Rac3 protein present in these cell lines was active (Fig. 2C). To demonstrate that consistent Rac3 activation is not limited to cell lines, we performed an initial screening of human metastatic breast cancer tissues and found active Rac3 in one of three samples, underlining the potential clinical relevance of the cellular findings (Fig. 2D).
Figure 1.
Differential growth rates of human breast cell lines. Human breast cell lines, including HMEC 184 (○), MDA-MB 231 (▵), Hs578T (□), MDA-MB 435 (●), T47D (▴), and MCF 7 (♦), were grown in 10% serum (A) or 0.5% serum (B) conditions. The cells were split in duplicate over 6-well plates at 5 × 105 cells per well and counted daily with a hemocytometer for 4 days. Data shown in A and B are representative of three independent experiments.
Figure 2.
Active Rac3 is present in highly proliferative cell lines and in human breast cancer tissue. (A and C) Cell lysates from serum-starved breast cancer cell lines without (A and C) or after (+) GTP[γS] loading (A) were incubated with 10 μg of GST-PBD. Active Rac proteins (PBD pulldown) were detected by immunoblot with anti-Rac1 (A) or anti-Rac3 antibodies (C). Blotting of PBD supernatants revealed the GDP-bound form of Rac3 in lysates. Equal amounts of Rac3 protein were detected by immunoblot (IB) in all cell lines. (B) A PBD-pulldown assay of extracts from HeLa cells expressing Myc-Rac3wt or -Rac3 mutants, followed by an anti-Myc immunoblot, detected only active Rac3 (GTP[γS] loading or Rac3V12). (D) PBD pulldown of lysates obtained from three different human metastatic breast cancer tissues, followed by anti-Rac1 and anti-Rac3 immunoblots, revealed active Rac3 in tissue 1. (E) PBD pulldown of lysates derived from MDA-MB 435 and MDA-MB 231 cells expressing LacZ control or Myc-Rac3wt without or after in vitro GTP[γS] loading. Consistent activation of Myc-Rac3wt occurred only in MDA-MB 435 cells. (F) Subcellular localization of Rac1 and Rac3. Cytosol (c) and membranes (m) were obtained after nitrogen cavitation and fractionation of breast cancer cell lines and immunoblotted with anti-Rac1 and anti-Rac3 antibodies. All blots are representative of at least three experiments.
Subcellular Localization and GTPase-Regulatory Factors Influence Rac3 Activity.
Constitutive activation of Ras proteins in cancer cells is often caused by activating point mutations at the switch I or II regions (29). cDNA cloning and complete sequence analysis of full-length Rac3 did not reveal any mutations in the breast cell lines studied and did not explain the observed Rac3 activation. GTPase-regulatory proteins such as GEFs and GAPs, which are usually regulated by upstream stimuli, control cycling between the active and inactive forms of Rac. To confirm the presence of an altered regulatory mechanism involved in Rac3 activation, we used the PBD-pulldown assay to analyze the activation state of Myc-tagged Rac3wt transfected into either MDA-MB 231, a cell line harboring only GDP-Rac3, or MDA-MB 435, a cell line that contains endogenous, active GTP-Rac3. Fig. 2E shows that activated Myc-Rac3 was detected only in the MDA-MB 435 cell line, confirming that the regulation of the GDP/GTP state of Rac3 was altered in these cells. We then investigated several upstream stimuli that have been shown to affect GTPase-regulatory proteins (28, 30–32). We excluded the possibility of an autocrine growth-stimulatory loop by culturing MDA-MB 231 cells with the conditioned medium from MDA-MB 435, which did not affect the Rac3 activation state (data not shown). Treatment of cell cultures with phosphatidylinositol 3-kinase or tyrosine kinase inhibitors, including wortmannin, LY294002, and genistein, did not decrease Rac3 activation (data not shown). At this point, we speculated that an oncogenic, Rac3-specific GEF is present in certain breast cancer cells. GEFs possess a pleckstrin homology domain that is essential for membrane localization and for their oncogenic properties (5, 33). Analysis of the subcellular localization of the Rac family members revealed that Rac3 is located in the membranes of breast epithelial cell lines, independently of its activation state (Fig. 2F). In contrast, endogenous Rac1 in its inactive GDP-bound state was essentially cytosolic (Fig. 2F). Thus, the distinct localization of Rac3 and Rac1 may contribute to their different activation states in certain breast cancer cell lines. It is conceivable that the highly proliferative cell lines (Fig. 1) express a constitutively active, membrane-bound Rho GEF that activates adjacent Rac3 protein. This hypothesis was further supported by using an hydroxymethylglutaryl-CoA reductase inhibitor, lovastatin, that interferes with isoprenoid synthesis and thereby with posttranslational processing of GTPases. Unprocessed Rac3 from lovastatin-treated MDA-MB 435 cells was predominantly cytosolic and inactive (GDP-Rac3) (data not shown). The requirement of membrane localization for consistent Rac3 activity was further supported by using a Rac3S189 mutant. Replacing cysteine-189 of the CAAX box with serine abolishes isoprenoid incorporation, rendering the GTPase cytosolic. This Rac3 mutant remained in its inactive GDP-bound state when transfected into MDA-MB 435 cells (data not shown).
