Abstract
Aims
To develop tables that report the life expectancy associated with levels of major modifiable risk factors for patients with type 2 diabetes.
Methods and results
A set of tables reporting life-expectancy stratified by age–sex groups for combinations of modifiable risk was constructed based on predictions from the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model. This model is based on a system of parametric proportional hazards risk equations for estimating mortality and vascular complications of diabetes that have been estimated from 3642 patients from the UKPDS. The tables show substantial potential gains in life expectancy within every age group from modifying major risk factors. The estimated life expectancy of men at age of 55 years with type 2 diabetes, 5 years after diagnosis, varies between 13.2 years for a patient who smokes, has systolic blood pressure of 180 mmHg, a total:HDL cholesterol ratio of 8, and HbA1c of 10%, and 21.1 years for a non-smoker with SBP of 120 mmHg, total/HDL ratio of 4, and HbA1c of 6%.
Conclusion
Life expectancy tables provide a potentially useful tool of conveying prognostic information to people with type 2 diabetes and suggest substantial scope for increasing longevity by improving modifiable risk factors.
Keywords: Risk factors, Cardiovascular disease, Type 2 diabetes, Life expectancy, Mathematical modelling and simulation, Education
Introduction
The increased risk of cardiovascular disease among people with type 2 diabetes is well understood and is increasingly recognized as one of the major hazards of type 2 diabetes. Cardiovascular disease is responsible for ∼70% of all mortality among patients with type 2 diabetes1 and is also a major contributor to diabetes-related healthcare costs.2
Adherence to therapies intended to control risk factors such as lipids or blood pressure for patients with type 2 diabetes has been shown to both reduce major cardiovascular complications and increase survival.3,4 One potential way to improve patients’ metabolic control is to help them understand the risks of the disease and the potential benefits of available therapy options.5 Research has shown that information on the potential benefits of improving modifiable risk factors may assist both doctors and patients in making treatment decisions6 and may increase patients’ willingness to accept management strategies recommended by their doctor.7,8 In fact, risk communication in its variety of formats (e.g. decision aids, leaflets, verbal advice, tables) increases patients’ knowledge, comprehension, and understanding.9
Information on potential benefits from better management of diabetes can be provided in many different ways. For example, typically results from trials are reported in terms of relative risk or absolute risk of events, or the numbers needed to treat to avoid events. Available cardiovascular information tools and guidelines usually report absolute risk of events, that is, the probability of an event occurring in a defined population over a specified period of time. However, these tools have limited use in a diabetic population as they either do not include diabetes as a risk factor (e.g. European-based Systematic Coronary Risk Evaluation (SCORE) risk charts10) or they are based on cohort studies that contain fewer diabetic subjects than most robust epidemiological studies require (e.g. Framingham risk charts). In fact, the Framingham risk charts, based on only 337 people with diabetes and currently used in the United States, Australia, and New Zealand,11 have been shown to underestimate the coronary heart disease (CHD) risk for people with diabetes.12–14 Furthermore, the Framingham study does not include risk factors such as HbA1c which are relevant to assessing outcomes of the diabetic population.
Another metric for representing the potential benefits from better management of diabetes is life expectancy, which has been suggested as a valuable comparative measure of benefits from medical care and public health interventions.15 In this study, we estimate life-expectancy stratified by common risk factors obtained from a simulation model designed specifically for patients with type 2 diabetes. We demonstrate that remaining life expectancy can be displayed for a wide range of different patient characteristics using relatively simple tables. We also show how such tables can be used to calculate the potential benefits of modifying diabetes-related risk factors.
Methods
United Kingdom Prospective Diabetes Study outcomes model
The United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model is a computer simulation model for forecasting the likely first occurrence of major diabetes-related complications and death in patients with diagnosed type 2 diabetes; a full description of the model has been published elsewhere.16 The UKPDS model is as a probabilistic discrete-time model with annual cycles based on a system of parametric proportional hazards risk equations that have been estimated from 3642 patients participating in the UKPDS.16 A summary of the characteristics of the UKPDS patients based on previously published information17,18 is shown in Table 1.
