Abstract
Species of the large family Orchidaceae display a spectacular array of adaptations and rapid speciations that are linked to several innovative features, including specialized pollination syndromes, colonization of epiphytic habitats, and the presence of Crassulacean acid metabolism (CAM), a water-conserving photosynthetic pathway. To better understand the role of CAM and epiphytism in the evolutionary expansion of tropical orchids, we sampled leaf carbon isotopic composition of 1,103 species native to Panama and Costa Rica, performed character state reconstruction and phylogenetic trait analysis of CAM and epiphytism, and related strong CAM, present in 10% of species surveyed, to climatic variables and the evolution of epiphytism in tropical regions. Altitude was the most important predictor of photosynthetic pathway when all environmental variables were taken into account, with CAM being most prevalent at low altitudes. By creating integrated orchid trees to reconstruct ancestral character states, we found that C3 photosynthesis is the ancestral state and that CAM has evolved at least 10 independent times with several reversals. A large CAM radiation event within the Epidendroideae, the most species-rich epiphytic clade of any known plant group, is linked to a Tertiary species radiation that originated 65 million years ago. Our study shows that parallel evolution of CAM is present among subfamilies of orchids, and correlated divergence between photosynthetic pathways and epiphytism can be explained by the prevalence of CAM in low-elevation epiphytes and rapid speciation of high-elevation epiphytes in the Neotropics, contributing to the astounding diversity in the Orchidaceae.
Crassulacean acid metabolism (CAM) is a taxonomically widespread photosynthetic pathway that has evolved in plants of CO2- and water-limited environments, including tropical forest canopies with intermittent or seasonal water availability, hot semiarid regions, and some aquatic environments. The CAM pathway is characterized by the temporal separation of carbon fixation between nocturnal CO2 fixation by phosphoenolpyruvate carboxylase in the cytosol and daytime decarboxylation of organic acids to release CO2 that is then refixed by Rubisco in the chloroplast (Ting, 1985). CAM photosynthesis is found in approximately 7% of vascular plant species from 34 families (Smith and Winter, 1996; Holtum et al., 2007). About 10% of all vascular plant species are estimated to be epiphytes (Benzing, 1989), many of which exhibit CAM (Lüttge, 2004). CAM vascular epiphytes (mostly orchids and bromeliads) are an important component of the biomass and species richness of tropical forest canopies (Benzing, 1987; Lüttge, 2004; Zotz, 2004). Bromeliads, aroids, and orchids are three of the very few flowering plant lineages that were able to successfully colonize epiphytic niches (Gentry and Dodson, 1987). Yet, orchids are particularly species-rich relative to these other epiphytic groups (Gravendeel et al., 2004), making Orchidaceae a prime subject for understanding mechanisms of evolutionary radiation and diversification. About 72% of orchid species are estimated to be epiphytic (Benzing, 1989; Gravendeel et al., 2004), with the majority of these being restricted to tropical regions. Tropical forest canopies are rich in epiphytic CAM plant diversity (Benzing, 1987; Winter and Smith, 1996; Lüttge, 2004). CAM has been found in 62% and 26% of epiphytic orchid species in Australian and New Guinean rainforests, respectively (Winter et al., 1983; Earnshaw et al., 1987), 42% of orchid species in a moist lowland forest site in Panama (Zotz and Ziegler, 1997), and up to 100% of the epiphytic flora in a Mexican dry forest (Mooney et al., 1989). The abundance of CAM species in such habitats is related to limited water availability. Within a single site, the percentage of CAM epiphytes increases with canopy height, from 7% in the forest understory, to 25% at intermediate heights, to 50% in exposed canopy sites (Zotz and Ziegler, 1997). CAM species are also found in contrasting habitats such as very arid and very moist sites, which provides evidence of the biochemical flexibility of this photosynthetic adaptation (Dodd et al., 2002). Several researchers have postulated that in addition to CAM, mechanisms such as production of dust-like seeds capable of long-distance dispersal, germination associations with mycorrhizae, absorptive velamentous photosynthetic root tissue capable of rapid water uptake, and reproductive features that promote specialized pollination syndromes have contributed to the diversification of epiphytic orchids (Benzing, 1987; Gravendeel et al., 2004; Peakall, 2007; Mondragón-Palomino and Theissen, 2008). Whether CAM is linked to epiphytic diversification and species radiations throughout evolutionary time remains poorly understood.
Orchid systematics is now at an advanced stage, allowing ancestral state reconstruction and correlated evolution analysis of key adaptive traits within the context of a highly resolved phylogeny (Chase et al., 2003). We used whole leaf tissue carbon isotopic composition (δ13C) as a rapid screening method to establish whether CO2 assimilation occurs predominantly by strong CAM or C3 photosynthesis. Strong CAM and C3 photosynthetic tissues exhibit distinct δ13C values because CAM plants are enriched in 13C relative to C3 plants (Rundel et al., 1979; Winter, 1979; Winter et al., 1983; Kluge et al., 1991; Zotz and Ziegler, 1997; Crayn et al., 2001, 2004; Zotz, 2004; Silvera et al., 2005), whereas weak CAM species show overlapping δ13C values with C3 species and measurable changes in day/night titratable acidity (Silvera et al., 2005). We present data on the prevalence of CAM in the largest family of angiosperms using the regional, highly diversified, and well-described tropical orchid flora of Panama and Costa Rica (Dressler, 1993; Hammel et al., 2003) and evaluate its relationship with climate and its role in the megadiversification of epiphytes. We used available phylogenetic information to determine the distribution of photosynthetic pathways among orchid species and relate these evolutionary patterns to climate-driven habitat preferences and epiphytism. We also used phylogenetic comparative methods to demonstrate statistically the evolutionary association between epiphytism and photosynthetic pathways and to evaluate the role of CAM in species radiations in the Orchidaceae.
