Abstract
Background Birth-size is a problematic proxy for the fetal environment, and regression models testing for associations between birth-size and blood pressure have been criticized.
Methods We modelled fetal environment as a latent variable determined by maternal height and arm fat area (AFA) during pregnancy using structural equation modelling. We tested for associations between latent fetal environment (LFE) and systolic blood pressure (SBP) while controlling for birth weight (BW) and current weight (CW). Data are from 1435 male and 1218 female young adult Filipinos (2005; mean age 21 years) enrolled in the Cebu Longitudinal Heath and Nutrition Survey, an ongoing, community-based study of a one-year birth cohort. Using AMOS 6.0, LFE was modelled as a determinant of BW, CW and SBP; CW was modelled as a determinant of SBP.
Results Overall model fit was excellent (χ2: 32.14, 27 df, P = 0.23). The estimated direct relationship between LFE and SBP was inverse for both males (−0.43 −0.26 −0.10) and females (−0.29 −0.18 −0.07).
Conclusions These results are consistent with the hypothesis that maternal height and AFA impact fetal development in a manner that is positively associated with fetal growth (as reflected by BW) and inversely associated with SBP in young adulthood.
Keywords: Blood pressure, birth weight, structural equation model, developmental origins
Evidence suggests that birth-size is inversely related to systolic blood pressure (SBP) in adulthood.1–3 This research is often interpreted as an effect of poor fetal environment on cardiovascular and/or kidney disease risk under the Developmental Origins of Health and Disease (DOHaD) paradigm. While plausible biological mechanisms explain how fetal environment could affect later SBP,2,4 and experimental animal evidence is strongly supportive of the hypothesis,2,4 the human epidemiological evidence is criticized.
One critique is of birth-size as an indicator of fetal environment. Misclassification occurs since despite an optimal fetal environment, a newborn may still be small because of a lower innate growth potential; conversely, a larger baby could have suffered from fetal malnutrition that prevented it from reaching its full growth potential. Fetal environment may also affect organ size5 or other aspects of development that lead to later disease without influencing birth-size.6 Because birth-size reflects multiple determinants, only some of which reflect aspects of fetal environment hypothesized to affect SBP,7 it does not represent a target for public health intervention in this context.
There is also debate regarding the appropriateness of adjusting for current-size in regressions of SBP on birth-size.8–11 Researchers typically find a null association between birth-size and SBP that ‘shifts’ inversely away from the null after current-size is controlled.1–3 There are at least three possible reasons for this shift:
Current-size suppresses12 the relationship between birth-size and SBP. While we hypothesize that small babies will have higher adult SBP (an inverse relationship), we also expect small babies to become small adults, who will have lower SBP (a direct relationship). Thus controlling this direct relationship could be revealing a true inverse relationship between birth-size and SBP, which is reflected by the birth-size coefficient's shift from the null.
With current-size in the regression, the birth-size coefficient can also be interpreted as an effect of growth, because decreasing birth-size by one unit, holding current-size constant, must result in a one unit increase in growth from birth to the current period.9,13 This change in interpretation could explain the shift in the birth-size coefficient, since growth is also a hypothesized determinant of SBP.1,9,14,15
Body composition, which affects SBP, may also be influenced by fetal environment.16–20 If body composition, reflected by current-size, is a mediator of the relationship between birth-size and SBP, and shares unmeasured determinants with SBP, controlling for it can create confounding of the birth-size–SBP relationship by these unmeasured variables within strata of current-size.21,22 This bias could then potentially explain the shift in the birth-size coefficient when current-size is controlled for.
Thus we must control current-size to account for possible suppression, but doing so has critical consequences for parameter interpretation and could introduce bias.23
Our goal was to improve on previous analyses by testing a series of structural equation models (SEMs) to explain how life-course influences on fetal environment [maternal height and arm fat area (AFA)] may affect offspring birth-weight (BW), current-weight (CW) and SBP in a birth cohort of young adult Filipinos. Through SEM, investigators can impose a hypothesized causal structure upon a set of measured variables in an attempt to explain their observed variances and covariances. This structure can include a variety of features not possible with regression methods, including latent variables and multiple linear equations.24 The SEMs we tested are based on the hypothesis that an underlying latent variable, which we have labelled latent fetal environment (LFE), is in part caused by maternal height and AFA, and is in turn inversely related to SBP and positively related to BW and CW.
These SEMs are not the ultimate solution to problems inherent in testing developmental hypotheses using observational data, though we do contend that our analysis is a step in the right direction. The first advantage of this analysis is that instead of testing the hypothesis that BW and SBP are associated, we are able to test a more specific hypothesis that attempts to explain why birth-size and SBP are related.
Second, because fetal environment is complex6,25,26 and cannot be directly observed in any singular way, modelling it as a latent variable with multiple causal indicators seems more realistic that using BW as a proxy measure. While numerous factors are hypothesized to impact fetal environment in a manner that leads to later disease, we used maternal height and AFA in this analysis. This is primarily based on work by Gluckman and Hanson,6 who have posited that life-course markers of maternal nutrition, particularly those related to maternal constraint of fetal growth via skeletal size, act as the key predictors of the future nutritional environment that signal the developing fetus to alter physiology in a manner that promotes survival in an energy poor environment at the possible expense of later disease in an energy rich environment. We used AFA because it is a known determinant of BW in this sample27,28 and acts as a maternal energy store that can be mobilized to support fetal growth in late pregnancy.29,30
Another advantage is that we can include CW in the SEM without invoking a growth interpretation, because a one-unit change in LFE, holding CW constant, does not imply that the individual grew any more or less. However, the parameter estimates from the SEMs we tested are only valid under the assumption that they are properly specified. With respect to the validity of the estimated relationship between LFE and SBP, this means that all potential confounders of that relationship are accounted for, as well as any shared determinants of CW and SBP. Thus, in an additional analysis, we also tested a SEM that added measures of socio-economic status (SES) as determinants of LFE, SBP and CW.
