Abstract
Heat shock (HS) is a common form of stress suffered by plants. It has been proposed that calmodulin (CaM) is involved in HS signal transduction, but direct evidence has been lacking. To investigate the potential regulatory function of CaM in the HS signal transduction pathway, T-DNA knockout mutants for AtCaM2, AtCaM3, and AtCaM4 were obtained and their thermotolerance tested. Of the three knockout mutant plants, there were no differences compared with wild-type plants under normal conditions. However, the AtCaM3 knockout mutant showed a clear reduction in thermotolerance after heat treatment at 45°C for 50 min. Overexpression of AtCaM3 in either the AtCaM3 knockout or wild-type background significantly rescued or increased the thermotolerance, respectively. Results from electrophoretic mobility-shift assays, real-time quantitative reverse transcription-polymerase chain reaction, and western-blot analyses revealed that, after HS, the DNA-binding activity of HS transcription factors, mRNA transcription of HS protein genes, and accumulation of HS protein were down-regulated in the AtCaM3 knockout mutant and up-regulated in the AtCaM3-overexpressing transgenic lines. Taken together, these results suggest that endogenous AtCaM3 is a key component in the Ca2+-CaM HS signal transduction pathway.
In most organisms, temperatures above those needed for normal growth act as a form of stress. In plants, heat shock (HS) affects growth and crop production. Most organisms, including higher plants, synthesize heat shock proteins (HSPs) in response to high-temperature stress (Vierling, 1991; Queitsch et al., 2000; Nieto-Sotelo et al., 2002; Charng et al., 2006; Yang et al., 2006). In eukaryotes, expression of HSPs is regulated by heat shock transcription factors (HSFs). Upon HS, HSFs become activated and initiate the transcription of HSPs through binding to heat shock promoter elements (HSEs) in the promoter regions of genes encoding HSPs (Nover et al., 2001; Mishra et al., 2002; Baniwal et al., 2004; Charng et al., 2007). Many HSFs and HSPs have been shown to be involved in improving plant thermotolerance (Queitsch et al., 2000; Mishra et al., 2002; Wunderlich et al., 2003; Sanmiya et al., 2004; Charng et al., 2006, 2007; Nishizawa et al., 2006). Although the downstream components (HSFs, HSEs, and HSPs) of heat stress response have been studied in detail, the signal transduction pathway through which HS influences the binding activity of HSFs to HSEs to regulate HSP expression is not well understood. The aim of this study was to identify possible upstream components of HS signal transduction.
Several hypotheses regarding the HS signal transduction pathway have been proposed. For example, Ananthan et al. (1986) suggested that the accumulation of proteins denatured by heat stress stimulates the expression of HSP genes. Alternatively, changes in membrane fluidity and calcium influx following heat stress have also been reported (Sangwan et al., 2002; Sung et al., 2003). More recently, other authors have described potential roles for the different signaling molecules (Larkindale and Huang, 2004; Vacca et al., 2004, 2006; Larkindale et al., 2005; Liu et al., 2006; Kotak et al., 2007).
In both animals and plants, data indicate that HS can elicit changes in the levels of intracellular Ca2+, one of the most ubiquitous cellular second messengers. Gong et al. (1998b) directly observed that HS caused a significant, transient increase in the intracellular concentration of free calcium ([Ca2+]i) in tobacco (Nicotiana plumbaginifolia). Increased [Ca2+]i was suggested to regulate the binding activity of HSFs to HSEs (Mosser et al., 1990; Li et al., 2004) and to protect against HS-induced oxidative damage in Arabidopsis (Arabidopsis thaliana; Larkindale and Knight, 2002). Our laboratory has reported evidence in wheat (Triticum aestivum) for an increase in [Ca2+]i as early as 1 min after HS, with a maximal [Ca2+]i at 4 or 10 min after HS in wheat or suspension-cultured Arabidopsis cells, respectively (Liu et al., 2003, 2006).
Calmodulin (CaM) is a well-studied intracellular calcium sensor that mediates Ca2+ signal transduction (Snedden and Fromm, 1998, 2001). Gong et al. (1997b) observed that CaM was up-regulated during HS in maize (Zea mays) seedlings. In our studies, the levels of CaM mRNA and protein increased during HS in wheat, and the increase of CaM mRNA for only 10 min preceded the expression of HSP genes, which was detected after 20 min of HS (Liu et al., 2003). We also reported that CaM antagonists, including CPZ, W7, and CaM antiserum, inhibited the DNA-binding activity of maize HSF during HS (Li et al., 2004). More recently, a CaM-binding protein kinase was identified as an important component in the Ca2+-CaM pathway involved in HS signal transduction (Liu et al., 2008). Collectively, available evidence suggests that AtCaM may participate as a key component in HS signal transduction. However, to date, there is no direct genetic evidence for CaM involvement in HS signaling.
Arabidopsis contains at least nine different CaM genes. AtCaM1 through AtCaM7 share a high level of sequence identity (>95%), differing from each other only by a few nucleic acids; AtCaM8 and AtCaM9 are more divergent from the other forms (Reddy et al., 2000; Luan et al., 2002). Our previous studies started with all nine members of the AtCaM gene family, but only AtCaM3 gene expression showed a significant up-regulation after a 37°C HS. Moreover, the temporal expression of the AtCaM3 and AtHsp18.2 genes demonstrated that up-regulation of AtCaM3 expression occurred earlier than that of AtHsp18.2 (Liu et al., 2005). These results suggested AtCaM3 may be involved in the HS signal transduction pathway. Also, based on the gene expression results (supplemental data) and the existing mutants, AtCaM2 and AtCaM4 were selected as the controls.
In this paper, we generated T-DNA knockout mutants and transgenic plants to provide direct molecular genetic evidence that endogenous AtCaM3 is a key component in HS signal transduction. We also confirmed the existence of a previously proposed, new Ca2+-CaM HS signal transduction pathway (Liu et al., 2003, 2008).