Several Rho GTPase-regulating GEFs have been identified (5), including the Rac1-specific GEF Tiam-1, which has been linked to tumors such as invasive T-lymphomas (34). Although Tiam-1 is expressed in virtually all tissues, no evidence of oncogenic truncations or alternative splicing of Tiam-1 transcripts has been found (35). A variation of Tiam-1 transcript levels in certain cancer cell lines might lead to overexpression and possibly activation of Tiam-1 protein. However, the activation state of Rac3 protein in the cell lines used in this study does not seem to correlate with Tiam-1 expression levels as reported by Habets et al. (35). Hyperactivity of Rac3 in cancer cells could also result from an absent or dysfunctional Rac3-specific GAP protein. By accelerating the intrinsic GTP hydrolysis rate, GAPs render the GTPase inactive and act as tumor suppressors. Deletion or mutations in the RasGAP gene NF1 and the RhoGAP homologs bcr and DLC-1 have been reported in cancer cells (36, 37).
Active Rac3 Drives Epithelial Cell Proliferation.
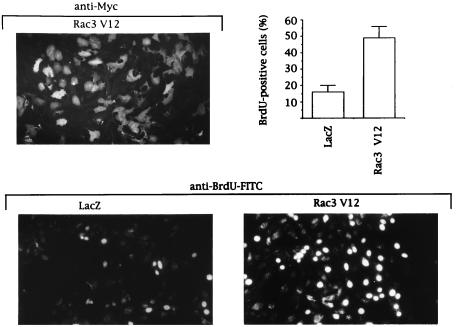
To study whether active Rac3 could account for the high proliferation rate of certain breast cancer cells, we expressed a constitutively active Rac3 mutant (Rac3V12) in normal mammary epithelial cells (HMEC 184) that contain only GDP-Rac3 (Fig. 2C). Rac3V12 expression significantly increased the incorporation of BrdUrd into nascent DNA (Fig. 3), emphasizing that transfection of active Rac3 drives epithelial cell proliferation.
Figure 3.
Rac3V12 induces DNA synthesis in human mammary epithelial cells. HMEC 184 cells, infected with recombinant LacZ or Rac3V12 Semliki Forest virus, were allowed to express protein for 14 h in serum-free medium containing 10 μM BrdUrd. Cells were fixed and stained with anti-Myc antibody for Myc-Rac3V12 expression level (Upper) or with FITC-conjugated anti-BrdUrd antibody for BrdUrd incorporation (Lower). The presence of bright fluorescent nuclei indicates BrdUrd-positive cells. The percentage was calculated after counting 400 cells in each of three independent experiments.
Hyperactive Pak and c-Jun Kinases in Cancer Cells.
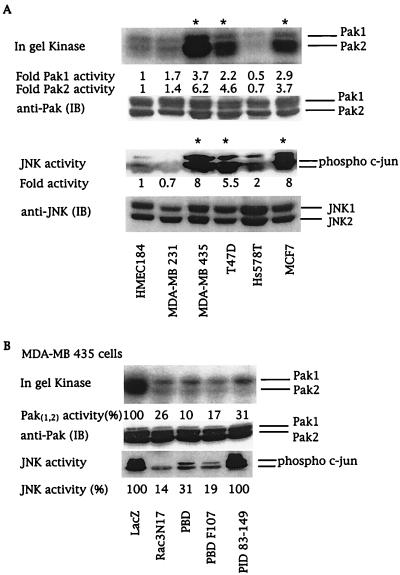
The signaling cascade utilized by Rac proteins to control cell proliferation still remains to be identified (1, 9), but might involve Paks. We analyzed Pak activity in cell lysates derived from serum-starved breast cancer cell lines by using in-gel kinase assays and by using in vitro kinase assays after immunoprecipitation with Pak-specific antibodies. Pak activity was increased 4- to 6-fold in the three cell lines containing active Rac3 (Fig. 4A). This increased kinase activity was mainly associated with the Pak2 isoform, which can phosphorylate and positively regulate Raf-1 activity, another key component in cell proliferation (38–40).
