Abstract
The glutathione-ascorbate redox pathway in chloroplasts is a complex network of spontaneous, photochemical, and enzymatic reactions for detoxifying hydrogen peroxide. This article presents a comprehensive sensitivity analysis of the system. A model has been constructed to simulate oxidative stress conditions, enabling steady-state concentrations of the metabolites involved in the pathway and photochemical and enzymatic fluxes to be calculated. The model includes an electron source whose flux is distributed among three competitive routes (photogeneration of  , photoreduction of NADP+ to NADPH, and photoreduction of monodehydroascorbate to ascorbate) and that allows the simulation of variations in NADPH concentration with time. Each enzyme considered is introduced in the model, taking into account its particular catalytic mechanism, including the inactivation of ascorbate peroxidase in the presence of low-ascorbate concentrations. Computer simulations pointed to the great sensitivity of the system to the ratio among fluxes corresponding to ascorbate and NADPH photoproduction and NADPH consumption by the Calvin cycle. Under oxidative stress conditions, the model shows a sequential depletion of antioxidant power in chloroplasts in the order NADPH, glutathione, ascorbate and their recovery in the reverse order. Decreasing levels of glutathione reductase, ascorbate peroxidase, and superoxide dismutase led to the irreversible photoinactivation of ascorbate peroxidase and the subsequent increase in hydrogen peroxide concentration, preceded by a maximum in dehydroascorbate reductase activity.
, photoreduction of NADP+ to NADPH, and photoreduction of monodehydroascorbate to ascorbate) and that allows the simulation of variations in NADPH concentration with time. Each enzyme considered is introduced in the model, taking into account its particular catalytic mechanism, including the inactivation of ascorbate peroxidase in the presence of low-ascorbate concentrations. Computer simulations pointed to the great sensitivity of the system to the ratio among fluxes corresponding to ascorbate and NADPH photoproduction and NADPH consumption by the Calvin cycle. Under oxidative stress conditions, the model shows a sequential depletion of antioxidant power in chloroplasts in the order NADPH, glutathione, ascorbate and their recovery in the reverse order. Decreasing levels of glutathione reductase, ascorbate peroxidase, and superoxide dismutase led to the irreversible photoinactivation of ascorbate peroxidase and the subsequent increase in hydrogen peroxide concentration, preceded by a maximum in dehydroascorbate reductase activity.
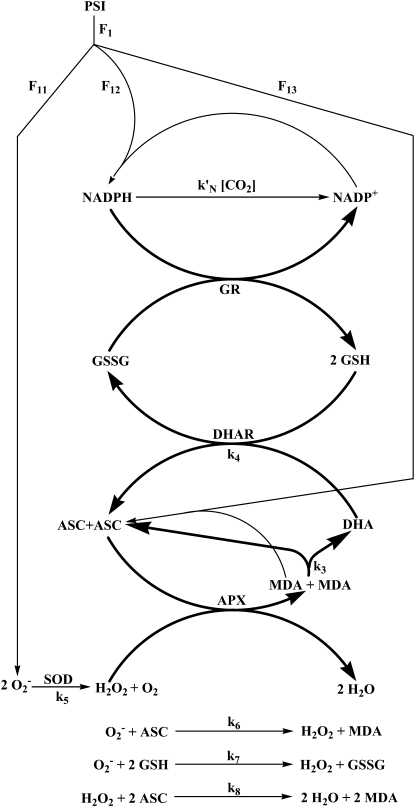
In plants, the main generation sites of reactive oxygen species (ROS) are the reaction centers of PSI and PSII in chloroplast thylakoids. Under steady-state conditions, the intracellular level of ROS is tightly buffered and controlled by detoxifying antioxidant systems formed of a network of enzymatic and nonenzymatic components (Noctor and Foyer, 1998; Mittler, 2002). Superoxide dismutase (superoxide:superoxide oxidoreductase [SOD]; EC 1.15.1.1) acts as the first line of defense against ROS by dismutating  to hydrogen peroxide (H2O2) and O2. In chloroplasts, the H2O2 thus generated is reduced to water by ascorbate (ASC) catalyzed with l-ascorbate peroxidase (l-ascorbate:hydrogen peroxide oxidoreductase [APX]; EC 1.11.1.11). This is the first step of the so-called glutathione-ascorbate (GSH-ASC) cycle, also named the water-water cycle (Asada, 1999, 2006). Dehydroascorbate reductase (glutathione:dehydroascorbate oxidoreductase [DHAR]; EC 1.8.5.1), monodehydroascorbate reductase (NADH:monodehydroascorbate oxidoreductase [MDAR]; EC 1.6.5.4), and glutathione reductase (glutathione:NADP+ oxidoreductase [GR]; EC 1.8.1.7) also participate in the pathway (Fig. 1), which uses ASC, GSH, and NADPH as electron donors (for an extensive review of the pathway, see Asada, 1999).
to hydrogen peroxide (H2O2) and O2. In chloroplasts, the H2O2 thus generated is reduced to water by ascorbate (ASC) catalyzed with l-ascorbate peroxidase (l-ascorbate:hydrogen peroxide oxidoreductase [APX]; EC 1.11.1.11). This is the first step of the so-called glutathione-ascorbate (GSH-ASC) cycle, also named the water-water cycle (Asada, 1999, 2006). Dehydroascorbate reductase (glutathione:dehydroascorbate oxidoreductase [DHAR]; EC 1.8.5.1), monodehydroascorbate reductase (NADH:monodehydroascorbate oxidoreductase [MDAR]; EC 1.6.5.4), and glutathione reductase (glutathione:NADP+ oxidoreductase [GR]; EC 1.8.1.7) also participate in the pathway (Fig. 1), which uses ASC, GSH, and NADPH as electron donors (for an extensive review of the pathway, see Asada, 1999).
Figure 1.
Schematic representation of the model to simulate the dynamic behavior of the GSH-ASC pathway in chloroplasts. ki values (i = 3–8) are apparent bimolecular rate constants for the spontaneous steps included in the model.
Numerous studies have been performed on the GSH-ASC cycle, and it is still a matter of intensive research (Noctor and Foyer, 1998; Asada, 1999, 2006). The computer modeling of metabolic systems is an increasingly used tool that has enhanced our understanding of the behavior of metabolic pathways. It was introduced to analyze pathways in chloroplasts in the early 1980s (Laisk and Walker, 1986; Hahn, 1987), and more recently, a very interesting paper has analyzed the network of redox reactions of the GSH-ASC cycle (Polle, 2001). The model was successfully used to simulate the functioning of the redox cycle in chloroplasts, making it possible to calculate steady-state concentrations of species and fluxes of steps involved in the reaction scheme proposed. However, the model has some limitations, such as the assumption of a constant NADPH concentration during the simulation time, the nonreduction of the NADP+ formed (leading to the erroneous accumulation of NADP+ in the chloroplast), and a constant 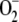 production rate during the simulation time. Furthermore, the fact that the kinetic behavior of the enzymes involved in the pathway was introduced with an identical equation, that corresponding to a ping-pong bi bi reaction mechanism (except SOD, which was treated as a bimolecular reaction), means that the model should be modified.
production rate during the simulation time. Furthermore, the fact that the kinetic behavior of the enzymes involved in the pathway was introduced with an identical equation, that corresponding to a ping-pong bi bi reaction mechanism (except SOD, which was treated as a bimolecular reaction), means that the model should be modified.