RESULTS
AtCaM3 T-DNA Insertion Mutant Decreases the Thermotolerance of Arabidopsis Seedlings
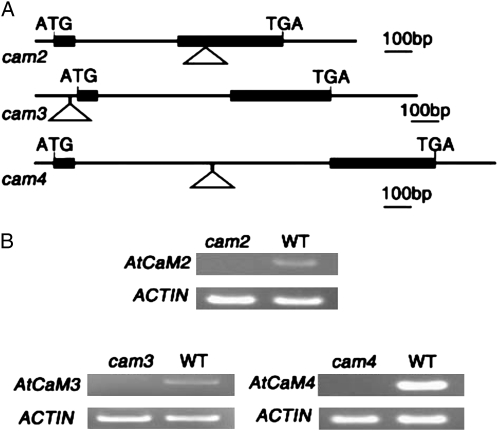
To determine whether AtCaM3 is involved in the HS signal transduction pathway, we first utilized a reverse genetic approach by screening Arabidopsis T-DNA insertion mutants effecting AtCaM family members. T-DNA insertion mutants for AtCaM genes were obtained from the Arabidopsis Biological Resource Center. Of all the mutants obtained, only the homozygous T-DNA insertion mutants cam2, cam3, and cam4, which separately affect AtCaM2, AtCaM3, and AtCaM4, respectively, were detected to be deficient in transcripts by reverse transcription (RT)-PCR analysis in this experiment (Fig. 1B). The positions of the individual T-DNA insertions are shown in Figure 1A.
Figure 1.
Isolation and identification of cam2, cam3, and cam4 T-DNA insertional mutants. A, The intron-exon organization of AtCaM2, AtCaM3, and AtCaM4 and the locations of the T-DNA insertions. The black boxes represent exons, lines indicate introns and untranslated regions, and the positions of the T-DNA insertions are indicated by triangles. B, RT-PCR analyses to detect AtCaM2, AtCaM3, and AtCaM4 transcripts in RNA extracts from 10-d-old wild-type (WT) and mutant seedlings. Actin expression levels were used as a control.
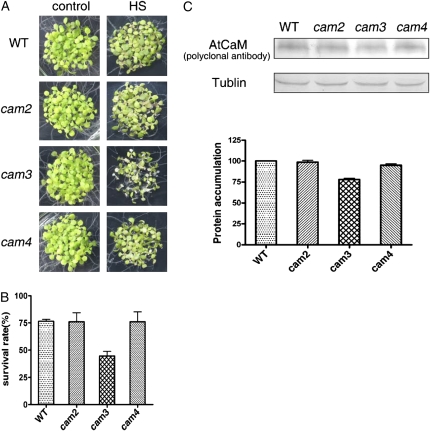
AtCaM2 and AtCaM4 were used as controls and compared with AtCaM3 in a thermotolerance assay. The cam2, cam3, and cam4 mutants exhibited no phenotypic differences compared with wild-type plants under normal growth conditions (Fig. 2A). However, the thermotolerance assay in which 6-d-old seedlings were heat shocked at 45°C for 50 min and then allowed to recover for 6 d at 22°C showed that thermotolerance of the cam2 and cam4 mutant seedlings was similar to that of the wild-type seedlings and that only the cam3 mutant exhibited a significant impairment in thermotolerance (Fig. 2A). The survival rate of the cam3 mutants was about 43% lower than that of the wild-type plants (Fig. 2B).
Figure 2.
cam3 mutant exhibited significantly decreased thermotolerance. A, cam2, cam3, and cam4 seedlings showed no differences from wild-type seedlings under normal growth conditions. However, when 6-d-old seedlings were exposed to 45°C for 50 min and then recovered at 22°C for another 6 d, only cam3 exhibited decreased thermotolerance as compared with cam2, cam4, and wild-type plants. B, Survival rate of the wild-type, cam2, cam3, and cam4 mutant plants after HS at 45°C for 50 min. Each value is the mean ± se of three biological replicates, with 30 seedlings per experiment. C, AtCaM protein levels in wild-type, cam2, cam3, and cam4 mutant plants after HS at 37°C for 2 h. Tubulin was used as an internal loading control. Quantification of the bands is shown below. WT, Wild type.
With the exception of gene expression analyses by RT-PCR (Fig. 1B), to further determine whether the reduction in AtCaM3 was responsible for the increased heat sensitivity, we examined the endogenous total AtCaM protein (translated by all AtCaM genes) levels in wild-type plants and in cam2, cam3, and cam4 mutants after HS at 37°C by western blot. As shown in Figure 2C, AtCaM protein was lower in the cam3 mutant than in wild-type plants or the cam2 and cam4 mutants.
Effects of HS on the Expression of AtCaM3 and the Expression Pattern of AtCaM3
The expression of AtCaM3 after HS was studied in Arabidopsis in order to gain insight into the function of its protein product. The AtCaM3 promoter was fused to the reporter gene GUS and introduced into Arabidopsis by Agrobacterium tumefaciens-mediated transformation. AtCaM3∷GUS transgenic plants were used to analyze the expression pattern of AtCaM3 under HS conditions. Four different transgenic lines were tested. Plants were grown at 22°C for 10 d and then treated at 37°C before they were harvested for GUS activity assays. Assay results showed that, compared with the activity at 0 h, HS treatment caused GUS activity to increase rapidly by 2-fold in the first 2 h. When heat treatment was extended to 4 h, GUS activity was slightly lower than was observed for the 2-h treatment but was still 1.8-fold higher than at 0 h (Fig. 3A). These GUS activity assays confirmed the results of previous real-time RT-PCR experiments, in which the expression of the AtCaM3 gene was significantly up-regulated after HS (Liu et al., 2005). Based on these results, 2 h of HS was used as a reference for increasing AtCaM3 in further experiments (Fig. 2C).
Figure 3.
Time course of AtCaM3 expression during HS and spatiotemporal expression of the AtCaM3 gene in Arabidopsis. A, Ten-day-old AtCaM3∷GUS seedlings were heat stressed at 37°C for 0 to 4 h. GUS activity was determined as described in “Materials and Methods.” Data are means ± se from four different AtCaM3∷GUS transgenic lines with three biological replicates. B, GUS staining showing the expression pattern of AtCaM3 in a 10-d-old seedling (a), a rosette leaf (b), a cauline leaf (c), a silique (d), an anthotaxy (e), a flower (f), a stamen (g), a petal (h), and a sepal (i). Bars = 1 mm in a to c, e, and f, and 0.1 mm in d and g to i.
We also examined the tissue- and organ-specific expression of AtCaM3 in Arabidopsis. As shown in Figure 3B, AtCaM3∷GUS was expressed ubiquitously in young seedlings as well as in rosette and cauline leaves (Fig. 3B, a–c). When plants entered the flowering stage, AtCaM3∷GUS expression became concentrated in the sepals, stigma, and filaments but not in the anthers or petals (Fig. 3B, e–h). AtCaM3∷GUS was also strongly expressed in the separation layer of the abscission zone of flower organs in which the petal, sepal, and stamen had fallen off after anthesis (Fig. 3B, d and i). These results are consistent with the results of previous microarray analyses (Zimmermann et al., 2004). GUS assays revealed that AtCaM3 was expressed in most organs and tissues of the plant, especially in the young seedling.