Participants and Methods
Participants
Data are from the Cebu Longitudinal Health and Nutrition Survey (CLHNS). The CLHNS is a community-based study of a one-year birth cohort (1983–84) from Metro Cebu, the second largest metropolitan area of Philippines. Using single-stage cluster sampling, pregnant women in 33 randomly selected communities were identified and invited to participate in the study. More than 95% of these women agreed to participate. A baseline survey was conducted during their second or third trimester (mean gestational week 30). The birth cohort included 3080 non-twin, live births. Follow-up surveys were conducted immediately after birth, bimonthly to age two, then in 1991, 1994, 1998, 2002 and 2005 (Table 1).
Table 1.
CLHNS sample characteristics (n = 2653)
| Maternal variables |
Offspring variables |
||||||
|---|---|---|---|---|---|---|---|
| HT (cm) | AFA (cm2) | BW (kg) | CW (kg) | SBP (mmHg)a | Baseline SES | 2005 SES | |
| Males | |||||||
| n | 1435 | 1434 | 1409 | 886 | 885 | 1434 | 915 |
| Mean | 150.73 | 14.93 | 3.08 | 56.23 | 112.13 | 0.07 | 0.04 |
| Var. | 25.15 | 32.98 | 0.16 | 89.46 | 113.31 | 4.51 | 7.34 |
| Range | 136.10–169.20 | 3.75–55.47 | 1.58–4.8 | 36–110.2 | 85.33–175.33 | −2.03–8.03 | −4.15–13.33 |
| Percentile | |||||||
| 10th | 144.2 | 9.01 | 2.55 | 46 | 100 | −1.77 | −2.90 |
| 25th | 147.25 | 11.04 | 2.8 | 50 | 106 | −1.37 | −1.95 |
| 50th | 150.55 | 13.66 | 3.06 | 55 | 110 | −0.70 | −0.41 |
| 75th | 154 | 17.52 | 3.32 | 60 | 120 | 0.88 | 1.25 |
| 90th | 157.15 | 22.24 | 3.62 | 69 | 126.67 | 3.16 | 4.00 |
| Females | |||||||
| n | 1218 | 1218 | 1200 | 737 | 737 | 1218 | 757 |
| Mean | 150.58 | 14.84 | 3.02 | 46.45 | 99.36 | 0.04 | 0.15 |
| SD | 25.02 | 33.23 | 0.16 | 67.09 | 104.95 | 4.31 | 6.94 |
| Range | 134.2–166.65 | 4.48–47.90 | 1.81–4.8 | 30–105 | 60.67–138 | −2.03–8.03 | −4.15–13.33 |
| Percentile | |||||||
| 10th | 144.45 | 9.01 | 2.5 | 38 | 88.67 | −1.77 | −2.77 |
| 25th | 147.3 | 11.05 | 2.72 | 41 | 91.33 | −1.39 | −1.86 |
| 50th | 150.475 | 13.66 | 3 | 45 | 100 | −0.70 | −0.20 |
| 75th | 153.75 | 17.52 | 3.26 | 50 | 105.33 | 0.81 | 1.46 |
| 90th | 157 | 22.24 | 3.51 | 57 | 111.33 | 3.00 | 3.61 |
aMean of three observed SBP values.
AFA, arm fat area; BW, birth weight; CW, current weight; HT, height; SBP, systolic blood pressure; SES, socio-economic status.
We use data from the baseline, birth and 2005 surveys. The participants were between the ages of 20 years and 22 years in 2005. We excluded pregnant females and participants who were born preterm (completed <37 weeks gestation), resulting in a sample of 2653 individuals (1218 females and 1435 males). Preterm births were excluded because they most likely experienced the modelled set of relationships differently than the rest of the cohort. This exclusion is consistent with other studies of the developmental effects of SBP.1,31
While complete data were available for 1597 individuals, we used full information maximum likelihood (FIML)32 estimation as implemented in AMOS 6.0 (Chicago, IL, USA) to include those with incomplete data in the analysis. The FIML estimator is consistent if the pattern of missing data are ‘missing at random’ (MAR). MAR data occur when, given the observed data, the mechanism resulting in missing data does not depend on the unobserved data. We know of no empirical test of the MAR assumption, particularly for longitudinal cohorts where most of the missing data is due to sample attrition.33 However, it is a less restrictive assumption than that needed for the list-wise deleted samples used in OLS regressions, which is that the data are ‘missing completely at random’ (MCAR). Since previous studies used list-wise or pair-wise deletion of cases and hence are assuming MCAR, our MAR assumption is less restrictive than previous analyses.
