Abstract
Aims
To determine the effects of omega-3 polyunsaturated fatty acids (omega-3 PUFAs) from fish on the incidence of recurrent ventricular arrhythmia in implantable cardioverter defibrillator (ICD) patients by combining results from published trials.
Methods and results
We searched in the Medline, EMBASE, and Cochrane databases and performed a meta-analysis on all three available trials on fish oil and ventricular arrhythmia. Furthermore, we pooled individual data of two of these randomized, double-blind, placebo-controlled trials (Raitt et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA 2005;293:2884–2891 and Brouwer et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA 2006;295:2613–2619). The main outcome was time to first confirmed ventricular fibrillation (VF) or ventricular tachycardia (VT) combined with death for the meta-analysis, and time to first spontaneous confirmed VF or VT for the pooled analysis. The meta-analysis (n = 1148) showed no convincing protective effect of fish oil (RR 0.90; 95% CI 0.67–1.22). The hazard ratio for the subgroup of patients with coronary artery disease at baseline (0.79; 0.60–1.06) tended towards a protective effect. The pooled analysis (n = 722) showed that time to appropriate ICD intervention was similar for fish oil and placebo treatment (log-rank P = 0.79).
Conclusion
These findings do not support a protective effect of omega-3 PUFAs from fish oil on cardiac arrhythmia in all patients with an ICD. Current data neither prove nor disprove a beneficial or a detrimental effect for subgroups of patients with specific underlying pathologies.
Keywords: ICD, Arrhythmia, Fish oil, Omega-3
Introduction
Observational studies strongly suggest that the intake of very long-chain omega-3 polyunsaturated fatty acids (omega-3 PUFAs) as found in algae and fish protects against fatal coronary heart disease.1,2 Trials in patients with myocardial infarction (MI) also indicate that fish or omega-3 PUFA reduce the incidence of fatal coronary heart disease.3,4 The effect seems most pronounced for fatal heart disease and sudden death.3–7 Furthermore, sudden death is in many cases preceded by life-threatening cardiac arrhythmia.8 This supports the hypothesis that omega-3 PUFA from fish oil may prevent cardiac arrhythmia. Animal and in vitro studies also indicate that omega-3 PUFA prevent fatal heart disease and sudden death by reducing susceptibility for ventricular arrhythmia.9–11 Three double-blind, randomized intervention studies in patients with implantable cardioverter defibrillators (ICDs) investigated the direct effects of fish oil on ventricular tachyarrhythmia. None of the three trials convincingly showed whether or not supplementation with omega-3 PUFA has preventive effects in ICD patients. Therefore, we combined the results of these three trials to assess the effect of fish oil on ventricular tachyarrhythmia in the total group of ICD patients and in subgroups with different disease history.
Methods
Literature search and selection
We performed a literature search in Medline, EMBASE, and the Cochrane Library. The search terms were fish oil or omega-3 AND implantable cardioverter defibrillator. We included only trials that investigated the effects of fish oil vs. placebo on spontaneous ventricular arrhythmia. This search resulted in 30 publications up until May 2008. Fourteen of these publications were reviews, 1 was a design paper, 1 was a cross-sectional analysis, 1 an uncontrolled experimental study, and 10 were opinions or letters to the editor. Only three were trials on the effects of fish oil on ventricular arrhythmia in patients with an ICD. These three were included in this analysis.
Use of data and quality
We used the data of all three trials on the effects of fish on ventricular arrhythmia that are currently available. In a meta-analysis, we combined the data of all three available studies. The studies were randomized, double-blind, placebo-controlled trials with high quality control standards. However, the non-compliance rate in the trial of Leaf et al.12 was rather high (∼35%). We had complete original individual data available for the Raitt et al.13 and Brouwer et al. studies.14 The data of Leaf et al. were extracted from the publication.12 The studies did not use the same clinical endpoints for all analyses. Therefore, we defined common clinical endpoints and re-analysed the data of Raitt et al. and Brouwer et al. before combining the outcomes with those of Leaf et al. Because two of the three studies lasted only 1 year, we used data from each study up to 1 year of intervention to standardize the duration of treatment across studies.