Table 1.
Baseline characteristics of patients used to estimate the UKPDS Outcomes Model
| Characteristics | Observational data (n = 3642) |
|---|---|
| Age (years) | 53 (8) |
| Proportion of men (%) | 60 (0.8) |
| HBA1c (%) | 7.1 (1.8) |
| Systolic blood pressure (mmHg) | 135 (19) |
| Low-density lipoprotein cholesterol (mmol/L) | 3.5 (1.0) |
| High-density lipoprotein cholesterol (mmol/L) | 1.06 (0.24) |
| Smokers (%)a | 31 (0.7) |
| Body mass index (kg/m2) | 27.7 (5.3) |
To undertake simulations the estimated set of equations are used to predict the absolute risk of first occurrence of different complications and death given the patient’s characteristics (such as age, ethnicity, sex and duration of diabetes) and time-varying risk factors (such as systolic blood pressure (SBP), HbA1c, lipid levels, smoking status, and history of previous complications). The model includes both macro-vascular (fatal and non-fatal myocardial infarction, ischaemic heart disease, renal failure, congestive heart failure, and fatal and non-fatal stroke) and micro-vascular complications, such as blindness, in order to estimate the total impact of the disease on morbidity and mortality. Simulated patients start with a given health status and can experience one or more non-fatal complications or die during any of the annual cycles as the simulation progresses. Holding everything else constant, the higher the values of risk factors such as systolic blood pressure the higher the absolute risk of a complication. The risk of many types of events also increases with the duration of diagnosed diabetes. If a non-fatal event occurs the simulated patient carries forward to the next cycle that history together with the updated risk factor values. The simulation continues until death or the end of the simulation period. The model can quantify outcomes in terms of risk, life expectancy, or quality-adjusted life expectancy. Estimates of the relative risk, holding everything else constant, for different macro-vascular complications and death associated with various risk factors are summarized in Table 2.
Table 2.
Increase/decrease in relative riska, holding everything else constant, for different types of macro-vascular events and death from a change in risk factors levels
| Risk factor (units) | Myocardial infarction, % | Other IHD, % | Congestive heart failure, % | Stroke, % | Event fatality, % | Diabetes mortality, % | Other mortality, % |
|---|---|---|---|---|---|---|---|
| HbA1c (1% increase) | 13 (7–18) | 13 (6–21) | 17 (5–31) | 14 (5–23) | 12 (1–24) | — | — |
| Systolic blood pressure (10 mmHg increase) | 11 (5–16) | 10 (3–19) | 12 (0.4–25) | 32 (21–43) | — | — | — |
| Total:HDL cholesterol (1 unit increase) | 24b (0–55) | 31b (0–74) | — | 12 (7–18) | — | — | 12 (2–22) |
| Smoking | 41 (17–71) | — | — | 43 (0.4–103) | — | — | 36 (3–79) |
| Body mass index (1 kg/m2 increase) | — | — | 7 (3–10) | — | — | — | — |
| Afro-Caribbean compared with Caucasian/Asian Indian | −73 (−86 to −47) | — | — | — | — | — | — |
Figures are means (95% confidence intervals).
aCalculated using hazard ratios from UKPDS 6816 where a positive(negative) value means increase(decrease) in relative risk.
