Abstract
Aims
The metabolic syndrome (MS) is associated with an increased cardiovascular risk. Patients with the MS have endothelial dysfunction, decreased circulating adiponectin, and a high expression of angiogenic inhibitors such as plasminogen activator inhibitor-1 (PAI-1). We hypothesized that such patients, in the event of a coronary occlusion, might exhibit a less developed collateral circulation.
Methods and results
Three hundred and eighty-seven consecutive patients with at least one coronary occlusion of a major coronary vessel at diagnostic angiography were prospectively enrolled. Collateral development was graded with validated angiographic methods. The MS was defined according to the ATP-III definition. Fasting glucose, adiponectin, insulin concentrations, and PAI-1 were measured at the time of angiography. MS was associated with less developed collateral vessels (P = 0.005). In multivariable analysis adjusting for potential confounding factors including the duration of coronary occlusion (P = 0.0001), fasting glycaemia (P = 0.0007), low adiponectin concentration (P = 0.01), insulin-resistance (HOMA-IR; P = 0.01), high circulating PAI-1 concentration (P = 0.01), and hypertension (P = 0.008) were independently associated with poor coronary collateral vessel development.
Conclusion
This study shows that in patients with coronary occlusion, collateral circulation is impaired in patients with the MS. This association is partly related to fasting glycaemia and to key parameters linked to insulin resistance.
Keywords: Angiogenesis, Collateral circulation, Coronary disease, Diabetes mellitus, Fibrinolysis, Insulin
Introduction
The metabolic syndrome (MS) is a cluster of risk factors such as elevated blood pressure, dyslipidaemia, and central obesity, associated with various biological manifestations, including hyperglycaemia and insulin resistance.1 Patients with the MS have an increased risk for cardiovascular morbidity and mortality.2
Development of coronary collateral circulation is an important adaptive mechanism occurring in patients with occlusive coronary artery disease. Well-developed coronary collaterals reduce the size of myocardial infarction, preserve left ventricular function and viability, and reduce the risk of death.3 Patients with the MS have endothelial dysfunction,4 decreased circulating adiponectin, and heightened expression of plasminogen activator inhibitor-1 (PAI-1), which may negatively influence vessels remodelling and growth.5,6 However, whether these patients have a maladaptive response to coronary occlusion, manifested as a less developed collateral circulation, remains a matter of debate. Indeed, in a recent study, patients with hypertension, but not patients with the MS had less developed coronary collaterals.7,8 However, in the studied population, only 24 patients had occluded coronary vessels and no information on the duration was available.7
As the growth of collaterals is a time-dependent process,9 occurring only in case of severe ischaemia secondary to tight stenoses or occluded vessels,10 we investigated the influence of the MS on collateral circulation in a population that comprised only patients with occluded coronary vessels, and after adjustment on the time elapsed since coronary occlusion. In addition, we investigated the potential relationship between biological variables related to the MS, such as PAI-1 and adiponectin plasma concentration, and the development of coronary collateral circulation. Because circulating endothelial progenitor cells (EPCs), including ‘early EPCs’, have been demonstrated to play a significant role in the growth of new vessels, we further investigated the potential relationship between the presence of the MS and the number of circulating ‘early EPCs’.
Methods
Study population
Between May 2000 and October 2001, 2050 patients scheduled for coronary angiography were prospectively enrolled in a cardiovascular registry. The Ethics Committee of the ‘Centre Hospitalier Régional Universitaire de Lille’ approved the study and each subject gave informed written consent the day before angiography. Patients who required unplanned coronary angiography (e.g. patients addressed for emergent angiography or for primary or rescue PCI) were not part of this registry.
We prospectively selected all patients who had at least one total occlusion of a major coronary vessel excluding only patients with a history of coronary artery bypass graft. Three hundred and eighty-seven patients fulfilled these criteria and made up the study population. The baseline clinical and angiographic characteristics as well as cardiovascular medications were prospectively recorded by trained cardiologists at the time of enrolment.
Patients were fasting and hypoglycaemic drugs were stopped for at least 12 h before angiography. Blood samples were drawn from the arterial sheath immediately after puncture for angiography. Insulin was measured with an immunometric assay. PAI-1 and adiponectin were measured by ELISA according to the manufacturer’s directions. In order to evaluate the potential relationship between the number of circulating ‘early EPCs’ and the presence of the MS, 30 consecutive patients underwent blood sampling to evaluate colony forming unit endothelial cells (CFU-ECs) from peripheral mononuclear cells.
Definitions
MS was defined according to the ATP-III criteria, including three or more of the following abnormalities including high blood pressure (≥130/85 mmHg or the use of antihypertensive drugs) and metabolic derangements such as hypertriglyceridemia [serum triglycerides ≥1.70 mmol/L (150 mg/dL)], low HDL cholesterol [serum HDL cholesterol <1.04 mmol/L (40 mg/dL) in men and <1.29 mmol/L (50 mg/dL) in women], high fasting glucose [fasting serum glucose ≥6.1 mmol/L (110 mg/dL)], and obesity. As waist circumference was not available, obesity was defined as a body mass index >30 kg/m2.1 Diabetes mellitus was defined as a fasting glucose ≥7 mmol/L on two separate occasions, the use of hypoglycaemic agents, or a history of physician-diagnosed diabetes mellitus. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the following formula: HOMA-IR= (fasting glucose × fasting insulin)/22.5.11
Angiography procedure and coronary collaterals grading
Selective coronary angiography was performed in multiple orthogonal projections by experienced investigators using a standardized procedure including: 6 Fr catheters (Cordis), administration of intracoronary nitrates, standardized contrast media (iohexol350), and injection method. All angiograms were recorded at 35 frames/s and stored on a high quality support. Ventricular function was evaluated in a single-plane right anterior oblique projection (30°), and left ventricular ejection fraction (LVEF) was calculated according to the area length method.