Measures
AFA was calculated from mid-upper arm circumference and triceps skinfold thickness. Maternal height was measured with a folding stadiometer. These measurements were taken during home visits during the baseline survey by trained field staff. Infants born at home (62%) were weighed by trained birth attendants with Salter hanging scales. The remainder, born at hospitals or clinics, were weighed on clinical scales. Gestational age was estimated from the mother's self-reported date of her last menstrual period. For cases where this date was unknown, when pregnancy complications occurred or when the infant was born weighing <2.5 kg, gestational age was clinically assessed using the Ballard method.34 SBP was measured in triplicate after a 10-min seated rest using a mercury sphygmomanometer. All three measurements were taken within 5–10 min by the same observer. CW was measured to the nearest kg using a digital scale during in-home visits. SES was derived from a principal components analysis (PCA) of housing quality and assets indicators measured at baseline and in 2005.35
Modelling and statistical methods
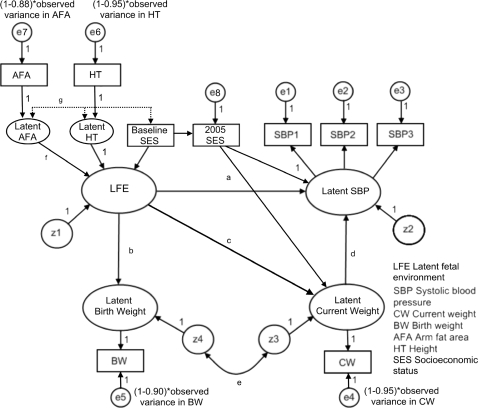
The base SEM is depicted in Figure 1. Ovals represent latent variables and boxes represent measured variables. Circles represent the latent error (e) and disturbance (z) terms. Error terms reflect random variation in measured variables, while disturbance terms represent variation in a latent variable not explained by other variables in the model. The variables are related by single-headed arrows that are hypothesized causal paths estimated by linear regression coefficients, and double headed arrows that are estimated covariances.
Figure 1.
Base SEM
Latent maternal height and AFA were modelled as determinants24,36,37 of a latent variable labelled fetal environment. They are not modelled reflectively (which is more common in SEM) because this is inconsistent with our hypothesis. They represent different aspects of the life-course nutrition of the mother hypothesized to impact fetal environment, not her diet during pregnancy. They are allowed to covary in the model. Because LFE is endogenous it has an associated disturbance term, z1; thus LFE is not simply a linear combination of maternal height and AFA.
SBP was modelled as a latent variable with three measured effect indicators. Each measured indicator has an associated error term unique to each measure (e1–e3), while the underlying latent SBP variable also has an associated disturbance term (z2).
AFA, height, BW and CW were modelled as single indicator latent variables. The error variances for the measured variables would not be identified if freely estimated. These values are fixed a priori using reliabilities (r) of 0.88, 0.95, 0.90 and 0.95, respectively38,39 and calculated as (1 − r) multiplied by the observed variance of the measured variable within gender. Latent BW and CW also have associated disturbance terms (z3 and z4).
LFE was modelled as a determinant of both latent BW and SBP. The disturbances for latent BW and CW were modelled as covaried to represent shared determinants exogenous to the model (e.g. genetics and environment), and latent CW was modelled as a determinant of latent SBP. Initial analyses indicated that overall model fit would be substantially improved by including a path from LFE to latent CW. The inclusion of this path was the only deviation from our original theoretical model.
LFE was scaled to maternal height by setting the respective coefficient to one. Latent SBP was scaled to the first SBP measurement; and latent maternal height, AFA, BW and CW were each scaled to their respective indicators in the same manner. We used empirical means (the non-singular information matrix and alternate starting values) to verify the model's identification.24
Previous studies have inconsistently reported gender differences in the estimated relationship between BW and SBP.1,40 To test for gender differences, we used a multi-group analysis that tested alternate models (A–G), each of which constrained one key path or covariance as equal for males and females (noted in Figure 1), and then compared their model fit to that of the base SEM for which every parameter was freely estimated within gender. For each model whose fit did not decline relative to the base SEM, we concluded that the there was no gender difference in the respective path tested, and these paths were all constrained in a subsequent model. To account for potential confounding by SES, we tested an additional SEM that added 2005 SES as a determinant of SBP and CW, and baseline SES as a determinant of LFE and 2005 SES (Figure 2).
Figure 2.
SEM with SES indicators added
To place this analysis in the context of previous studies, we used OLS regression (STATA 9.2; College Station, TX, USA) to estimate the crude and adjusted (for CW) relationship between BW and SBP (using the average of the three available measures taken for each individual) in a reduced sample with complete data for all variables included in the full SEMs (726 females and 874 males).
Where appropriate, parameter estimates and their respective 95% confidence intervals (CI) are given as LOWER LIMIT Estimate UPPER LIMIT.41
Results
SEM fit
Table 2 presents model fit indices for the base SEM, models A–G, and the uncorrelated variable model (which assumes that the variables are unrelated to each other). Briefly, SEM parameters are estimated in a manner that attempts to best reproduce the observed variances and covariances of the model's measured variables. The model's χ2 tests the hypothesis that the model implied variances and covariances are equal to those of the observed data. This hypothesis is not rejected for any of the models in Table 2. Models A–G are nested forms of the base model, which permits χ2 difference tests of each model to the base model by subtracting the χ2 and degrees of freedom (df) of the models. Based on these χ2 tests, only model C has a worse fit than the base SEM.
Table 2.