bEstimated from the UKPDS outcomes model using a patient aged 55 years with the average risk factor levels of the UKPDS population.17
The UKPDS Outcomes Model’s forecasts have been shown to closely match the observed occurrence of all-cause mortality during the UKPDS follow-up period,16 as well as diabetes-related complications in observational studies and the impact of interventions assessed through randomized controlled trials.19,20 For example, the UKPDS model predicted the 4-year total event rate of acute coronary events reported in the CARDS trial.19 Also, the UKPDS model estimated a relative risk of 0.87 at 3 years with respect to a composite of all-cause mortality, non-fatal myocardial infarction, and stroke, well within the 95% CI (0.72–0.98) seen in the PROactive study.20
Outcome tables for major risk factors for diabetes
For illustrative purposes, the Outcomes Model was used to predict the life expectancy of patients with type 2 diabetes, 5 years after diagnosis, stratified into the following risk groups: age (55, 65, and 75 years old), sex (male, female), systolic blood pressure (120, 140, 160, and 180 mmHg), HbA1c (6, 8, and 10%), ratio of total:HDL cholesterol (4–8), and smoking status (never smoked, current smoker). The chosen age groups, smoking status, and the range of values for SBP and ratio of total:HDL cholesterol were similar to those reported in previous cardiovascular risk prediction charts.10,21 The HBA1c values were selected to capture a large proportion of people with type 2 diabetes.22 The number of years since diagnosis was similar to the average duration reported in recent large-scale type 2 diabetes studies.22–24 To demonstrate the variation in life expectancy due to duration of diabetes, we also report the life expectancy of 65-year-old male and female patients at 1, 3, and 7 years after diagnosis (see Supplementary material online). The modifiable risk factors were assumed to remain constant over time as is common practice in published 5 and 10 year absolute risk tables.10,13,21 For each of the risk groups, the model simulated the future life course of a hypothetical person with type 2 diabetes, diagnosed 5 years previously, without prior history of diabetes-related complications, and with a body mass index (BMI) of 30 kg/m2 (men) or 33 kg/m2 (women). Although the reduction of obesity is a major therapeutic aim in type 2 diabetes, other modifiable risk factors such as HBA1c, SBP, and cholesterol have a more significant impact on diabetes-related complications than BMI (Table 2). As a result, we used levels of BMI that are representative of the average levels for non-insulin treated patients with type 2 diabetes in the community.25 As the model is probabilistic, it was run 10 000 times to obtain stable point-estimates; the model can also calculate confidence intervals around these estimates (see Supplementary material online). As the tables are primarily for clinical use no discount rate has been applied to the estimated life expectancy.
Results
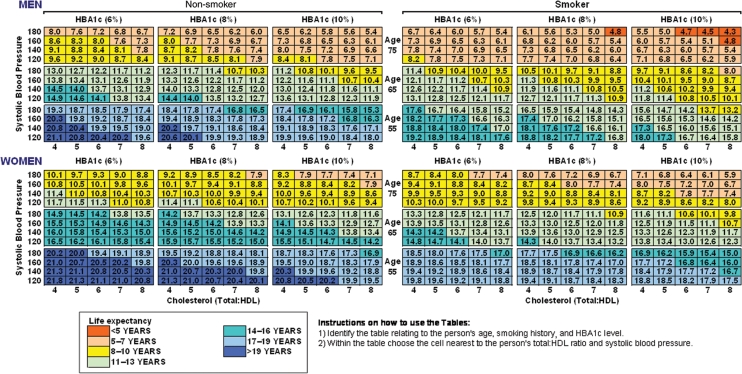
The life-expectancy estimates for men and women are reported in Figure 1, which demonstrates that there is a substantial gradient in survival across risk factors. For example, the estimated age-specific life expectancy of men with type 2 diabetes varies between 13.2 (CI 95%: 11.3–15.2) and 21.1 (CI 95%: 18.1–23.5) years at age 55 for patients in the highest to lowest risk groups. The comparative range of life expectancy is between 8.0 (CI 95%: 6.4–8.9) and 14.9 (CI 95%: 13.4–16.7) years at age 65, and between 4.3 (CI 95%: 3.4–5.4) and 9.6 (CI 95%: 8.0–11.2) years at age 75, for different combinations of risk factors. This compares with the life expectancy of the UK general male population of 24.7, 16.6, and 10.0 years at ages 55, 65, and 75 years, respectively (Government Actuary Department). In terms of duration of diabetes, life expectancy in years for men aged 65 in the highest to lowest risk groups varies between 10.0 and 18.1 at 1 year post-diagnosis, 8.9 and 16.5 at 3 years post–diagnosis, and 7.1 and 13.4 at 7 years post-diagnosis (see Supplementary material online).
Figure 1.
Assessment of life expectancy in men and women with Type 2 diabetes. These men and women were assumed to have no previous diabetes-related complications, diagnosed with the disease 5 years previously, and a body mass index of 30 and 33 kg/m2, respectively.