Two independent observers, blinded to patient characteristics, graded the severity of coronary artery disease and evaluated the collateral flow. These evaluations were used for the calculation of the inter-observer variability. In the case of disagreement, a new reading involving the two observers acting as a panel was performed and the resulting agreement was accepted as the final grading (see Supplementary material online, data 1 for details).12 Values of kappa weighted for multiple categories (wκ) were computed to calculate the agreement between observers in grading ‘collateral flow grade’ and ‘recipient filling grade’.13 Agreement between the two observers in grading the ‘recipient filling grade’ (wκ = 0.739) or the ‘collateral flow grade’ (wκ = 0.867) was very good.
Variability of the measurement overtime was analysed in 21 patients in whom the following conditions were met: (i) no revascularization was performed after the initial angiography and (ii) a repeated angiography was performed within 10 days. Agreement between the two sets of angiography in grading the ‘Recipient flow grade’ (wκ = 0.844) or the ‘collateral flow grade’ (wκ = 0.874) was very good.
Time elapsed since coronary occlusion
For each patient, the time elapsed since coronary occlusion was evaluated based on a review of clinical data and after review of all available ECGs. This was performed by a physician who was not involved in grading coronary collateral flow. Patients were classified into five groups based on the estimated duration of coronary occlusion: (1) <15 days; (2) 15 days to 1 month; (3) 1–6 months; (4) >6 months, and (5) unknown but no evidence of an event during the month preceding angiography. In patients in whom the duration of coronary occlusion was <15 days, the median time between symptoms and angiography was 5 (3–9) days.
Isolation, culture and phenotyping of colony forming unit endothelial cells
There are at least two morphological and functionally distinct EPC populations among circulating mononuclear cells involved in the angiogenic process.14 The early spindle-like outgrowth cells (‘early EPCs’) possess a relatively low proliferative capacity and are considered to act partly indirectly through the production of high levels of angiogenic cytokines. Late ‘outgrowth cells’ show a high proliferative potential and are considered as circulating angioblasts. In the present study, the relation between MS and EPCs has been assessed by investigating ‘early EPCs’.
Isolation and culture of ‘early EPCs’ (CFU-ECs) were performed according to previously described methods (see Supplementary material online, data 2 for details).15,16 Demonstration of VEGF secretion was performed by the measurement of VEGF concentration of supernatant (478 ± 154 pg/106 cells) as previously described.17
Statistical analysis
Continuous variables are presented as mean ± SD [or median (inter-quartile range) when data were skewed]. Discrete variables are presented as absolute number and percentages. Comparison between patients with/without the MS was performed using Students’ t-test (the Mann–Whitney rank-sum test for skewed variables) or the χ2 analysis when appropriate. Relationships between the ‘collateral flow grade’ or ‘recipient filling grade’ and continuous variables were assessed using Spearman’s correlation coefficient, whereas relationships with discrete variables were assessed using the Mantel–Haenszel linear tendency test.
Multivariable analyses were performed with a general linear model. To assess the association between collateral flow grade and LVEF, a multivariable analysis was performed adjusting on age, sex, smoking, diabetes mellitus, total cholesterol, clinical symptoms, number of vessels with total occlusion, collateral flow grade, time since coronary occlusion, and cardiovascular medications. To evaluate the relation between ‘collateral flow grade’ and the demographic and clinical characteristics of the subjects, the multivariable model included age, sex, smoking, total cholesterol, time since coronary occlusion, clinical symptoms, use of ACE-inhibitors, the use of statins and the number of diseased coronary vessels. These variables were selected on the basis of previous publications on development of collateral circulation in experimental and clinical settings18–22 and on variables associated with ‘collateral flow grade’ at P < 0.1 in the present study. The same adjustment variables were used to assess the relation between ‘collateral flow grade’ and MS or its components separately (high blood pressure, hypertriglyceridaemia, low HDL cholesterol, high fasting glucose, obesity, HOMA-IR, PAI-1, and adiponectin concentration) in additional multivariable analyses. Similar analyses were performed with ‘recipient filling grade’. All hypotheses were two-tailed with a 0.05 type I error.
Results
Baseline characteristics according to the presence of the metabolic syndrome
The baseline characteristics of the subjects are presented Table 1. One hundred and eighty-one patients (47%) met the criteria for the MS. Patients with the MS were more likely to be women (P = 0.01) and diabetic (P = 0.0004). Total cholesterol (P = 0.01), insulin (P = 0.0001), HOMA-IR (P = 0.0001), HbA1c (P = 0.0001), and PAI-1 (P = 0.0008) levels were higher and adiponectin (P = 0.0002) concentration was lower in patients with the MS. The differences observed between subjects with or without the MS remained statistically significant after exclusion of diabetic patients (data not shown). Patient with the MS had also more severe coronary artery disease (P = 0.02) and less developed collateral circulation as estimated by the ‘recipient filling grade’ or the ‘collateral flow grade’ (P = 0.005).
Table 1.