RESULTS
A total of 1,022 Panamanian and Costa Rican orchid species from 147 genera covering 802 sites, and a total of 1,103 species-site combinations, were analyzed. Our study covered 4% of the total number of orchids (24,910 species) as described by Chase et al. (2003) and about 68% of the total estimated number of species from Panama and Costa Rica (approximately 1,500 species) as described by Dressler (1993). Variation in whole tissue δ13C values ranged from −37.1‰ to −11.4‰, with an overall mean of −27.7‰. The isotopic values among orchid species showed a bimodal distribution, with the majority of species showing values near −28‰, indicative of the C3 photosynthetic pathway, and a smaller mode near −16‰, indicative of the CAM pathway. We found that 924 species (90%) belong to the cluster of mainly C3 photosynthesis and had δ13C values more negative than −22‰, whereas 98 species (10%) belong to the cluster of mainly CAM and had δ13C values less negative than −22‰.
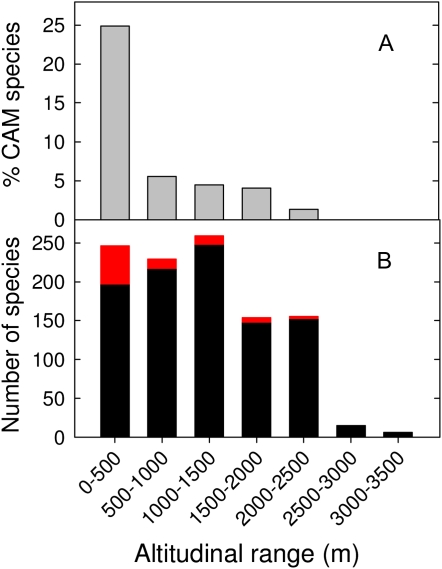
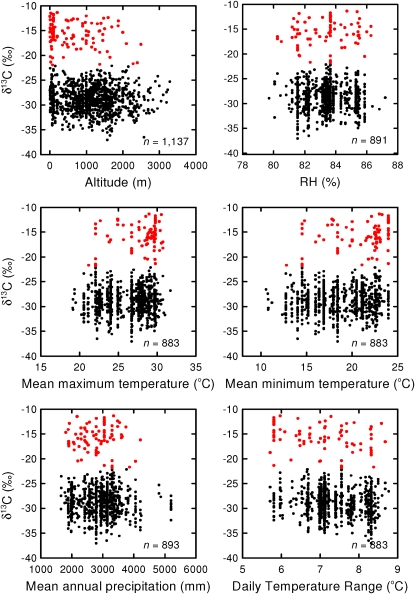
The percentage of CAM species showed a steady decline with increasing altitude, with the largest number of CAM species at sites from 0 to 500 m altitude, with no strong CAM species being observed above 2,400 m (Fig. 1A). The number of orchid species was lowest at high elevations (3,000–3,500 m; Fig. 1B), with the greatest number of species at mid elevation (1,000–1,500 m; Fig. 1B). Based on bivariate regression, δ13C was most strongly related to daily temperature range (DTR; r2 = 0.176, P ≤ 0.0001; Fig. 2), mean annual rainfall (r2 = 0.07, P = 0.0392), altitude (r2 = 0.05, P ≤ 0.0001), mean maximum temperature (Tmax; r2 = 0.05, P ≤ 0.0001), and mean minimum temperature (Tmin; r2 = 0.05, P ≤ 0.0001) but was not significantly related to mean monthly relative humidity (RH; r2 = 0.0004, P = 0.8549). Multiple regression analysis showed that when all variables were taken into account, δ13C was most strongly related to altitude (P = 0.0013), followed by RH (P = 0.0065), Tmax (P = 0.0157), Tmin (P = 0.0156), and DTR (P = 0.0164), but was not significantly related to mean annual rainfall. The best fit multiple regression model for the effects of environmental variables on δ13C included altitude, RH, Tmax, Tmin, and DTR (F = 14.47, r2 = 0.0709, P ≤ 0.0001, degrees of freedom = 882).
Figure 1.
A, Percentage of CAM species as a function of altitude. B, Total number of species as a function of altitude. Black bars represent C3 photosynthesis species, and red bars represent CAM species.
Figure 2.
Relationship between δ13C and climatic variables. Each point represents a species-site combination. Black points represent C3 photosynthesis species, and red points represent CAM species.
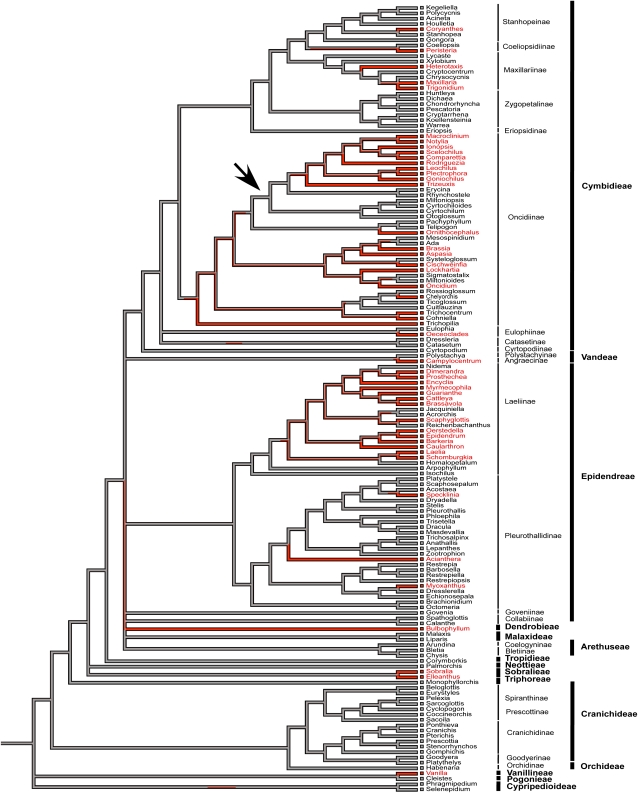
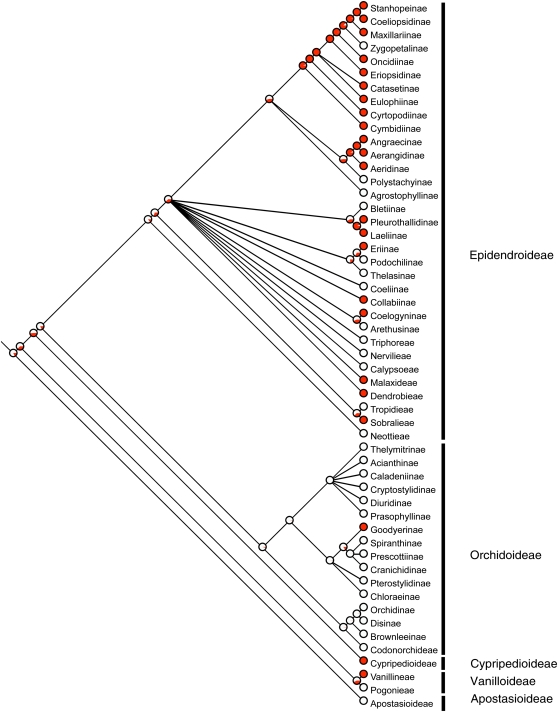
Our phylogenetic analyses support multiple origins of CAM within the orchid family (Figs. 3 and 4). CAM is present in 43% of orchid subtribes worldwide (23 of 53 subtribes show CAM; Fig. 4) and has evolved at least 10 independent times among Neotropical orchid genera, with two large CAM radiation events, one within the subtribe Oncidiinae and a second one within the subtribe Laeliinae, both belonging to the large epiphytic subfamily Epidendroideae (Fig. 3). Across the entire Orchidaceae family, maximum-likelihood estimates suggest that C3 photosynthesis is the ancestral state and that CAM has evolved multiple times, with a large radiation event in the Epidendroid group (20 of 33 subtribes show CAM; Fig. 4). Our data also indicate the possibility of several reversal