The data of Raitt et al. and Brouwer et al. were also used in a pooled analysis.
Patients
Patients in all studies had an ICD and prior malignant ventricular tachycardia (VT) or ventricular fibrillation (VF). Raitt et al. excluded patients using class I and class III anti-arrhythmic medication; Brouwer et al. and Leaf et al. included both patients using and not using these medications. Raitt et al. and Brouwer et al. excluded patients with high fish intake at baseline; patients of Leaf et al. were asked not to consume more than two fish meals per month during the trial.
Intervention
Patients randomly received either placebo or fish oil capsules in all three trials; the dose of very long-chain omega-3 PUFAs (main forms were EPA+DHA) from fish oil was 1.3 g/day in the study of Raitt et al. (n = 200) in the form of ethyl esters in 2 g of oil, 0.9 g/day in the form of triglycerides in 2 g of oil in the study of Brouwer et al. (n = 546), and 2.6 g/day in the form of ethyl esters in 4 g of oil in the study of Leaf et al. (n = 402).
Main outcome measurement
The primary outcome of the meta-analysis was time to first confirmed spontaneous ventricular tachyarrhythmia (VT or VF) or death from any cause. To optimize the meta-analysis of three trials, we adjusted the definition of clinical outcomes and subgroups of patients for the studies of Raitt et al. and Brouwer et al. We analysed relevant subgroups for which there was information available for all three trials. These subgroups consisted of patients having a left ventricular ejection fraction of ≤30 or >30, patients with coronary artery disease or ischaemic heart disease, and patients entering the trial having had a spontaneous VT. We re-analysed the data of Raitt et al. and Brouwer et al. in such a way that the subgroups and outcome variables were the same as for Leaf et al. For Raitt et al., data were used only up to 1 year of intervention. The main outcome measurement of the pooled analysis of the studies of Raitt et al. and Brouwer et al. was appropriate ICD treatment—shock or pacing—for a spontaneous VF or VT, as confirmed by judgement by cardiologists.
Statistical analysis
We performed statistical analyses on intention-to-treat data. We dealt with missing data by using the last available ICD reading. Participants without any ICD data or information on survival did not contribute to the person-time.
Meta-analyses
Meta-analyses were performed with STATA version 10. Combined estimates of treatments effects were calculated by applying a random effects model on hazard ratios (HRs) from the individual studies (Cox proportional hazards). We used the published data from Leaf et al., and for the other two trials, we adjusted the analysis (as described earlier) so as to fit with the subgroups and outcome variables as reported for Leaf et al. The random effects analysis accounts for heterogeneity among trials in any condition that may influence the true underlying effect. From each study, we used the subgroup outcomes corrected for baseline characteristics of patients to calculate the overall outcome of the meta-analysis. Statistical significance level was defined as α < 0.05 at two-sided tests.
Pooled analyses
Pooled analyses were performed with SAS software version 9 (SAS Institute Inc., Cary, NC, USA). Time-to-first-event graphs were generated using the Kaplan–Meier method. We created log cumulative hazard plots and used these plots to visually check the proportional hazards assumption. We checked parallelism across the two strata to be sure that the proportional hazards assumption was met. The statistical significance of observed differences between treatments was determined using log-rank tests. Cox proportional hazard models were used to assess outcomes when controlling for relevant baseline characteristics. We included a variable named ‘trial’ to take into account differences between studies/trials such as supplemented dose into account. Other variables were age, gender, ejection fraction (squared because of skewness of the distribution), current smoking, NYHA class for angina pectoris, NYHA class for dyspnoea, valvular heart disease, prior MI, cardiomyopathy, VT as index arrhythmia, VF as index arrhythmia, and use of anti-arrhythmic medication at baseline. The adjusted models were based on subjects with complete data on the characteristics included in the model.
