Abstract
Aims
Cardiac dyssynchrony is common in patients with heart failure, whether or not they have ischaemic heart disease (IHD). The effect of the underlying cause of cardiac dysfunction on the response to cardiac resynchronization therapy (CRT) is unknown. This issue was addressed using data from the CARE-HF trial.
Methods and results
Patients (n = 813) were grouped by heart failure aetiology (IHD n = 339 vs. non-IHD n = 473), and the primary composite (all-cause mortality or unplanned hospitalization for a major cardiovascular event) and principal secondary (all-cause mortality) endpoints analysed. Heart failure severity and the degree of dyssynchrony were compared between the groups by analysing baseline clinical and echocardiographic variables. Patients with IHD were more likely to be in NYHA class IV (7.5 vs. 4.0%; P = 0.03) and to have higher NT-proBNP levels (2182 vs. 1725 pg/L), indicating more advanced heart failure. The degree of dyssynchrony was more pronounced in patients without IHD (assessed using mean QRS duration, interventricular mechanical delay, and aorta-pulmonary pre-ejection time). Left ventricular ejection fraction and left ventricular end-systolic volume improved to a lesser extent in the IHD group (4.53 vs. 8.50% and −35.68 vs. –58.52 cm3). Despite these differences, CRT improved all-cause mortality, NYHA class, and hospitalization rates to a similar extent in patients with or without IHD.
Conclusion
The benefits of CRT in patients with or without IHD were similar in relative terms in the CARE-HF study but as patients with IHD had a worse prognosis, the benefit in absolute terms may be greater.
Keywords: Dyssynchrony, Aetiology, Ischaemic, Resynchronization, CARE-HF
Introduction
The overall goal with the treatment of heart failure patients is to improve quality of life and symptoms such as tiredness and dyspnoea, reduce morbidity leading to hospitalization, and improve survival. Despite the remarkable advances in pharmacological treatment of this condition, many patients remain symptomatic with a poor prognosis.1,2 QRS prolongation, thought to denote intraventricular conduction abnormalities, is present in ∼25% of patients with heart failure and has been associated with a poor prognosis.3,4 The development of a pacemaker with an extra third lead placed epicardially on the left ventricle via the coronary sinus has provided an effective treatment for dyssynchrony. Cardiac resynchronization therapy (CRT) has been shown in several trials to reduce dyssynchrony and improve symptoms, quality of life, and exercise capacity in patients with heart failure.5 The COMPANION trial demonstrated a reduction in the combined endpoint mortality and hospitalizations,6 although a statistically significant reduction in mortality could only be demonstrated when CRT was combined with a defibrillator. The CARE-HF study demonstrated that CRT alone could reduce the risk of death and unplanned hospitalization substantially compared with optimal pharmacological treatment for heart failure (hazard ratio 0.64, 95% confidence interval 0.48–0.85, P < 0.002).7 However, the response to CRT is variable, and selecting patients who will respond to this treatment is of concern to patients, doctors, and those who provide health-care funding.8 Clinically and mechanically, patients with ischaemic heart disease (IHD) differ from patients with dilated cardiomyopathy.9,10 Response to CRT treatment may depend on the underlying myocardial pathology, especially regional variations in function and the extent of scar. The aim of the present study was to examine whether patients with and without IHD differed with regard to the response to CRT by analysing primary and secondary endpoints in the CARE-HF trial.