The Expression of AtCaM3 in the cam3 Mutant Rescues Heat Sensitivity
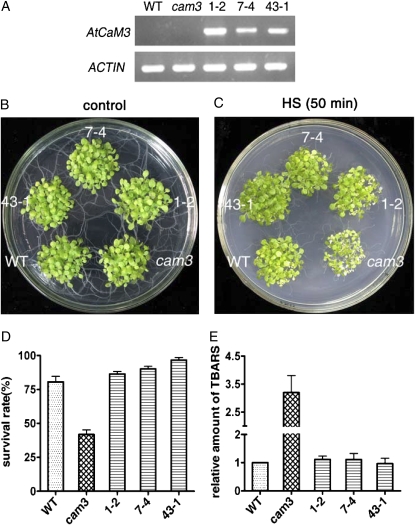
To confirm whether the suppression of AtCaM3 gene expression in cam3 was responsible for the phenotype of impaired thermotolerance, we also performed complementation analyses. A binary vector containing the coding region of AtCaM3 under the control of the 35S promoter was constructed and used to transform the cam3 mutant by the floral-dip method. Due to the more than 95% nucleic acid sequence identity of the seven AtCaM members, to ensure the specificity of the primers, the NOS terminator region of the binary vector was used to design the reverse primer used to identify the transgenic plants. RT-PCR showed that ectogenous AtCaM3 expression was only found in the complemented transgenic lines. By contrast, ectogenous AtCaM3 cDNA was not amplified in the wild-type or cam3 plants (Fig. 4A). These data confirmed that the cam3 mutant was successfully transformed with the AtCaM3 cDNA. Six homozygous lines of complemented transgenic seedlings (cam3/AtCaM3) were obtained and identified. Three of these lines (1-2, 7-4, and 43-1) were chosen for thermotolerance studies. In these experiments, none of the cam3/AtCaM3 transgenic plants showed variant phenotypes under normal growth conditions as compared with the wild-type and cam3 plants (Fig. 4B). However, the cam3/AtCaM3 transgenic lines completely rescued the heat-hypersensitive phenotype of the cam3 mutants (Fig. 4C) and exhibited survival rates that were similar to those of the wild-type plants (Fig. 4D).
Figure 4.
Transformation of the cam3 mutant with 35S∷AtCaM3 rescued the decreased thermotolerance. A, RT-PCR analysis of ectogenous AtCaM3 transcripts in wild-type, cam3 mutant, and three cam3/AtCaM3 transgenic lines (1-2, 7-4, and 43-1). Actin was used as an internal control. B and C, Wild-type, cam3 mutant, and three cam3/AtCaM3 transgenic lines under normal (B) and HS (C) conditions. For the thermotolerance assay, 6-d-old seedlings grown at 22°C were shifted to 45°C for 50 min and then returned to 22°C for 6 d. D, Survival rate for wild-type, cam3 mutant, and three cam3/AtCaM3 transgenic lines (1-2, 7-4, and 43-1) after HS at 45°C for 50 min. Each value is the mean ± se of three biological replicates, with 30 seedlings per experiment. E, TBARS levels for wild-type, cam3 mutant, and three cam3/AtCaM3 transgenic lines (1-2, 7-4, and 43-1). Plants were heat stressed at 45°C for 60 min and then returned to 22°C for 2 d for recovery. TBARS levels were determined relative to the wild-type controls. Each value is the mean ± se of three biological replicates. WT, Wild type.
It has been reported that heat induces oxidative damage, and considerable interlinking between heat and oxidative stress responses have been suggested (Gong et al., 1997a, 1998a; Dat et al., 1998). As an additional test of heat sensitivity, the cam3 mutants and complemented transgenic lines were assayed for the accumulation of thiobarbituric acid-reactive substances (TBARS) following heat stress. Plant tissues used in TBARS assays were harvested 2 d after HS at 45°C for 1 h (Larkindale and Knight, 2002; Larkindale et al., 2005). Damage to seedlings, including bleached leaves and plant bleaching, suggested that the damage caused by heating may have been due to oxidative stress occurring during the recovery phase. TBARS levels in three cam3/AtCaM3 transgenic lines were similar to those detected in wild-type plants (Fig. 4E). However, TBARS levels in cam3 mutant plants, which showed decreased thermotolerance, were 250% higher than those detected in wild-type plants (Fig. 4E).
Overexpression of AtCaM3 Improves the Thermotolerance of Arabidopsis Seedlings
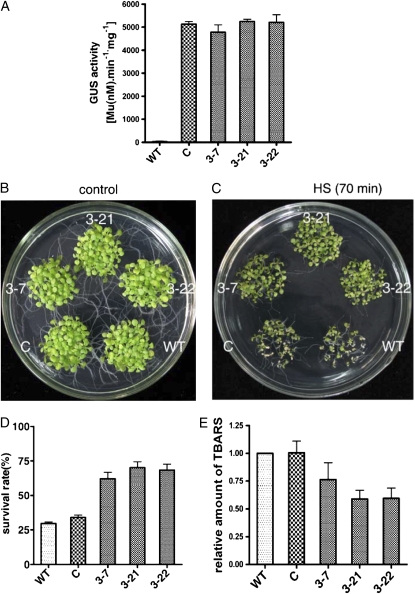
To further confirm the function of AtCaM3 in thermotolerance, transgenic plants were generated by transformation of the AtCaM3 gene fused to GUS under the control of the 35S promoter (35S∷AtCaM3-GUS) in the ecotype Columbia background. Because of the high level of nucleic acid sequence identity among AtCaM multigene family members, no specific primers could be used to determine the expression level of AtCaM3 by real-time RT-PCR. Thus, GUS was used as a label to show AtCaM3 expression levels in the different transgenic lines. After testing the level of AtCaM3 expression (as indicated by GUS activity) of seven independent T3 homozygous transgenic lines, three lines (3-7, 3-21, and 3-22) with strong GUS activity compared with wild-type control plants (Fig. 5A) were used in the following experiments. Transgenic plants carrying 35S∷GUS were used as a vector control.
Figure 5.
Overexpression of AtCaM3 improves thermotolerance. C, Vector control; WT, wild type; 3-7, 3-21, and 3-22 are three AtCaM3-GUS-overexpressing transgenic lines transformed with 35S∷AtCaM3-GUS. A, GUS activity in wild type, vector control, and three transgenic lines. Each value is the mean ± se of three biological replicates. B and C, Wild-type, vector control, and three transgenic lines under normal (B) and HS (C) conditions. For the thermotolerance assay, 6-d-old seedlings grown at 22°C were shifted to 45°C for 70 min and then returned to 22°C for 6 d, at which time they were photographed. D, Survival rates for the wild-type, vector control, and three transgenic lines. Survival was determined 6 d after heat stress at 45°C for 70 min. Each value is the mean ± se of three biological replicates, 30 seedlings per experiment. E, TBARS levels of wild-type, vector control, and three transgenic lines. Plants were heat stressed at 45°C for 60 min and then allowed 2 d at 22°C for recovery. TBARS levels were normalized relative to the wild-type control. Each value is the mean ± se of three biological replicates.