Results
The main baseline characteristics of patients in the three trials were similar except for the use of antiarrhythmic medication (Table 1). This was expected, as Raitt et al. excluded patients who used specific antiarrhythmic medication, and the other two trials did not. In all three trials, subjects were on average just above 60 years of age, and ∼85% of them were male. Seventy to 80 per cent of the patients reported a history of coronary artery disease or ischaemic heart disease, and the mean ejection fraction was in the order of 35% (Table 1).
Table 1.
Baseline characteristics of the patients included in three published implantable cardioverter defibrillator trials
| Interventions | Leaf et al. |
Raitt et al. |
Brouwer et al. |
|||
|---|---|---|---|---|---|---|
| Fish oil (n = 200) | Placebo (n = 202) | Fish oil (n = 100) | Placebo (n = 100) | Fish oil (n = 273) | Placebo (n = 273) | |
| Age (years) mean (SD) | 66 (12) | 65 (12) | 63 (13) | 62 (13) | 61 (13) | 62 (11) |
| Gender (male) | 169 (85) | 165 (82) | 86 (86) | 86 (86) | 231 (85) | 228 (84) |
| Smoking (current smoker) | 30 (15) | 23 (11) | 13 (13) | 24 (24) | 44 (16) | 23 (8) |
| Ejection fraction (%) mean (SD) | 33 (14) | 34 (15) | 34 (34) | 36 (36) | 37 (15) | 37 (15) |
| Ejection fraction ≤30% | 102 (51) | 99 (49) | 46 (46) | 37 (37) | 87 (32) | 95 (35) |
| Medical history | ||||||
| CAD/IHD | 151 (76) | 163 (81) | 75 (75) | 71 (71) | 187 (73) | 197 (79) |
| MI | Not reported | Not reported | 55 (55) | 56 (56) | 167 (65) | 175 (70) |
| VT at entry | 93 (47) | 82 (41) | 64 (64) | 69 (69) | 205 (75) | 206 (76) |
| Medications | ||||||
| Amiodarone | 31 (16) | 31 (15) | 0 (0) | 0 (0) | 59 (22) | 50 (18) |
| Sotalol | 23 (12) | 33 (16) | 0 (0) | 0 (0) | 21 (8) | 15 (5) |
| Beta-blocking agent | 132 (66) | 118 (58) | 74 (74) | 73 (73) | 145 (53) | 155 (57) |
| ACE-inhibitor | 121 (61) | 114 (56) | 66 (66) | 66 (66) | 151 (77) | 160 (82) |
| Calcium channel blocker | 16 (8) | 15 (7) | 9 (9) | 13 (13) | 15 (8) | 13 (7) |
| Diuretics | 104 (52) | 99 (49) | 52 (52) | 54 (54) | 109 (56) | 116 (60) |
Values are numbers of patients (% of total group), except when indicated otherwise.
CAD, coronary artery disease; IHD, ischaemic heart disease; VT, ventricular tachycardia.