The aim of this article was to extend the above approach in several aspects in an attempt to improve the model and to bring it closer to what occurs in reality. In this way, the model will allow a better scientific understanding of the spontaneous and enzymatic reactions involved in the pathway and may contribute to the prediction of results in the design of experiments. (1) The electron flux from PSI has been considered as being distributed among three competitive routes involving the photogeneration of 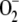 from O2, the photoreduction of NADP+ to NADPH, and the photoreduction of monodehydroascorbate radicals (MDA) to ASC. In this way, NADP+ is recycled to NADPH and the electron flux related to the production of
from O2, the photoreduction of NADP+ to NADPH, and the photoreduction of monodehydroascorbate radicals (MDA) to ASC. In this way, NADP+ is recycled to NADPH and the electron flux related to the production of 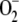 is changeable and dependent on the availability of NADPH and MDA, making the model much more flexible, since the three fluxes must be adapted to each other to respond to stress conditions. (2) Each enzymatic activity involved in the cycle has been introduced in the model, taking into account the particular catalytic mechanism of each enzyme. In addition, an inactivation step for APX in the presence of H2O2 (Miyake et al., 2006) has been included along with a route for the de novo synthesis of the protein. This last step represents one of the main defense mechanisms of plants to mitigate oxidative stress, so the recovery of APX inactivated by an excess of H2O2 can be simulated. (3) An additional step representing NADPH consumption in the Calvin cycle has been added, so that the competition between the NADPH consumed by the ROS-detoxifying pathway and the NADPH consumed by the Calvin cycle is included. (4) The model has been constructed based on the ordinary differential equations (ODE) system corresponding to the network of spontaneous and enzymatic steps involved in the cycle. Computer simulations have been performed by numerical integration of this set of ODE using the easily accessible software MATLAB. This software has several ODE solver routines for nonstiff and stiff (as in the present case) problems, so users can choose the best option for their specific problem.
is changeable and dependent on the availability of NADPH and MDA, making the model much more flexible, since the three fluxes must be adapted to each other to respond to stress conditions. (2) Each enzymatic activity involved in the cycle has been introduced in the model, taking into account the particular catalytic mechanism of each enzyme. In addition, an inactivation step for APX in the presence of H2O2 (Miyake et al., 2006) has been included along with a route for the de novo synthesis of the protein. This last step represents one of the main defense mechanisms of plants to mitigate oxidative stress, so the recovery of APX inactivated by an excess of H2O2 can be simulated. (3) An additional step representing NADPH consumption in the Calvin cycle has been added, so that the competition between the NADPH consumed by the ROS-detoxifying pathway and the NADPH consumed by the Calvin cycle is included. (4) The model has been constructed based on the ordinary differential equations (ODE) system corresponding to the network of spontaneous and enzymatic steps involved in the cycle. Computer simulations have been performed by numerical integration of this set of ODE using the easily accessible software MATLAB. This software has several ODE solver routines for nonstiff and stiff (as in the present case) problems, so users can choose the best option for their specific problem.
DESCRIPTION OF THE MODEL
The system being studied is depicted in Figure 1. There is a constant electron source in the model, PSI, whose flux is divided among three competitive routes: (1) the photoproduction of 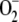 ; (2) the photoreduction of NADP+; and (3) the photoreduction of MDA. The last two processess are ferredoxin (Fd) dependent, although Fd has not been introduced as a variable in the present model, and only electron fluxes have been considered. The same holds for the electron donor species involved in the photogeneration of
; (2) the photoreduction of NADP+; and (3) the photoreduction of MDA. The last two processess are ferredoxin (Fd) dependent, although Fd has not been introduced as a variable in the present model, and only electron fluxes have been considered. The same holds for the electron donor species involved in the photogeneration of 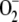 (in the same way, [O2] and [CO2] have also been considered constants). For the notation and definitions used for the fluxes, see Table I. F13 has been considered as directly proportional to [MDA], k13 being the proportionality constant. Factor 2 in F12 indicates the fact that the reduction of NADP+ to NADPH is a two-electron process. F11 is the remaining flux from PSI. In this way F11, F12, and F13 are variable and dependent on the [NADP+] and [MDA] present in chloroplasts. This imparts great flexibility to the model and a greater ability to study stress conditions in chloroplasts.
(in the same way, [O2] and [CO2] have also been considered constants). For the notation and definitions used for the fluxes, see Table I. F13 has been considered as directly proportional to [MDA], k13 being the proportionality constant. Factor 2 in F12 indicates the fact that the reduction of NADP+ to NADPH is a two-electron process. F11 is the remaining flux from PSI. In this way F11, F12, and F13 are variable and dependent on the [NADP+] and [MDA] present in chloroplasts. This imparts great flexibility to the model and a greater ability to study stress conditions in chloroplasts.
Table I.
Notation and definitions of fluxes used in the present model
| Notation | Definition | Mathematical Expression |
|---|---|---|
| F1 | Electron flow from PSI | F1 = F11 + F12 + F13 |
| F11 | Electron flow related to the one-electron photoreduction of 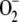
|
F1 − 2k12[NADP+] − k13[MDA] |
| F12 | Electron flow related to the two-electron photoreduction of NADP+ to NADPH | 2k12[NADP+] |
| F13 | Electron flow related to the one-electron reduction of MDA to ASC | k13[MDA] |
| FN | NADPH flow related to the Calvin cycle | k′N[CO2][NADPH] |
| F5 | Rate of de novo synthesis of APX | k5APX([APX]0 − [APX] − [CoI] − [CoII]) |
Enzymes Involved in the Model
SOD
The 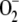 photogenerated directly by the PSI complex is mainly disproportionated to H2O2 and O2 catalyzed by SOD. This reaction has a 10,000-fold faster rate than spontaneous dismutation (Bowler et al., 1992). In almost all plants, chloroplasts contain CuZn-SOD as the major isoform of the enzyme (Asada, 1999). These enzymes are known as superefficient
photogenerated directly by the PSI complex is mainly disproportionated to H2O2 and O2 catalyzed by SOD. This reaction has a 10,000-fold faster rate than spontaneous dismutation (Bowler et al., 1992). In almost all plants, chloroplasts contain CuZn-SOD as the major isoform of the enzyme (Asada, 1999). These enzymes are known as superefficient 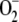 scavengers with pseudo first-order (kcat/Km) catalytic rates as high as 2 × 109 m−1 s−1 in water and 2 × 108 m−1 s−1 in the stroma, owing to its high viscosity (Ogawa et al., 1995). Taking this into account and the fact that [
scavengers with pseudo first-order (kcat/Km) catalytic rates as high as 2 × 109 m−1 s−1 in water and 2 × 108 m−1 s−1 in the stroma, owing to its high viscosity (Ogawa et al., 1995). Taking this into account and the fact that [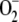 ] inside chloroplasts is on the order of micromolar, first-order kinetics can be assumed for the rate of SOD with respect to [
] inside chloroplasts is on the order of micromolar, first-order kinetics can be assumed for the rate of SOD with respect to [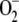 ]. So the following rate equation was used in our model:
]. So the following rate equation was used in our model:
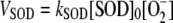 |
(1) |
APX
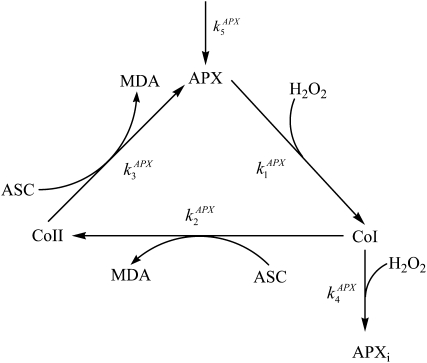
The H2O2 produced via the SOD-catalyzed disproportionation of  is reduced to water by ASC, catalyzed with APX. This enzyme is a class I heme peroxidase (Welinder, 1992) whose catalytic mechanism is reminiscent of that observed in other peroxidases and involves the formation of an oxidized compound I (CoI) intermediate, which is subsequently reduced by substrate in two sequential, single-electron-transfer steps (Fig. 2). A characteristic property of APX, especially of the chloroplastic isoform, is its lability in the absence of its electron donor ASC (Miyake et al., 2006), since it is inactivated by the interaction of CoI with H2O2. To account for this fact, the step has been included in the catalytic cycle of the enzyme, along with a step corresponding to the de novo synthesis of APX. This last step must be included in the mechanism for the APX activity to attain the steady-state rate under nonstress conditions. The rate of the biosynthetic route (F5) is considered directly proportional to the amount of initial enzyme present in a nonactive form (Table I). So, at t = 0, [APX] = [APX]0, [CoI] = [CoII] = [APXi] = 0, and then F5 becomes F5 = k5APX (0) = 0 (i.e. the biosynthetic route does not work at t = 0 since there is no inactive enzyme present). The more APX in the inactive form, the higher the term ([APX]0 − [APX] − [CoI] − [CoII]), so the rate of the biosynthetic route increases as the amount of active enzyme decreases. It is evident that the de novo synthesis in vivo is largely controlled by many factors other than APX, but we have chosen a simplified formula, since it is not possible to take into account all of these factors in the computer simulation. However, this fact does not change the general concept of our model. Taking into account the complexity of the APX mechanism and the great interest in simulating its kinetic behavior at the physiological level, it has been included in full in the model (Supplemental Equations S9–S12).