Model fit indices for the Base SEM and Models A through G using birth cohort data from the CLHNS (n = 2653)
| χ2; df; P* | RMSEA | p-close | CFI | IFI | TLI | BIC | ||
|---|---|---|---|---|---|---|---|---|
| Base SEM | 18.41; 20; 0.56 | 0.000 | 1.000 | 1.000 | 1.000 | 1.000 | −139.26 | |
| Model A | LFE→SBPa | 19.12; 21; 0.58 | 0.000 | 1.000 | 1.000 | 1.000 | 1.001 | −146.44 |
| Model B | LFE→BWa | 18.61; 21; 0.61 | 0.000 | 1.000 | 1.000 | 1.000 | 1.001 | −146.95 |
| Model C | LFE→CWa | 31.71; 21; 0.06 | 0.014 | 1.000 | 0.999 | 0.999 | 0.998 | −133.85 |
| Model D | CW→SBPa | 18.59; 21; 0.61 | 0.000 | 1.000 | 1.000 | 1.000 | 1.001 | −146.97 |
| Model E | Z4↔Z3a | 19.04; 21; 0.58 | 0.000 | 1.000 | 1.000 | 1.000 | 1.001 | −146.52 |
| Model F | AFA→LFEa | 19.92; 21; 0.53 | 0.000 | 1.000 | 1.000 | 1.000 | 1.000 | −145.64 |
| Model G | AFA↔HTa | 21.67; 21; 0.42 | 0.002 | 1.000 | 1.000 | 1.000 | 1.000 | −143.89 |
| Uncorrelated variables model | 11491.11; 56; 0.00 | 0.277 | 0.000 | 0.000 | 0.000 | 0.000 | 114249.64 |
*All P-values are two sided.
aPath constrained as equal for males and females.
It is common practice to report multiple fit indices when presenting SEM results, as the validity of a given fit index can be situational and the relative usefulness of the various indices is still debated (e.g. references 42,43). In addition to the χ2 test, we report other commonly used fit indices (see reference 24 for their detailed descriptions). Root mean square error of approximation (RMSEA) values <0.05 indicate close fit. P-close is the degree of confidence in concluding that the true RMSEA is <0.05. Values for the comparative fit index (CFI), incremental fit index (IFI) and Tucker–Lewis index (TLI) range from 0 to 1.00 (ideal fit). For the Bayesian information criterion (BIC = χ2 − [df × ln (n)]), more negative values favour the hypothesized model over the fully saturated one (for which there is an estimated parameter directly linking all observed variables to one another resulting in 0 df). Model fit based on these indices is consistent with the χ2 test: all models have excellent fit and model C is the only one that seems to differ from the base SEM.
Based on these results we tested three additional models (Table 3). Model H constrained every key parameter as equal for males and females. Model I constrained every key parameter as equal for males and females with the exception of the path from LFE to CW (the path constrained in model C). Fit for model H was poor. Model I fit the data fairly well, but not as well as the base SEM. However, it is the most parsimonious model. Identifying the best model is subjective; we have decided to report parameters estimated from the base SEM. However, it is important to note that while the parameters from this model are freely estimated for each gender, our analyses suggest that, with the exception of the path between LFE and CW, there is a great deal of similarity in these estimates between genders.
Table 3.
Model fit indices for models H, I, and J using birth cohort data from the CLHNS (n = 2653)
| χ2; df; P* | RMSEA | p-close | CFI | IFI | TLI | BIC | |
|---|---|---|---|---|---|---|---|
| Model Ha | 45.02; 27; 0.02 | 0.016 | 1.000 | 0.999 | 0.998 | 0.997 | −167.83 |
| Model Ib | 31.92; 26; 0.20 | 0.009 | 1.000 | 0.999 | 1.000 | 0.999 | −173.05 |
| Model Jc | 52.43; 38; 0.06 | 0.013 | 1.000 | 0.999 | 0.999 | 0.997 | −247.14 |
*All P-values are two sided.
aAll key paths constrained.
bAll key paths constrained except latent fetal environment→CW.
cSame as base SEM, but with SES controls added (see Figure 2).
For model J we added SES measures to the base SEM. While model fit was poor, we also report its parameter estimates so they can be directly compared with those from the base SEM.
Key parameter estimates
Estimated paths, covariances and variances for the base SEM are given in Table 4. Those from model J are given in Table 5. Non-standardized path coefficients are interpreted as the unit change in the dependant variable associated with a one-unit increase in the predictor variable. Standardized coefficients represent the same relationship in units of standard deviation. Non-standardized coefficients should be used to compare estimates between the males and females, while the standardized results can be used to compare the relative size of estimated effects within gender.
Table 4.