events within the subtribes Oncidiinae and Laeliinae (Fig. 3). However, more carbon isotope sampling in these clades is required in order to confirm the presence or absence of CAM in species within the respective genera. The majority of species in this study are epiphytic (88%) compared with terrestrial (10.5%), with a low percentage showing both growth forms (1.5%). When using maximum likelihood to trace epiphytism as a character state across the orchid phylogeny, we found that the terrestrial habit is the ancestral state within tropical orchids and, similar to CAM, the epiphytic habit is derived (data not shown).
Figure 3.
Orchidaceae tree showing the relationship among 147 tropical genera. Tribes (in boldface) and subtribes are shown. Photosynthetic pathways based on δ13C (this study) and titratable acidity derived from Silvera et al. (2005) were mapped onto the cladogram using Mesquite version 2.5. Those lineages that show C3 photosynthesis are represented by black lines, and those with CAM are highlighted in red. The arrow indicates node 68, which shows a large contribution index to divergence correlation. These data support the multiple, independent evolutionary origins of CAM.
Figure 4.
Orchidaceae tree showing the relationship among 53 subtribes. Black vertical lines represent five major lineages recognized as subfamilies. Presence and absence of CAM based on this study and published information derived from Silvera et al. (2005) and Smith and Winter (1996) were mapped onto the cladogram using Mesquite version 2.5. Those lineages that show CAM are depicted in red. The red area within each pie chart indicates the relative support for different ancestor states. These data support the multiple, independent evolutionary origins of CAM.
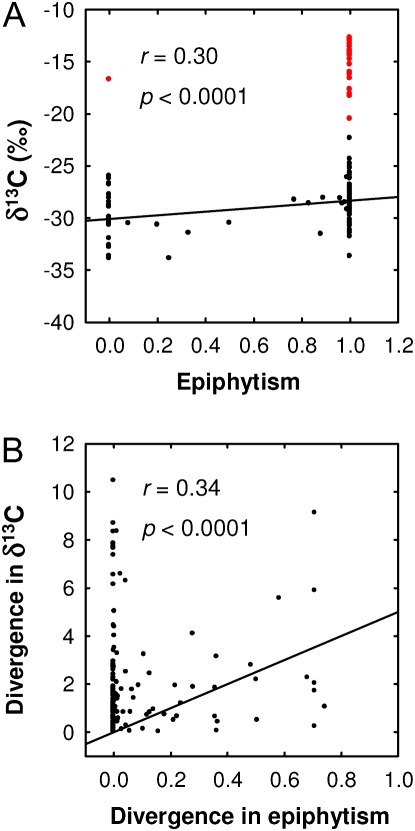
Across all genera, a positive relationship between the presence of epiphytism and δ13C was observed (r = 0.30, P < 0.0001; Fig. 5A), explained by the increased use of CAM among epiphytes. Similar to the intergeneric relationship, we found a significant positive relationship between epiphytism and δ13C using divergence analysis (r = 0.34, P < 0.0001; Fig. 5B), indicating correlated evolution of photosynthetic pathway properties associated with less discrimination against 13CO2 during photosynthesis, and the epiphytic habit. Correlated divergence analysis indicates that the positive cross-genera relationship between epiphytism and δ13C is driven by radiation events that occurred deep within the phylogenetic genera tree. Node 68 (Fig. 3), belonging to the subtribe Oncidiinae, showed the highest contribution index value due to a prominent split between CAM genera and C3 genera within closely related species. All species of 10 of 16 genera within this node are CAM and epiphytes (Macroclinium, Notylia, Ionopsis, Scelochilus, Comparettia, Rodriguezia, Leochilus, Plectophora, Goniochilus, and Trizeus). Similarly, clade Oeceoclades-Eulophia and clade Vanilla-Cleistes (Fig. 3) showed significantly high divergence width and contribution index values. Each of these radiations contributed to the observed positive significant cross-genera divergence.
Figure 5.
Pairwise correlation between δ13C and epiphytism (A), and divergences in δ13C and epiphytism among radiations in the tree for orchid genera (B). Epiphytism is represented as a gradient in which 0 represents terrestrial forms (no epiphytism) and 1 represents epiphytic forms. Values between 0 and 1 represent genera with species that have both growth forms. Black points represent C3 photosynthesis genera, and red points represent CAM genera.