is reduced to water by ASC, catalyzed with APX. This enzyme is a class I heme peroxidase (Welinder, 1992) whose catalytic mechanism is reminiscent of that observed in other peroxidases and involves the formation of an oxidized compound I (CoI) intermediate, which is subsequently reduced by substrate in two sequential, single-electron-transfer steps (Fig. 2). A characteristic property of APX, especially of the chloroplastic isoform, is its lability in the absence of its electron donor ASC (Miyake et al., 2006), since it is inactivated by the interaction of CoI with H2O2. To account for this fact, the step has been included in the catalytic cycle of the enzyme, along with a step corresponding to the de novo synthesis of APX. This last step must be included in the mechanism for the APX activity to attain the steady-state rate under nonstress conditions. The rate of the biosynthetic route (F5) is considered directly proportional to the amount of initial enzyme present in a nonactive form (Table I). So, at t = 0, [APX] = [APX]0, [CoI] = [CoII] = [APXi] = 0, and then F5 becomes F5 = k5APX (0) = 0 (i.e. the biosynthetic route does not work at t = 0 since there is no inactive enzyme present). The more APX in the inactive form, the higher the term ([APX]0 − [APX] − [CoI] − [CoII]), so the rate of the biosynthetic route increases as the amount of active enzyme decreases. It is evident that the de novo synthesis in vivo is largely controlled by many factors other than APX, but we have chosen a simplified formula, since it is not possible to take into account all of these factors in the computer simulation. However, this fact does not change the general concept of our model. Taking into account the complexity of the APX mechanism and the great interest in simulating its kinetic behavior at the physiological level, it has been included in full in the model (Supplemental Equations S9–S12).
Figure 2.
APX mechanism introduced in the model, including the inactivation of the enzyme by reaction of CoI with H2O2 and a continuous input of APX into the system by the de novo synthesis of the protein. APXi is the inactive enzyme.
MDA is the primary product of the APX-catalyzed reduction of H2O2 by ASC, whose recovery is essential for maintaining the activity of the ROS-scavenging system. MDA spontaneously disproportionates to dehydroascorbate (DHA) and ASC in a pH-dependent way (Bielski et al., 1971). It is also directly reduced to ASC by photoreduced ferredoxin (redFd) in thylakoids (Miyake and Asada, 1994). The redFd competes between MDA and NADP+, but the reduction rate of MDA is 34-fold higher than that of NADP+ (Miyake and Asada, 1994; Asada, 1999). Thus, redFd is preferably used to reduce MDA rather than NADP+, suggesting that MDA is mainly photoreduced via Fd but not via NAD(P)H with MDAR, at least in the thylakoidal scavenging system. Taking this into account, MDAR is not included in the present model, which does not change the general concept of our model.
DHAR
This enzyme catalyzes the reduction of DHA to ASC, with GSH as the electron donor, in a ter bi reaction. The regeneration of ASC from DHA by GSH also occurs spontaneously, but the rate is too slow to account for the observed photoreduction of DHA in chloroplasts (Asada, 1999). Kinetic analysis of the enzymatic reaction (Shimaoka et al., 2003) suggested that the reaction proceeds by means of a bi uni uni uni ping-pong mechanism (Segel, 1975), in which the binding of DHA to the free, reduced form of the enzyme was followed by the binding of GSH. Solving the ODE system corresponding to this mechanism for the forward reaction leads to the following rate equation under steady-state conditions, which has been used in our model:
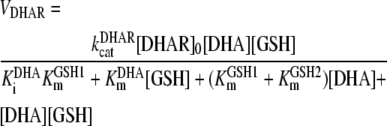 |
(2) |
This equation contains an independent term in the denominator, KiDHAKmGSH1, which we have grouped as K. The first expression, KiDHA, is the equilibrium constant between DHA, DHAR, and the enzyme-substrate DHAR-DHA complex. KmGSH1 and KmGSH2 are the values of Km for the first-binding and the second-binding molecules of GSH, respectively. The sum KmGSH1 + KmGSH2 has also been grouped as KmGSH in our model, since it has not been possible to calculate these values separately in kinetic assays (Shimaoka et al., 2003).
Note that Equation 2 can be simplified to the following expression:
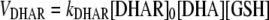 |
(3) |
when the following condition is fulfilled:
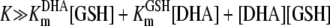 |
(4) |
being:
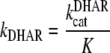 |
(5) |
GR
The supply of GSH is regenerated in an NADPH-dependent reaction catalyzed by GR. Kinetic studies performed with this enzyme have revealed a bi ter ping-pong mechanism (Vanoni et al., 1990). The rate equation corresponding to this mechanism under steady-state conditions for the forward reaction is the following, used in our model:
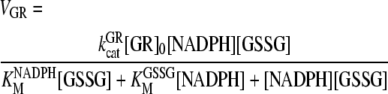 |
(6) |
Other Steps Involved in the Model
An additional route of NADPH consumption by the Calvin cycle (and other electron-consuming reactions) has also been added to the model, although in a simplified way, as a flux with an apparent rate constant, kN = k′N[CO2]. Other nonenzymatic reactions participating in the cycle and included in our model are indicated in Figure 1.
The ODE system that describes the mechanism shown in Figure 1 is shown in the supplemental data. The values for the kinetic constants and the initial conditions used to run the model are listed in Tables II and III, respectively. The rate constants corresponding to spontaneous steps are apparent bimolecular rate constants. Values for the kinetic parameters involved in the model have been taken from Asada (1999), Polle (2001), or from references describing kinetic studies of the target reaction. Other numerical values used were arbitrary but chosen to furnish reasonable data.
Table II.
Kinetic constants used to run the present model
| Kinetic Constant | Value | Unit | Reference |
|---|---|---|---|
| F1 | 640 | μm s−1 | Polle (2001) |
| kcatGR | 595 | s−1 | Polle (2001) |
| kcatDHAR | 142 | s−1 | Polle (2001) |
| kSOD | 200 | μm−1 s−1 | Asada (1999) |
| k1APX | 12 | μm−1 s−1 | Asada (1999) |
| k2APX | 50 | μm−1 s−1 | Asada (1999) |
| k3APX | 2.1 | μm−1 s−1 | Asada (1999) |
| k4APX | 0.7 | μm−1 s−1 | Asada (1999) |
| k5APX | 0.01 | s−1 | – |
| k3 | 0.5 (pH 7.0) | μm−1 s−1 | Bielski et al. (1971) |
| k4 | 0.1 | μm−1 s−1 | Hausladen and Kunert (1990) |
| k5 | 0.2 (pH 7.0) | μm−1 s−1 | Fridovich (1998) |
| k6 | 0.2 | μm−1 s−1 | Asada and Takahashi (1987) |
| k7 | 0.7 | μm−1 s−1 | Asada and Takahashi (1987) |
| k8 | 2.0 × 10−6 | μm−1 s−1 | Polle and Junkermann (1996) |
| k12 | 1.3 | s−1 | – |
| k13 | 42.5 | s−1 | – |
| kN = k′N[CO2] | 0.5 | s−1 | – |
| KmNADPH | 3 | μm | Polle (2001) |
| KmGSSG | 2.0 × 102 | μm | Polle (2001) |
| KmDHA | 70 | μm | Polle (2001) |
| KmGSH | 2.5 × 103 | μm | Polle (2001) |
| K | 5.0 × 105 | μm2 | – |
Table III.