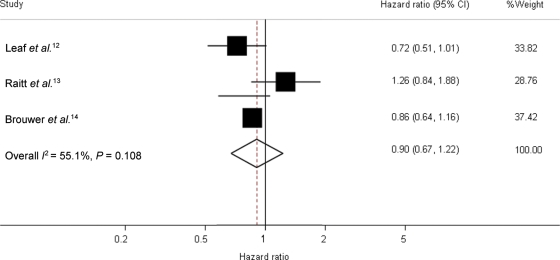
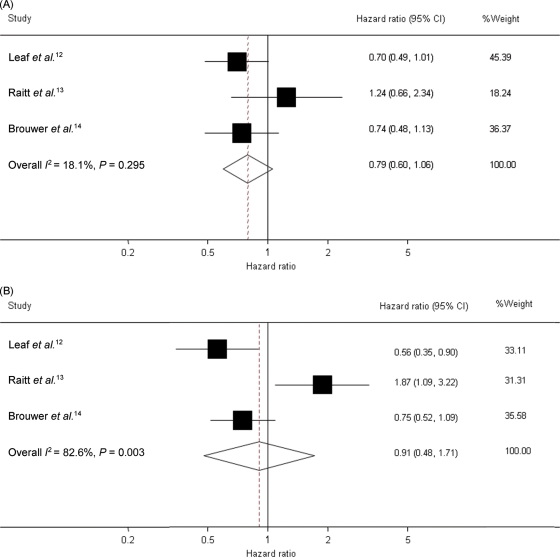
The meta-analysis of 1148 patients in the three trials showed a combined HR of fish oil vs. placebo of 0.90 [95% confidence interval (CI) 0.67–1.22] for time to first ICD intervention for a confirmed ventricular tachyarrhythmia or death (Figure 1). Meta-analyses in subgroups of patients (Figure 2) also revealed no statistically significant differences in risks of fish oil vs. placebo. The HR of fish oil vs. placebo in patients with an ejection fraction ≤30 was 0.80 (95% CI 0.58–1.12), and in patients with ejection fraction >30, it was 1.18 (95% CI 0.64–2.18). Heterogeneity in the last group was rather high (I2 = 70.1%, P = 0.035). The HR was 0.79 (95% CI 0.60–1.06) in patients with coronary artery disease at baseline (Figure 2B).
Figure 1.
Forest plot of hazard ratios and overall estimate of the effect of fish oil of time to first confirmed tachyarrhythmia or death in the studies. Leaf et al. and Brouwer et al. lasted 1 year and Raitt et al. 2 years; data refer only to the first year of intervention. Shaded squares indicate the point estimates for each trial (hazard ratios), with the size of the square proportional to the contribution (inverse variance random effects weight) of the study to the overall estimate. Heterogeneity χ2 P = 0.108. I2 (variation in ES attributable to heterogeneity) = 55.1%. The overall pooled estimate and 95% CI are indicated by the dotted line and the clear diamond.
Figure 2.
Forest plot of hazard ratios and overall estimate of the effect of fish oil on the first occurrence of tachyarrhythmia or death after inclusion in the studies until 1 year of intervention in the subgroups of patients with a history of coronary heart disease or ischaemic heart disease (A) and in a subgroup of patients with ventricular tachycardia at entry (B). Leaf et al. and Brouwer et al. lasted 1 year and Raitt et al. 2 years; data refer only to the first year of intervention. Shaded squares indicate the point estimates for each trial (hazard ratios adjusted for baseline characteristics), with the size of the square proportional to the contribution (inverse variance random effects weight) of the study to the overall estimate. The overall pooled estimate and 95% confidence interval are indicated by the dotted line and the clear diamond. (A) Subgroup of patients with coronary heart disease/ischaemic heart disease at entry. Heterogeneity χ2 P = 0.295. I2 (variation in ES attributable to heterogeneity) = 18.1%. (B) Subgroup of patients with ventricular tachycardia at entry. Heterogeneity χ2 P = 0.003. I2 (variation in ES attributable to heterogeneity) = 82.5%.
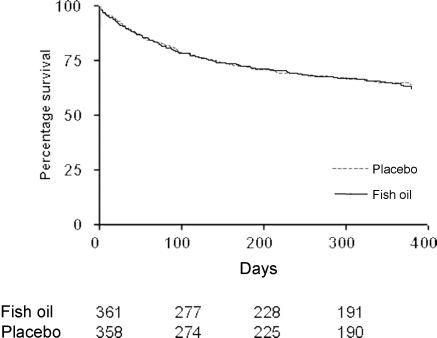
The pooled analysis on 746 patients from Raitt et al. and Brouwer et al. studies showed that time to first appropriate ICD intervention was similar in the treatment and placebo groups (Figure 3, log-rank P = 0.79). The primary endpoint of appropriate ICD intervention for ventricular tachyarrhythmia or death occurred in 126 (34%) patients receiving fish oil and in 121 (32%) patients taking placebo [crude HR 1.04, 95% CI 0.81–1.33; n = 722]. Figure 4 shows the adjusted value.