Figure 4.
Rac3 activates Pak and JNK by two different pathways. (A) Breast cancer cell lysates from serum-starved cells were analyzed for Pak and JNK activities. Pak activities in cell lysates were analyzed by in-gel kinase assays. JNK activity was determined by using phospho-specific c-Jun antibody. The numbers at the bottom of each lane give the increase above basal kinase activity levels in HMEC 184 cells. (B) Pak and JNK activities in MDA-MB 435 cells expressing LacZ control; dominant-negative Rac3N17; or the Pak fragments PBD, PBD F107, or PID 83–149. The numbers at the bottom of each lane give the relative percentage of kinase activity when compared with LacZ-transfected MDA-MB 435 cells. Quantitation was performed by PhosphorImager (Paks) or by densitometer (JNK) (Molecular Dynamics). Immunoblots (IB) show that equivalent amounts of kinases were present in each cell line. Asterisks indicate cell lines containing active Rac3. All blots are representative of at least three experiments.
Intracellular Rac-regulated signaling pathways impinge on distinct mitogen-activated protein kinase cascades. Constitutively active Rac has been shown to positively regulate the activity of the stress-activated kinases JNK and p38 (1). Moreover, ERK activity can be indirectly stimulated by Rac or mediated by crosstalk between the distinct mitogen-activated protein kinase cascades (1, 41). Determination of distinct mitogen-activated protein and stress-activated protein kinase activities in the breast cell lines studied here showed that consistent Rac3 and Pak kinase activities were associated with enhanced JNK activity (Fig. 4A). In contrast, no correlation existed between p38 or ERK kinase activities and active Rac3 or Pak (data not shown).
Rac3 Triggers Pak and JNK Activities by Separate Pathways.
To determine whether the highly proliferative phenotype of breast cancer cells depends directly on a consistently active Rac3-Pak-JNK cascade, we used virus-mediated protein expression in MDA-MB 435 cells to examine the ability of Rac3 and Paks to control JNK activation and cellular proliferation. The importance of Pak as an effector protein in Rac-mediated activation of JNK is still controversial and seems to be cell-type-dependent (42). Expression of the PBD domain, which controls the activity of both Rac and Pak (21), completely inhibited Pak and JNK stimulation (Fig. 4B). The mutation of leucine to phenylalanine at position 107 of the PBD domain suppresses the autoinhibitory function of the PBD (21). Thus, PBD F107 will act only to sequester active Rac3 and blocks its ability to bind and activate endogenous effectors. Expression of either dominant-negative Rac3N17 or PBD F107 almost completely blocked Pak and JNK activities, demonstrating that Rac3 is upstream of these proteins (Fig. 4B). Moreover, Pak kinase activity can be inhibited independently of Rac3 by overexpressing the kinase autoinhibitory domain, PID, which does not interact with Rac (21, 43). Transfection of PID into MDA-MB 435 cells dramatically inhibited Pak activity as expected, but did not decrease JNK activation (Fig. 4B). Our results indicate that in MDA-MB 435 cells, consistent stimulation of JNK by Rac3 is independent of PAK activity and that Rac3 initiates two different pathways involving Pak and JNK, respectively.
Rac3 and Pak Are Both Required for Breast Cancer Cell Proliferation.
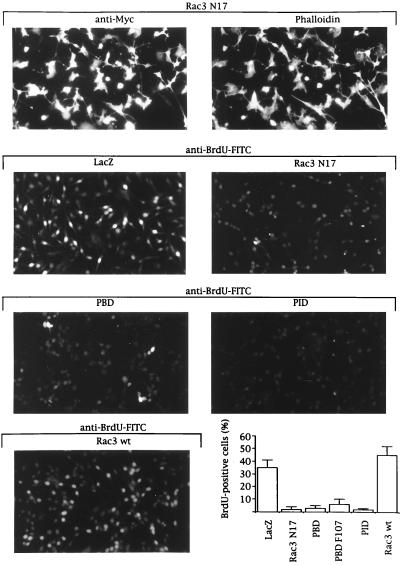
We subsequently determined which of these two Rac3 pathways promoted the increased cell proliferation in breast cancer cell lines with hyperactive Rac3. We studied the consequence of expressing inhibitory Rac mutants or Pak fragments on DNA synthesis. LacZ-expressing MDA-MB 435 cells still proliferated in low-serum conditions and 35% incorporated BrdUrd (Fig. 5). This percentage increased to 50% when Rac3wt, which will be partially activated in these cells (Fig. 2E), is expressed (Fig. 5 Bottom Right). In contrast, expression of inhibitory proteins, including Rac3N17 or the PBD that suppressed Pak and JNK activation (Fig. 4B), almost completely blocked S-phase entry, as indicated by the absence of BrdUrd incorporation (Fig. 5). Expression of the PID that inhibited Pak kinase activity without affecting JNK stimulation (Fig. 4B) also arrested proliferation in MDA-MB 435 cells (Fig. 5). These experiments emphasize the crucial role of active Rac3 for DNA synthesis in breast cancer cell lines and demonstrate that Pak kinase activity is necessary for Rac3-induced proliferation.