Initial concentrations used to run the model
These values were chosen to be similar to those used by Polle (2001), although we have preferred the system to synthesize 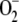 and H2O2 by itself at the beginning of the reaction as a function of the initial conditions. The initial concentrations of
and H2O2 by itself at the beginning of the reaction as a function of the initial conditions. The initial concentrations of 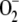 , H2O2, and other species, therefore, were set to zero.
, H2O2, and other species, therefore, were set to zero.
| Species | Initial Concentration |
|---|---|
| μm | |
| GR | 1.4 |
| DHAR | 1.7 |
| SOD | 50 |
| APX | 70 |
| NADPH | 150 |
| GSH | 4,000 |
| ASC | 10,000 |
RESULTS
Kinetic Behavior of the GSH-ASC Cycle as a Substrate Cycle
At the enzymatic level (i.e. without considering any spontaneous steps involved in the cycle or the electron source from PSI, except for the spontaneous dismutation of the MDA necessary for DHAR), the GSH-ASC cycle can be seen as two coupled substrate cycles involving the participation of APX, DHAR, and GR (Fig. 1, boldface steps). Substrate cycles are powerful metabolic tools involving two enzymes acting in opposite directions, whereby a target metabolite is reversibly interconverted into another chemical species without being consumed (Newsholme et al., 1984; Valero and García-Carmona, 1996; Valero et al., 2000). This is accompanied by the concomitant accumulation of other noncycling products at each turn of the cycle. The physiological explanation proposed for this wasteful cycling is that it is mainly a way of amplifying a metabolic response to a change in a metabolic concentration, thus greatly improving the sensitivity of metabolic regulation.
One of the main advantages of computer simulation is that it allows the dynamic behavior of a metabolic pathway to be studied by introducing the desired steps in the model until it is understood. The great amplification capacity of the GSH-ASC cycle can be checked by computer simulation of the boldface steps in Figure 1. The results obtained showed that an initial concentration of H2O2 as high as 10 μm, which has been reported to inhibit photosynthesis by 50% (Kaiser, 1979), was rapidly removed by the cyclic system in less than 0.02 s (half-time = 0.83 ms) at the expense of the equivalent consumption of NADPH.
When the spontaneous steps shown in Figure 1 were added to the model, the results obtained were very similar (data not shown), the most noteworthy differences being the lower accumulation of DHA in the first instants of the reaction, the shorter recovery time of the reducing species (GSH and ASC), an increased VGR, and a decreased VDHAR. In contrast, if [GR]0, [DHAR]0, and [APX]0 are made equal to zero and only spontaneous steps are operative in the system, the data obtained point to the slower removal of H2O2 (half-time = 34.65 s) and low ASC recovery. GSH cannot recover simply by means of these spontaneous reactions.
Kinetic Behavior of the System in Unstressed Chloroplasts
In the chloroplast, the cycle is not metabolically isolated; so now that we know how the cycle functions, we can introduce its immediate environment. Photoreduction of molecular oxygen in the PSI of chloroplasts generates 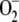 continuously in daylight, which means that the H2O2 must be continuously removed by the GSH-ASC cycle, for which the level of NADPH must recover. This process occurs in the photochemical phase of photosynthesis in PSI, although it is also important to take into account the consumption of NADPH in the Calvin cycle in the presence of CO2. All of these steps, as well as the photoreduction of MDA, have been included in our model.
continuously in daylight, which means that the H2O2 must be continuously removed by the GSH-ASC cycle, for which the level of NADPH must recover. This process occurs in the photochemical phase of photosynthesis in PSI, although it is also important to take into account the consumption of NADPH in the Calvin cycle in the presence of CO2. All of these steps, as well as the photoreduction of MDA, have been included in our model.
Unstressed chloroplasts generally show low, stationary levels of ROS and high ratios for the reduced form/oxidized form of the redox pairs involved in the cycle. The conditions indicated in Tables II and III have been chosen to mimic the behavior of the GSH-ASC cycle in unstressed chloroplasts. Under these conditions, a steady state was rapidly achieved by the system, in which metabolite concentrations and fluxes remained constant. The steady-state concentrations thus obtained were as follows: [NADPH]ss = 84.95 μm, [GSH]ss = 3.98 mm, [ASC]ss = 9.99 mm, [DHA]ss = 42.9 nm, [MDA]ss = 5.85 μm, 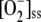 = 8.9 nm, and [H2O2]ss = 0.16 μm. These values are close to the ranges estimated for healthy tissues (Asada, 1994). The [MDA]ss is a little high, perhaps because MDAR is not included in our model. However [DHA]ss is very low in comparison with the levels usually found in plants (Polle, 1997; Kwon et al., 2003). Any inactivation of APX during integration time was negligible under these conditions, since ASC levels remained high throughout the reaction.
= 8.9 nm, and [H2O2]ss = 0.16 μm. These values are close to the ranges estimated for healthy tissues (Asada, 1994). The [MDA]ss is a little high, perhaps because MDAR is not included in our model. However [DHA]ss is very low in comparison with the levels usually found in plants (Polle, 1997; Kwon et al., 2003). Any inactivation of APX during integration time was negligible under these conditions, since ASC levels remained high throughout the reaction.
An 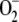 steady-state production rate of 222.2 μm s−1 was obtained, which is within the range previously mentioned by other authors (Asada, 1999; Polle, 2001), while the conversion rate of MDA into ASC (248.7 μm s−1) was slightly higher and the photochemical recycling of NADPH (169.1 μm s−1) was slightly slower, reflecting the preference of redFd to reduce MDA rather than NADP+. The most active enzymes were APX and SOD followed by GR and DHAR, since the low glutathione oxidized concentration ([GSSG]) and [DHA] in these conditions limits their velocity. A maximum in VSOD and VAPX was appreciated at the start of the reaction, since the enzymes must work hard to lower the accumulation of
steady-state production rate of 222.2 μm s−1 was obtained, which is within the range previously mentioned by other authors (Asada, 1999; Polle, 2001), while the conversion rate of MDA into ASC (248.7 μm s−1) was slightly higher and the photochemical recycling of NADPH (169.1 μm s−1) was slightly slower, reflecting the preference of redFd to reduce MDA rather than NADP+. The most active enzymes were APX and SOD followed by GR and DHAR, since the low glutathione oxidized concentration ([GSSG]) and [DHA] in these conditions limits their velocity. A maximum in VSOD and VAPX was appreciated at the start of the reaction, since the enzymes must work hard to lower the accumulation of 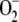 and H2O2, respectively, in the initial phase (Supplemental Fig. S1).
and H2O2, respectively, in the initial phase (Supplemental Fig. S1).
Kinetic Behavior of the System in Stressed Chloroplasts
Oxidative stress is characterized by the disruption of cellular homeostasis, leading to the enhanced production of ROS and changes in the redox state of chloroplasts. Many environmental perturbations, including intense light, temperature stress, air pollutants, and herbicides, affect the efficiency of photosynthetic electron transport. To study the dynamic behavior of the system under these conditions, F1 was increased to 2,450 μm s−1. NADPH levels fell very rapidly in the first instants of the reaction, followed by GSH. ASC levels remained high as long as NADPH and GSH were present in the chloroplasts, although afterward they decreased sharply to reach limiting concentrations. The disappearance of ASC was followed by the rapid inactivation of APX, reflecting what occurs in reality (Miyake and Asada, 1996), accompanied by a sharp increase in [APXi]. Once APX has been inactivated, H2O2 is accumulated in the chloroplast at a very high rate, since it is continuously produced from 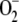 , spontaneously and enzymatically catalyzed by SOD. A minimum in [MDA] and in F13 could also be observed, after which [MDA] recovered by spontaneous steps. Consequently, a maximum in F11 and hence in [
, spontaneously and enzymatically catalyzed by SOD. A minimum in [MDA] and in F13 could also be observed, after which [MDA] recovered by spontaneous steps. Consequently, a maximum in F11 and hence in [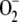 ] and VSOD appeared. The increase in [ASC] once APX has been inactivated is due to the spontaneous redox steps involved in the model. However, it must be taken into account that all of these recoveries observed after APX inactivation are produced as a result of [H2O2] increasing above a life-compatible level, so they have no physical meaning. F12 and VGR reached a steady state, which was not affected by APX inactivation. FN sharply decreased near the start of the reaction as a consequence of NADPH depletion in the chloroplast (Supplemental Fig. S2).