Demography, biology, and angiography with respect to the presence of the metabolic syndrome
| Controls (n = 206) | Metabolic syndrome (n = 181) | P‐value | |
|---|---|---|---|
| Age (years) | 62 ± 12 | 63 ± 11 | 0.71 |
| Female sex, n (%) | 29 (14) | 43(24) | 0.01 |
| Risk factors, n (%) | |||
| Smoking | 164 (79) | 130 (72) | 0.20 |
| Diabetes mellitus | 42 (24) | 93 (44) | 0.0001 |
| Familial history of CAD | 67 (33) | 68 (38) | 0.33 |
| Total cholesterol (mg/dL) | 177 (150–203) | 193 (155–217) | 0.01 |
| LDL cholesterol (mg/dL) | 115 (89–138) | 118 (93–144) | 0.28 |
| Biological variables related to the metabolic syndrome | |||
| Insulin (mUI/L) | 5.9 (4.0–8.7) | 8.9 (5.8–13.4) | 0.0001 |
| HOMA-IR | 1.45 (0.90–2.32) | 2.59 (1.52–4.56) | 0.0001 |
| HbA1C (%) | 5.5 (5.2–5.9) | 6.4 (5.6–8.1) | 0.0001 |
| C-reactive protein (mg/L) | 3.50 (1.76–11.30) | 6.04 (2.11–12.32) | 0.12 |
| Fibrinogen (g/L) | 3.82 (3.17–4.67) | 4.11 (3.36–4.81) | 0.10 |
| PAI-1 (ng/mL) | 22 (15–32) | 25 (19–35) | 0.0008 |
| Adiponectin (ng/mL) | 7982 (4176–12424) | 5567 (3213–9110) | 0.0002 |
| Cardiovascular medications, n (%) | |||
| Oral antiplatelet | 183 (89) | 149 (84) | 0.18 |
| ACE-inhibitors | 112 (54) | 103 (58) | 0.56 |
| Beta-blockers | 126 (61) | 116 (65) | 0.48 |
| Nitrates | 122 (59) | 107 (60) | 0.94 |
| Calcium antagonists | 49 (24) | 45 (25) | 0.82 |
| Statins | 116 (56) | 109 (61) | 0.35 |
| Clinical symptoms, n (%) | |||
| Stable symptoms | 125 (61) | 101 (56) | 0.47 |
| Unstable symptoms | 81 (39) | 80 (44) | |
| Angiographic data | |||
| Number of vessels with 50% stenosis, n (%) | |||
| One vessel | 66 (32) | 44 (24) | 0.02 |
| Two vessels | 77 (37) | 62 (34) | |
| Three vessels | 63(31) | 75 (42) | |
| Number of vessels with total occlusion, n (%) | |||
| One vessel | 180 (87) | 142 (79) | 0.02 |
| Two vessel | 26 (13) | 39 (21) | |
| Time since coronary occlusion, n (%) | |||
| <15 days | 54 (26) | 39 (22) | 0.61 |
| 15 days to 1month | 10 (5) | 13 (7) | |
| 1–6 months | 26 (13) | 22 (12) | |
| Unknown >1 month | 47 (23) | 42 (23) | |
| >6 months | 69 (33) | 65 (36) | |
| LVEF (%) | 52.5 ± 15.0 | 49.4 ± 15.4 | 0.15 |
| Collateral flow grade | 2.84 ± 1.04 | 2.22 ± 1.19 | 0.005 |
| Recipient filling grade | 3.15 ± 1.00 | 2.63 ± 1.20 | 0.005 |
Data are presented as percent of patients, mean value ± SD or median (inter-quartile range). CAD, coronary artery disease; BMI, body mass index.
Coronary collateral circulation evaluated by angiography and relation with left ventricular ejection fraction
The two grading scores were highly correlated to each other (R=0.76, P = 0.0001). A significant and positive correlation was observed between LVEF and the ‘collateral flow grade’ (P = 0.005, Table 2) or the ‘recipient filling grade’ (P = 0.01). In multivariable analyses adjusting for age, gender, smoking, diabetes, total cholesterol, clinical symptoms, number of vessels with total occlusion, time since coronary occlusion, and cardiovascular medications, the coronary collateral circulation estimated by the ‘collateral flow grade’ (P = 0.005) or by the ‘recipient filling grade’ (P = 0.01) was highly associated with LVEF.
Table 2.
Demography, biology, and angiography with respect to the coronary collateral circulation
| Collateral flow grade | 0 (n = 23) | 1 (n = 45) | 2 (n = 102) | 3 (n = 140) | 4 (n = 77) | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 65 ± 10 | 59 ± 13 | 63 ± 11 | 61 ± 11 | 65 ± 12 | 0.42 |
| Female sex, n (%) | 5 (21) | 10 (22) | 24 (24) | 24 (17) | 12 (9) | 0.08 |
| Risk factors, n (%) | ||||||
| Smoking | 20 (87) | 35 (78) | 76 (74) | 107 (76) | 56 (73) | 0.19 |
| Diabetes mellitus | 13 (56) | 18 (40) | 42 (41) | 38 (27) | 24 (31) | 0.005 |
| Familial history of CAD | 4 (17) | 21 (46) | 29 (28) | 47 (34) | 34 (44) | 0.14 |
| Total cholesterol (mg/dL) | 184 (158–216) | 199 (169–225) | 187 (160–211) | 182 (145–206) | 174 (144–216) | 0.08 |
| LDL cholesterol (mg/dL) | 126 (107–137) | 125 (105–146) | 118 (93–140) | 111 (89–140) | 111 (84–147) | 0.04 |
| Cardiovascular medications, n (%) | ||||||
| Oral antiplatelet | 21 (91) | 37 (82) | 89 (87) | 120 (86) | 65 (84) | 0.68 |
| ACE-inhibitors | 13 (57) | 23 (51) | 66 (65) | 72 (51) | 41 (53) | 0.47 |
| Beta-blockers | 17 (74) | 25 (56) | 65 (64) | 85 (61) | 50 (65) | 0.92 |
| Nitrates | 19 (83) | 31 (69) | 56 (55) | 72 (51) | 51 (66) | 0.26 |