The three 35S∷AtCaM3-GUS homozygous transgenic lines, a vector control line, and wild-type plants were plated together on the same Murashige and Skoog plate. There were no significant phenotypic differences between the transgenic and wild-type plants under normal growth conditions (Fig. 5B). The seedlings were grown at 22°C for 6 d followed by exposure to 45°C for 70 min, then they were allowed to recover at 22°C for another 6 d. As shown in Figure 5C, most of the wild-type and vector control plants could not survive under this lethal-level heat stress. The cotyledons of these plants were bleached and dried. However, although they all exhibited a delay in growth, seedlings overexpressing AtCaM3 survived, as demonstrated by their green cotyledons and the presence of young leaves (Fig. 5C). The overall survival rate of the AtCaM3-GUS-overexpressing transgenic lines was 40% to 60% higher than in either the wild-type or vector control plants (Fig. 5D). In the three AtCaM3-GUS-overexpressing transgenic lines, TBARS levels were 24% to 40% lower than those in either the wild-type plants or the 35S∷GUS vector control plants (Fig. 5E).
Effect of AtCaM3 on DNA-Binding Activity of HSF and Expression of HSP Genes during HS
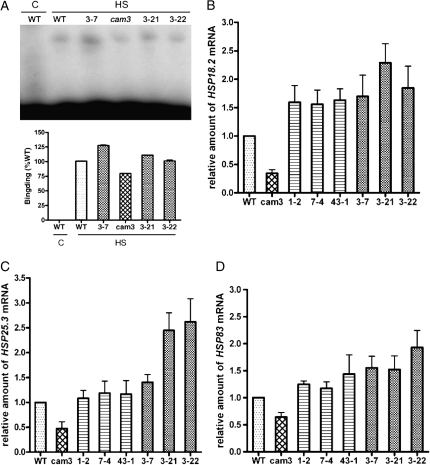
In order to understand the underlying mechanism of AtCaM3-induced thermotolerance in Arabidopsis, the binding activity of HSF to HSE in AtCaM3 knockout mutant, AtCaM3-GUS-overexpressing, and wild-type plants was analyzed by electrophoretic mobility-shift assay. Results indicate that after HS the binding activity of HSF to HSE in the cam3 mutant plants was weaker than that detected in the wild-type plants. In the three AtCaM3-GUS-overexpressing transgenic lines (3-7, 3-21, and 3-22), the binding activity was much stronger than in the wild-type plants (Fig. 6A). As a control, there was no binding when the wild-type plant was not heated, which suggested that the band shift was induced specifically by heat (Fig. 6A). These data indicate that changes in AtCaM3 expression influence the binding activity of HSF to HSE.
Figure 6.
Effect of AtCaM3 on DNA-binding activity of HSF and expression of HSP genes. A, Results of an electrophoretic mobility-shift binding assay using whole cell extracts from 10-d-old Arabidopsis plants incubated for 1 h at 22°C (C, control) or 37°C (HS). Whole cell extract was obtained from wild-type plants, three different AtCaM3-GUS-overexpressing transgenic lines (3-7, 3-21, and 3-22), and the cam3 mutant. Equal amounts (30 μg each) of whole cell protein extract were used in all lanes. Quantification of the bands is presented below the gel. B to D, Real-time RT-PCR analyses for expression of HSP genes in wild-type, cam3 mutant, three cam3/AtCaM3 transgenic lines (1-2, 7-4, and 43-1), and three AtCaM3-GUS-overexpressing transgenic lines (3-7, 3-21, and 3-22). B, Expression of AtHSP18.2. C, Expression of AtHSP25.3. D, Expression of AtHSP83. Ten-day-old seedlings were heat stressed at 37°C for 1 h. actin was used as an internal control. Samples from wild-type plants were used for normalization, with the expression level of these samples set to 1. Data are means ± se from three biological replicates. WT, Wild type.
The effect of AtCaM3 on the transcriptional regulation of HSPs was also examined by real-time quantitative RT-PCR. AtHSP18.2, AtHSP25.3, and AtHSP83 were chosen as marker genes. After HS at 37°C for 1 h, levels of AtHsp18.2 mRNA were 66% lower in the cam3 mutants than in the wild-type plants, while levels in the three cam3/AtCaM3 transgenic lines were rescued such that they were 56% to 63% higher than in the wild-type plants (Fig. 6B). Thus, overexpression of AtCaM3 increased the expression of AtHsp18.2. The relative level of AtHsp18.2 expression in the AtCaM3-GUS-overexpressing transgenic lines was 70% to 130% higher than in the wild-type plants (Fig. 6B).
Similar results were obtained for the expression of AtHsp25.3 and AtHsp83 in the mutant and different transgenic lines. After HS at 37°C for 1 h, AtHsp25.3 and AtHsp83 mRNA levels in the cam3 mutants were reduced to 47% and 64% of levels detected in the wild-type plants (Fig. 6, C and D), while complemented transgenic lines showed fully restored AtHsp25.3 and AtHsp83 expression (Fig. 6, C and D). Similarly, the expression of AtHsp25.3 and AtHsp83 in the AtCaM3-GUS-overexpressing transgenic lines was 40% to 160% higher than in the wild-type plants (Fig. 6, C and D).