Methods
The design and results of the CARE-HF study have been reported elsewhere.11 Briefly, heart failure patients were included if they fulfilled the following criteria: >18 years of age; recent NYHA class III or IV symptoms despite optimal pharmacological therapy; left ventricular (LV) ejection fraction (EF) ≤35%; LV end-diastolic diameter (LVEDD) ≥30 mm/m2; and QRS width ≥120 ms. If QRS width was 120–149 ms, two out of three criteria of dyssynchrony were also required: an aortic pre-ejection delay >140 ms; an interventricular mechanical delay (IVMD) >40 ms; or, delayed activation of the posterolateral LV wall. A total of 813 patients were randomly assigned to either medical therapy alone or medical therapy in combination with CRT and followed for mean 29.4 months (range 18–44.7 months). In this analysis, patients were grouped according to heart failure aetiology: IHD n = 339 (41.8%) and non-IHD n = 474 (58.2%) for the analysis of primary composite endpoint (all-cause mortality or unplanned hospitalization for a major cardiovascular event) and secondary endpoint (death from any cause). Baseline characteristics of the two patient groups were compared and an evaluation of differences in outcome conducted using: the Minnesota living with heart failure questionnaire (MLWHF); NYHA class; QRS duration—at 18 months; echocardiographic variables [mitral regurgitation, LVEDD, LV end-systolic diameter (LVESD), LVEF, and IVMD] at 3 and 18 months of assigned therapy. Patients were defined as having IHD if they had a history of myocardial infarction, and/or a coronary angiogram indicating major disease and/or a history of coronary artery by-pass graft and/or angioplasty. Previous reports have defined aetiology by the principal diagnosis but any diagnosis of IHD was used in this analysis, which accounts for minor discrepancies in numbers of patients between reports.7
Statistical methods
All statistical tests were two sided, and an α value of 5% was considered to be nominally statistically significant. No adjustment was made for multiple testing. Median values were compared using the Kruskal–Wallis test, difference in means was assessed using generalized mixed models with an identity link and normal error, incorporating baseline values as patient level covariates and the investigational sites as random effects. Interaction terms to describe the relationship between a patient level characteristic of interest and the effects of treatment were estimated in the multivariable models through fitting terms describing the main effects of the characteristic of interest and a treatment, and an interaction term. Survival functions were contrasted using approximate frailty models, which included investigational sites as grouped frailties. These models were also used to estimate interaction effects for the primary and secondary outcomes. All analyses were conducted in SAS 9.1 (SAS Institute, Cary, NC, USA).
The authors had full access to the data and take responsibility of its integrity. All authors have read and agreed to the manuscript as written.
Results
Of 339 patients with IHD, 186 (54.9%) were assigned to CRT and 153 (45.1%) to the control group, and of patients with dilated cardiomyopathy or other causes of heart failure (non-IHD), 223 (47.2%) were assigned to CRT and 250 (52.9%) to the control group. Baseline characteristics of the patients are presented in Table 1. The clinical characteristics in the two patient groups differed in many aspects. Patients with IHD were older (P < 0.001) and were more often men (P < 0.001). High-dose furosemide (56 vs. 34%, respectively; P < 0.001) was more frequently used in those with IHD, whereas beta-blockers (67 vs. 76%, respectively; P = 0.003) and angiotensin-converting enzyme-inhibitors (92.9 vs. 96.2%, respectively; P = 0.06) were used more frequently in patients without IHD (Table 1). More patients with IHD were in NYHA class IV, a difference that was statistically significant in both the CRT and medical therapy group (Table 1). Furthermore, median NT-proBNP levels were higher at baseline in patients with IHD, both for CRT and medically treated patients (Table 1).
Table 1.