DISCUSSION
The challenge presented by the Orchidaceae is to understand how this group evolved with the large array of adaptive characteristics that has allowed a multitude of species-rich radiations and colonization of diverse terrestrial ecosystems worldwide. Our data demonstrate patterns of CAM evolution across the Orchidaceae, including multiple independent origins of CAM, several reversals indicating the evolutionary flexibility of CAM (Figs. 3 and 4), and parallel evolution of CAM across subfamilies (Fig. 4). Our data also indicate that divergences in photosynthetic pathway and epiphytism have been consistently correlated through evolutionary time (Fig. 5), related to the prevalence of CAM epiphytic species in lower elevations and abundant species diversification of high-elevation epiphytes.
The δ13C bimodal distribution found in this study is consistent with other studies (Griffiths and Smith, 1983; Pierce et al., 2002; Holtum et al., 2004; Silvera et al., 2005; Motomura et al., 2008) showing disruptive selection on C3 photosynthesis and strong CAM δ13C values with very few intermediate δ13C values. Strong CAM is present in about 10% of orchid species studied, and the largest proportion is distributed at lower altitude. We found a weak relationship between climatic variables and the photosynthetic pathway in this study, partly because Panama and Costa Rica are concentrated in a high-precipitation region and partly because the coarse climate variables analyzed do not fully describe the microclimate of epiphytic habitats. Nonetheless, our data demonstrate that CAM has contributed to the exploitation of epiphytic habitats through mid-elevation tropical montane environments. Orchid species richness in our study, with the largest peak around 1,000 to 1,500 m of altitude, is consistent with the rule of the mid-altitudinal bulge. The mechanism creating this bulge has been explained by the interaction between temperature and water gradients caused by increasing altitude (Whittaker and Niering, 1975; Peet, 1978; Zhao et al., 2005). Higher moisture availability from elevations around 1,000 m are likely to provide a more suitable environment for epiphytic lineages (Gentry and Dodson, 1987; Cardelús et al., 2006), whereas sites above 2,000 m might have reduced canopy height with less habitat for epiphyte colonization. Increases in δ13C with increasing altitude among C3 photosynthesis groups (Fig. 2), due to the reduced photosynthetic carbon isotope discrimination at high elevation, independent of CAM (Körner et al., 1988), can contribute to the δ13C-epiphytism relationship. However, previous studies have shown that the presence of weak CAM can also contribute to a second peak of abundance within the C3 photosynthesis isotopic cluster (Winter and Holtum, 2002; Silvera et al., 2005). Evaluation of the evolutionary role of weak CAM will require extensive in situ measurements of dawn/dusk titratable acidity, an endeavor limited by the accessibility of live specimens in the field. Nevertheless, the contribution of CAM-independent factors in determining the δ13C of high-elevation epiphytes and the role of weak CAM as an evolutionary reservoir for further CAM radiations in changing environments deserve further study.
We provide evidence that C3 photosynthesis is the ancestral state in the Orchidaceae and that CAM has evolved multiple independent times (Figs. 3 and 4). There is strong evidence that evolutionary progression of photosynthesis in plants has been from C3 photosynthetic ancestors to derived weak CAM to strong CAM modes (Pilón-Smits et al., 1996; Crayn et al., 2004), paralleled by progression from terrestrial ancestors to epiphytic growth forms. Phylogenetic tree construction using phosphoenolpyruvate carboxylase sequences has shown that sequences from CAM species within the Oncidiinae clade cluster together and differ from CAM sequences of species in the Vanillineae clade, providing evidence that these paralogous genes probably arose from one or more ancient duplications, thus adding an additional line of evidence for the multiple origins of CAM (K. Silvera, L. Rodriguez, R.L. Albion, K.M. Neubig, M.W. Whitten, N.H. Williams, K. Winter, and J.C. Cushman, unpublished data). A recent study using the genus Cymbidium concluded that weak CAM was the ancestral state and that C3 and strong CAM were derived (Motomura et al., 2008). However, the results were restricted to species within one genus and limit the conclusions that can be applied at the family level. The change from terrestrial to epiphytic habitat is paralleled by a change from monopodial to sympodial growth form, a common feature in epiphytic orchids often associated with pseudobulbs, which provide tolerance to drought stress. Our study also demonstrates parallel evolution of CAM among four of the five subfamilies of orchids (Epidendroideae, Orchidoideae, Cypripedioideae, and Vanilloideae). The two largest orchid subfamilies, Orchidoideae and Epidendroideae, are considered to have diversified early in the Tertiary (Ramírez et al., 2007). The progressive aridification and declining CO2 concentrations during the Tertiary (Pearson and Palmer, 2000) are likely factors that have contributed to the large CAM radiation in Epidendroideae, which has produced the most species-rich epiphytic clades of any known plant group. CAM and epiphytism in orchids are likely to have originated about 65 million years ago. The CAM radiation events within the subfamily Epidendroideae (Fig. 4), and especially within the Neotropical subtribes Oncidiinae and Laeliinae (Fig. 3), are thus in line with paleoenvironmental conditions that favored the evolution of CAM (Monson, 1989; Ehleringer and Monson, 1993; Raven and Spicer, 1996).
Epiphytism in orchids is a pantropical phenomenon, and our study shows significant correlation between photosynthetic pathways and epiphytism (Fig. 5), indicating that throughout evolutionary time, divergence in δ13C is consistently accompanied by divergence in epiphytism and demonstrating a functional relationship between these traits. Correlated divergence between the photosynthetic pathway and epiphytism is likely an important factor contributing to the burst of speciation that occurred in diverse epiphytic orchid clades (subtribe Oncidiinae and Laeliinae; Fig. 3). These clades are species rich due to colonization of epiphytic habitats, and species within them were able to colonize wider habitat ranges compared with other plant species, due to the development of CAM as a water-conserving mode of photosynthesis, ultimately contributing to the large floristic diversity found in the Neotropics. Correlated divergence between photosynthetic pathways also indicates that epiphytic orchid species developed CAM convergently to other epiphytic or terrestrial plant species under the selectional pressure arising from the search for light in rainforest habitats. Our study shows that strong CAM species are restricted to epiphytic habitats, except for Oeceoclades maculata, a terrestrial invasive orchid species native to Africa with recent distribution in the Neotropics from Argentina to Florida (Stern, 1988; Cohen and Ackerman, 2009). The presence of strong CAM in this genus is intriguing, and whether or not the presence of CAM contributes to invasiveness in this species is unknown.