] and VSOD appeared. The increase in [ASC] once APX has been inactivated is due to the spontaneous redox steps involved in the model. However, it must be taken into account that all of these recoveries observed after APX inactivation are produced as a result of [H2O2] increasing above a life-compatible level, so they have no physical meaning. F12 and VGR reached a steady state, which was not affected by APX inactivation. FN sharply decreased near the start of the reaction as a consequence of NADPH depletion in the chloroplast (Supplemental Fig. S2).
Kinetic Response of the System against an Increasing Electron Flux
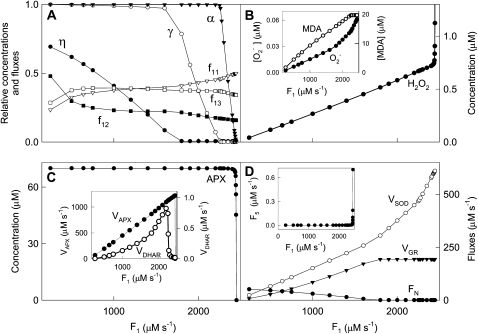
Intense light exposure causes oxidative stress in plants, but a high electron flux through the water-water cycle provides a way to dissipate excess light energy (Asada, 1999). To address the response of the system under study to this increasing oxidative stress, F1 was varied from 250 to 2,450 μm s−1 (i.e. until APX underwent photoinactivation by excess of H2O2). Steady-state concentrations of metabolites involved in the mechanism under study and the fluxes thus obtained are shown in Figure 3. The model shows a progressive decrease in the steady-state levels of antioxidants as F1 increases, NADPH being the first to be depleted in the chloroplast, followed by GSH and ASC. The [MDA]ss, 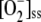 , and [H2O2]ss increased linearly as long as F1 was low. At higher F1, [MDA]ss remained constant, the accumulation of
, and [H2O2]ss increased linearly as long as F1 was low. At higher F1, [MDA]ss remained constant, the accumulation of 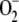 was more pronounced (steeper slope of the plot), and [H2O2]ss sharply increased owing to the induced photoinactivation of APX. APX falls in a very narrow range of F1 (Fig. 3C) when its inactivation exceeds its de novo synthesis, although its enzymatic activity continues increasing almost until the end, with the aim of eliminating as much H2O2 as possible. The rate of de novo synthesis of APX (F5; Fig. 3D, inset) sharply increased with the inactivation of APX, which represents a defense against ROS.
was more pronounced (steeper slope of the plot), and [H2O2]ss sharply increased owing to the induced photoinactivation of APX. APX falls in a very narrow range of F1 (Fig. 3C) when its inactivation exceeds its de novo synthesis, although its enzymatic activity continues increasing almost until the end, with the aim of eliminating as much H2O2 as possible. The rate of de novo synthesis of APX (F5; Fig. 3D, inset) sharply increased with the inactivation of APX, which represents a defense against ROS.
Figure 3.
Dependence of steady-state concentrations and fluxes on F1 variations. The values of the rate constants and initial conditions used were those shown in Tables II and III, except that F1 was varied between 250 and 2,450 μm s−1. Relative concentrations and fluxes are defined in “Materials and Methods.”
The relative distribution of F1 among the three routes considered under these conditions is also shown in Figure 3A. It can be seen that at high F1, only the 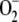 production rate increased. FN decreased owing to the lower availability of NADPH (Fig. 3D), although VGR increased steadily to reach a constant value because of increasing levels of GSSG in the chloroplast. VSOD increased in a parallel way with
production rate increased. FN decreased owing to the lower availability of NADPH (Fig. 3D), although VGR increased steadily to reach a constant value because of increasing levels of GSSG in the chloroplast. VSOD increased in a parallel way with 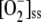 , and VDHAR showed a maximum, after which it abruptly fell, pointing to the next photoinactivation of APX.
, and VDHAR showed a maximum, after which it abruptly fell, pointing to the next photoinactivation of APX.
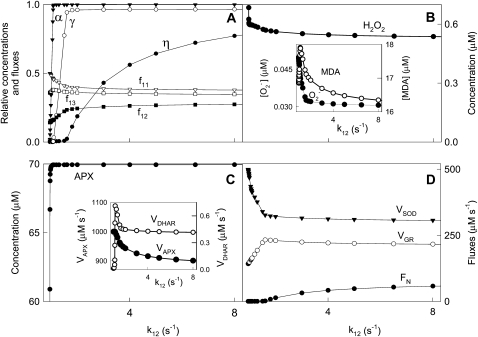
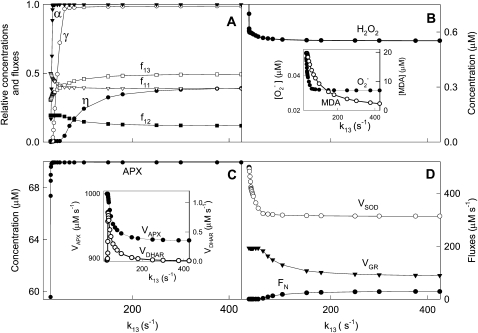
Effects on the System of Varying k12 and k13
The detoxifying efficiency of the mechanism under study is probably best evaluated by the relative distribution of F1 among the three competitive routes through the system. To do this, we have introduced a constant value of F1 = 2,000 μm s−1, and computer simulations were performed by varying k12 and k13. The system is extremely sensitive to low values of k12 and k13 when the photochemical recovery of NADPH and ASC is inoperative and the photoproduction rate of 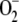 is higher (Figs. 4 and 5). For this reason, antioxidants were rapidly depleted and APX suffered inactivation in a very low range of k12 and k13. Therefore, sharp increases in [NADP+]ss, [GSSG]ss, [DHA]ss,
is higher (Figs. 4 and 5). For this reason, antioxidants were rapidly depleted and APX suffered inactivation in a very low range of k12 and k13. Therefore, sharp increases in [NADP+]ss, [GSSG]ss, [DHA]ss, 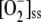 , and [H2O2]ss and increasing VSOD and VAPX values are observed at low k12 and k13. The MDA plot, however, shows a maximum at low k12, which is related to APX photoinactivation when ASC concentration is limiting. This maximum in [MDA]ss is not seen at low k13, since the electron flux deviated by the F13 branch for the photorecycling of ASC continuously increases to reach a nearly constant value.
, and [H2O2]ss and increasing VSOD and VAPX values are observed at low k12 and k13. The MDA plot, however, shows a maximum at low k12, which is related to APX photoinactivation when ASC concentration is limiting. This maximum in [MDA]ss is not seen at low k13, since the electron flux deviated by the F13 branch for the photorecycling of ASC continuously increases to reach a nearly constant value.
Figure 4.
Dependence of steady-state concentrations and fluxes on k12 variation. The values of the rate constants and initial conditions used were those shown in Tables II and III, except that F1 = 2,000 μm s−1 and k12 was varied between 0.95 and 250 s−1.
Figure 5.
Dependence of steady-state concentrations and fluxes on k13 variation. The values of the rate constants and initial conditions used were those shown in Tables II and III, except that F1 = 2,000 μm s−1 and k13 was varied between 31.1 and 500 s−1.
It is also possible to observe in Figure 4A an increase in F12 when k12 increased at low values, essentially at the expense of F11. In contrast, F12 remained constant when k13 was increased at low values (Fig. 5A). In this last plot, F13 increased basically at the expense of F11; once F11 attained a nearly constant value, F13 continued increasing at the expense of F12. Note that at higher k12 and k13 values, the distribution of the electron flux from PSI became almost constant (i.e. the system was saturated). Figures 4D and 5D show a similar dependence of FN as k12 and k13 were increased, in agreement with the greater availability of NADPH. NADPH almost completely recovered at relatively high values of k12, but not by increasing k13 under the conditions used here. The dependence of VGR on k12 and k13 was parallel to its respective F12 plot, and a maximum in VDHAR was also obtained in both cases at low k12 and k13, again pointing to the next photoinactivation of APX.