Baseline characteristics in the 813 patients enrolled in CARE-HF
| CRT group |
Medical therapy group |
IHD vs. non-IHD (P-value) | |||
|---|---|---|---|---|---|
| IHD n = 186 | Non-IHD n = 223 | IHD n = 153 | Non-IHD n = 250 | ||
| Male sex, n (%) | 154 (82.80) | 150 (67.26) | 132 (86.27) | 161 (64.40) | <0.0001 |
| Age (years), mean (SD) | 67.69 (9.02) | 63.73 (10.72) | 67.94 (8.82) | 63.44 (10.41) | <0.0001 |
| NYHA class IV, n (%) | 14 (7.53) | 9 (4.04) | 14 (9.15) | 13 (5.20) | 0.039 |
| Systolic blood pressure (mmHg), mean (SD) | 116.69 (16.44) | 118.21 (17.41) | 115.45 (18.17) | 118.70 (17.89) | 0.12 |
| LVEF (%), mean (SD) | 25.30 (5.72) | 25.47 (6.19) | 26.20 (6.01) | 25.27 (6.15) | 0.19 |
| LVESV (cm3), mean (SD) | 120.86 (42.66) | 129.98 (52.83) | 123.70 (40.09) | 130.81 (54.79) | 0.22 |
| Mitral regurgitation, median (IQR)a | 23.57 (14.37) | 23.59 (16.15) | 23.11 (13.18) | 24.51 (15.33) | 0.95 |
| ACE-I/ARB, n (%) | 175 (94.09) | 212 (95.07) | 140 (91.50) | 243 (97.20) | 0.052 |
| Digoxin, n (%) | 75 (40.32) | 90 (40.36) | 54 (35.29) | 127 (50.80) | 0.031 |
| β-Blocker, n (%) | 124 (66.67) | 174 (78) | 102 (66.67) | 186 (74.40) | 0.003 |
| Loop diuretics ≥80 mg per day furosemide or equivalent, n (%) | 101 (54.30) | 74 (33.18) | 89 (58.17) | 88 (35.20) | <0.0001 |
| GFR (mL/min), median (IQR) | 56.93 (43.74–70.63) | 61.43 (49.41–75.61) | 52.51 (42.75–65.84) | 64.16 (49.99–77.54) | <0.0001 |
| NT-proBNP (pg/mL), median (IQR) | 2182 (888–4888) | 1725 (651–3992) | 2064 (1057–4787) | 1658 (592–3611) | 0.0021 |
| QRS (ms), mean (SD) | 163.47 (18.95) | 167.41 (20.85) | 161.84 (20.33) | 166.25 (17.57) | 0.0012 |
| IVMD >40, n (%) | 64 (38.10) | 117 (59.39) | 43 (31.85) | 144 (61.28) | <0.0001 |
| IVMD (ms), mean (SD) | 41.44 (26.30) | 56.70 (29.61) | 37.86 (24.55) | 54.64 (26.75) | <0.0001 |
| Aorta pre-ejection time ≥140 ms, n (%) | 101 (58.72) | 166 (82.59) | 77 (55.40) | 193 (80.75) | <0.0001 |
| Pulmonary pre-ejection time ≥140 ms, n (%) | 25 (14.88) | 45 (22.61) | 23 (17.04) | 51 (21.43) | 0.0471 |
ACE-I, angiotensin-converting enzyme-inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; IVMD, interventricular mechanical delay; NT-proBNP, brain natriuretic peptide.
aThe area of the mitral regurgitation jet was calculated as the area of the colour flow Doppler regurgitant jet divided by the area of the left atrium in systole, both in square centimetres.
The proportion of patients with echocardiographic signs of dyssynchrony at baseline, including IVMD >40 ms, aorta pre-ejection time >140 ms, and a QRS duration ≥150 ms at baseline, was greater in patients without IHD.
The effect of CRT on echocardiographic variables by aetiology and assigned treatment at 3 months are shown in Table 2. At this time, reductions in IVMD, mitral regurgitation, and LV end-systolic volume and improvement in LVEF with CRT were already substantial and no clear difference in the magnitude of effect of CRT between patients with and without IHD had appeared.
Table 2.
Echocardiographic and quality of life outcome differences at 3 months’ follow-up
| CRT group |
Medical therapy group |
P-value for interactiona | |||
|---|---|---|---|---|---|
| IHD | Non-IHD | Non-IHD | IHD | ||
| Mitral regurgitation index, median (IQR) | 16.75 (9.73–26.13) | 13.37 (5.48–24.63) | 19.03 (11.51–31.90) | 18.05 (9.51–28.77) | 0.1866 |
| LVEDD (cm), mean (SD) | 7.15 (0.99) | 6.90 (1.25) | 7.20 (1.11) | 7.26 (1.05) | 0.2858 |
| LVESV (cm3), mean (SD) | 193.99 (69.36) | 194.01 (104.74) | 233.18 (98.36) | 231.54 (86.05) | 0.0354 |
| LVEF (%), mean (SD) | 29.08 (6.90) | 30.59 (8.19) | 26.56 (6.92) | 26.31 (6.50) | 0.3550 |
| IVMD (ms), mean (SD) | 22.38 (20.03) | 32.54 (22.52) | 52.86 (29.24) | 39.30 (27.07) | 0.3570 |
| MLWHF, mean (SD) | 31.29 (19.74) | 41.50 (20.49) | 35.56 (21.68) | 30.25 (22.00) | 0.1542 |
IHD, ischaemic heart disease; IVMD, interventricular mechanical delay; LVEDD, left ventricular end-diastolic diameter; LVESV, left ventricular end-systolic volume.
aBetween effect of treatment and ischaemia.