| Calcium antagonists | 3 (13) | 10 (22) | 25 (25) | 34 (24) | 22 (29) | 0.28 |
| Statins | 13 (57) | 26 (58) | 55 (54) | 83 (59) | 48 (62) | 0.40 |
| Clinical symptoms, n (%) | ||||||
| Stable symptoms | 9 (39) | 14 (31) | 55 (54) | 94 (67) | 54 (70) | 0.0001 |
| Unstable symptoms | 14 (61) | 31 (69) | 47 (46) | 46 (33) | 23 (30) | |
| Time since coronary occlusion, n (%) | ||||||
| <15 days | 14 (61) | 22 (49) | 32 (31) | 16 (11) | 9 (12) | 0.0001 |
| 15 days to 1 month | 2 (9) | 1 (2) | 10 (10) | 8 (6) | 2 (3) | |
| 1–6 months | 4 (17) | 3 (7) | 13 (13) | 21 (15) | 7 (9) | |
| Unknown >1 month | 1 (4) | 9 (20) | 19 (19) | 42 (30) | 18 (23) | |
| >6 months | 2 (9) | 10 (22) | 28 (27) | 53 (38) | 41 (53) | |
| Angiographic data | ||||||
| Number of vessels with 50% stenosis, n (%) | ||||||
| One vessel | 7 (31) | 13 (29) | 40 (39) | 37 (26) | 13 (17) | 0.01 |
| Two vessels | 10 (43) | 16 (35) | 36 (35) | 54 (39) | 23 (30) | |
| Three vessels | 6 (26) | 16 (36) | 26 (26) | 49 (35) | 41 (53) | |
| Number of vessels with total occlusion, n (%) | ||||||
| One vessel | 22 (96) | 41 (91) | 90 (88) | 112 (80) | 57 (74) | 0.04 |
| Two vessels | 1 (4) | 4 (9) | 12 (12) | 28 (20) | 20 (26) | |
| LVEF (%) | 46.9 ± 13.3 | 48.9 ± 13.9 | 51.5 ± 11.4 | 52.0 ± 16.6 | 53.0 ± 16.0 | 0.005 |
Analysis of the association between metabolic syndrome and coronary collateral circulation
As analyses of the relation between ‘collateral flow grade’ and ‘recipient filling grade’ yielded similar results, only those relative to ‘collateral flow grade’ are presented.
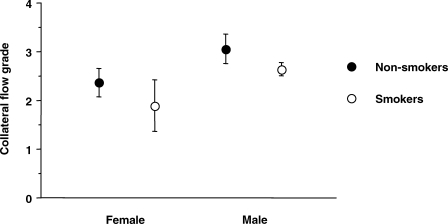
Coronary collateral circulation was significantly less developed in patients with diabetes (P = 0.005) and raised LDL cholesterol levels (P = 0.04), whereas it was more developed in patients with an old occlusions (P = 0.0001) and more diffuse coronary artery disease (P = 0.01, Table 2). The association between collateral flow grade and clinical variables (age, sex, smoking, total cholesterol, time since coronary occlusion, clinical symptoms, use of ACE-inhibitors, use of statins, and number of diseased coronary vessels) was tested in a multivariable analysis. In this model, a shorter time since coronary occlusion (β =+0.297; P = 0.0001), female sex (β =−0.483; P = 0.003), and smoking (β =−0.446; P = 0.003) were associated with a significantly lower grade of collateral flow (Figure 1).
Figure 1.
Female sex (P = 0.003) and smoking (P = 0.003) are associated with a lower ‘collateral flow grade’.
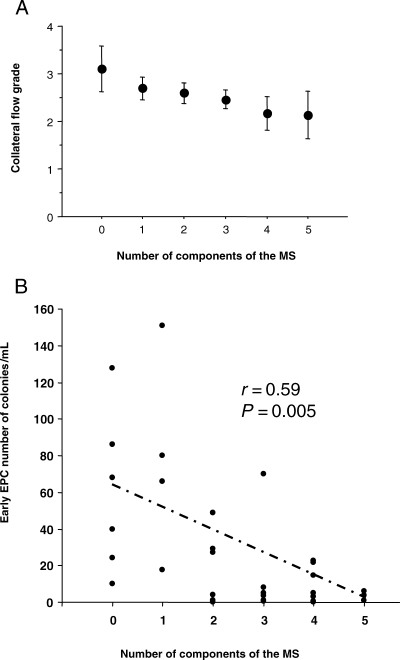
In univariable analyses, MS was associated with a lower grade of collateral flow (P = 0.005, Table 3). The collateral flow grade was inversely correlated to the number of components of the MS (P = 0.002), high blood pressure (P = 0.04), fasting glycaemia >6.1 mmol/L (P = 0.002), insulin concentration (P = 0.04), HOMA-IR (P = 0.03), and PAI-1 concentration (P = 0.02) and positively correlated to adiponectin concentration (P = 0.001). In order to further assess the relationship between coronary collateral flow grade and the MS, multivariable analyses were performed (Table 4). First, the association between the MS and collateral flow grade was assessed by using the MS as a quantitative data (from 0 to 5 according to the number of components of the MS) in a model adjusted for potential confounding factors (age, sex, smoking, total cholesterol, time since coronary occlusion, clinical symptoms, use of ACE-inhibitors, use of statins, and number of diseased coronary vessels). In this model, the MS was associated with a lower collateral flow grade (P = 0.0003, Model 1; Table 4, Figure 2). Similar results were observed when the MS was entered as a qualitative data (yes vs. no, β =−381; P = 0.001) adjusted for the same confounding factors. Finally, as an alternative to MS, the relation between individual components of the MS (high blood pressure, hypertriglyceridaemia, low HDL cholesterol, high fasting glucose, obesity, HOMA-IR, adiponectin, and PAI-1), and collateral flow grade was assessed. High fasting glycaemia (P = 0.0007), high blood pressure (P = 0.008), HOMA-IR (P = 0.01), adiponectin (P = 0.01), and PAI-1 (P = 0.01) were significantly associated with collateral flow grade (Model 2, Table 4). Similar associations were observed when analyses were restricted to patients with only one coronary occlusion (data not shown).
Table 3.