These tables can also be used to calculate the potential gains from altering one or more modifiable risk factors through primary and secondary prevention, by estimating the years lost relative to the lowest risk type within each age–sex group. For example, for a 65-year-old male smoker (SBP 180 mmHg, HBA1c 8%, total:HDL cholesterol 7), holding all else constant, a reduction of HBA1c from 8 to 6% leads to a gain of 0.9 life years (LYs), a reduction of total:HDL cholesterol from 7 to 4 gains 1.5 LYs, a reduction of SBP from 180 to 120 mmHg results in a gain of 2.2 LYs, and stopping smoking will result in a gain 1.6 LYs. The impact of interventions affecting several risk factors can also be estimated. For example, a reduction of both HBA1c from 8 to 6% and total:HDL cholesterol from 7 to 4 due to a hypothetical intervention, increases life expectancy by 2.3 LYs. Finally, if all the combined risk factors are successfully reduced, life expectancy will increase by 5.8 LYs.
Discussion
Research has shown that diabetic patients have a shorter life expectancy than non-diabetic individuals and that this excess mortality is largely attributable to cardiovascular causes.26–28 Our results show that there is substantial variation in life expectancy among people with diabetes depending on their levels of commonly measured risk factors, such as HbA1c as well as duration of diabetes across the different age groups.
The results presented here for life-expectancy are in a format similar to the cardiovascular risk tables that are widely available, where various combinations of columns and rows allow a specific absolute risk to be read from the table.10,13,21 Such tables can provide important information on a patient’s prognosis as well as the potential health gains that could be achieved if common risk factors were to be reduced. In effect, our results confirm that there is substantial scope for increasing a patient’s longevity by improving modifiable risk factors. It would be possible to generate and present additional tables for other ranges and gradations of the risk factors as well as different duration of diabetes. An alternative approach would be to design a computer-based tool enabling the production of user-defined tables. These could be tailored to the patient’s current risk factors and inform on a range of potential life-expectancy gains achievable by improving metabolic control.
Practice guidelines based on absolute risks provide clear quantitative information to clinicians and patients but they do not convey the length of life lost due to premature death. There is strong evidence that the way information is provided has important implications in terms of the patient’s willingness to accept a treatment recommendation. For example, spending extra time with a patient explaining chronic conditions such as diabetes and available treatments led to improved self-management and a significant increase on the patients’ confidence and expectation to follow the chosen treatments.6,8 However, there is comparatively little evidence on the most appropriate methods of informing individuals about their health risks.5,9,29 Overall, more research is needed on which formats to use (e.g. decision aids, tables, video) and on ways to communicate risk to patients, which are easy to comprehend. Here, we have developed information on life expectancy as a way of supplementing the existing information on risks in diabetes. This may be helpful as there is evidence that information about the postponement of adverse health outcomes (e.g. heart attack, hip fracture) can be used to convey information about treatment effectiveness to lay people.30,31 In addition, helping patients understand their projected life expectancy may help them make better decisions about their future. This is particularly relevant as O’Brien et al.32 reported that the general population underestimated their projected life expectancy by over 5 years and that high-risk people (e.g. smokers) appeared to understate their own risk. We would envisage that the main users of the life-expectancy tables will be clinicians, patients, and other healthcare policy decision makers. The colour-coded tables provide a ready means of communicating prognostic information and may improve patient understanding of their prognosis and how it could be altered. However, it is important to recognize that such tables require interpretation and clinicians and patients may not be familiar with quantifying outcomes in terms of life expectancy. It would be useful to investigate the utility of information on life expectancy in a clinical setting, for example, in helping improve adherence to therapies by showing patients their potential long-term benefits. Further research on the benefit of life expectancy and other available cardiovascular risk tables as a decision tool is required, as well as on different ways of presenting information, such as the appropriate number and range of risk factor categories as well as the representation of uncertainty around the point estimates.