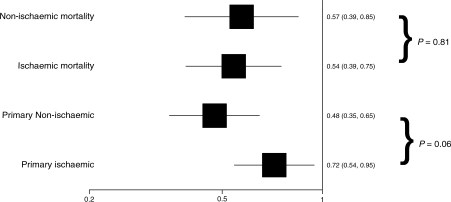
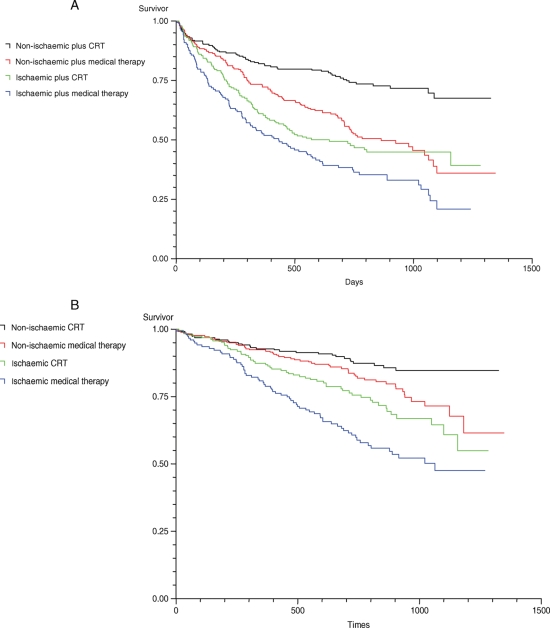
Clinical outcome variables measured during the study at 18 months’ follow-up are presented in Table 3. Mean QRS duration at 18 months was shortened and quality of life and NYHA class were improved by CRT in both patient groups. There was a trend for a greater effect of CRT on the primary outcome measure of all-cause mortality and cardiovascular hospitalization, in patients without IHD (hazard ratio 0.48; 95% CI 0.35–0.65) when compared with those with IHD (hazard ratio 0.72; 95% CI 0.54–0.95) (Table 4, Figures 1 and 2). However, the interaction term comparing these two hazard functions was not statistically significant, indicating that the apparent difference could plausibly be explained by chance (P = 0.06). The effect of CRT on all-cause mortality was very similar [hazard ratio 0.60 (95% CI 0.42–0.86) and 0.59 (95% CI 0.37–0.92) for IHD and no IHD, respectively].
Table 3.
Outcome at 18 months’ follow-up
| Value | Estimate | 95% CI | P-value | P-value for interactiona |
|---|---|---|---|---|
| Difference in mean NYHA | ||||
| Non-IHD | −0.53 | −0.74 to −0.33 | <0.0001 | 0.30 |
| IHD | −0.69 | −0.69 | <0.0001 | |
| End of study status (odds ratio) | ||||
| Non-IHD | 0.52 | 0.35 to 0.76 | 0.0009 | 0.49 |
| IHD | 0.42 | 0.27 to 0.66 | 0.0001 | |
| Difference in mean MR | ||||
| Non-IHD | 4.77 | 1.12 to 8.42 | 0.0109 | 0.83 |
| IHD | 4.16 | −0.44 to 8.75 | 0.0755 | |
| LVEF (%) | ||||
| Non-IHD | 8.50 | 10.14 to 6.85 | <0.0001 | 0.003 |
| IHD | 4.53 | 6.24 to 2.82 | <0.0001 | |
| LVESV (cm3) | ||||
| Non-IHD | −58.52 | 45.56 to 71.49 | <0.0001 | 0.01 |
| IHD | −35.68 | 20.80 to 50.56 | <0.0001 | |
| NT-proBNP | ||||
| Non-IHD | −1231.24 | −1992.53 to −469.96 | 0.0016 | 0.97 |
| IHD | −1137.89 | −2441.17 to 165.38 | 0.0866 | |
| Difference in mean QRS | ||||
| Non-IHD | −19.8 | −25.5 to −14.1 | <0.0001 | 0.03 |
| IHD | −9.7 | −16.4 to −2.9 | 0.0052 | |
IHD, ischaemic heart disease; MLWHF, Minnesota living with heart failure questionnaire; MR, mitral regurgitation.
aBetween effect of treatment and ischaemia.