CONCLUSION
This study demonstrates several patterns of CAM evolution across the Orchidaceae, including multiple independent origins of CAM, several reversal events indicating the evolutionary flexibility of CAM, and parallel evolution of CAM across subfamilies. Divergences in the photosynthetic pathway and epiphytism have been consistently correlated through evolutionary time and are related to the prevalence of CAM epiphytic species in lower elevations and abundant species diversification of high-elevation epiphytes. Overall, our study reveals biochemical underpinnings and evolutionary interactions between CAM as a water-saving mode of photosynthesis and colonization of epiphytic habitats that have contributed to some of the most substantial plant speciations known to exist.
MATERIALS AND METHODS
Site Description
Panama and Costa Rica are equatorial tropical countries located between 7° to 11° N and 77° to 80° W. The Panamanian isthmus serves as a land bridge between North and South America and fosters a rich intermixture of plant and animal life that has migrated between the continents. The two principal mountain ranges, the Tabasará Mountains (Cordillera Central) in the west and the Cordillera de San Blas in the east, divide the country into Atlantic- and Pacific-facing slopes. Costa Rica is similarly divided into Caribbean and Pacific slopes by the Cordillera Central and the Cordillera de Talamanca. Two distinct regional seasons driven by latitudinal movement of the intertropical convergence zone produce a dry season from December to May with shorter, less intense dry seasons in sites with greater annual precipitation (Dressler, 1993). Annual precipitation is generally greater on the Caribbean coast (1,500–5,550 mm per year) than on the Pacific coast (1,140–2,290 mm per year). We present data from sites ranging in mean annual precipitation from 1,652 to 5,204 mm per year, mean annual temperatures ranging from 14.9°C to 27.6°C, mean RH ranging from 79.7% to 89.2%, and altitude from sea level to 3,290 m.
Carbon Isotope Analysis
Small fragments (2–5 mg) of leaf tissue were collected from a combination of 12 live specimens from Selby Botanical Gardens and 1,091 species from five herbaria: Missouri Botanical Gardens Herbarium, Marie Selby Botanical Gardens Herbarium, University of Florida Herbarium, University of Panama Herbarium, and the Smithsonian Tropical Research Institute Herbarium. Leaf samples were analyzed for carbon stable isotopic composition (δ13C) at the Center for Stable Isotope Biogeochemistry at the University of California, Berkeley, with an isotope ratio mass spectrometer (Finnigan-MAT Delta Plus XT). 13C/12C ratios were calculated relative to the Pee Dee belemnite standard using the relationship:
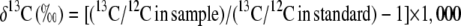 |
We categorized species into C3 or CAM based on leaf δ13C values commonly observed for C3 photosynthesis plants ranging from −33‰ to −22‰ and with δ13C values typical of CAM plants ranging from −22‰ to −12‰ (Ehleringer and Osmond, 1989).
Climatic Data
We used altitude and geographic coordinates to determine climate variables for each herbarium specimen sampled for carbon isotope analysis. For each specimen, we recorded species name, herbarium code and collection number, growth form (epiphytic, terrestrial, or both), collection location, elevation, and coordinates. We used average altitude for species in which a range was given. For species with missing entries, verbal descriptions of location were converted to altitude and coordinates using the online Global Gazette Version 2.0 (http://www.fallingrain.com/world/) for Panama and Costa Rica. Coordinate information for each specimen was then used to generate mean annual temperature, mean RH, mean annual precipitation, and mean annual DTR using the Climatic Research Unit application CRU_CL_2.0 (New et al., 2002; http://www.cru.uea.ac.uk/cru/data/tmc.htm). From DTR, we calculated mean Tmin and Tmax. Simple and multiple regression analyses were used to determine relationships between climatic variables and δ13C using SAS statistics software (version 9.1.3).
Orchid Phylogeny and Species Nomenclature
We constructed two tree hypotheses for the Orchidaceae (genera and subtribes) using Phylomatic 2 (Webb et al., 2008) and Mesquite version 2.5 (Maddison and Maddison, 2008). The relationship among genera and overall tree topology followed the strict consensus tree resulting from a combined analysis of psaB + rbcL gene sequences (Cameron, 2004). The relationship among subtribes and overall topology followed the summary tree of the revised classification of Orchidaceae (Chase et al., 2003). Resulting subtribe and genera trees retained existing polytomies. The overall tree topology differs between these two trees because of different taxa and regions sampled within each publication. Because of the large size of the Orchidaceae, relationships among genera were further expanded in the genera tree using recent publications and nomenclatural changes to the Oncidiinae (Williams et al., 2001a, 2001b; Dressler and Williams, 2003), Laeliinae (Higgins, 1997; van den Berg et al., 2000; Dressler, 2002; Dressler and Higgins, 2003), Pleurothallidinae (Pridgeon and Chase, 2001; Pridgeon et al., 2001; Luer, 2004), Cranichideae (Salazar et al., 2003), and Maxillarieae (Whitten et al., 2000, 2007; Ojeda et al., 2005). Overall, our nomenclature is consistent with recent publications on nomenclatural changes presented above, the Field Guide to the Orchids of Costa Rica and Panama (Dressler, 1993), and the Royal Botanic Gardens Kew World Checklist of Monocotyledons nomenclatural database and associated authority files (http://apps.kew.org/wcsp/home.do).