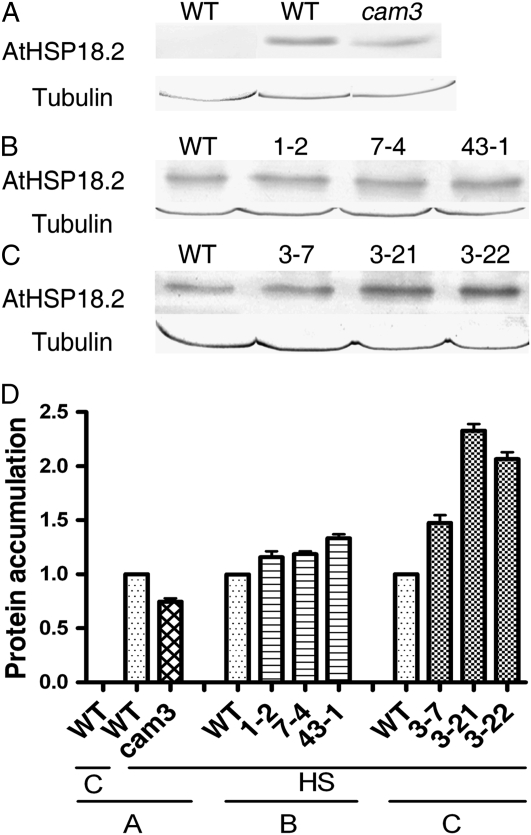
The effect of AtCaM3 on the accumulation of AtHSP18.2 was further documented through examination of protein changes by western blot. Ten-day-old seedlings were heat treated at 37°C for 2 h or maintained at 22°C and cellular protein was extracted. AtHSP18.2, a heat-induced protein, was not detected in plants incubated at the normal growth temperature of 22°C. HS at 37°C for 2 h rapidly induced the expression of AtHSP18.2 protein (Fig. 7A). Similar to changes seen in RNA transcriptional regulation, AtHSP18.2 accumulation in the cam3 mutants was lower than in the wild-type plants (Fig. 7A). In the three cam3/AtCaM3 transgenic lines (1-2, 7-4, and 43-1), AtHSP18.2 accumulation was rescued as compared with the cam3 mutant, with AtHSP18.2 levels that were similar to those in the wild-type plants (Fig. 7B). AtHSP18.2 levels in the three AtCaM3-GUS-overexpressing lines (3-7, 3-21, and 3-22) were higher than in the wild-type plants (Fig. 7C). In these experiments, tubulin protein expression was used to ensure equal sample loading. These results suggest a link between changes in the expression and accumulation of HSPs in both cam3 mutant and transgenic plants and changes in plant thermotolerance.
Figure 7.
Effect of AtCaM3 on the accumulation of AtHSP18.2. Ten-day-old seedlings were heat stressed at 37°C for 2 h or maintained at 22°C as a control. Total protein was extracted, separated by SDS-PAGE, and analyzed by western blotting. Tubulin was used as an internal quantification control. A, Wild-type plants at 22°C; wild type plants and cam3 mutants after HS. B, Wild-type plants and three independent cam3/AtCaM3 transgenic seedlings (1-2, 7-4, and 43-1) exposed to 37°C. C, Wild-type plants and three AtCaM3-GUS-overexpressing transgenic seedlings (3-7, 3-21, and 3-22) exposed to 37°C. D, Quantification of the bands in A to C. C, Control; WT, wild type.
DISCUSSION
The Effect of AtCaM3 on Thermotolerance in Arabidopsis
As one of the most conserved cellular proteins, CaM has been well studied in its role as an intracellular Ca2+ sensor. CaM participates in numerous signaling pathways, and expression of CaM genes in plants is regulated by many types of environmental stresses, including wind, touch, wounding, osmotic stress, pathogens, and cold stress (Braam et al., 1997; Jang et al., 1998; Bergey and Ryan, 1999; Heo et al., 1999; Reddy, 2001; Yamakawa et al., 2001; Townley and Knight, 2002; Yang and Poovaiah, 2003; McCormack et al., 2005). It has also been reported that HS could induce the accumulation of CaM (Gong et al., 1997b). In our previous studies, we provided preliminary, indirect evidence for the involvement of CaM in HS signal transduction (Liu et al., 2003, 2005; Li et al., 2004). In Arabidopsis, the AtCaM gene family contains nine members that share a high level of nucleic acid and amino acid sequence identity (Snedden and Fromm, 2001; Luan et al., 2002). Based on previous results regarding the expression of nine AtCaM genes and temporal expression of AtCaM3 and AtHsp18.2 (Liu et al., 2005), our attention was focused on AtCaM3. Here, compared with a no-heat control, we analyzed the expression time course of AtCaM2, AtCaM3, and AtCaM4 by real-time quantitative RT-PCR. The results indicated that the level of AtCaM3 mRNA increased and reached its maximum after 20 min of HS at 37°C, then decreased slowly after 30 min. The levels of AtCaM2 and AtCaM4 did not increase after HS treatment (Supplemental Fig. S4), so AtCaM2 and AtCaM4 were selected as the controls. The results in Supplemental Figure S4 and the data shown in Figure 3A together suggest that AtCaM3 is significantly induced by HS. Thus, AtCaM3 may be a component in HS signal transduction.
To obtain direct molecular genetic evidence for the function of AtCaM3 in HS signaling pathways, AtCaM3 T-DNA knockout mutants, complemented transgenic plants, and AtCaM3-GUS-overexpressing plants were generated. Thermotolerance testing indicated that knockout of AtCaM3 by T-DNA insertion clearly decreased the thermotolerance of Arabidopsis, whereas complementary transformants were able to completely rescue the decreased thermotolerance of the cam3 mutant (Fig. 4). Moreover, overexpression of AtCaM3 resulted in transgenic plants with a thermotolerance much higher than that observed in wild-type plants (Fig. 5).
As shown in Figure 2, single AtCaM gene knockout (including the cam3 mutant) did not exhibit any phenotypic differences as compared with the wild-type plants under normal growth conditions. These data suggested the existence of functional redundancy between different AtCaM members for plant growth. But the absence of a single AtCaM3 gene or overexpression could change thermotolerance under HS conditions. As shown in AtCaM3∷GUS activity tests (Fig. 3A) and western-blot analyses (Fig. 2C), because AtCaM3 was heat induced, AtCaM3 protein in the AtCaM3 T-DNA insertion mutant was not up-regulated after HS. Using a polyclonal antibody, the cam3 mutant showed lower total AtCaM protein levels than the wild type and other mutant (cam2 and cam4) plants. These results suggested that higher AtCaM3 protein levels were linked to high thermotolerance.
Even though AtCaM2 and AtCaM4 have a high amino acid sequence identity with AtCaM3 (Supplemental Fig. S3; Supplemental Table S1), the cam2 and cam4 mutants did not show obvious differences in thermotolerance compared with wild-type plants after HS at 45°C, unlike the cam3 mutant (Fig. 2A). The reason for this phenotypic difference may be that different CaM genes are differentially regulated by distinct cis-regulatory elements under differing stress conditions. Moreover, even CaM genes encoding the same protein can be differentially regulated in response to different external stimuli, such as disease resistance and cold (Takezawa et al., 1995; Zielinski, 1998; Heo et al., 1999; Townley and Knight, 2002; Yang and Poovaiah, 2003). Because we did not obtain knockout mutants of all of the AtCaM genes, we cannot conclude that only AtCaM3 is involved in HS signal transduction. Nonetheless, our data provide strong evidence for AtCaM3 as one of the major components participating in the pathway involving Ca2+-CaM in HS signal transduction in Arabidopsis.