Table 4.
Primary and secondary outcomes in patients with and without ischaemic heart diseasea
| Value | Hazard ratio | 95% CI | P-value | P-value for interactionb |
|---|---|---|---|---|
| Primary outcome | ||||
| Non-IHD | 0.46 | 0.35–0.63 | <0.0001 | 0.06 |
| IHD | 0.71 | 0.54–0.94 | 0.018 | |
| All-cause mortality | ||||
| Non-IHD | 0.56 | 0.37–0.83 | 0.004 | 0.86 |
| IHD | 0.54 | 0.39–0.76 | 0.0003 | |
IHD, ischaemic heart disease.
aPrimary outcome combined mortality and hospitalization. Secondary outcome, all-cause mortality.
bBetween effect of treatment and ischaemia.
Figure 1.
Hazard ratio and 95% confidence interval for ischaemic and non-ischaemic subgroups, primary outcome, and all-cause mortality.
Figure 2.
Kaplan–Meier estimates of the time to the primary endpoint (A) and the secondary endpoint death from any cause (B) according to aetiology.
Discussion
Previous reports and theoretical considerations have led many to believe that the benefits of CRT are less in patients with IHD compared with those without IHD. This analysis of CARE-HF suggests that the long-term effects of CRT on symptoms, quality of life, morbidity, and mortality are similar in patients with and without IHD. These benefits were observed even though patients with IHD had less evidence of dyssynchrony, lower arterial pressure, worse renal function, higher doses of diuretic therapy, more severe cardiac dysfunction, more severe heart failure, and a worse overall prognosis. Accordingly, patients with IHD should not be denied CRT on the grounds of the aetiology of their LV dysfunction alone. Of note, the severity and complexity of heart failure in patients with IHD was associated with lower uptake of guideline-indicated therapy at baseline, such as beta-blockers.
The initial improvement in cardiac function, both in terms of reduced mitral regurgitation and improved LVEF, was similar in patients with and without IHD, although trends favoured patients without IHD. Patients with IHD had less dyssynchrony and this might have accounted for the observed trends although it is uncertain whether the degree of dyssynchrony predicts the extent of improvement in LV function or of mitral regurgitation.12–16 However, in the long-term, as reported elsewhere, LV function improved to a lesser extent in patients with IHD, presumably reflecting the relative inability of ventricular scar tissue to remodel favourably.17 This observation is further corroborated by echocardiographic findings in the MIRACLE study.18 In that study, although LV volumes were reduced compared with baseline values after 12 months of CRT, the percentage of patients demonstrating progressive reduction of LV size decreased from 6 to 12 months. Further, LVEF improved and LV mass regressed. The changes observed were greater in non-IHD patients.18 The trend for a greater effect of CRT in non-IHD might be explained by a quantitative effect on more viable tissue (cardiomyocytes) in the non-IHD group and thus a better substrate for response.19
This suggests that CRT may exert additional effects on prognosis than merely improving LV function. The combination of IHD and a low LVEF predisposes to ventricular arrhythmias, and CRT reduced sudden cardiac death during long-term follow-up, suggesting a favourable effect on the arrhythmic substrate.14
Whether or not specific patterns or extent of ventricular scar can predict CRT response has been the subject of several small observational studies that await confirmation.14 It may be harder to find an effective pacing site in patients with extensive or postero-lateral scar, and these patients might get less improvement in ventricular function and possibly symptoms. However, the studies provide no adequate data on major morbidity or mortality. Without a control group, it may be difficult to gauge whether the amount of scar really influences the benefits of CRT.12 Maybe, these patients will do badly with CRT but much worse without. Only randomized controlled trials can address such issues robustly. No attempt was made to define the extent or distribution of scar in the CARE-HF study. It is possible that the effect of CRT was heterogeneous within the group of patients with IHD. It is known that patients with IHD and extensive myocardial viability are more likely to show a large improvement in LV function in response to beta-blockers and there are some similar data with CRT.20 Whether these greater benefits in LV function translate into greater benefits on morbidity and mortality is not clear and it would be premature to jump to such a conclusion.