Parameter estimates for the base model using birth cohort data from the CLHNS (n = 2653)
| 1218 females |
1435 males |
||||
|---|---|---|---|---|---|
| Estimatea | Standardized | Estimatea | Standardized | ||
| Paths | |||||
| a | LFE→SBP (mmHg/cm)b | −0.29 − 0.18 −0.07 | −0.28 | −0.43 − 0.26 −0.10 | −0.30 |
| b | LFE→BW (kg/cm) | 0.009 0.013 0.017 | 0.54 | 0.010 0.014 0.018 | 0.44 |
| c | LFE→CW (kg/cm) | 0.18 0.28 0.38 | 0.57 | 0.46 0.57 0.69 | 0.74 |
| d | CW→SBP (mmHg/kg) | 0.51 0.68 0.85 | 0.53 | 0.41 0.62 0.84 | 0.48 |
| f | AFA→LFE (cm/cm2) | 0.55 1.00 1.46 | 0.34 | 0.48 0.71 0.93 | 0.32 |
| HT→LFE (cm/cm)c | 1 | 0.30 | 1 | 0.41 | |
| SBP→SBP1 (mmHg/mmHg)c | 1 | 0.99 | 1 | 0.99 | |
| SBP→SBP2 (mmHg/mmHg) | 0.99 1.00 1.02 | 0.99 | 1.00 1.01 1.03 | 0.99 | |
| SBP→SBP3 (mmHg/mmHg) | 0.97 0.99 1.00 | 0.99 | 0.99 1.00 1.02 | 0.99 | |
| Covariances | |||||
| e | z4↔z3 | −0.72 − 0.15 0.42 | −0.07 | −1.02 − 0.48 0.06 | −0.23 |
| g | AFA↔HT | 4.03 5.68 7.33 | 0.22 | 2.13 3.63 5.13 | 0.14 |
| Variances | |||||
| AFA (cm2) | 26.59 29.32 31.87 | 26.56 28.99 31.40 | |||
| HT (cm) | 21.77 23.75 25.74 | 22.05 23.89 25.73 | |||
| z1 (LFE), (cm) | 9.08 191.51 4.63 | 25.45 98.30 171.15 | |||
| z2 (SBP), (mmHg) | 74.59 84.77 94.96 | 84.99 95.26 105.52 | |||
| z3 (CW), (kg) | 27.87 43.09 58.32 | 15.40 38.69 62.00 | |||
| z4 (BW) (kg) | 0.08 0.10 0.13 | 0.10 0.12 0.14 | |||
| e1 (SBP1), (mmHg) | 1.63 1.97 2.31 | 2.26 2.62 2.98 | |||
| e2 (SBP2), (mmHg) | 2.50 2.91 3.32 | 2.25 2.62 2.99 | |||
| e3 (SBP3), (mmHg) | 1.51 1.84 2.17 | 1.59 1.92 2.24 | |||
| e4 (CW), (kg)c | 3.35 | 4.47 | |||
| e5 (BW), (kg)c | 0.016 | 0.016 | |||
| e6 (HT), (cm)c | 1.25 | 1.25 | |||
| e7 (AFA), (cm2)c | 3.97 | 3.97 | |||
aDisplayed with 95% confidence interval as LOWER LIMIT Estimate UPPER LIMIT.
bFetal environment is scaled to maternal height, thus its unit is in centimeters.
cValue set a priori.
Table 5.
Parameter estimates for model J using birth cohort data from the CLHNS (n = 2653)
| 1218 females |
1435 males |
||||
|---|---|---|---|---|---|
| Estimatea | Standardized | Estimatea | Standardized | ||
| Paths | |||||
| a | LFE→SBP (mmHg/cm)b | −0.29 − 0.17 −0.06 | −0.27 | −0.39 − 0.23 −0.08 | −0.27 |
| b | LFE→BW (kg/cm) | 0.009 0.013 0.017 | 0.55 | 0.010 0.014 0.018 | 0.46 |
| c | LFE→CW (kg/cm) | 0.19 0.29 0.40 | 0.58 | 0.40 0.51 0.62 | 0.68 |
| d | CW→SBP (mmHg/kg) | 0.51 0.68 0.86 | 0.53 | 0.41 0.61 0.80 | 0.53 |
| f | AFA→LFE (cm/cm2) | 0.57 1.02 1.46 | 0.35 | 0.42 0.65 0.89 | 0.29 |
| HT→LFE (cm/cm)c | 1 | 0.31 | 1 | 0.40 | |
| SES83→LFE (cm/ses)d | −1.01 − 0.30 0.41 | −0.02 | −0.52 − 0.03 0.46 | −0.001 | |
| SES83→SES05 (ses/ses)d | 0.54 0.62 0.70 | 0.49 | 0.59 0.66 0.73 | 0.52 | |
| SES05→SBP (mmHg/ses)d | −0.38 − 0.11 0.16 | −0.03 | −0.50 − 0.22 0.07 | −0.06 | |
| SES05→CW (kg/ses)d | −0.28 − 0.06 0.16 | −0.02 | 0.49 0.71 0.94 | 0.21 | |
| SBP→SBP1 (mmHg/mmHg)c | 1 | 0.99 | 1 | 0.99 | |
| SBP→SBP2 (mmHg/mmHg) | 0.99 1.00 1.02 | 0.99 | 1.00 1.01 1.02 | 0.99 | |
| SBP→SBP3 (mmHg/mmHg) | 0.97 0.99 1.00 | 0.99 | 0.99 1.00 1.02 | 0.99 | |
| Covariances | |||||
| e | z4↔z3 | −0.79 − 0.18 0.42 | −0.09 | −1.06 − 0.47 0.11 | −0.22 |
| SES↔HT | 1.27 1.86 2.45 | 0.18 | 1.25 1.81 2.37 | 0.18 | |
| AFA↔SES | 2.73 3.43 4.13 | 0.31 | 2.95 3.61 4.27 | 0.32 | |
| g | AFA↔HT | 4.03 5.68 7.33 | 0.22 | 2.13 3.63 5.13 | 0.14 |
| Variances | |||||
| AFA (cm2) | 26.79 29.23 31.67 | 26.57 28.99 31.40 | |||
| HT (cm) | 21.77 23.75 25.74 | 22.05 23.89 25.73 | |||
| Baseline SESd | 3.97 4.31 4.65 | 4.18 4.51 4.84 | |||
| z1 (LFE), (cm) | 7.69 190.63 373.58 | 20.59 106.22 191.85 | |||
| z2 (SBP), (mmHg) | 74.85 84.98 95.11 | 85.34 95.52 105.70 | |||
| z3 (CW), (kg) | 26.08 42.13 58.18 | 17.58 39.88 62.18 | |||
| z4 (BW), (kg) | 0.07 0.10 0.13 | 0.10 0.12 0.14 | |||
| e1 (SBP1), (mmHg) | 1.63 1.97 2.31 | 2.26 2.62 2.98 | |||
| e2 (SBP2), (mmHg) | 2.50 2.91 3.32 | 2.25 2.62 2.99 | |||
| e3 (SBP3), (mmHg) | 1.51 1.84 2.17 | 1.59 1.92 2.24 | |||
| e4 (CW), (kg)c | 3.35 | 4.47 | |||
| e5 (BW), (kg)c | 0.016 | 0.016 | |||
| e6 (HT), (cm)c | 1.25 | 1.25 | |||
| e7 (AFA), (cm2)c | 3.97 | 3.97 | |||
| e8 (2005 SES)d | 4.82 5.36 5.90 | 4.84 5.33 5.82 | |||
aDisplayed with 95% confidence interval as LOWER LIMIT Estimate UPPER LIMIT.