Figure 5.
Rac3 mediates proliferation in MDA-MB 435 cells by a Pak-dependent pathway. MDA-MB 435 cells growing in 0.5% FBS were infected with Semliki Forest virus encoding for LacZ, Rac3N17, Pak1-PBD, Pak1-PBD F107, Pak1-PID, or Rac3wt. After 12 to14 h of protein expression in serum-free medium, 20 μM BrdUrd was added for 20 min before the cells were fixed and stained with anti-Myc antibody and phalloidin for expression (Top) or with FITC-conjugated anti-BrdUrd antibody for BrdUrd incorporation (Lower five micrographs). The presence of bright fluorescent nuclei indicates BrdUrd-positive cells. The percentage was calculated after counting 400 cells in each of four independent experiments.
Our results establish the persistent activation of a small Rho GTPase, Rac3, and the effector kinase Pak in human breast cancer cells. In contrast to Rac1, endogenous Rac3 is localized at the plasma membrane in both guanine nucleotide states. It seems likely that a Rac3 regulatory protein is altered or deleted in highly proliferating cancer cells, and that its specificity toward Rac3 results from the adjacent location of both proteins at the membrane and/or from discrete Rac3 domains, which convey a specific interaction. The cytoskeletal phenotypes of serum-starved breast cancer cells, such as ruffles or lamellipodia typical of Rac1 protein activation, did not seem to correlate with the GDP versus GTP state of endogenous Rac3. This may suggest that Rac family members are specialized in certain cellular functions, as already reported for Rac2 in leukocyte phagocytosis (44) and now demonstrated by us for Rac3 in cancer cell proliferation. Our studies establish further that endogenous, active Rac3 is essential for breast cancer cell proliferation via a Pak-dependent pathway. Paks have been shown to directly phosphorylate Raf kinase, which binds to retinoblastoma protein and regulates its function (45), and to interact with cyclin-dependent kinases to up-regulate cyclin D1 expression (46). Initial screening of various human cancer-derived cell lines revealed the presence of hyperactive Rac3 and Pak kinase in other types of highly proliferating tumors (data not shown). Further investigations, primarily in animal models and clinical settings, will be necessary to assess whether loss of Rac3 and Pak regulation correlates with certain breast tumor stages and is accompanied by specific alterations in cell-cycle regulators. Approaches to inhibit Rac3 or Pak activity would then open a new avenue for cancer therapeutics.
Acknowledgments
We thank M. Stampfer for the HMEC 184 cell line, J. H. Jackson for human metastatic breast cancer tissues, J. A. Badwey for p47phox peptide, A. Reilly and W.-K. Kwan for providing technical assistance, H. Daniels and G. M. Bokoch for critical comments on the manuscript, and A. Lestelle for editorial assistance. This work was supported by Institut Lilly, France (J.-P.M.), the Arthritis Foundation (V.B.), the National Institutes of Health and National Cancer Institute (J.G.), the U.S. Army Breast Cancer Research Program (L.C.S.), and the Breast Cancer Fund of the State of California (U.G.K.). This is manuscript 12527-IMM of the Scripps Research Institute.