Phylogenetic and Statistical Analyses
Presence and absence of CAM were traced onto the genera tree based on δ13C (this study) and titratable acidity measurements (Silvera et al., 2005). Genera were labeled as CAM if at least one species within the genus was known to conduct CAM. Character state reconstruction was performed by maximum likelihood in Mesquite version 2.5 (Maddison and Maddison, 2008), using a marginal probability reconstruction with Asymmetrical parameter Markov-k model of evolution, with an estimated forward rate of 0.1020 and backward rate of 0.2007 corresponding to gain or loss of CAM, respectively. Root state frequency in this analysis was set to be the same as equilibrium, and we specified a bias of less than 1 indicating that backward changes (loss of CAM) are more probable than forward changes. This model was chosen over parsimony or the Mk1 model (gain and loss of CAM are equally weighted) based on the prior assumption that the gain of CAM is less probable because it requires the appearance and coordination of multiple genes, whereas the loss of CAM could result from a single loss-of-function mutation. This prior assumption would likely avoid the false rejection of Dollo's law when reconstructing a character (Goldberg and Igic, 2008). Because both trees were the result of the compilation of different phylogenies, branch lengths were not available and analyses were performed with branch lengths set as equal to 1. Presence and absence of CAM based on δ13C were traced onto the orchid subtribe tree using the same reconstruction method as the orchid genera tree. Subtribes were labeled as CAM if at least one genus within the subtribe was known to conduct CAM based on the information presented in this paper and on previously published information (Smith and Winter, 1996; Silvera et al., 2005). The results of analyses using two different assumptions about the probability of each character state at the root (state frequency same as equilibrium and state frequency equal) gave nearly identical outputs for both genera and subtribe trees. The results were displayed as likelihood states reported as the proportion of total likelihood and represented as pie diagrams for the subtribe tree and as proportional to weights in each branch for the genera tree.
To analyze the relationship between the photosynthetic pathway and epiphytism, leaf δ13C was coded for each genus as the mean for all species of that genus. Epiphytism was coded for each species as 0 for terrestrial and 1 for epiphytic and for the genus as the mean for all species of that genus. For simplicity, both epiphytes and lithophytes were considered as epiphytes. Trait correlation between leaf δ13C and epiphytism was evaluated with Pearson product-moment correlation coefficients across orchid genera. The same genus-level coding was used to perform correlated divergence analysis between δ13C and epiphytism in the Analysis of Traits module in Phylocom (Felsenstein, 1985; Webb et al., 2008) using the genera tree for Orchidaceae. Branch lengths were estimated by calibrating the tree for orchid genera at a single point (first bifurcation, 76 million years ago) using the age estimate for the Orchidaceae from Ramírez et al. (2007). We then used the branch length adjustment utility in Phylocom (BLADJ) to perform age interpolations for undated nodes and produce phylogenetic distances. Node ages were treated as approximations. Analysis of Traits calculates a standardized divergence of extant taxa and handles polytomies by ranking species based on the value of the independent variable when the median is used to create two groups (Pagel, 1992). Correlated divergence analysis was performed by constructing a 0-intercept linear regression between divergence in trait 1 (δ13C) and divergence in trait 2 (epiphytism) among sister groups throughout the genera tree, so that divergence width was based on differences in traits between descendent lines (Garland et al., 1993; Garland and Díaz-Uriarte, 1999; Webb et al., 2008). We used divergence width instead of independent contrasts as a measure of absolute trait radiation because the sd (divergence width) can be used when polytomies are present in the phylogeny (Moles et al., 2005; Beaulieu et al., 2007). We also used contribution index as a measure of the amount of present-day variation within a trait that is attributed to a focal clade (Moles et al., 2005).
Acknowledgments
Special thanks go to Dr. Bruce Holst (Selby Botanical Gardens); Kent Perkins, Dr. Mark Whitten, and Dr. Norris Williams (University of Florida Herbarium); Dr. Jim Solomon (Missouri Botanical Gardens Herbarium); and Mireya Correa (University of Panama Herbarium) for assisting with herbarium collections. We gratefully acknowledge Dr. Mark Whitten, Kurt Neubig, and Lorena Endara (University of Florida), the Santiago laboratory (University of California, Riverside), and two anonymous reviewers for comments to improve this paper; Dr. Todd Dawson and Dr. Stefania Mambelli (University of California, Berkeley) for assisting with isotopic analysis; Dr. Karen Schlauch (University of Nevada, Reno) for statistical advice; Dr. Doug Altshuler (University of California, Riverside) for Mesquite advice; Cristina Milsner (University of Nevada, Reno) and Michael O'Leary (University of California, Riverside) for assisting with database entry; and Vanessa Boukili (University of California, Berkeley) and Becky Albion (University of Nevada, Reno) for assistance in the laboratory.
This work was supported by the Environmental Protection Agency (Greater Research Opportunities Graduate Program Assistance Agreement no. MA 91685201 to K.S.), the National Science Foundation (grant nos. IOB–0543659 to J.C.C. and DEB–0706813 to L.S.S.), the National Institutes of Health (grant no. P20 RR–016464 from the Idea Network of Biomedical Research Excellence Program of the National Center for Research Resources supporting the Nevada Genomics, Proteomics, and Bioinformatics Center), the Andrew W. Mellon Foundation through the Smithsonian Tropical Research Institute (to K.W.), and the Nevada Agricultural Experiment Station (as publication no. NAES 03087114).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Katia Silvera (silverak@unr.nevada.edu).
Open Access articles can be viewed online without a subscription.