No attempt was made to measure intraventricular dyssynchrony in CARE-HF, reflecting the then existing and now persisting lack of consensus on how it should be done. The large trials of CRT have not relied on measures of intraventricular dyssynchrony and yet shown benefit. Small observational trials suggest that such measures might be useful in patients selection but lack control groups to prove their hypothesis. Moreover, there is evidence that dyssynchrony changes with physiological stress and over time.12,21 Many more patients with heart failure experience dyssynchrony than appears the case in simple cross-sectional studies of patients at rest. The feasibility of an approach dependent on repeated stress-dyssynchrony studies, which might be what is required for thorough assessment, has not been explored. Indeed, dyssynchrony might be a near-universal phenomenon in patients with severe LV dilatation and dysfunction.16 Certainly, no adequately powered randomized controlled trial has yet identified a group of patients with heart failure in whom CRT is not effective in improving ventricular function, symptoms, morbidity, and mortality. How to measure dyssynchrony and whether it is truly the substrate for CRT are likely to remain controversial for some time.12
It is possible that the reasons why patients with IHD respond to CRT are different even if the ultimate effect is similar. In a PET study performed by Linde and co-workers,22 myocardial perfusion improved both patients with and without IHD. Improvement in perfusion may be of greater importance to patients with flow-limiting coronary disease and an extensive collateral circulation than to patients with unobstructed coronary arteries. Further, the relation between the degree of dyssynchrony and QRS width is weak and cannot explain the differences in response to CRT between the two groups. Mitral valve insufficiency may be due to damage to the papillary apparatus in patients with IHD as well as due to dilatation of the mitral annulus, and this could account for differences in response.23 However, as improvement in mitral regurgitation may be critically dependent on the site and timing of the ventricular stimuli, the aetiology of heart failure may not be an overriding factor in clinical practice, as suggested by this study.24–28
Inevitably, data on mechanistic outcomes used here to attempt to describe any differences between the ischaemic and non-ischaemic groups in response to treatment are not available for all subjects. It was possible to determine ischaemic status in all but one subject in the trial. Ejection fraction, end-systolic volume index, and intraventricular mechanical delay were available in >80% of surviving subjects at 3 months. Ejection fraction and end-systolic volume index were also available in nearly 70% of subjects at 18 months. Difficulties in measurement led to only 66% of available subjects providing a mitral regurgitation index score at 3 months, and 52% at 18 months. Left ventricular end-diastolic diameter was subject to considerable missing data (21% of surviving subjects provided a measure at 3 months). Outcome data were available on a substantially higher proportion of subjects, with 3 month Minnesota Living with Heart Failure data available on 86% of subjects, and at 18 months, 94% of subjects provided NYHA data and 100% provided end of study status. All randomized subjects provided data on mortality and the primary outcome.
In conclusion, despite smaller long-term improvements in LV function in patients with IHD, the relative benefits of CRT on morbidity and mortality are similar in patients with and without IHD. However, as patients with IHD generally have a worse prognosis, the absolute reduction in mortality tends to be greater in this group. This dissociation between improvement in LV function and reduction in mortality suggests that CRT may exert effects beyond LV remodelling, perhaps by reducing the arrhythmic substrate. These data suggest that improvement in LV function may not be a good surrogate for the effects on mortality unless the aetiology of LV dysfunction is taken into account.
Funding
C.B.L. has received a research grant from Medtronic. Funding to pay open access publication charges for this article was provided by Professor G. Wikstrom's research grants provided by Uppsala University funds.
Conflict of interest: J.G.F.C. and N.F. were steering committee members for the trial and received payment for work done and have also received speaker's honoraria from Medtronic. G.W. has received speaker's honoraria from Medtronic.
Acknowledgements
The authors would like to thank the CARE-HF team for their contribution in the planning and running of the CARE-HF study and for their facilitation in the preparation of this manuscript.