bFetal environment is scaled to maternal height, thus its unit is in centimetres.
cValue set a priori.
dSES units derived from principle components analysis.
Base model
Because LFE is scaled to maternal height, its metric is in centimetres. The path from LFE to SPB was inverse (males: −0.43 −0.26 −0.10 mmHg/cm; females: −0.29 −0.18 −0.07 mmHg/cm). The other path coefficients are also in line with our expectations. LFE was a positive predictor of BW (males: 0.010 0.014 0.018 kg/cm; females: 0.009 0.013 0.017 kg/cm) and CW (males: 0.46 0.57 0.69 kg/cm; females: 0.18 0.28 0.38 kg/cm). CW was a positive predictor of SBP (males: 0.41 0.62 0.84 mmHg/kg; females: 0.51 0.68 0.85 mmHg/kg).
Model J
Model J is equivalent to the base SEM, but with the previously described SES measures added. 2005 SES was included as a determinant of SBP and CW, while baseline SES was added as a determinant of LFE and 2005 SES. Parameter estimates present in both models I and J were virtually identical (see Tables 4 and 5). Baseline SES was related to maternal height (covariance: 1.17 1.74 2.31; correlation: 0.17) and AFA (covariance: 0.31 0.39 0.47; correlation: 0.28), was not a predictor of LFE in either gender (males: −0.53 −0.01 0.51 cm/ses; females: −0.76 −0.11 0.53 cm/ses), but was a positive predictor of 2005 SES (males: 0.59 0.66 0.73 ses/ses; females: 0.54 0.62 0.70 ses/ses). 2005 SES was not a predictor of SBP in either gender (males: −0.44 0.19 0.07 mmHg/ses; females: −0.37 −0.11 −0.16 mmHg/ses). 2005 SES was a positive predictor of CW in males (0.50 0.72 0.95 kg/ses) but not in females (−0.29 0.06 0.16 kg/ses).
OLS regression
Using the list-wise deleted sample with complete data (n = 1597), OLS regression estimates of the unadjusted relationship between BW and SBP were 2.19 −0.33 −0.73 mmHg/kg for females and −2.53 −0.73 1.06 mmHg/kg for males. Adjustment for CW shifted these coefficients further from null (females: −4.47 −2.69 −0.91 mmHg/kg; males: −3.92 −2.20 −0.47 mmHg/kg). These results are similar in direction and magnitude to those commonly seen in the literature.1,2,8,44 We also tested the same regression model as a SEM, using FIML estimation and the full sample with missing data; the results were nearly identical.
Discussion
We sought to explain the observed variances and covariances of a subset of maternal and offspring variables collected for the CLHNS. These variables were maternal height and AFA, and offspring BW, CW and SBP. To explain how these variables are related, we used SEM to impose a hypothetical structure on their relationships. Our hypothesis is based on the DOHaD paradigm, which generally posits that the fetal environment can have long-term effects on disease risk. Specifically, we hypothesized that maternal height and AFA were determinants of an unobserved latent variable that was directly related to offspring BW and CW, and inversely related to offspring SBP. The results from our analysis failed to reject this hypothesis. While better fitting models can and often do exist,45 overall model fit was excellent, indicating that our theoretical model adequately explained the observed relationships among the model's observed variables. Based on the similarity between our reported OLS regression results and those commonly seen in the literature, we conclude that these results are not likely due to sample idiosyncrasies. However, the disturbance variances for SBP in both males and females (95.26 and 84.77 mmHg, respectively) are still fairly large, indicating that a large proportion of the observed variance in SBP is still left unexplained by the model.
Additionally, we utilized a multi-group analysis to test for differences in parameter estimates by gender, finding that males and females similarly experienced most key relationships with the exception of that of LFE and CW, which was stronger in the males. A model which also included measures of SES to control potential confounding had poor fit relative to the base model, and did not result in any substantial changes in the estimated parameters. Lastly, only trivial differences in model fit and parameter estimates were found in unreported sensitivity analyses that used a range of different a priori error variances for BW, CW, maternal height and AFA; that excluded potential outliers; that used more normal transformations of CW and AFA; that controlled the gestational week of maternal measurement and the age of the offspring; and that used a list-wise deleted sample with no missing data.