Relationship between components of the metabolic syndrome as well as related biological variables with the extent of coronary collateral circulation
| Collateral flow grade | 0 (n = 23) | 1 (n = 45) | 2 (n = 102) | 3 (n = 140) | 4 (n = 77) | P-value |
|---|---|---|---|---|---|---|
| Metabolic syndrome | ||||||
| No | 8 (35) | 18 (42) | 57 (56) | 77 (55) | 46 (60) | 0.005 |
| Yes | 15 (65) | 27 (58) | 45 (44) | 63 (45) | 31 (40) | |
| Number of components of MS | ||||||
| 0–1 | 2 (9) | 10 (22) | 23 (23) | 34 (24) | 23 (30) | |
| 2–3 | 10 (43) | 21 (47) | 56 (55) | 84 (60) | 40 (52) | 0.002 |
| 4–5 | 11 (48) | 14 (31) | 23 (22) | 22 (16) | 14 (18) | |
| Components of the metabolic syndrome | ||||||
| Obesity (BMI > 30 kg/m2) | 12 (52) | 18 (42) | 23 (23) | 39 (28) | 23 (30) | 0.06 |
| High blood pressure | 17 (74) | 24 (53) | 62 (61) | 68 (48) | 39 (50) | 0.04 |
| Low HDL cholesterol | 19 (83) | 32 (71) | 75 (73) | 111 (79) | 54 (70) | 0.41 |
| High triglycerides | 6 (27) | 45 (49) | 41 (40) | 57 (41) | 26 (34) | 0.33 |
| Fasting glycaemia >6.1 mmol/L | 18 (78) | 24 (56) | 49 (48) | 55 (39) | 33 (43) | 0.002 |
| Other biological variables | ||||||
| Insulin (mUI/L) | 9.60 (6.80–16.10) | 8.90 (6.90–12.60) | 6.70 (4.80–10.70) | 6.60 (4.50–11.70 | 6.50 (4.50–10.00 | 0.04 |
| HOMA-IR | 3.15 (1.97–4.41) | 2.53 (1.78–4.74) | 1.93 (1.01–3.14) | 1.61 (1.10–3.10) | 1.70 (1.08–2.71) | 0.03 |
| HbA1C (%) | 6.1 (5.4–6.6) | 5.8 (5.3–7.1) | 5.8 (5.3–7.2) | 5.6 (5.3–6.5) | 5.8 (5.3–6.6) | 0.37 |
| C-reactive protein (mg/L) | 6.99 (1.00–11.90) | 6.75 (1.88–13.12) | 6.23 (2.31–16.62) | 3.44 (1.71–8.20) | 3.44 (2.10–14.65) | 0.16 |
| Fibrinogen (g/L) | 3.96 (2.69–4.51) | 4.09 (3.30–5.20) | 4.22 (3.36–4.81) | 3.81 (3.14–4.74) | 4.03 (3.37–4.77) | 0.45 |
| Adiponectin (ng/mL) | 5170 (2462–8057) | 5480 (3385–8358) | 6648 (3421–10834) | 6807 (3674–11533) | 7529 (3664–12269) | 0.001 |
| PAI-1 (ng/mL) | 33 (19–44) | 27 (18–40) | 23 (17–35) | 22 (16–34) | 21 (16–33) | 0.01 |
MS, metabolic syndrome; BMI, body mass index. Trends were tested using Spearman’s correlations and Mantel–Haenszel linear tendency tests.
Table 4.
Association between metabolic syndrome and ‘collateral flow grade’: multivariable analyses
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | |
| Time since coronary occlusion | +0.299 | +0.219 to +0.365 | 0.0001 | +0.281 | +0.187 to +0.372 | 0.0001 |
| Female sex | −0.402 | −0.790 to −0.090 | 0.01 | −0.351 | −0.739 to −0.071 | 0.03 |
| Smoking | −0.443 | −0.784 to −0.188 | 0.002 | −0.447 | −0.789 to −0.179 | 0.003 |
| Age (10 years increase) | −0.043 | −0.155 to +0.070 | 0.46 | −0.039 | −0.154 to −0.075 | 0.49 |
| Total cholesterol (10 mg/dL increase) | +0.006 | −0.018 to +0.030 | 0.64 | +0.008 | −0.015 to +0.031 | 0.51 |
| Number of diseased coronary vessels | +0.084 | −0.067 to +0.235 | 0.27 | +0.057 | −0.095 to +0.210 | 0.46 |
| Unstable symptoms | −0.020 | −0.298 to +0.258 | 0.89 | −0.059 | −0.340 to +0.222 | 0.67 |
| Use of ACE-inhibitors | +0.001 | −0.210 to +210 | 0.99 | +0.009 | −0.197 to +228 | 0.89 |
| Use of statins | −0.008 | −0.223 to +207 | 0.94 | −0.002 | −0.219 to +215 | 0.99 |
| Number of components of the metabolic syndrome (0–5) | −0.148 | −0.229 to −0.075 | 0.0003 | — | — | — |
| Fasting glycaemia > 6.1 mmol/L | — | — | — | −0.397 | −0.639 to −0.160 | 0.0007 |
| High blood pressure | — | — | — | −0.283 | −0.508 to −0.078 | 0.008 |
| PAI-1 (10 ng/mL increase) | — | — | — | −0.030 | −0.069 to −0.009 | 0.01 |
| HOMA-IR (1 U increase) | — | — | — | −0.031 | −0.058 to −0.008 | 0.01 |
| Adiponectin (103mg/mL increase) | — | — | — | +0.027 | +0.005 to +0.051 | 0.01 |
| High Triglycerides | — | — | — | −0.028 | −0.273 to +0.216 | 0.81 |
| Low HDL cholesterol | — | — | — | +0.043 | −0.216 to +0.303 | 0.74 |
| Obesity | — | — | — | −0.136 | −0.377 to +0.104 | 0.26 |
Model 1 included age, sex, smoking, total cholesterol, time since coronary occlusion, clinical symptoms, use of ACE inhibitors, use of statins, number of diseased coronary vessels and the number of components of the metabolic syndrome(0 to 5).