The Mechanism for the Effect of AtCaM3 on Thermotolerance
In eukaryotes, HSFs are the downstream components of the HS signal transduction chain, which regulates the expression of genes encoding HSPs. HSPs, in turn, are known to contribute to thermotolerance (Kotak et al., 2007). Nover et al. (2001) identified 21 open reading frames for HSFs in the Arabidopsis genome. It was demonstrated that HSFA1a acts as a major regulator of the heat stress response in tomato (Solanum lycopersicum; Mishra et al., 2002). HSF phosphorylation has been proposed to play an important role in regulating the activity of this group of proteins (Schöffl et al., 1998; Dai et al., 2000; Hashikawa and Sakurai, 2004; Wang et al., 2006). Wang et al. (2004) found that Arabidopsis CaM-binding protein kinase3 (AtCBK3) binds to CaM in a Ca2+-dependent manner and that this binding was directly regulated by the kinase activity of AtCBK3. Our recent work further showed that, as a CaM target protein, AtCBK3 activity could be stimulated by AtCaM (Supplemental Fig. S2). AtCBK3 phosphorylated AtHSFA1a, thus regulating the binding activity of HSF to HSE (Liu et al., 2008). Here, we have provided evidence that the DNA-binding activity of HSFs was increased in Arabidopsis cells overexpressing AtCaM3 but decreased in the AtCaM3 knockout mutant (Fig. 6A). Therefore, AtCaM3 may modulate the thermotolerance of Arabidopsis by regulating the phosphorylation status affected by AtCBK3 and thus the activity of HSFs.
HSF binding to HSE activates the transcription of HSP genes (Baniwal et al., 2004; Hahn et al., 2004; Yamamoto et al., 2005), which are thought to encode molecular chaperones responsible for protein folding, assembly, translocation, and degradation (Miernyk, 1999; Hartl and Hayer-Hartl, 2002; Nollen and Morimoto, 2002; Mayer and Bukau, 2005). These proteins are classified based on their molecular masses and include HSP100, HSP90, HSP70, HSP60, and small HSPs. An important class is the small HSPs. These small HSPs are probably critical for survival of heat stress and for specific developmental processes in plants (Waters et al., 1996). Hence, we selected two small HSPs and one HSP gene of the HSP90 family. Our previous work using CaM antagonist experiments suggested that CaM affected the expression of HSP genes (Liu et al., 2003). In this study, using transgenic plants, we examined the effect of AtCaM3 on the expression of two genes encoding small HSPs, HSP18.2 (Takahashi and Komeda, 1989) and HSP25.3 (Osteryoung et al., 1993), and one gene belonging to the HSP90 family, HSP83 (Takahashi et al., 1992). Our results indicate that down-regulation of AtCaM3 expression in mutant plants led to reduced expression of these HSP genes, while complementary transformants of the cam3 mutant showed restored expression of these genes to the levels observed in wild-type plants. Furthermore, overexpression of AtCaM3 increased HSP expression following HS (Fig. 6). HSP expression in the cam2 and cam4 mutants was not changed, which was consistent with the lack of change in thermotolerance experiments compared with wild-type plants (Supplemental Fig. S1). Furthermore, results of AtHSP18.2 protein accumulation analyses in Arabidopsis seedlings were similar to those obtained for the expression of the AtHSP18.2 (Figs. 6C and 7). Taken together, the mechanism by which AtCaM3 affects plant thermotolerance may involve regulation of the DNA-binding activity of HSFs and HSP gene expression and protein accumulation.
AtCaM3 Is a Key Component in the Ca2+-CaM Pathway of HS Signal Transduction
Several HS signal transduction pathways have been proposed. Ananthan et al. (1986) suggested that the accumulation of heat stress-denatured proteins stimulates the expression of HSP genes. In this model, the chaperone HSP70 is thought to form a protein complex with HSF under normal conditions. After HS, the denatured proteins produced by HS in cytoplasm bind to HSP70, releasing HSF from the complex and freeing it to activate HSP expression. The involvement of membrane fluidity and calcium-dependent signaling molecules in plant HS signal transduction has also been proposed. In this putative pathway, HS induces a change in membrane fluidity, Ca2+ influx, and cytoskeletal remodeling. The increased cytosolic calcium concentration was suggested to activate calcium-dependent protein kinase (or mitogen-activated protein kinase) cascades and subsequently initiate the HS signal transduction pathway (Sangwan et al., 2002; Sung et al., 2003). Recently, other authors have described potential roles for many different signaling molecules, including ethylene, abscisic acid, inositol 1,4,5-triphosphate, salicylic acid, and hydrogen peroxide, in HS signaling pathways (Larkindale and Huang, 2004; Vacca et al., 2004; Larkindale et al., 2005; Liu et al., 2006; Volkov et al., 2006; Kotak et al., 2007).
We have proposed a Ca2+-CaM pathway involved in HS signal transduction (Liu et al., 2003). Our laboratory and others have reported that HS-induced [Ca2+]i influx, as a primary response, regulated the binding of HSF to HSE and the subsequent expression of HSPs (Mosser et al., 1990; Gong et al., 1998b; Li et al., 2004; Liu et al., 2006). More recently, we found that AtCBK3 is an important AtCaM downstream component of the HS signal transduction pathway. So AtCaM could be the key connection between upstream [Ca2+]i increases and downstream AtCBK3 activation in this pathway, but direct genetic evidence for the participation of AtCaM is still missing. In this study, we used T-DNA knockout and AtCaM3-overexpressing plants to provide molecular and genetic evidence showing that AtCaM3 affects plant thermotolerance by regulating the DNA-binding activity of HSFs and the gene expression and protein accumulation of HSPs. Therefore, our data fill the gap between upstream changes in Ca2+ concentration and downstream activation of CaM-binding protein kinases, thus confirming the Ca2+-CaM pathway of HS signal transduction in Arabidopsis.
According to this model, the HS signal is perceived by an as yet unidentified receptor, leading to an increase in the cytosolic concentration of Ca2+, perhaps through the regulation of inositol 1,4,5-triphosphate/phospholipase C. This elevated [Ca2+]i directly activates AtCaM3 and, in turn, stimulates AtCBK3, which ultimately regulates the phosphorylation and DNA-binding activity of HSFs. By binding to HSEs, HSFs may initiate the transcription of HSP genes as part of the plant's adaptation to environmental heat stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of Arabidopsis (Arabidopsis thaliana ecotype Columbia) were surface sterilized and plated on Murashige and Skoog medium containing 1.0% (w/v) Suc and 0.3% (w/v) Phytagel (Sigma). The seeds were vernalized at 4°C for 3 d and then grown under long-day conditions (16 h of light/8 h of dark) at 22°C.