However, regardless of how well the model fit the data, or how robust it was to the sensitivity analyses, the crux of this analysis is interpreting the variable labelled LFE. We have labelled the variable as such because it is in line with our hypothesis, but we must consider the possibility that this latent variable is not what we think it is. The most likely alternative interpretation is that the LFE variable represents aspects of SES that we could reasonably expect to be associated with ‘healthier’ outcomes like lower blood pressure and larger birth-size. This is partly our rationale for testing the model that included SES measures, though we cannot exclude the possibility that our SES measures are inadequate. However, the prevalence of hypertension (>140 mmHg SBP) is only 6% in this sample and it is unlikely that individuals in this sample are modifying their behaviours due to a perceived health problem; no individual in this study is being treated for hypertension. Furthermore, western, atherogenic diets tend to be associated with affluence in this context and thus SES seems more likely to be positively associated with hypertension in this sample. These points are consistent with our analysis and make an SES interpretation seem less appropriate.
In the introduction we noted three reasons why OLS regression coefficients estimating the ‘effect’ of birth-size on SBP shift inversely away from the null once current-size is controlled. We now return to these three points in the context of the tested SEM. First, because we have included an indirect path between SBP, via CW, we have appropriately controlled the potentially suppressive path that could otherwise obscure any inverse relationship between LFE and SBP (reason 1). Second, because a one-unit change in LFE, holding CW constant, does not imply that the individual grew any more or less, the path from LFE to SBP is free of any growth interpretation (reason 2).
However, the SEM is limited in that if CW is a mediator of the relationship between LFE and SBP, and it shares unmeasured determinants with SBP then bias can still occur. As with any statistical model, the estimates given by this SEM are only unbiased to the degree that the model is properly specified (reason 3). Most researchers understand that the estimated effect of LFE on SBP will be biased if they share a confounder that is not controlled. Less well understood is that the estimated relationship between LFE and SBP can also be biased if there is an unaccounted for confounder between SBP and CW.21,22 To account for this possibility, we tested an SEM that included baseline SES as a confounder of the LFE–SBP relationship and 2005 SES as a confounder of the CW–SBP relationship. As noted above, this did not result in any change to the parameters of interest, though it is of course impossible to empirically disprove the presence of residual confounding.
While the tested SEM is a step in the right direction, there are several important improvements we intend to implement in subsequent analyses. Treating the SES variables derived from PCA as measured variables is not ideal. However, SEM is particularly well suited to account for latent constructs such as SES46—which we intend to treat more appropriately in future models. We will also decompose weight into multiple dimensions of body size such as adiposity and height, and use latent growth curves47 to account for the potential effects of postnatal growth. Lastly, future analyses will include multiple hypothesized dimensions of fetal environment, including maternal diet, age and parity. We look forward to hearing ideas from other researchers in this field on how to further improve this analysis.
While the DOHaD paradigm has rapidly grown, both in terms of its scope and acceptance among public health researchers and practitioners, epidemiological methods to test developmental hypotheses have not kept pace. While elegant studies of animals have suggested biological mechanisms that may explain relationships between fetal environment and health, observational studies in humans are still overly focused on birth-size ‘effects’. While we are limited by the observational nature of our data, we are not maximizing its potential to test developmental hypotheses. Through the use of prospective birth cohort studies with detailed data on the mother and offspring, and statistical methods such as SEM [recently highlighted as a useful method in epidemiology and life-course research (e.g. references 22,48,49–53)], we strongly believe that DOHaD researchers will be able to meet this challenge.
Funding
The National Institutes of Health (NICHD 5-R01-HD38700); the Fogarty International Center (5-R01-TW05596); the National Science Foundation (NSF SES 0617276); Carolina Population Center predoctoral traineeship (T32-HD07168 to D.D.).
Acknowledgements
The authors would like to thank the following people for their contributions: Daniel Adkins and Dr William Ware for advice given during the formative stages of the reported research; Drs Jay Kaufman and Mark Gilthorpe for their helpful comments on a draft of the paper; and the Office of Population Studies at the University of San Carlos for their collaboration in survey design and data collection.
Conflict of interest: None declared.
References
- 1.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 2.Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annu Rev Nutr. 2005;25:407–34. doi: 10.1146/annurev.nutr.25.050304.092538. [DOI] [PubMed] [Google Scholar]
- 3.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–41. [PubMed] [Google Scholar]
- 4.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 5.McDade TW, Adair LS. Defining the “urban” in urbanization and health: a factor analysis approach. Soc Sci Med. 2001;53:55–70. doi: 10.1016/s0277-9536(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW. Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol. 2002;31:294–99. [PubMed] [Google Scholar]
- 8.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–65. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 9.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. BMJ. 1999;319:245–49. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu YK, Gilthorpe MS. Revisiting the relation between change and initial value: a review and evaluation. Stat Med. 2007;26:443–57. doi: 10.1002/sim.2538. [DOI] [PubMed] [Google Scholar]
- 11.Tu YK, Gilthorpe MS, Ellison GT. What is the effect of adjusting for more than one measure of current body size on the relation between birthweight and blood pressure? J Hum Hypertens. 2006;20:646–57. doi: 10.1038/sj.jhh.1002044. [DOI] [PubMed] [Google Scholar]