The life expectancy estimates were based on results from the UKPDS Outcome Model, a computer simulation model that was specifically designed for estimating outcomes for people with type 2 diabetes. The forecasts of this model have been shown to closely predict events in other diabetic populations such as patients participating in the Collaborative Atorvastatin Diabetes Study19 and in the PROactive study.20 Conversely, previous published risk charts, based on Framingham risk equations, appear to underestimate CHD risk for people with diabetes.12–14
Computer simulation models are increasingly used as a tool for evaluating therapies in diabetes, as they provide a way of translating changes in risk factors into long-term outcomes.19 It is important to recognize that the predicted outcomes from these models are based on risks that were estimated from studies that have long-term follow-up data on diabetic populations such as patients participating in the UKPDS study recruited between 1977 and 1982. However, some limitations should be noted concerning the UKPDS study. Patients with recent major cardiovascular events were excluded from the study17 resulting in a sample that differs at least in the first follow-up years from newly diagnosed patients. Nevertheless, the differences in outcomes between UKPDS and the general diabetic populations arising from the selection restriction are likely to be small as the model was based on up to 20 years of follow-up information on both risk factors and events. More importantly, outcomes may differ in other diabetic populations due to factors such as changes over time in healthcare practice and different competing risks between populations. For example, new treatments, such as statins, became common practice, as did other curative treatments, such as coronary surgery, which are credited with improvements in survival following diabetes-related events. It would therefore be useful to examine the degree to which the rates of mortality predicted by the model apply in other diabetic populations as this would assist in assessing whether the existing tables could be applied, or whether some form of modification (e.g. calibration of mortality risk) is required. Nevertheless, our estimates of the reduction in life expectancy are similar to those observed in long-term follow-up studies in other populations with diabetes. For example, in the UKPDS Outcomes Model, diabetic men aged 55 years were predicted to live 3.6–11.5 years less than the general population. British and US studies reported this gap to range on average between 5 and 9.27,28,33,34
In conclusion, the life expectancy tables presented here provide a ready means of conveying potentially useful prognostic information to people with type 2 diabetes. The variation in life expectancy suggests substantial scope for increasing longevity by improving modifiable risk factors.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The Health Economics Research Centre is grateful to the National Institute of Health Research for some of its funding. P.M.C.’s participation in this project is partly supported by a Diabetes Australia DART research grant and a National Health and Medical Research Council project grant (512463).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
Earlier versions of these tables and their rationale were presented at the 19th World Diabetes Congress in Cape Town in December 2006, and we are grateful to participants for constructive comments and suggestions.
References
- 1.Laakso M. Hyperglycemia and cardiovascular disease in Type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 2.Brown JB, Pedula KL, Bakst AW. The progressive cost of complications in type 2 diabetes mellitus. Arch Intern Med. 1999;159:1873–1880. doi: 10.1001/archinte.159.16.1873. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 5,963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. [Google Scholar]
- 4.ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 5.Epstein RM, Alper BS, Quill TE. Communicating evidence for participatory decision making. JAMA. 2004;291:2359–2366. doi: 10.1001/jama.291.19.2359. [DOI] [PubMed] [Google Scholar]
- 6.Edwards A, Elwyn G, Hood K, Atwell C, Robling M, Houston H, Kinnersley P, Russell I. Patient-based outcome results from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract. 2004;21:347–354. doi: 10.1093/fampra/cmh402. [DOI] [PubMed] [Google Scholar]