Figure 3.
Time to first ventricular tachycardia (sustained ventricular tachycardia or ventricular fibrillation) for all patients in the pooled analysis (Raitt et al. and Brouwer et al.) (log-rank P = 0.79).
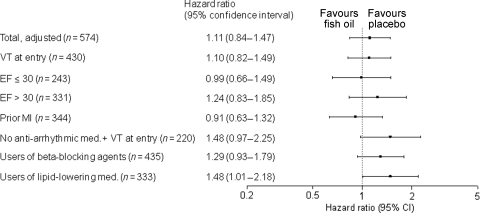
Figure 4.
Hazard ratios of fish oil treatment for time to first ventricular tachyarrhythmia in the pooled analyses (Raitt et al. and Brouwer et al.) in the entire study population (primary analysis) and subgroups (subgroup analyses). Analyses are adjusted for age, gender, ejection fraction (squared because of skewness of the distribution), current smoking, New York Heart Association class for angina pectoris, New York Heart Association class for dyspnoea, valvular heart disease, prior myocardial infarction, cardiomyopathy, ventricular tachycardia as index arrhythmia, ventricular fibrillation as index arrhythmia, and use of anti-arrhythmic medication at baseline.
Subgroup analyses did not show profound effects for any of the subgroups of patients, but there was a suggestion that some groups of patients may respond differently towards the use of fish oil than other groups (Figure 4). In 220 patients who did not use class I or III anti-arrhythmic medication and entered the study with a VT, the adjusted HR for the use of fish oil was 1.48 (0.97–2.25). In a post hoc analysis in 333 patients in whom the use of lipid-lowering medication was confirmed, the adjusted HR for the use of fish oil was 1.48 (1.01–2.18). In total, 423 patients had experienced an MI in the past. The HR for the crude effect of fish oil in these patients was 0.84 (0.61–1.17). The HR of the fully adjusted model for 344 patients with a previous MI was 0.91 (0.63–1.32).
Discussion
This combined analysis of three large randomized, controlled trials in the USA and Europe does not support a protective effect of omega-3 PUFAs from fish oil on recurrent life-threatening cardiac arrhythmia in patients with an implanted cardioverter defibrillator. This is line with the findings of an earlier published combined analysis of these studies.15 In contrast to the study of Jenkins et al.,15 we had access to the original data of two of the three studies which enabled us to pool the data of two studies and standardize the outcome variables across the three studies. Furthermore, we could analyse the effects in the subgroups of patients.
Although considerable heterogeneity existed between the trials, the similarity in designs and in baseline characteristics allowed a combination of the data. We used a random-effects model that took heterogeneity into account. The major difference between the studies was in the dose of fish oil: 900 mg/day fish fatty acids in Brouwer et al., 1300 mg/day in Raitt et al., and 2600 mg/day in Leaf et al. However, we think that this cannot explain the absence of a significant effect in the combined analysis. The lowest applied dose of 900 mg in Brouwer et al. was as high as the dose shown to have a clear protective effect on sudden death in the GISSI trial of patients with a previous MI.4 Furthermore, the outcomes of the individual ICD trials do not show a pattern that is consistent with a dose effect relationship in the 900–2600 mg/day range.
Both our pooled analysis and our meta-analysis showed that fish oil was not efficacious in patients who entered the studies with a VT. However, there was considerable heterogeneity between the patient populations that entered the studies with a VT. Therefore, it is difficult to interpret the results. In our pooled analysis, the risk of VT or VF in patients with a previous VT who did not use anti-arrhythmic medication tended to be increased in patients using fish oil (HR 1.48; 95% CI 0.97–2.25). This is in line with the finding in the study of Raitt et al., where the effect was even statistically significant. Although the effect was not statistically significant in this pooled analysis and confined to Raitt et al., fish oil supplementation might not be advisable for this group of patients.