The T-DNA insertion lines for AtCaM3 (SALK_001357) and AtCaM2 (SALK_114166) were obtained from the Arabidopsis Biological Resource Center. The T-DNA insertion line for AtCaM4 (GABI_309E09) was ordered from GABI-Kat. T-DNA insertion lines were screened and isolated as described by the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/tdna_FAQs.html). Homozygous mutants were used in further analyses.
Construction of Transgenic Lines
The 35S cauliflower mosaic virus promoter (restriction enzymes PstI and XbaI) and the reporter gene GUS (restriction enzymes BamHI and SacI) were cloned into the vector pCAMBIA1300, yielding pCAMBIA1300M.
To generate AtCaM3∷GUS transgenic plants, a fragment of the AtCaM3 promoter containing 1.2 kb of the upstream region of the gene was amplified by PCR using the forward primer 5′-AACTGCAGAGGAGGTAAAGGCCAACAC-3′ and the reverse primer 5′-GCTCTAGACCTTGTCGAATAAGCTGAAAG-3′. The amplified fragment was cloned into pCAMBIA1300M using the PstI and XbaI restriction sites, thus replacing the 35S promoter and generating the AtCaM3∷GUS construct.
To generate 35S∷AtCaM3 for complementation experiments, the AtCaM3 coding region was amplified by PCR from the AtCaM3 cDNA using the primers CaM3F1 (5′-CTCTAGAATGGCGGATCAGCTCACCGA-3′) and CaM3R1 (5′-CGAGCTCTCACTTAGCCATCATGACCTTAAC-3′). The amplified products (450 bp) were cloned into the binary vector pCAMBIA1300M using the XbaI and SacI restriction sites, thus replacing GUS.
To generate 35S∷AtCaM3-GUS for plants overexpressing AtCaM3 in the wild-type background, the AtCaM3 cDNA was amplified by RT-PCR using the primers CaM3F1 (5′-CTCTAGAATGGCGGATCAGCTCACCGA-3′) and CaM3R2 (5′-CGGATCCCCTTAGCCATCATGACCTTAAC-3′). The 35S∷AtCaM3-GUS fusion construct was generated by cloning the 450-bp PCR product into pCAMBIA1300M using the XbaI and BamHI restriction sites (underlined).
Transformation of the different constructs into Arabidopsis (ecotype Columbia) was carried out by the floral dipping method (Clough and Bent, 1998). Transformants were selected on plates containing hygromycin (25 mg mL−1). The number of T-DNA insertion loci was determined in the T2 generation based on the segregation ratio of hygromycin resistance. After three generations of selection, homozygous transgenic lines were used in the experiments.
GUS Staining and Activity Assay
Histochemical staining for GUS expression was performed according to the method of Jefferson (1987). Plants were incubated in buffer (50 mm Na3PO4, pH 7.0, 10 mg mL−1 X-Gluc, and 0.02% Triton X-100) at 37°C for times ranging from 2 h to overnight. The plant cells were stained and then washed three times with 70% ethanol to remove chlorophyll.
GUS activity was quantified according to the method of Jefferson (1987). Briefly, plant tissues were ground in liquid nitrogen, the crude plant extracts were prepared with extraction buffer (0.1% Triton X-100, 0.1% sarcosyl, 10 mm EDTA, 10 mm 2-mercaptoethanol, and 50 mm Na3PO4 buffer, pH 7.0), and GUS enzyme activity was assayed fluorometrically using 4-methylumbelliferyl-β-d-glucoronide (Sigma) as the substrate.
RT-PCR Analysis
Total RNA was isolated from 10-d-old seedlings using the Trizol reagent (Invitrogen). Transcript abundance in wild-type and mutant plants was determined by RT-PCR using a one-step RT-PCR Kit (TaKaRa). The AtCaM3 (At3g56800) transcript was amplified using the forward primer 5′-CGTACCCGATAAATACGGTTG-3′ and the reverse primer 5′-GACCTAATTTGCATTTCACAAAACC-3′. The AtCaM2 (At2g41110) transcript was amplified using the forward primer 5′-TTCGTTTTTCCCTTTTTCTCC-3′ and the reverse primer 5′-CAAGAATCAGGTTCTGAACCTG-3′. The AtCaM4 (At1g66410) transcript was amplified using the forward primer 5′-CCAAAGAAACGAGAAGAAGAAGC-3′ and the reverse primer 5′-GGCTCAAATCAAACCCAAGAC-3′. The actin (At2g37620) transcript served as a control and was amplified using the forward primer 5′-AGGCACCTCTTAACCCTAAAGC-3′ and the reverse primer 5′-GGACAACGGAATCTCTCAGC-3′. PCR products were analyzed by agarose gel electrophoresis and stained with ethidium bromide.
Thermotolerance Test
All plants were grown under the same conditions, and seeds were harvested at the same time for use in thermotolerance assays. For thermotolerance testing, 6-d-old seedlings were exposed to 45°C for 50 min (to kill CaM mutants) or 70 min (to kill wild-type plants) and then allowed to recover at 22°C for an additional 6 d (Larkindale et al., 2005). Seedlings that were still green and continued to produce new leaves were scored as survivors. For real-time quantitative RT-PCR analysis, 10-d-old seedlings were heated at 37°C for 1 h for HSP gene expression. For western-blot analysis, 10-d-old seedlings were kept at 37°C for 2 h for HSP accumulation (Liu et al., 2005).
TBARS Assay
TBARS was assayed according to the method of Heath and Packer (1968). Seedlings were heated at 45°C for 60 min and then allowed to recover at 22°C for 2 d. Tissues were harvested, ground in liquid nitrogen, and then mixed with buffer (0.5% [w/v] thiobarbituric acid in 20% [v/v] trichloroacetic acid and 0.25 mL of 175 mm NaCl in 50 mm Tris, pH 8). The resulting extracts were boiled for 15 min and then centrifuged at 14,000g for 20 min. The absorbance of the supernatant was measured at 450, 532, and 600 nm. TBARS levels were calculated according to C = 6.45 (OD532 − OD600) − 0.56 OD450, where OD532 is the optical density at 532 nm. The TBARS levels in extracts from mutant or transgenic plants were determined relative to the level in wild-type extracts.