References
- Beaulieu JM, Moles AT, Leitch IJ, Bennett MD, Dickie JB, Knight CA (2007) Correlated evolution of genome size and seed mass. New Phytol 173 422–437 [DOI] [PubMed] [Google Scholar]
- Benzing DH (1987) Vascular epiphytism: taxonomic participation and adaptive diversity. Ann Mo Bot Gard 74 183–204 [Google Scholar]
- Benzing DH (1989) The evolution of epiphytism. In U Lüttge, ed, Vascular Plants as Epiphytes: Evolution and Ecophysiology, Vol 76. Springer-Verlag, Berlin, pp 15–41
- Cameron KM (2004) Utility of plastid psaB gene sequences for investigating intrafamilial relationships within Orchidaceae. Mol Phylogenet Evol 31 1157–1180 [DOI] [PubMed] [Google Scholar]
- Cardelús CL, Colwell RK, Watkins JE (2006) Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak. J Ecol 94 144–156 [Google Scholar]
- Chase MW, Cameron KM, Barrett RL, Freudenstein JV (2003) DNA data and Orchidaceae systematics: a new phylogenetic classification. In KW Dixon, SP Kell, RL Barrett, PJ Cribb, eds, Orchid Conservation. Natural History Publications, Kota Kinabalu, Borneo, pp 69–89
- Cohen IM, Ackerman JD (2009) Oeceoclades maculata, an alien tropical orchid in the Caribbean rainforest. Ann Bot (Lond) (in press) [DOI] [PMC free article] [PubMed]
- Crayn DM, Smith JAC, Winter K (2001) Carbon-isotope ratios and photosynthetic pathways in the Rapateaceae. Plant Biol 3 569–576 [Google Scholar]
- Crayn DM, Winter K, Smith JAC (2004) Multiple origins of Crassulacean acid metabolism and the epiphytic habit in the neotropical family Bromeliaceae. Proc Natl Acad Sci USA 101 3703–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (2002) Crassulacean acid metabolism: plastic, fantastic. J Exp Bot 53 569–580 [DOI] [PubMed] [Google Scholar]
- Dressler RL (1993) Field Guide to the Orchids of Costa Rica and Panama. Cornell University Press, Ithaca, NY
- Dressler RL (2002) New species and combinations in Costa Rican orchids. II. Lankesteriana 3 25–29 [Google Scholar]
- Dressler RL, Higgins WE (2003) Guarianthe, a generic name for the “Cattleya” skinneri complex. Lankesteriana 7 37–38 [Google Scholar]
- Dressler RL, Williams NH (2003) New combinations in Mesoamerican Oncidiinae (Orchidaceae). Selbyana 24 44–45 [Google Scholar]
- Earnshaw MJ, Winter K, Ziegler H, Stichler W, Cruttwell NEG, Kerenga K, Cribb PJ, Wood J, Croft JR, Carver KA, et al (1987) Altitudinal changes in the incidence of Crassulacean acid metabolism in vascular epiphytes and related life forms in Papua New Guinea. Oecologia 73 566–572 [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK (1993) Evolutionary and ecological aspects of photosynthetic pathway variation. Annu Rev Ecol Syst 24 411–439 [Google Scholar]
- Ehleringer JR, Osmond BO (1989) Stable isotopes. In RW Pearcy, J Ehleringer, HA Mooney, PW Rundel, eds, Plant Physiological Ecology. Chapman & Hall, London, pp 255–280
- Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125 1–15 [Google Scholar]
- Garland T, Díaz-Uriarte R (1999) Polytomies and phylogenetically independent contrasts: examination of the bounded degrees of freedom approach. Syst Biol 48 547–558 [DOI] [PubMed] [Google Scholar]
- Garland T, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer-simulation. Syst Biol 42 265–292 [Google Scholar]
- Gentry AH, Dodson CH (1987) Diversity and biogeography of neotropical vascular epiphytes. Ann Mo Bot Gard 74 205–233 [Google Scholar]
- Goldberg EE, Igic B (2008) On phylogenetic tests of irreversible evolution. Evolution Int J Org Evolution 62 2727–2741 [DOI] [PubMed] [Google Scholar]
- Gravendeel B, Smithson A, Slik FJW, Schuiteman A (2004) Epiphytism and pollinator specialization: drivers for orchid diversity? Philos Trans R Soc Lond B Biol Sci 359 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Smith JAC (1983) Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM. Oecologia 60 176–184 [DOI] [PubMed] [Google Scholar]
- Hammel BE, Grayum MH, Herrera C, Zamora N (2003) Manual de Plantas de Costa Rica. III. Monocotiledóneas (Orchidaceae-Zingiberaceae), Vol 93. Missouri Botanical Garden Press, St. Louis
- Higgins WE (1997) A reconsideration of the genus Prosthechea (Orchidaceae). Phytologia 82 370–383 [Google Scholar]
- Holtum JAM, Aranda J, Virgo A, Gehrig HH, Winter K (2004) δ13C values and Crassulacean acid metabolism in Clusia species from Panama. Trees Struct Funct 18 658–668 [Google Scholar]
- Holtum JAM, Winter K, Weeks MA, Sexton TR (2007) Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae). Am J Bot 94 1670–1676 [DOI] [PubMed] [Google Scholar]
- Kluge M, Brulfert J, Ravelomanana D, Lipp J, Ziegler H (1991) Crassulacean acid metabolism in Kalanchoë species collected in various climatic zones of Madagascar: a survey by δ13C analysis. Oecologia 88 407–414 [DOI] [PubMed] [Google Scholar]
- Körner C, Farquhar GD, Roksandic Z (1988) A global survey of carbon isotope discrimination in plants from high-altitude. Oecologia 74 623–632 [DOI] [PubMed] [Google Scholar]
- Luer CA (2004) Pleurothallis subgenus Acianthera and three allied subgenera: a second century of new species of Stelis of Ecuador. Epibator, Ophidion, Zootrophion. Addenda to Brachionidium, Dracula, Lepanthes, Platystele, Pleurothallis, Porroglossum, and Masdevallia: new genera and combinations. Monogr Syst Bot Missouri Bot Gard 95 1–265 [Google Scholar]
- Lüttge U (2004) Ecophysiology of Crassulacean acid metabolism (CAM). Ann Bot (Lond) 93 629–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2008) Mesquite: a modular system for evolutionary analysis, version 2.5. The Mesquite Project. http://mesquiteproject.org (January 12, 2009)
- Moles AT, Ackerly DD, Webb CO, Tweddle JC, Dickie JB, Westoby M (2005) A brief history of seed size. Science 307 576–580 [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Theissen G (2008) MADS about the evolution of orchid flowers. Trends Plant Sci 13 51–59 [DOI] [PubMed] [Google Scholar]