References
- 1.Cleland JG, Clark AL. Delivering the cumulative benefits of triple therapy to improve outcomes in heart failure: too many cooks will spoil the broth. J Am Coll Cardiol. 2003;42:1234–1237. doi: 10.1016/s0735-1097(03)00948-3. [DOI] [PubMed] [Google Scholar]
- 2.Khand A, Gemmel I, Clark AL, Cleland JG. Is the prognosis of heart failure improving? J Am Coll Cardiol. 2000;36:2284–2286. doi: 10.1016/s0735-1097(00)00995-5. [DOI] [PubMed] [Google Scholar]
- 3.Kalra PR, Sharma R, Shamim W, Doehner W, Wensel R, Bolger AP, Genth-Zotz S, Cicoira M, Coats AJ, Anker SD. Clinical characteristics and survival of patients with chronic heart failure and prolonged QRS duration. Int J Cardiol. 2002;86:225–231. doi: 10.1016/s0167-5273(02)00270-x. [DOI] [PubMed] [Google Scholar]
- 4.Baldasseroni S, Gentile A, Gorini M, Marchionni N, Marini M, Masotti G, Porcu M, Maggioni AP Italian Network on Congestive Heart Failure Investigators. Intraventricular conduction defects in patients with congestive heart failure: left but not right bundle branch block is an independent predictor of prognosis. A report from the Italian Network on Congestive Heart Failure (IN-CHF database) Ital Heart J. 2003;4:607–613. [PubMed] [Google Scholar]
- 5.Stahlberg M, Braunschweig F, Gadler F, Karlsson H, Linde C. Three year outcome of cardiac resynchronization therapy: a single center evaluation. Pacing Clin Electrophysiol. 2005;28:1013–1017. doi: 10.1111/j.1540-8159.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM Comparison of Medical Therapy, Pacing, Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, Bryan S CARE-HF Investigators. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J. 2005;26:2681–2688. doi: 10.1093/eurheartj/ehi662. [DOI] [PubMed] [Google Scholar]
- 9.Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005;10:211–222. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- 10.de Sisti A, Toussaint JF, Lavergne T, Ollitrault J, Abergel E, Paziaud O, Ait Said M, Sader R, Le Heuzey JY, Guize L. Determinants of mortality in patients undergoing cardiac resynchronization therapy: baseline clinical, echocardiographic, and angioscintigraphic evaluation prior to resynchronization. Pacing Clin Electrophysiol. 2005;28:1260–1270. doi: 10.1111/j.1540-8159.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Klein W, Tavazzi L CARE-HF Study Steering Committee Investigators. The CARE-HF study (Cardiac Resynchronisation in Heart Failure study): rationale, design and end-points. Eur J Heart Fail. 2001;3:481–489. doi: 10.1016/s1388-9842(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 12.Cleland JG, Nasir M, Tageldien A. Cardiac resynchronization therapy or atrio-biventricular pacing—what should it be called? Nat Clin Pract Cardiovasc Med. 2007;4:90–101. doi: 10.1038/ncpcardio0794. [DOI] [PubMed] [Google Scholar]
- 13.Fruhwald FM, Fahrleitner-Pammer A, Berger R, Leyva F, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, Daubert JC, Cleland JG. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony. Eur Heart J. 2007;28:1592–1597. doi: 10.1093/eurheartj/ehl505. [DOI] [PubMed] [Google Scholar]
- 14.Ypenburg C, Roes S, Bleeker B, Kaandorp T, de Roos A, Schalij M, van der Wall E, Bax J. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol. 2007;99:657–660. doi: 10.1016/j.amjcard.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 15.Agricola E, Oppizzi M, Galderisi M, Pisani M, Meris A, Pappone C, Margonato A. Role of regional mechanical dyssynchrony as determinant of functional mitral regurgitation in patients with left ventricular systolic dysfunction. Heart. 2006;92:1390–1395. doi: 10.1136/hrt.2005.