- 7.Halvorsen PA, Selmer R, Kristiansen IS. Different ways to describe the benefits of risk-reducing treatments: a randomized trial. Ann Intern Med. 2007;146:848–856. doi: 10.7326/0003-4819-146-12-200706190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17:243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevena LJ, Davey HM, Barratt A, Butow P, Caldwell P. A systematic review on communicating with patients about evidence. J Eval Clin Pract. 2006;12:13–23. doi: 10.1111/j.1365-2753.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 10.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, Backer GD, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoel H on behalf of the SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 11.Eichler K, Puhan MA, Steurer J, Bachmann LM. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J. 2007;153:722–731. doi: 10.1016/j.ahj.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Stevens RJ, Retnakaran R, Holman RR. Framingham, SCORE, and DECODE Risk Equations Do Not Provide Reliable Cardiovascular Risk Estimates in Type 2 Diabetes. Diabetes Care. 2007;30:1292–1294. doi: 10.2337/dc06-1358. [DOI] [PubMed] [Google Scholar]
- 13.Sheridan S, Pignone M, Mulrow C. Framingham-based tools to calculate the global risk of coronary heart disease: a systematic review of tools for clinicians. J Gen Intern Med. 2003;18:1039–1052. doi: 10.1111/j.1525-1497.2003.30107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo WW, Yeo KR. Predicting CHD risk in patients with diabetes mellitus. Diabet Med. 2001;18:341–344. doi: 10.1046/j.1464-5491.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 15.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions–standardizing data on outcomes. N Engl J Med. 1998;339:380–386. doi: 10.1056/NEJM199808063390606. [DOI] [PubMed] [Google Scholar]
- 16.Clarke PM, Gray AM, Briggs A, Farmer A, Fenn P, Stevens R, Matthews D, Stratton IM, Holman R. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;24:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 17.Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR on behalf of the UKPDS Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 19.The Mount Hood 4 Modeling Group. Computer modelling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care. 2007;30:1638–1646. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- 20.Holman RR, Retnakaran R, Farmer A, Stevens R. PROactive study. Lancet. 2006;367:25. doi: 10.1016/S0140-6736(06)67914-2. [DOI] [PubMed] [Google Scholar]
- 21.Jackson R. Updated New Zealand cardiovascular disease risk-benefit prediction guide. BMJ. 2000;320:709–710. doi: 10.1136/bmj.320.7236.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ADVANCE Collaborative Group. ADVANCE—Action in Diabetes and Vascular Disease: patient recruitment and characteristics of the study population at baseline. Diabet Med. 2005;22:882–888. doi: 10.1111/j.1464-5491.2005.01596.x. [DOI] [PubMed] [Google Scholar]
- 23.The FIELD Study Investigators. Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: baseline characteristics and short-term effects of fenofibrate. Cardiovasc Diabetol. 2005;4:13. doi: 10.1186/1475-2840-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomason MJ, Colhoun HM, Livingstone SJ, Mackness MI, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Fuller JH. Baseline characteristics in the Collaborative AtoRvastatin Diabetes Study (CARDS) in patients with Type 2 diabetes. Diabet Med. 2004;21:901–905. doi: 10.1111/j.1464-5491.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- 25.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dale AC, Nilsen TI, Vatten L, Midthjell K, Wiseth R. Diabetes mellitus and risk of fatal ischaemic heart disease by gender: 18 years follow up of 74 914 individuals in the HUNT 1 Study. Eur Heart J. 2007;28:2924–2929. doi: 10.1093/eurheartj/ehm447. [DOI] [PubMed] [Google Scholar]
- 27.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U. S. population, 1971–1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 28.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ. 2001;322:1389–1393. doi: 10.1136/bmj.322.7299.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 30.Dahl R, Gyrd-Hansen D, Kristiansen IV, Nexoe J, Nielsen JB. Can postponement of an adverse outcome be used to present risk reductions to a lay audience? A population survey. BMC Med Inform Decis Mak. 2007;7:8. doi: 10.1186/1472-6947-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen PM, Brosen K, Brixen K, Andersen M, Kristiansen IS. A randomized trial of laypersons’ perception of the benefit of osteoporosis therapy: Number needed to treat versus postponement of hip fracture. Clin Ther. 2003;25:2575–2585. doi: 10.1016/s0149-2918(03)80318-1. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien C, Fenn P, Diacon S. How long do people expect to live? Results and implications. CRIS Research Report 2005-1, March 2005. 13 March 2008 http://www.nottingham.ac.uk/business/cris/papers/crisresearchreport2005-1.pdf .
- 33.Narayan KMV, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 34.Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In: Harris MI, editor. Diabetes In America. 2nd ed. National Institutes of Health Publication No. 95–1468; 1995. pp. 233–258. http://www.niddk.nih.gov/health/diabetes/dia. (9 September 2008) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.