- Monson RK (1989) On the evolutionary pathways resulting in C4 photosynthesis and Crassulacean acid metabolism (CAM). Adv Ecol Res 19 57–92 [Google Scholar]
- Mooney HA, Bullock SH, Ehleringer JR (1989) Carbon isotope ratios of plants of a tropical forest in Mexico. Funct Ecol 3 137–142 [Google Scholar]
- Motomura H, Yukawa T, Ueno O, Kagawa A (2008) The occurrence of Crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. J Plant Res 121 163–177 [DOI] [PubMed] [Google Scholar]
- New M, Lister D, Hulme M, Makin I (2002) A high-resolution data set of surface climate over global land areas. Clim Res 21 1–25 [Google Scholar]
- Ojeda I, Fernández-Concha GC, Romero-González GA (2005) New species and combinations in Heterotaxis lindley (Orchidaceae: Maxillariinae). Novon 15 572–582 [Google Scholar]
- Pagel MD (1992) A method for the analysis of comparative data. J Theor Biol 156 431–442 [Google Scholar]
- Peakall R (2007) Speciation in the Orchidaceae: confronting the challenges. Mol Ecol 16 2834–2837 [DOI] [PubMed] [Google Scholar]
- Pearson PN, Palmer MR (2000) Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406 695–699 [DOI] [PubMed] [Google Scholar]
- Peet RK (1978) Forest vegetation of the Colorado Front Range: patterns of species diversity. Vegetatio 37 65–78 [Google Scholar]
- Pierce S, Winter K, Griffiths H (2002) Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytol 156 75–83 [Google Scholar]
- Pilón-Smits EAH, t'Hart H, Brederode J (1996) Evolutionary aspects of Crassulacean acid metabolism in the Crassulaceae. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer-Verlag, Berlin, pp 349–359
- Pridgeon AM, Chase MW (2001) A phylogenetic reclassification of Pleurothallidinae (Orchidaceae). Lindleyana 16 235–271 [Google Scholar]
- Pridgeon AM, Solano R, Chase MW (2001) Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. Am J Bot 88 2286–2308 [PubMed] [Google Scholar]
- Ramírez SR, Gravendeel B, Singer RB, Marshall CR, Pierce NE (2007) Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature 448 1042–1045 [DOI] [PubMed] [Google Scholar]
- Raven JA, Spicer RA (1996) The evolution of Crassulacean acid metabolism. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer-Verlag, Berlin, pp 360–385
- Rundel PW, Rundel JA, Ziegler H, Stichler W (1979) Carbon isotope ratios of central Mexican Crassulaceae in natural and glasshouse environments. Oecologia 38 45–50 [DOI] [PubMed] [Google Scholar]
- Salazar GA, Chase MW, Arenas MAS, Ingrouille M (2003) Phylogenetics of Cranichideae with emphasis on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA sequences. Am J Bot 90 777–795 [DOI] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Winter K (2005) Distribution of Crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Funct Plant Biol 32 397–407 [DOI] [PubMed] [Google Scholar]
- Smith JAC, Winter K (1996) Taxonomic distribution of Crassulacean acid metabolism. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer-Verlag, Berlin, pp 427–436
- Stern WL (1988) The long distance dispersal of Oeceoclades maculata. Am Orchid Soc Bull 57 960–971 [Google Scholar]
- Ting IP (1985) Crassulacean acid metabolism. Annu Rev Plant Physiol 36 595–622 [Google Scholar]
- van den Berg C, Higgins WE, Dressler RL, Whitten WM, Soto Arenas MA, Culham A, Chase MW (2000) A phylogenetic analysis of Laeliinae (Orchidaceae) based on sequence data from internal transcribed spacers (ITS) of nuclear ribosomal DNA. Lindleyana 15 96–114 [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution, version 4.0.1. The Phylodiversity Network. http://www.phylodiversity.net/phylocom (September 30, 2008) [DOI] [PubMed]
- Whittaker RH, Niering WA (1975) Vegetation of Santa Catalina Mountains, Arizona. V. Biomass, production and diversity along elevation gradient. Ecology 56 771–790 [Google Scholar]
- Whitten WM, Blanco MA, Williams NH, Koehler S, Carnevali G, Singer RB, Endara L, Neubig KM (2007) Molecular phylogenetics of Maxillaria and related genera (Orchidaceae: Cymbidieae) based on combined molecular data set. Am J Bot 94 1860–1889 [DOI] [PubMed] [Google Scholar]
- Whitten WM, Williams NH, Chase MW (2000) Subtribal and generic relationship of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. Am J Bot 87 1842–1856 [PubMed] [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM (2001. a) Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae). Lindleyana 162 113–139 [Google Scholar]
- Williams NH, Chase MW, Whitten WM (2001. b) Phylogenetic position of Miltoniopsis, Caucaea, a new genus, Cyrtochiloides, and relationship of Oncidium phymatochilum based on nuclear and chloroplast DNA sequence data (Orchidaceae: Oncidiinae). Lindleyana 16 272–285 [Google Scholar]
- Winter K (1979) δ13C values of some succulent plants from Madagascar. Oecologia 40 103–112 [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM (2002) How closely do the δ13C values of Crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiol 129 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Smith JAC (1996) Crassulacean acid metabolism: current status and perspectives. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer-Verlag, Berlin, pp 389–426
- Winter K, Wallace BJ, Stocker GC, Roksandic Z (1983) Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 57 129–141 [DOI] [PubMed] [Google Scholar]
- Zhao CM, Chen WL, Tian ZQ, Xie ZQ (2005) Altitudinal pattern of plant species diversity in Shennongjia Mountains, central China. J Integr Plant Biol 47 1431–1449 [Google Scholar]
- Zotz G (2004) How prevalent is Crassulacean acid metabolism among vascular epiphytes? Oecologia 138 184–192 [DOI] [PubMed] [Google Scholar]
- Zotz G, Ziegler H (1997) The occurrence of Crassulacean acid metabolism among vascular epiphytes from central Panama. New Phytol 137 223–229 [DOI] [PubMed] [Google Scholar]