- 12.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1:173–81. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu YK, Ellison GTH, Gilthorpe MS. Growth, current size and the role of the ‘reversal paradox’ in the foetal origins of adult disease: an illustration using vector geometry. Epidemiologic Perspectives & Innovations. 2006;3:9. doi: 10.1186/1742-5573-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41:451–56. doi: 10.1161/01.HYP.0000054212.23528.B2. [DOI] [PubMed] [Google Scholar]
- 15.Robinson SM, Barker DJ. Coronary heart disease: a disorder of growth. Proc Nutr Soc. 2002;61:537–42. doi: 10.1079/pns2002189. [DOI] [PubMed] [Google Scholar]
- 16.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 17.Remacle C, Bieswal F, Reusens B. Programming of obesity and cardiovascular disease. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S46–53. doi: 10.1038/sj.ijo.0802800. [DOI] [PubMed] [Google Scholar]
- 18.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 19.Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WP. Fetal programming of appetite and obesity. Mol Cell Endocrinol. 2001;185:73–79. doi: 10.1016/s0303-7207(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 20.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–77. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 21.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S, Brumback B. An overview of relations among causal modelling methods. IEA; 2002. pp. 1030–37. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg CR. Invited commentary: Barker meets Simpson. Am J Epidemiol. 2005;161:33–35. doi: 10.1093/aje/kwi003. ; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 24.Bollen KA. Structural Equations with Latent Variables. New York: Wiley; 1989. [Google Scholar]
- 25.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71(Suppl 5):1344S–52S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 26.Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–13. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 27.Adair LS, Popkin BM. Birth weight, maturity and proportionality in Filipino infants. Hum Biol. 1988;60:319–39. [PubMed] [Google Scholar]
- 28.Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. Am J Clin Nutr. 2003;77:960–66. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- 29.King JC, Bronstein MN, Fitch WL, Weininger J. Nutrient utilization during pregnancy. World Rev Nutr Diet. 1987;52:71–142. doi: 10.1159/000415196. [DOI] [PubMed] [Google Scholar]
- 30.Hediger ML, Scholl TO, Schall JI, Healey MF, Fischer RL. Changes in maternal upper arm fat stores are predictors of variation in infant birth weight. J Nutr. 1994;124:24. doi: 10.1093/jn/124.1.24. [DOI] [PubMed] [Google Scholar]
- 31.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, et al. Fetal, infant, and childhood growth and adult blood pressure a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–92. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 32.Enders C, Bandalos D. The relative performance of full information maximum likelihood estimation of missing data in structural equations models. Structural Equations Modeling. 2001;8:430–57. [Google Scholar]
- 33.Potthoff RF, Tudor GE, Pieper KS, Hasselblad V. Can one assess whether missing data are missing at random in medical studies? Stat Methods Med Res. 2006;15:213. doi: 10.1191/0962280206sm448oa. [DOI] [PubMed] [Google Scholar]
- 34.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95(5 Pt 1):769–74. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 35.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bollen KA. Multiple indicators: Internal consistency or no necessary relationship? Quality and Quantity. 1984;18:377–85. [Google Scholar]
- 37.Bollen K, Lennox R. Conventional wisdom on measurement: a structural equation perspective. Psycholl Bull. 1991;110:305–14. [Google Scholar]
- 38.Alsop-Shields L, Alexander H. A study of errors that can occur when weighing infants. J Adv Nursing. 1997;25:587–94. doi: 10.1046/j.1365-2648.1997.1997025587.x. [DOI] [PubMed] [Google Scholar]
- 39.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. British. J Nutr. 2007;82:165–77. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 40.Lawlor DA, Ebrahim S, Davey Smith G. Is there a sex difference in the association between birth weight and systolic blood pressure in later life? Findings from a meta-regression analysis. Am J Epidemiol. 2002;156:1100–4. doi: 10.1093/aje/kwf154. [DOI] [PubMed] [Google Scholar]
- 41.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2008 doi: 10.1093/biostatistics/kxn014. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulaik S. There is a place for approximate fit in structural equation modelling. Personality and Individual Differences. 2007;42:883–91. [Google Scholar]
- 43.Barrett P. Structural equation modelling: adjudging model fit. Personality and Individual Differences. 2007;42:815–24. [Google Scholar]
- 44.Schluchter MD. Publication bias and heterogeneity in the relationship between systolic blood pressure, birth weight, and catch-up growth—a meta analysis. J Hypertens. 2003;21:273–79. doi: 10.1097/00004872-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 45.MacCallum RC, Wegener DT, Uchino BN, Fabrigar LR. The problem of equivalent models in applications of covariance structure analysis. Psychol Bull. 1993;114:185–99. doi: 10.1037/0033-2909.114.1.185. [DOI] [PubMed] [Google Scholar]
- 46.Bollen KA, Glanville JL, Stecklov G. Socioeconomic status and class in studies of fertility and health in developing countries. Annu Rev Sociol. 2001;27:153–85. [Google Scholar]
- 47.Bollen KA, Curran PJ. Latent Curve Models: A Structural Equation Perspective. Hoboken, NJ: Wiley-Interscience; 2006. [Google Scholar]
- 48.De Stavola BL, Nitsch D, dos Santos Silva I, et al. Statistical Issues in Life Course Epidemiology. American Journal of Epidemiology. 2006;163:84–96. doi: 10.1093/aje/kwj003. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. IEA; 2002. pp. 285–93. [PubMed] [Google Scholar]
- 50.Singh-Manoux A, Clarke P, Marmot M. Multiple measures of socio-economic position and psychosocial health: proximal and distal measures. IEA; 2002. pp. 1192–99. [DOI] [PubMed] [Google Scholar]
- 51.Maldonado G, Greenland S. Estimating causal effects. IEA; 2002. pp. 422–29. [PubMed] [Google Scholar]
- 52.Der G. Commentary: structural equation modelling in epidemiology: some problems and prospects. IEA; 2002. pp. 1199–200. [Google Scholar]
- 53.Cheung YB. Commentary: fetal origins of social situations? Medicalization of social life?. IEA; 2004. pp. 856–57. [DOI] [PubMed] [Google Scholar]