Model 2 included age, sex, smoking, total cholesterol, time since coronary occlusion, clinical symptoms, use of ACE inhibitors, use of statins, and number of diseased coronary vessel, plus high blood pressure, hypertriglyceridemia, low HDL cholesterol, high fasting glucose, obesity, HOMA-IR, adiponectin and PAI-1 concentration.
Figure 2.
Effect of the number of components of the metabolic syndrome on the ‘collateral flow grade’ (A; P = 0.0005) and on the number of circulating ‘early endothelial progenitor cells’ (B; P = 0.005).
Metabolic syndrome and endothelial progenitor cells
Median CFU-ECs, as measured in 30 consecutive patients, was 12.2 (3.0–29.0) colonies/mL. Median CFU-ECs was lower in smokers [5.2 (1.0–21.7), n = 21] than in non-smokers [27.0 (20.5–72.5), n = 9; P = 0.004]. It was also lower in patients with the MS [3.0 (0.6–12.8), n = 12] than in those without [25.5 (4.9–53.3), n = 18; P = 0.01]. Interestingly, the number of CFU-ECs was inversely related to the number of components of the MS present (r = 0.59, P = 0.005; Fig. 2B). Among the individual components of the MS, the strongest inverse relationship was found with fasting glycaemia >6.1 mmol/L (P = 0.01) and high blood pressure (P = 0.05). Among the other biological parameters related to the MS, a significant and inverse relationship was found with adiponectin concentration (r = −0.51, P = 0.005) and HOMA-IR (r = −0.36, P = 0.05). No significant relationship was found with PAI-1 concentration or cardiovascular medications.
Discussion
To our knowledge, this study is the first to demonstrate a strong and inverse relationship between the presence of the MS and the extent of the development of the collateral circulation in patients. It also demonstrates that hyperglycaemia, insulin resistance (HOMA-IR), hypertension, circulating PAI-1, and adiponectin concentration are independently associated with poor coronary collateral vessel development. These findings could partly account for the poor clinical outcome reported in patients with coronary artery disease who have the MS.2
Factors associated with the development of ‘chronic’ coronary collateral circulation and the need for adjustment for these factors
Previous studies have clearly demonstrated that myocardial ischaemia related to tight stenoses or occluded coronary vessels plays a major role in the development of coronary collaterals.10 In the present study, performed in patients with occluded coronary vessels, the time elapsed since occurrence of coronary occlusion is the most powerful correlate of well developed coronary collateral vessels, in agreement with earlier hypotheses.9 Therefore, the importance of the ischaemic stimuli and a sufficient time are necessary to develop collateral circulation. Furthermore, these results clearly shows that these factors should be taken into account when analysing other correlates of coronary collateral vessel development.
Interestingly, our study also identifies, to our knowledge for the first time, smoking and female sex as independent correlates of poor coronary collateral circulation. The poor coronary collateral circulation observed in smokers is consistent with studies in animals demonstrating that exposure to cigarette smoke impairs angiogenesis in response to ischaemia by inhibiting VEGF through a decreased expression of HIF-1alpha.19 Our observation as well as that of others16 that smokers have less circulating ‘early EPCs’ also suggest an important role of this mechanism.
The poor coronary collateral circulation observed in women may be related to the fact that 96% of the female population was older than 50 years and therefore post-menopausal. Evidence suggests that oestrogen directly modulates angiogenesis via effects on endothelial cells under physiological and pathophysiological conditions and that its loss in post-menopausal women is associated with a decreased angiogenic response.20
Consideration of the results from previous studies investigating the impact of diabetes and components of the metabolic syndrome on coronary collateral circulation
We found a clear and inverse association between the extent of collateral formation and the presence of diabetes mellitus, the MS, or markers of insulin resistance. The results of earlier studies have been conflicting; some suggested that diabetes had no impact on collateral development,23,24 others that diabetes was associated with decreased collaterals,25–27 and one reported that diabetes was associated with increased collateral development28 (see Supplementary material online, Table S3 for details). Recently, a group reported that patients with hypertension, but not patients with the MS, had less developed coronary collaterals.7,8
With respect to hypertension, our study confirms that it is associated with less coronary collaterals in ischaemic myocardium.8 The effect of hypertension could be related partly to a detrimental effect on the number of ‘early EPCs’, as suggested by our results, and on the migratory properties of EPCs.16 It could also be related to a profound downregulation of an important endothelial growth factor, hepatocyte growth factor, as demonstrated in spontaneous-hypertensive rats.
The apparent discrepancies regarding the relationship between diabetes/MS and collateral circulation may be related to: (i) differences in the severity of CAD among the populations studied; (ii) the lack of adjustment for major known confounding factors; and (iii) the small sample size of most of these studies (see Supplementary material online, Table S3 for details). Indeed, most studies, including the most recent,7 enrolled a broad range of patients with coronary artery disease where only a minority of patients had severely stenosed or occluded coronary vessels.25,28,29 Furthermore, apart from a small study that included 90 patients,27 the analyses were not adjusted to account for the time elapsed since coronary occlusion,24,26 and confounders such as sex or smoking were not taken into account.
We sought to avoid these pitfalls by studying a relatively large population of 387 patients, exclusively including individuals with occluded coronary vessels and adjusting for major confounding factors such as time elapsed since coronary occlusion, smoking, and sex.
The metabolic syndrome and lack of coronary collateral development: biological associations
Our study demonstrates that in patients with coronary occlusion, those with the MS have less developed coronary collateral vessels. In addition, it appears that the extent of collateral vessel development and the number of circulating ‘early EPCs’ are inversely related to the number of components of the MS present. Furthermore, multivariable analysis including clinical and biological variables suggest that hyperglycaemia, insulin resistance (HOMA-IR), hypertension, low adiponectin concentration, and high PAI-1 concentration in blood are independently associated with poor coronary collateral vessel development.