Burr et al.16 suggested that interaction between fish oil and medication could play a role in determining the effect of fish oil. They showed in their population of patients with stable angina that excess mortality—both total and cardiac death—in the fish group was restricted to the men who were not taking beta-blockers.16 In contrast, in our analysis, ICD patients taking beta-blockers tended to have a higher risk on fish oil (HR 1.29; 95% CI 0.93–1.79). Although not significant, this would point in the direction of excess risk for patients using rather than not using beta-blocking agents. In any case, the available evidence shows no consistent interaction between fish oil and beta-blocking agents.
The pooled analysis also suggested a higher risk of ventricular tachyarrhythmia (adjusted HR 1.48; 95% CI 1.01–2.18) for users of fish oil on lipid-lowering medication, e.g. statins. However, separate analyses of the data of the two trials showed that this effect was only seen in patients in the Portland trial (HR 2.24; 95% CI 1.18–4.3). Risk in the same patients in the SOFA trial was essentially the same on fish oil and placebo. Therefore, this might be a chance finding. Nevertheless, it would be prudent to address this possible interaction between fish oil and statins in future studies.
Intake of fish or fish oil prevented fatal coronary heart disease in two earlier open-label trials in patients with a recent previous MI.4,17 In addition, the Japanese JELIS trial in hypercholesterolaemic patients showed a significant reduction of non-fatal cardiovascular events, although not of fatal events.18 In the DART 2 trial patients with stable angina, intake of fish oil did not show protective effects.19 The present pooled analysis on 423 patients with a previous MI also did not show a significant protective effect, although the observed difference and its CI cannot definitely exclude the benefit of fish oil in this patient group (HR 0.91; 95% CI 0.63–1.32). Furthermore, the meta-analysis of all three studies on outcomes in patients with previous coronary artery disease or ischaemic heart disease (Figure 2A) may also be interpreted as a trend towards a protective effect (HR 0.79; 95% CI 0.60–1.06). Animal and cell studies support the notion that the effects of fish oil on cardiac electrophysiology and arrhythmia may be dependent on the nature of the underlying disease.20–22 Dietary fish oil promoted arrhythmia during acute regional myocardial ischaemia in isolated pig hearts.20 This study showed a moderate increase in inexcitable ventricular tissue after fish oil feeding, which favoured the onset of re-entrant arrhythmias. In other circumstances, fish oil showed anti-arrhythmic properties.22–24 On the basis of these experimental studies plus the findings in our combined analyses of subgroups, we speculate that fish oil could be harmful in patients vulnerable for life-threatening arrhythmia based on re-entry, whereas it might be protective in patients with a recent prior MI or acute myocardial ischaemia. In patients with a recent prior MI, arrhythmias may have been based on triggered activity and prolonged action potentials.21 This is the type of arrhythmias that are probably prevented by fish oil.21,23,25,26 A differential effect of fish oil dependent on disease history and consequent cardiac electrophysiological condition might explain why fish oil caused a major reduction in death rates in the GISSI trial of patients with a recent MI4 and an increased risk in the DART 2 trial of patients with stable angina.19
In conclusion, combined data from the three available trials to date indicate that fish oil does not confer protection against recurrent life-threatening ventricular arrhythmia in patients with an ICD. However, the data suggest that underlying diseases might determine whether patients may benefit from fish oil or not. Furthermore, fish oil supplementation might not be advisable for all patients.
Author contributions
I.A.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: I.A.B., M.H.R., P.L.Z., M.B.K., W.E.C., A.J.C., E.G.S., J.M.
Analysis and interpretation of the data: I.A.B., M.H.R., C.D., P.L.Z., W.E.C., J.M.
Drafting of the manuscript: I.A.B.
Critical revision of the manuscript for important intellectual content: I.A.B., M.H.R., C.D., D.F.K., P.L.Z., C.M., M.B.K., W.E.C., A.J.C., E.G.S., J.M.
Statistical analysis: I.A.B., C.D., D.F.K.