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caso P, D’Andrea A, Martiniello AR, Severino S, Cioppa C, Iengo R, Di Salvo G, Ascione L, Scherillo M, Calabro R. Myocardial systolic activation delay in patients with left bundle branch block and either normal or impaired left ventricular function. Echocardiography. 2006;23:14–23. doi: 10.1111/j.1540-8175.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghio S, Freemantle N, Cleland JGF, Serio A, Magrini G, Scelsi L, Pasotti M, Stegeman B, Tavazzi L. Long-term ventricular reverse remodelling with cardiac resynchronisation therapy. Results from the CARE-HF Trial. (Abstract) Circulation. 2005;112(suppl.):II-672. doi: 10.1093/eurjhf/hfp034. [DOI] [PubMed] [Google Scholar]
- 18.St John Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology. Circulation. 2006;113:266–272. doi: 10.1161/CIRCULATIONAHA.104.520817. [DOI] [PubMed] [Google Scholar]
- 19.van Campen CM, Visser FC, van der Weerdt AP, Knaapen P, Comans EF, Lammertsma AA, de Cock CC, Visser CA. Eur J Nucl Med Mol Imaging. 2007;34:309–315. doi: 10.1007/s00259-006-0235-y. Published online ahead of print 26 September 2006. [DOI] [PubMed] [Google Scholar]
- 20.Bellenger NG, Rajappan K, Rahman SL, Lahiri A, Raval U, Webster J, Murray GD, Coats AJ, Cleland JG, Pennell DJ CHRISTMAS Study Steering Committee and Investigators. Effects of carvedilol on left ventricular remodelling in chronic stable heart failure: a cardiovascular magnetic resonance study. Heart. 2004;90:760–764. doi: 10.1136/hrt.2003.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Alamgir MF, Nikitin NP, Clark AL, Cleland JG. Prevalence of intraventricular dyssynchrony increases with stress. (Abstract) Circulation. 2005;112(suppl.):II-403. [Google Scholar]
- 22.Braunschweig F, Sorensen J, von Bibra H, Olsson A, Ryden L, Langstrom B, Linde C. Effects of biventricular pacing on myocardial blood flow and oxygen consumption using carbon-11 acetate positron emission tomography in patients with heart failure. Am J Cardiol. 2003;92:95–99. doi: 10.1016/s0002-9149(03)00479-x. [DOI] [PubMed] [Google Scholar]
- 23.Uemura T, Otsuji Y, Nakashiki K, Yoshifuku S, Maki Y, Yu B, Mizukami N, Kuwahara E, Hamasaki S, Biro S, Kisanuki A, Minagoe S, Levine RA, Tei C. Papillary muscle dysfunction attenuates ischemic mitral regurgitation in patients with localized basal inferior left ventricular remodeling: insights from tissue Doppler strain imaging. J Am Coll Cardiol. 2005;46:113–119. doi: 10.1016/j.jacc.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 24.Bleeker GB, Schalij MJ, Nihoyannopoulos P, Steendijk P, Molhoek SG, van Erven L, Bootsma M, Holman ER, van der Wall EE, Bax JJ. Left ventricular dyssynchrony predicts right ventricular remodeling after cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2264–2269. doi: 10.1016/j.jacc.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 25.Ghio S, Freemantle N, Serio A, Magrini G, Scelsi L, Pasotti M, Cleland JG, Tavazzi L. Baseline echocardiographic characteristics of heart failure patients enrolled in a large European multicentre trial (Cardiac Resynchronisation Heart Failure study) Eur J Echocardiogr. 2006;7:373–378. doi: 10.1016/j.euje.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Squire I. Neurohormonal intervention to reduce sudden cardiac death in heart failure: what is the optimal pharmacologic strategy? Heart Fail Rev. 2004;9:337–345. doi: 10.1007/s10741-005-7301-6. Discussion 347–351. [DOI] [PubMed] [Google Scholar]
- 27.Hummel JP, Lindner JR, Belcik JT, Ferguson JD, Mangrum JM, Bergin JD, Haines DE, Lake DE, DiMarco JP, Mounsey JP. Extent of myocardial viability predicts response to biventricular pacing in ischemic cardiomyopathy. Heart Rhythm. 2005;2:1211–1217. doi: 10.1016/j.hrthm.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, Iskandrian AE. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]