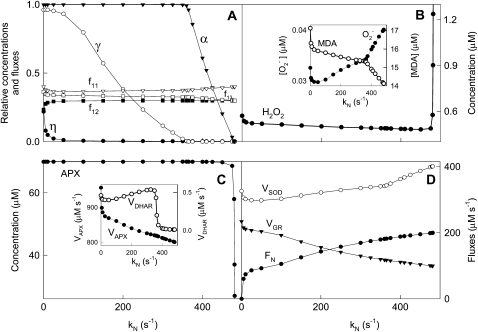
Effects on the System of Varying kN
We have introduced the photoproduction of NADPH and its consumption during the CO2 fixation in a simplified way through the FN flux step. Thus, kN is a kinetic parameter related to the Calvin cycle and other electron-consuming reactions under conditions of constant [CO2]. Computer simulations pointed to the great sensitivity of the model to the k12/kN ratio. Figure 6 shows the results obtained when varying kN for a fixed value of k12 = 2 s−1. Minimums in 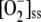 , VSOD, and F11 can be seen at low kN, meaning variations in kN can lead to the system adapting to light intensity, giving rise to minimal ROS levels. Therefore, an appropriate Calvin cycle rate (e.g. by means of an adequate level of CO2) might provide protection against ROS.
, VSOD, and F11 can be seen at low kN, meaning variations in kN can lead to the system adapting to light intensity, giving rise to minimal ROS levels. Therefore, an appropriate Calvin cycle rate (e.g. by means of an adequate level of CO2) might provide protection against ROS.
Figure 6.
Dependence of steady-state concentrations and fluxes on kN variation. The values of the rate constants and initial conditions used were those shown in Tables II and III, except that F1 = 2,000 μm s−1, k12 = 2 s−1, and kN was varied between 0 and 487 s−1.
As kN was increased, the antioxidant concentration in the chloroplast gradually decreased, in the order NADPH, GSH, ASC, so that their respective oxidized species concentrations increased, although [MDA]ss decreased due to the lower availability of ASC to spontaneously react with 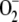 to produce it. [H2O2]ss showed a gradual decrease in spite of the increase in
to produce it. [H2O2]ss showed a gradual decrease in spite of the increase in 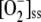 once NADPH was nearly depleted, which was probably related to spontaneous steps since [APX]ss, and its activity also decreased. Once ASC became limiting, APX was inactivated, so that [H2O2] increased abruptly.
once NADPH was nearly depleted, which was probably related to spontaneous steps since [APX]ss, and its activity also decreased. Once ASC became limiting, APX was inactivated, so that [H2O2] increased abruptly.
With regard to the electron fluxes, F12 increased as long as NADPH was left in the chloroplast but remained nearly constant when NADPH was nearly depleted. F11 and F13 decreased at low kN and after F11 increased at the expense of F13. A maximum in VDHAR prior to the photoinactivation of APX could be seen again. VGR decreased with increasing kN as a consequence of the lower availability of NADPH, whereas FN evidently increased.
Effects on the System of Varying Antioxidant Enzyme Concentrations
Antioxidant enzymes are critical components in preventing oxidative stress in plants. Manipulation of the expression of these enzymes by gene transfer technology has provided new insight into their role in chloroplasts by allowing direct investigation of their functions and interactions (Foyer et al., 1994; Allen et al., 1997). Mathematical modeling is also a powerful tool that can be used to predict the response of the system to changes in the activities and concentrations of the enzymes involved in the pathway. Therefore, computer simulations were performed here at different initial concentrations of the four enzymes considered in our model.
Decreasing levels of [GR]0 (data not shown) led to the irreversible photoinactivation of APX, again announced by a maximum in VDHAR, resulting in considerable increases in [GSSG]ss, [DHA]ss, [H2O2]ss, and 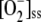 , accompanied by increases in F11, VSOD, and VAPX. However, NADPH consumption was lower at low [GR]0, and an increase in FN was observed.
, accompanied by increases in F11, VSOD, and VAPX. However, NADPH consumption was lower at low [GR]0, and an increase in FN was observed.
It has been reported that plants overexpressing GR are more tolerant of ROS-inducing herbicides such as methyl viologen, since they are able to maintain the reduction state of their ascorbate pools more effectively than control plants (Aono et al., 1991; Foyer et al., 1991). The numerical data obtained here agree with these results. Increasing levels of [GR]0 led to the recovery of ASC and APX and the partial recovery of GSH, although NADPH decreased due to its greater consumption by GR. In the same way, [GSSG]ss, [DHA]ss, [H2O2]ss, and 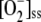 decreased to reach nearly constant values. [MDA]ss showed a maximum at low [GR]0, and also reached a nearly constant value when [GR]0 increased. F12 and F13 increased at low [GR]0 as a consequence of the decrease in F11, while VGR only increased at low [GR]0, also reaching a nearly constant value.
decreased to reach nearly constant values. [MDA]ss showed a maximum at low [GR]0, and also reached a nearly constant value when [GR]0 increased. F12 and F13 increased at low [GR]0 as a consequence of the decrease in F11, while VGR only increased at low [GR]0, also reaching a nearly constant value.
Unlike changes in [GR]0, variations in [DHAR]0 in our model had no noticeable effect on the steady-state concentrations of metabolites or on the fluxes through the GSH-ASC pathway (data not shown), even when [DHAR]0 = 0. Only a very slight decrease in [DHA]ss accompanied by a slight increase in [ASC]ss could be observed as [DHAR]0 increased, with a subsequent decrease in the rate of the spontaneous reaction between GSH and DHA. This means that this spontaneous step could be sufficient to cope with DHA removal, as suggested by Polle (2001). However, these results must be interpreted in the context of the present model, and they do not mean that DHAR is a dispensable enzyme in reality, in agreement with data reported by Chen and Gallie (2006). When computer simulations were performed in the absence of spontaneous DHA reduction (k4 = 0), a threshold [DHAR]0 was necessary if ASC levels were to recover and to prevent APX inactivation. It was also observed that under these conditions, VDHAR was higher than usually observed when k4 ≠ 0.
When [APX]0 was increased, a threshold value of [APX]0 of 3.37 μm was found to be necessary for the system to reach a steady state. Below this value, APX was irreversibly inactivated, since insufficient protein was supplied by de novo synthesis, and consequently, [H2O2] increased to toxic amounts. However, no appreciable effects on the steady state of the antioxidant pools, [MDA]ss, and 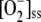 or on the fluxes and velocities of the other enzymes involved in the system were observed, even when [APX]0 = 0 (data not shown). These results indicate that high [H2O2] might coexist with high [GSH] and [ASC], although they should be treated with caution, since other possible pernicious effects of H2O2 in the cell have not been included in the model. Above the previously mentioned threshold value of [APX]0, the plots remained constant and parallel to the abscissa axis, the only changes observed being a decrease in [H2O2]ss, an increase in [APX]ss, and a rapid increase in VAPX until it reached a constant value.
or on the fluxes and velocities of the other enzymes involved in the system were observed, even when [APX]0 = 0 (data not shown). These results indicate that high [H2O2] might coexist with high [GSH] and [ASC], although they should be treated with caution, since other possible pernicious effects of H2O2 in the cell have not been included in the model. Above the previously mentioned threshold value of [APX]0, the plots remained constant and parallel to the abscissa axis, the only changes observed being a decrease in [H2O2]ss, an increase in [APX]ss, and a rapid increase in VAPX until it reached a constant value.
When k5APX (the apparent rate constant for the de novo synthesis of APX) was increased from 0.01 to 1 s−1, the system showed greater tolerance against oxidative stress conditions and the inactivation of the peroxidase enzyme was less abrupt (data not shown). This result agrees with the experimentally observed increased tolerance to methyl viologen damage reported in tobacco (Nicotiana tabacum) plants overexpressing cytosolic APX (Pitcher et al., 1994), although it is in apparent contradiction with data obtained by the same authors (Torsethaugen et al., 1997), indicating that overproduction of APX in tobacco chloroplasts does not provide protection against O3-induced stress. This may be due to the above-mentioned fact that VAPX remained nearly constant above a certain value (about 10 μm under the conditions used here) even when the [APX] was increased.