A potential role for hyperglycaemia has been previously suggested, based on studies in animals with chronic hyperglycaemia.30 This could partly reflect a decreased expression of VEGF in the myocardium, a decreased sensitivity to VEGF stimulation,31 or a direct inhibitory effect of hyperglycaemia on ‘early EPCs’ as suggested by our results and by previous in vitro studies.32
The reported effect of insulin resistance on collateral circulation and ‘early EPCs’ number is also supported by the results of studies in animal.33 They demonstrate that insulin is an important regulator of the angiogenic pathway and that rat models of insulin resistance are associated with a decreased expression of both VEGF and Akt leading to a decreased vascular density in myocardium in response to ischaemia.33
Adiponectin is an adipocyte-derived cytokine downregulated in patients with MS and known to have angiogenic properties in animals.34 By demonstrating that a high adiponectin plasma level is independently associated with a good coronary collateral circulation, our results confirm these observations in the clinical situation. Adiponectin is considered to promote angiogenesis by promoting endothelial cell proliferation and migration and by suppressing apoptosis.34 Interestingly, our results also suggest a role of adiponectin on circulating ‘early EPCs’. This supports the previous observation that adiponectin stimulate proliferation of human EPCs35 in vitro.
A high concentration of PAI-1 in blood is one of the features of the MS.4 Our study is the first to our knowledge to demonstrate a negative relationship between plasma PAI-1 concentration and the development of collateral circulation in humans. Our observation extends previous reports demonstrating that decreasing PAI-1 expression in animals stimulate vessel growth in response to myocardial ischaemia.6 The lack of relationship between PAI-1 concentration and the number of circulating ‘early EPCs’ in the present report is also consistent with findings in pre-clinical studies demonstrating that PAI-1 alters the angiogenic response at the level of ECs36 and EPCs6 migration. It should be also noted that the inverse relationship between plasma PAI-1 concentration and coronary collateral circulation, demonstrated in our study, could partly explain the previously reported increased risk of cardiovascular events in subjects with a high concentration of PAI-1 in blood.37
Limitations
Our study was a single-centre study and patient referral and medical management may have influenced the results. However, it comprised a consecutive series of patients referred for a diagnostic coronary angiography, and it is the largest study to date focusing only on patients with coronary occlusion. In addition, careful adjustment for the duration of coronary occlusion and other potential risk factors strengthen our results. Analysis of coronary collateral circulation by angiography because it is a semi-quantitative method, requires a very strict methodology to provide reliable results. To comply with that request, several measures were taken: first, a standardized method for performing and recording angiography was used. Second, two complementary and previously validated grading systems were used: one investigating the size of collateral vessels (‘collateral flow grade’) and one the quality of the filling of the occluded vessel by the collaterals (‘recipient filling grade’). Third, the grading process was performed by two independent observers and demonstrated a very good inter-observer and overtime reproducibility. Internal consistency among our results is also a guarantee for the quality of the data: there was indeed a strong correlation between the results of the two grading systems (R = 0.76), whereas univariate and multivariable analyses identified similar correlates (data not shown).
Pressure- and Doppler-wire-derived measurements may also be considered more physiologically relevant than angiographically derived grading. However, previous studies have demonstrated a good correlation between wire-derived and angiographic analyses of collateral circulation.38 In our study, the physiological relevance of the angiographically derived grading is confirmed by the observation that collateral circulation by angiography is highly associated with LVEF. In addition, because the wire-derived techniques require crossing the coronary occlusion with a device, they can only be used in patients referred for a PCI of the occluded vessel. In that regard, it is important to note that analyses restricted to patients referred for a PCI of the occluded vessel in our population demonstrated that this group was not representative of the overall population in terms of duration of coronary occlusion, severity of coronary artery disease, and LVEF (data not shown). Analysis of the baseline patients’ characteristics of the largest study performed with the use of wire-derived measurements confirmed this observation.27 The use of angiography therefore appears more suitable than wire-derived methods to investigate collateral circulation in a large population with coronary occlusion while avoiding major selection biases.
The present study did not find a relationship between the use of cardiovascular medications and the development of coronary collaterals. To investigate this issue, a specifically designed study is needed.
Although analysis of the relationship between MS and ‘early EPCs’ provided interesting findings, it was performed in a small subgroup and these exploratory results need confirmation.
Conclusion
Our results demonstrate that patients with the MS and an occlusion in a major epicardial coronary vessel have less developed coronary collaterals than those without MS. This observation may explain the well-documented higher risk of cardiac events in patients with the MS.2 It suggests that patients with the MS may constitute a particularly relevant group in which future investigation of pharmacological, or other, strategies favouring the development of coronary collaterals can be studied. Our results suggest also that novel biological mediators such as PAI-1 or adiponectin may be reasonable targets to stimulate coronary collateral circulation.
Supplementary material
Supplementary material is available at European Heart Journal Online.
Funding
This study was funded by the “programme hospitalier de recherche clinique (PHRC)”, the University of Lille II (EA2693) and the “Conseil Régional Nord-Pas de Calais”. Funding to pay the Open Access publication charges for this article was provided by the Association pour la Recherche Expérimentale Hématologique et Cardiovasculaire à Lille.
Conflict of interest: none declared.