Abbreviations
- Pak
p21-activated kinase
- JNK
c-Jun N-terminal kinase
- PBD
p21-binding domain
- PID
Pak-inhibitory domain
- GEF
guanine nucleotide exchange factor
- GAP
GTPase-activating protein
- GST
glutathione S-transferase
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
- LacZ
β-galactosidase
- wt
wild type
References
- 1.Van Aelst L, D'Souza-Schorey C. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 2.Haataja L, Groffen J, Heisterkamp N. J Biol Chem. 1997;272:20384–20388. doi: 10.1074/jbc.272.33.20384. [DOI] [PubMed] [Google Scholar]
- 3.Wu W J, Cerione R A, Manor D. J Biol Chem. 1998;273:16655–16658. doi: 10.1074/jbc.273.27.16655. [DOI] [PubMed] [Google Scholar]
- 4.Joneson T, Bar-Sagi D. J Biol Chem. 1998;273:17991–17994. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- 5.Cerione R A, Zheng Y. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead I P, Campbell S, Rossman K L, Der C J. Biochim Biophys Acta. 1997;1332:F1–F23. doi: 10.1016/s0304-419x(96)00040-6. [DOI] [PubMed] [Google Scholar]
- 7.Casey P J, Seabra M C. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 8.Seabra M C. Cell Signalling. 1998;10:167–172. doi: 10.1016/s0898-6568(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 9.Boettner B, Van Aelst L. Prog Mol Subcell Biol. 1999;22:136–158. doi: 10.1007/978-3-642-58591-3_7. [DOI] [PubMed] [Google Scholar]
- 10.Manser E, Leung T, Salihuddin H, Zhao Z-S, Lim L. Nature (London) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 11.Daniels R H, Bokoch G M. Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 12.Hall A. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 13.Zohn I M, Campbell S L, Khosravi-Far R, Rossman K L, Der C J. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]
- 14.Qiu R G, Chen J, Kirn D, McCormick F, Symons M. Nature (London) 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs J B, Chernoff J, Field J. Mol Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Yu J, Field J. Mol Cell Biol. 1999;19:1881–1891. doi: 10.1128/mcb.19.3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson M F, Ashworth A, Hall A. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 19.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestell R G, Der C J. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenstroem P, Bridges T, Chant J, Hall A. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z-S, Manser E, Chen X Q, Chong C, Leung T, Lim L. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manser E, Loo T-H, Koh C-G, Zhao Z-S, Tan I, Leung T, Lim L. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 23.Sells M A, Boyd J T, Chernoff J. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benard V, Bohl B J, Bokoch G M. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 25.Sanders L C, Matsumura F, Bokoch G M, de Lanerolle P. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 26.Ding J U, Knaus U G, Lian J P, Bokoch G M, Badwey J A. J Biol Chem. 1996;271:24869–24873. doi: 10.1074/jbc.271.40.24869. [DOI] [PubMed] [Google Scholar]
- 27.Knaus U G, Morris H-J, Dong J, Chernoff J, Bokoch G M. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 28.Ennis B W, Valverius E M, Bates S E, Lippman M E, Bellot F, Kris R, Schlessinger J, Masui H, Goldenberg A, Mendelsohn J, et al. Mol Endocrinol. 1989;3:1830–1838. doi: 10.1210/mend-3-11-1830. [DOI] [PubMed] [Google Scholar]
- 29.Mangues R, Pellicer A. Semin Cancer Biol. 1992;3:229–239. [PubMed] [Google Scholar]
- 30.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 31.Nimnual A S, Yatsula B A, Bar-Sagi D. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 32.Bustelo X R, Barbacid M. Science. 1992;256:1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Zangrilli D, Cerione R A, Eva A. J Biol Chem. 1996;271:19017–19020. doi: 10.1074/jbc.271.32.19017. [DOI] [PubMed] [Google Scholar]
- 34.Michiels F, Habets G G M, Stam J C, Van der Kammen R A, Collard J G. Science. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 35.Habets G G M, Van der Kammen R A, Stam J C, Michiels F, Collard J G. Oncogene. 1995;10:1371–1376. [PubMed] [Google Scholar]
- 36.Hall A. Cell. 1992;69:389–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- 37.Yuan B-Z, Miller M J, Keck C L, Zimonjic D B, Thorgeirsson S S, Popescu N C. Cancer Res. 1998;58:2196–2199. [PubMed] [Google Scholar]
- 38.King A J, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall M S. Nature (London) 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 39.Kerkhoff E, Rapp U R. Oncogene. 1998;17:1457–1462. doi: 10.1038/sj.onc.1202185. [DOI] [PubMed] [Google Scholar]
- 40.Sherr C J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 41.Frost J A, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P E, Cobb M H. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manser E, Lim L. Prog Mol Subcell Biol. 1999;22:115–133. doi: 10.1007/978-3-642-58591-3_6. [DOI] [PubMed] [Google Scholar]
- 43.Frost J A, Khokhlatchev A, Stipper S, White M A, Cobb M A. J Biol Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- 44.Roberts A W, Kim C, Zhen J B, Lowe J B, Petryniak B, Spaetti A, Pollock J D, Borneo J B, Bradford G B, Atkinson S J, et al. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Ghosh R N, Chellappan S P. Mol Cell Biol. 1998;18:7487–7498. doi: 10.1128/mcb.18.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tjandra H, Compton J, Kellogg D. Curr Biol. 1998;8:991–1000. doi: 10.1016/s0960-9822(07)00419-8. [DOI] [PubMed] [Google Scholar]