Finally, [SOD]0 was varied from 9 nm to 1 mm (Fig. 7). A value of less than 9 nm led to depletion of antioxidants, the inactivation of APX, and an excessive accumulation of H2O2 even if [SOD]0 = 0. However 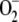 was not accumulated, since most of the
was not accumulated, since most of the 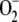 photogenerated in the chloroplast disappeared through spontaneous dismutation and reaction with ASC and GSH, yielding H2O2.
photogenerated in the chloroplast disappeared through spontaneous dismutation and reaction with ASC and GSH, yielding H2O2.
Figure 7.
Dependence of steady-state concentrations and fluxes on [SOD]0. The values of the rate constants and initial conditions used were those shown in Tables II and III, except that F1 = 2,000 μm s−1 and [SOD]0 was varied between 9 nm and 1 mm.
Computer simulations performed at low values of [SOD]0 yielded increased levels of NADP+, GSSG, DHA, MDA, H2O2, and 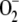 , indicating that a certain level of SOD is necessary for the plant to maintain its reduction potential against oxidative stress. These responses of the system disappeared when [SOD]0 was increased, since GSH levels began to recover and ASC was almost totally recovered. The inactivation of APX was negligible under these conditions, and [MDA]ss,
, indicating that a certain level of SOD is necessary for the plant to maintain its reduction potential against oxidative stress. These responses of the system disappeared when [SOD]0 was increased, since GSH levels began to recover and ASC was almost totally recovered. The inactivation of APX was negligible under these conditions, and [MDA]ss, 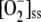 , [H2O2]ss, and VAPX decreased as a consequence of the lower amount of H2O2 accumulated. Again, a maximum in VDHAR appeared. The increase in F11 was accompanied by an increase in VSOD. This illustrates the balance between
, [H2O2]ss, and VAPX decreased as a consequence of the lower amount of H2O2 accumulated. Again, a maximum in VDHAR appeared. The increase in F11 was accompanied by an increase in VSOD. This illustrates the balance between 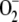 production and elimination in the metabolism. That is, when the chloroplast has insufficient
production and elimination in the metabolism. That is, when the chloroplast has insufficient 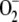 because of an excess of SOD (or for any other reason), part of the electron flux from PSI is directed to the production of more
because of an excess of SOD (or for any other reason), part of the electron flux from PSI is directed to the production of more 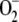 , so that ASC and NADPH photochemical recovery fall.
, so that ASC and NADPH photochemical recovery fall.
These data demonstrate that SOD is a critical component of the ROS-scavenging system of chloroplasts and indicate that overexpression of this enzyme in transgenic plants may improve plant stress tolerance. Transgenic plants with high levels of the three types of SOD have been developed and characterized by several research groups. The results obtained showed that, in many cases, high levels of SOD in a variety of cellular compartments lead to detectable increases in cellular protection from oxidative stress (Allen et al., 1997), in agreement with the theoretical data obtained here.
DISCUSSION
A new mathematical model has been developed for the kinetic study of the complex network of redox reactions involved in the GSH-ASC pathway for detoxifying ROS in chloroplasts. The model is based on another one reported previously by Polle (2001), although we have included several modifications that considerably improve it, bringing the results obtained nearer to reality.
We have introduced into the model the ODE corresponding to NADPH and NADP+, taking into account the balance between the photochemical recovery of NADPH, its consumption by the Calvin cycle, and the GR-catalyzed reaction. Polle's model (2001) could not contemplate this important subject from a physiological point of view because of excessive run times in the computer, so the corresponding equations were simplified. The consequence was that NADPH could not be recovered (it was assumed as a constant value), leading to the continuous accumulation of NADP+ in the chloroplast. However, as shown here, H2O2 is detoxified at the expense of the reducing power of NADPH. In its evolution since the appearance of oxygen in the atmosphere, the cell has developed a very efficient defense tool against oxygen toxicity, based on two coupled substrate cycles, although it needs a supply of NADPH. The waste of NADPH can be understood as the cost that chloroplasts must pay to swiftly detoxify H2O2. The present model is able to simulate this.
The pathway under study is very interesting, since the same daylight that gives rise to the synthesis of 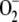 toxic radicals also generates NADPH and ASC to detoxify the H2O2 arising from
toxic radicals also generates NADPH and ASC to detoxify the H2O2 arising from 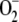 dismutation. Therefore, it is very important to know the relative weight of each route in the growth conditions of the plant. This is taken into account in the present model by introducing a constant electron flux from PSI distributed among three competitive branches: the photoproduction of
dismutation. Therefore, it is very important to know the relative weight of each route in the growth conditions of the plant. This is taken into account in the present model by introducing a constant electron flux from PSI distributed among three competitive branches: the photoproduction of 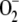 , the photoreduction of NADP+, and the photoreduction of MDA. Electron fluxes through these routes must adjust to each other as a function of the growth conditions of the plant. The fit between the three branches is a better way to understand the kinetic behavior of the pathway and the physiological response of chloroplasts in the face of a given stimulus, as has been shown by means of the computer simulations performed here.
, the photoreduction of NADP+, and the photoreduction of MDA. Electron fluxes through these routes must adjust to each other as a function of the growth conditions of the plant. The fit between the three branches is a better way to understand the kinetic behavior of the pathway and the physiological response of chloroplasts in the face of a given stimulus, as has been shown by means of the computer simulations performed here.
Each enzyme involved in our model has been introduced into the ODE, taking into account its particular catalytic mechanism. This is especially important in the case of APX, since it is the most H2O2 sensitive. SOD, DHAR, and GR are also inactivated, but only after long illumination in the presence of paraquat (Iturbe-Ormaetxe et al., 1998). Polle's model (2001) did not take into account the photoinactivation of APX, so a steady state was always attained. Instead, in our model, a steady state is only attained under nonstress conditions, when reducing power in the chloroplast is high and APX is not inactivated. The introduction of a step corresponding to the de novo synthesis of APX plays a double role in the model: on the one hand, it is necessary so that the system can reach a steady state under nonstress conditions; on the other hand, the results obtained are more realistic, since this is one of the main defense mechanisms of plants to mitigate oxidative stress. The present model is able to predict the rapid biosynthesis of the protein in response to harsh oxidative stress conditions.
The electron flux in the thylakoids also supplies NADPH for the Calvin cycle, so we included this in our model. There is a competition between the NADPH consumed by the ROS-detoxifying pathway and the NADPH consumed by the Calvin cycle. The results obtained here illustrate the importance of the fine regulation of the cycle, since the plots show a minimum of ROS production at low values of the apparent rate constant for the Calvin cycle (kN; Fig. 6B, inset). It is known that plants can regulate temporary mechanisms of stomatal closure in response to abiotic stress, thus limiting the entrance of CO2 into chloroplasts and decreasing the amount of NADPH consumed by the Calvin cycle (Fig. 6D). The model developed here is able to simulate, to our knowledge for the first time, this interesting physiological response of plants and therefore should contribute to its better understanding.
CONCLUSION
In conclusion, the mathematical model developed here is a very powerful tool for improving our theoretical understanding of the kinetic behavior of the ROS-detoxifying pathway in chloroplasts. The model, besides including new physiologically important aspects, such as the balance between the photogeneration and consumption of NADPH and APX inactivation, can easily be expanded to include new steps, such as regulatory devices for enzymes, dark-light interactions, the peroxiredoxin system (Dietz, 2003; Dietz et al., 2006), etc. It may also allow the guidance of future experimental work to evaluate those parameters involved in the cycle that have not been evaluated yet. In this way, the approach offers a new strategy for studying plant defense mechanisms against oxidative stress and can be used to predict the response of the plant to different stress conditions and where the limits of resistance can be expected.