Supplementary Material
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Koerselman J, van der Graaf Y, de Jaegere PP, Grobbee DE. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation. 2003;107:2507–2511. doi: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 4.Sobel BE. The metabolic syndrome vis-a-vis syndrome of insulin resistance: the perfect squall. Coron Artery Dis. 2005;16:461–463. [Google Scholar]
- 5.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–2221. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 6.Xiang G, Schuster MD, Seki T, Kocher AA, Eshghi S, Boyle A, Itescu S. Down-regulation of plasminogen activator inhibitor 1 expression promotes myocardial neovascularization by bone marrow progenitors. J Exp Med. 2004;200:1657–1666. doi: 10.1084/jem.20040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olijhoek JK, Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y, Visseren FL. Presence of the metabolic syndrome does not impair coronary collateral vessel formation in patients with documented coronary artery disease. Diabetes Care. 2005;28:683–689. doi: 10.2337/diacare.28.3.683. [DOI] [PubMed] [Google Scholar]
- 8.Koerselman J, de Jaegere PP, Verhaar MC, van der Graaf Y, Grobbee DE. High blood pressure is inversely related with the presence and extent of coronary collaterals. J Hum Hypertens. 2005;19:809–817. doi: 10.1038/sj.jhh.1001917. [DOI] [PubMed] [Google Scholar]
- 9.Werner GS, Ferrari M, Betge S, Gastmann O, Richartz BM, Figulla HR. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation. 2001;104:2784–2790. doi: 10.1161/hc4801.100352. [DOI] [PubMed] [Google Scholar]
- 10.Cohen M, Sherman W, Rentrop KP, Gorlin R. Determinants of collateral filling observed during sudden controlled coronary artery occlusion in human subjects. J Am Coll Cardiol. 1989;13:297–303. doi: 10.1016/0735-1097(89)90502-0. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CM, Ryan K, Sparano A, Moynihan JL, Rizzo M, Kelley M, Marble SJ, Laham R, Simons M, McClusky TR, Dodge JT., Jr Angiographic methods to assess human coronary angiogenesis. Am Heart J. 1999;137:169–179. doi: 10.1016/s0002-8703(99)70473-4. [DOI] [PubMed] [Google Scholar]
- 13.Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303–308. doi: 10.1148/radiol.2282011860. [DOI] [PubMed] [Google Scholar]
- 14.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, Finkel T. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res. 1999;59:5875–5877. [PubMed] [Google Scholar]
- 16.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 17.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 18.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Michaud SE, Menard C, Guy LG, Gennaro G, Rivard A. Inhibition of hypoxia-induced angiogenesis by cigarette smoke exposure: impairment of the HIF-1alpha/VEGF pathway. FASEB J. 2003;17:1150–1152. doi: 10.1096/fj.02-0172fje. [DOI] [PubMed] [Google Scholar]
- 20.Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 21.Fabre JE, Rivard A, Magner M, Silver M, Isner JM. Tissue inhibition of angiotensin-converting enzyme activity stimulates angiogenesis in vivo. Circulation. 1999;99:3043–3049. doi: 10.1161/01.cir.99.23.3043. [DOI] [PubMed] [Google Scholar]
- 22.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilia R, Carmel S, Gueron M. Patients with coronary collaterals and normal left ventricular systolic function: clinical, hemodynamic, and angiographic characteristics. Angiology. 1998;49:631–635. doi: 10.1177/000331979804900807. [DOI] [PubMed] [Google Scholar]
- 24.Kornowski R. Collateral formation and clinical variables in obstructive coronary artery disease: the influence of hypercholesterolemia and diabetes mellitus. Coron Artery Dis. 2003;14:61–64. doi: 10.1097/00019501-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 26.Kilian JG, Keech A, Adams MR, Celermajer DS. Coronary collateralization: determinants of adequate distal vessel filling after arterial occlusion. Coron Artery Dis. 2002;13:155–159. doi: 10.1097/00019501-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Werner GS, Richartz BM, Heinke S, Ferrari M, Figulla HR. Impaired acute collateral recruitment as a possible mechanism for increased cardiac adverse events in patients with diabetes mellitus. Eur Heart J. 2003;24:1134–1142. doi: 10.1016/s0195-668x(03)00187-8. [DOI] [PubMed] [Google Scholar]
- 28.Melidonis A, Tournis S, Kouvaras G, Baltaretsou E, Hadanis S, Hajissavas I, Tsatsoulis A, Foussas S. Comparison of coronary collateral circulation in diabetic and nondiabetic patients suffering from coronary artery disease. Clin Cardiol. 1999;22:465–471. doi: 10.1002/clc.4960220706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilia R, Carmel S, Cafri C, Gueron M. Coronary collaterals in patients with normal and impaired left ventricular systolic function. Int J Cardiol. 1998;63:151–153. doi: 10.1016/s0167-5273(97)00297-0. [DOI] [PubMed] [Google Scholar]
- 30.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 33.He Z, Opland DM, Way KJ, Ueki K, Bodyak N, Kang PM, Izumo S, Kulkarni RN, Wang B, Liao R, Kahn CR, King GL. Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000209500.15801.4e. [DOI] [PubMed] [Google Scholar]
- 34.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 36.McIlroy M, O’Rourke M, McKeown SR, Hirst DG, Robson T. Pericytes influence endothelial cell growth characteristics: role of plasminogen activator inhibitor type 1 (PAI-1) Cardiovasc Res. 2006;69:207–217. doi: 10.1016/j.cardiores.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Wiman B, Andersson T, Hallqvist J, Reuterwall C, Ahlbom A, deFaire U. Plasma levels of tissue plasminogen activator/plasminogen activator inhibitor-1 complex and von Willebrand factor are significant risk markers for recurrent myocardial infarction in the Stockholm Heart Epidemiology Program (SHEEP) study. Arterioscler Thromb Vasc Biol. 2000;20:2019–2023. doi: 10.1161/01.atv.20.8.2019. [DOI] [PubMed] [Google Scholar]
- 38.Yamada T, Okamoto M, Sueda T, Hashimoto M, Kajiyama G. Relation between collateral flow assessed by Doppler guide wire and angiographic collateral grades. Am Heart J. 1995;130:32–37. doi: 10.1016/0002-8703(95)90232-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.