Funding
The SOFA study was funded by the Wageningen Centre for Food Sciences (WCFS) with an additional grant from the European Union (SEAFOODplus integrated project No. 506359). The Portland study was funded by the National Institutes of Health (grant R01HL61682), Public Health Service (grant 5 M01 RROO334), and Hoffman-LaRoche Inc. The meta-analysis and pooled analysis were funded by Top Institute Food and Nutrition. TI Food and Nutrition, formerly known as WCFS, is a public/private partnership. Partners are major Dutch food companies and research organizations.
Conflict of interest: P.L.Z. is employed by Unilever, a company that produces foods enriched with omega-3 fatty acids.
Acknowledgement
The authors would like to thank all the members of the SOFA Study Group and all the co-workers involved in the SOFA Study and the Portland Study.
References
- 1.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 2.Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93:1119–1123. doi: 10.1016/j.amjcard.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 4.Investigators GISSI-Prevenzione. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 5.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 7.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 8.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 9.Billman GE, Kang JX, Leaf A. Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids. 1997;32:1161–1168. doi: 10.1007/s11745-997-0149-2. [DOI] [PubMed] [Google Scholar]
- 10.Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLennan PL. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids. 2001;36(Suppl. S):S111–S114. doi: 10.1007/s11745-001-0692-x. [DOI] [PubMed] [Google Scholar]
- 12.Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 13.Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, McClelland J, Cook J, MacMurdy K, Swenson R, Connor SL, Gerhard G, Kraemer DF, Oseran D, Marchant C, Calhoun D, Shnider R, McAnulty J. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A, Buschler H, Schouten EG. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins DJ, Josse AR, Beyene J, Dorian P, Burr ML, LaBelle R, Kendall CW, Cunnane SC. Fish-oil supplementation in patients with implantable cardioverter defibrillators: a meta-analysis. CMAJ. 2008;178:157–164. doi: 10.1503/cmaj.070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burr ML, Dunstan FD, George CH. Is fish oil good or bad for heart disease? Two trials with apparently conflicting results. J Membr Biol. 2005;206:155–163. doi: 10.1007/s00232-005-0784-1. [DOI] [PubMed] [Google Scholar]
- 17.Burr ML, Fehily AM, Rogers S, Welsby E, King S, Sandham S. Diet and reinfarction trial (DART): design, recruitment, and compliance. Eur Heart J. 1989;10:558–567. doi: 10.1093/oxfordjournals.eurheartj.a059528. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 19.Burr ML, Ashfield WP, Dunstan FDJ, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NAA, Elwood PC. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 20.Coronel R, Wilms-Schopman FJ, Den Ruijter HM, Belterman CN, Schumacher CA, Opthof T, Hovenier R, Lemmens AG, Terpstra AH, Katan MB, Zock P. Dietary n-3 fatty acids promote arrhythmias during acute regional myocardial ischemia in isolated pig hearts. Cardiovasc Res. 2007;73:386–394. doi: 10.1016/j.cardiores.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Den Ruijter HM, Berecki G, Opthof T, Verkerk AO, Zock PL, Coronel R. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res. 2007;73:316–325. doi: 10.1016/j.cardiores.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Verkerk AO, van Ginneken AC, Berecki G, den Ruijter HM, Schumacher CA, Veldkamp MW, Baartscheer A, Casini S, Opthof T, Hovenier R, Fiolet JW, Zock PL, Coronel R. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res. 2006;70:509–520. doi: 10.1016/j.cardiores.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 24.Den Ruijter HM, Verkerk AO, Berecki G, Bakker D, van Ginneken AC, Coronel R. Dietary fish oil reduces the occurrence of early afterdepolarizations in pig ventricular myocytes. J Mol Cell Cardiol. 2006;41:914–917. doi: 10.1016/j.yjmcc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Baartscheer A, Schumacher CA, Belterman CN, Coronel R, Fiolet JW. SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc Res. 2003;58:99–108. doi: 10.1016/s0008-6363(02)00854-4. [DOI] [PubMed] [Google Scholar]
- 26.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]