MATERIALS AND METHODS
Simulated progress curves were obtained by numerical solution of the nonlinear set of differential equations corresponding to the mechanism shown in Figure 1 (Supplemental Equations S1–S13). Numerical integration was performed using the ode15s function from MATLAB software version 7.0 (http://www.mathworks.com). This is a variable-order solver based on numerical differentiation formulas and is a multistep solver recommended for stiff problems (Shampine and Reichelt, 1997). The file including the ODE and the kinetic constant values used is available to interested readers on request. Fluxes and enzymatic activities were calculated from data thus obtained by a computer program made by us using the Transform tool in the SigmaPlot scientific graphing system for Windows version 8.02 (http://www.spss.com), which is also available to interested readers on request. Data thus obtained were plotted using the same software. Steady-state values for metabolite concentrations and fluxes plotted in Figures 3 to 7 were obtained from the linear portion of progress curves given by the computer. The points represent theoretical data obtained in the computer, and the solid lines are straight lines linking them. Relative concentrations of antioxidants were defined as follows: α = [ASC]/[ASC]0, γ = [GSH]/[GSH]0, and η = [NADPH]/[NADPH]0. Relative fluxes were defined as follows: f11 = F11/F1, f12 = F12/F1, and f13 = F13/F1.
These programs were run on a PC-compatible computer based on a Pentium IV 2-GHz processor with 512 MB of RAM. The time consumed by this computer to obtain each particular numerical solution was on the order of a few seconds or even less in those cases in which a steady state was reached.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Simulated progress curves corresponding to the species involved in the mechanism shown in Figure 1, in unstressed chloroplasts.
Supplemental Figure S2. Simulated progress curves corresponding to the species involved in the mechanism shown in Figure 1, in stressed chloroplasts.
Supplemental Equations S1 to S13. Differential equations system corresponding to the mechanism shown in Figure 1.
Supplementary Material
Acknowledgments
We thank Dr. F. Sevilla from the Centro de Edafología y Biología Aplicada del Segura (Murcia, Spain) for her constructive criticism during the course of this work.
This work was supported by the Junta de Comunidades de Castilla-La Mancha (grants no. PAI–05–036 and PAI08–0175–8618 and a predoctoral fellowship to M.I.G.-S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Edelmira Valero (edelmira.valero@uclm.es).
The online version of this article contains Web-only data.
References
- Allen RD, Webb RP, Schake SA (1997) Use of transgenic plants to study antioxidant defenses. Free Radic Biol Med 23 473–479 [DOI] [PubMed] [Google Scholar]
- Aono M, Kubo A, Saji H, Natori T, Tanaka K, Kondo N (1991) Resistance to active oxygen toxicity of transgenic Nicotiana tabacum that express the gene for glutathione reductase from Escherichia coli. Plant Cell Physiol 32 691–697 [Google Scholar]
- Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In CH Foyer, PM Mullineaux, eds, Causes of Photooxidative Stress and Amelioration of Defense System in Plants. CRC Press, Boca Raton, FL, pp 77–103
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K, Takahashi M (1987) Production and scavenging of active oxygen species in photosynthesis. In D Kyle, C Osmond, C Arntzen, eds, Photoinhibition. Elsevier Science Publishers, New York, pp 227–287
- Bielski BHJ, Comstock DA, Bowen RA (1971) Ascorbic acid free radicals. I. Pulse radiolysis study of optical absorption and kinetic properties. J Am Chem Soc 93 5624–5629 [Google Scholar]
- Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43 83–116 [Google Scholar]
- Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ (2003) Plant peroxiredoxins. Annu Rev Plant Biol 54 93–107 [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Jacob S, Oelze ML, Laxa M, Tognetti V, De Miranda SMN, Baier M, Finkemeier I (2006) The function of peroxiredoxins in plant organelle redox metabolism. J Exp Bot 57 1697–1709 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17 507–523 [Google Scholar]
- Foyer CH, Lelandais M, Galap C, Kunert KJ (1991) Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol 97 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I (1998) Oxygen toxicity: a radical explanation. J Exp Biol 201 1203–1209 [DOI] [PubMed] [Google Scholar]
- Hahn BD (1987) A mathematical model of photorespiration and photosynthesis. Ann Bot (Lond) 60 157–169 [Google Scholar]
- Hausladen A, Kunert KJ (1990) Effects of artificially enhanced levels of ascorbate and glutathione on the enzymes monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase in spinach (Spinacia oleracea). Physiol Plant 79 384–388 [Google Scholar]
- Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M (1998) Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol 116 173–181 [Google Scholar]
- Kaiser WM (1979) Reversible inhibition of the Calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta 145 377–382 [DOI] [PubMed] [Google Scholar]
- Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160 347–353 [DOI] [PubMed] [Google Scholar]
- Laisk A, Walker DA (1986) Control of phosphate turnover as a rate-limiting factor and possible causes of oscillations in photosynthesis: a mathematical model. Proc R Soc Lond B Biol Sci 227 281–302 [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7 405–410 [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction of monodehydro-ascorbate radical in spinach thylakoids. Plant Cell Physiol 34 539–549 [Google Scholar]
- Miyake C, Asada K (1996) Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37 423–430 [Google Scholar]
- Miyake C, Shinzaki Y, Nishioka M, Horiguchi S, Tomizawa K (2006) Photoinactivation of ascorbate peroxidase in isolated tobacco chloroplasts: Galdieria partita APX maintains the electron flux through the water-water cycle in transplastomic tobacco plants. Plant Cell Physiol 47 200–210 [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Challiss RAJ, Crabtree B (1984) Substrate cycles: their metabolic, energetic and thermic consequences in man. Trends Biochem Sci 9 277–280 [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49 249–279 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Kanematsu S, Takabe K, Asada K (1995) Attachment of CuZn-superoxide dismutase to thylakoid membranes at the site of superoxide generation (PSI) in spinach chloroplasts: detection by immunogold labeling after rapid freezing and substitution method. Plant Cell Physiol 36 565–573 [Google Scholar]
- Pitcher LH, Repetti P, Zilinskas BA (1994) Overproduction of ascorbate peroxidase protects transgenic tobacco against oxidative stress (abstract no. 623). Plant Physiol (Suppl) 105 S-116 [Google Scholar]
- Polle A (1997) Defense against photo-oxidative damage in plants. In JG Scandalios, ed, Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 623–666
- Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling: computer simulations as a step towards flux analysis. Plant Physiol 126 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A, Junkermann W (1996) Inhibition of apoplastic and symplastic peroxidase activity from Norway spruce by the photooxidant hydroxymethyl hydroperoxide. Plant Physiol 104 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel IH (1975) Steady-state kinetics of multireactant enzymes. In Enzyme Kinetics. John Wiley & Sons, New York, pp 684–687
- Shampine LF, Reichelt MW (1997) The MATLAB ODE suite. SIAM J Sci Comput 18 1–22 [Google Scholar]
- Shimaoka T, Miyake C, Yokota A (2003) Mechanism of the reaction catalyzed by dehydroascorbate reductase from spinach chloroplasts. Eur J Biochem 270 921–928 [DOI] [PubMed] [Google Scholar]
- Torsethaugen G, Pitcher LH, Zilinskas BA, Pell EJ (1997) Overproduction of ascorbate peroxidase in the tobacco chloroplast does not provide protection against ozone. Plant Physiol 114 529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero E, García-Carmona F (1996) Optimizing enzymatic cycling assays: spectrophotometric determination of low levels of pyruvate and l-lactate. Anal Biochem 239 47–52 [DOI] [PubMed] [Google Scholar]
- Valero E, Varón R, García-Carmona F (2000) Kinetics of a self-amplifying substrate cycle: ADP-ATP cycling assay. Biochem J 350 237–243 [PMC free article] [PubMed] [Google Scholar]
- Vanoni MA, Wong KK, Ballou DP, Blanchard JS (1990) Glutathione reductase: comparison of steady-state and rapid reaction primary kinetic isotope effects exhibited by the yeast, spinach, and Escherichia coli enzymes. Biochemistry 29 5790–5796 [DOI] [PubMed] [Google Scholar]
- Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol 2 388–393 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.