Electrophoretic Mobility-Shift Assays
The HSE (Scharf et al., 1990, 1998; Hübel and Schöffl, 1994; Li et al., 2004) oligonucleotides (5′-TCGAGGATCCTAGAAGCTTCCAGAAGCTTCTAGAAGCAGATC-3′ and 5′-TCGAGATCTGCTTCTAGAAGCTTCTGGAAGCTTCTAGGATCC-3′) were annealed and labeled with [γ-32P]ATP using T4 polynucleotide kinase (TaKaRa). Ten-day-old seedlings were ground in liquid nitrogen and extracted with buffer (10 mm Tris, pH 8.0, 1 mm EDTA, 10 mm boric acid, and 0.1 mm phenylmethylsulfonyl fluoride). After centrifugation, the supernatants were used as whole cell extracts. These extracts were heat shocked at 37°C for 1 h. Electrophoretic mobility-shift assays were carried out according to the method of Li et al. (2004).
Real-Time Quantitative RT-PCR Analysis
Total RNA (500 ng) isolated using the Trizol reagent (Invitrogen) from 10-d-old seedlings was used together with the PrimeScript RT Reagent Kit (TaKaRa) for first-stand cDNA synthesis according to the manufacturer's instructions. For RT-PCR, SYBR Premix Ex Taq (TaKaRa) was used. The PCR program was as follows: initial polymerase activation for 10 s at 95°C, and 40 cycles of 95°C for 5 s followed by 60°C for 31 s. The reactions were carried out using an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Primer pairs were designed using Primer Express (Applied Biosystems). The primers used for AtHsp18.2 (At5g59720) were 5′-TCGTGATGTGGCAGCGTTTA-3′ (forward) and 5′-AAGTCCGCTTTGAACACATGTG-3′ (reverse); those for AtHsp25.3 (At4g27670) were 5′-GACGTCTCTCCTTTCGGATTGT-3′ (forward) and 5′-CTCCACTTCCTCCTCTGTTTCTTC −3′ (reverse); and those for AtHsp83 (At5g52640) were 5′-GCTGCTAGGATTCACAGGATG-3′ (forward) and 5′-TCCTCCATCTTGCTC TCTTCA-3′ (reverse). The primers used for the internal control actin (At2g37620) were 5′-TGTGCTCAGTGGTGGAACCA-3′ (forward) and 5′-GGAGCCAAAGCAGTGATCTCTT-3′ (reverse).
Western Blot Analyses
Ten-day-old seedlings were kept at 37°C for 2 h and then ground in liquid nitrogen. Total protein was extracted using extraction buffer (10 mm HEPES, pH 7.9, 0.4 m NaCl, 0.5 mm dithiothreitol, 0.1 mm EDTA, 5% glycerol, and 0.5 mm phenylmethylsulfonyl fluoride), and the extracts were clarified by centrifugation at 14,000g for 20 min at 4°C. Supernatants were transferred to fresh tubes, and the protein content was determined (Bradford, 1976). Total proteins (40 μg) were separated by SDS-PAGE and then transferred onto polyvinylidene difluoride membranes. Polyvinylidene difluoride membranes were blocked for at least 2 h and then probed with rabbit antiserum against AtHsp18.2 and mouse antiserum against the loading control tubulin (Sigma). After extensive washing, membranes were incubated with the appropriate secondary antibodies conjugated to alkaline phosphatase. BCIP/NBT (Amresco) was used for immunodetection.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of AtCaM3 on the expression of HSP genes and the accumulation of AtHSP18.2 in cam2 and cam4 mutants.
Supplemental Figure S2. The activation of additive CaM on the activity of AtCBK3.
Supplemental Figure S3. Alignment of the amino acid sequences of three AtCaM proteins; amino acid sequence identities are shown in red.
Supplemental Figure S4. The expression pattern of AtCaM2, AtCaM3, and AtCaM4 during HS at 37°C.
Supplemental Table S1. Protein data comparison for AtCaM2, AtCaM3, and AtCaM4.
Supplemental Table S2. Primer used for real-time quantitative RT-PCR.
Supplementary Material
Acknowledgments
We thank Dr. Wen-Qiang Tang (Department of Plant Biology, Carnegie Institution of Washington) for critical reading of the manuscript and comments and Drs. Su-Juan Cui, Ying Sun, Yi Guo, and Jun-Feng Zhao (Institute of Molecular Cell Biology, Hebei Normal University) for technical assistance.
This work was supported by the National Basic Research Program of China (grant nos. 2006CB100101 and 2006CB910600) and the Natural Science Foundation of China (grant no. 90208004).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Da-Ye Sun (dayesun@gmail.com).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ananthan J, Goldberg AL, Voellmy R (1986) Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232 522–524 [DOI] [PubMed] [Google Scholar]
- Baniwal SK, Bharti K, Chan KY, Ganguli A, Kotak S, Mishra SK, Port M, Scharf KD, Tripp J, Weber C, et al (2004) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29 471–487 [DOI] [PubMed] [Google Scholar]
- Bergey DR, Ryan CA (1999) Wound- and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol Biol 40 815–823 [DOI] [PubMed] [Google Scholar]
- Braam J, Sistrunk ML, Polisensky DH, Xu W, Purugganan MM, Antosiewicz DM, Campbell P, Johnson KA (1997) Plant responses to environmental stress: regulation and functions of the Arabidopsis TCH genes. Planta (Suppl) 203: S35–S41 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT (2007) A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS (2006) Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol 140 1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dai R, Frejtag W, He B, Zhang Y, Mivechi NF (2000) JNK targeting and phosphorylation of heat shock factor-1 suppress its transcriptional activity. J Biol Chem 275 18210–18218 [DOI] [PubMed] [Google Scholar]
- Dat JF, Foyer CH, Scott IM (1998) Changes in salicylic acid and antioxidants during induction of thermotolerance in mustard seedlings. Plant Physiol 118 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Chen SN, Song YQ, Li ZG (1997. a) Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. J Plant Physiol 24 371–379 [Google Scholar]
- Gong M, Li YJ, Chen SZ (1998. a) Abscisic acid induced thermotolerance in maize seedlings is mediated by Ca2+ and associated with antioxidant systems. J Plant Physiol 153 488–496 [Google Scholar]
- Gong M, Li YJ, Dai X, Tian M, Li ZG (1997. b) Involvement of calcium and calmodulin in the acquisition of heat-shock induced thermotolerance in maize. J Plant Physiol 150 615–621 [Google Scholar]
- Gong M, van der Luit AH, Knight MR, Trewavas AJ (1998. b) Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol 116 429–437 [Google Scholar]
- Hahn JS, Hu Z, Thiele DJ, Iyer VR (2004) Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24 5249–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295 1852–1858 [DOI] [PubMed] [Google Scholar]
- Hashikawa N, Sakurai H (2004) Phosphorylation of the yeast heat shock transcription factor is implicated in gene-specific activation dependent on the architecture of the heat shock element. Mol Cell Biol 24 3648–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125 189–198 [DOI] [PubMed] [Google Scholar]
- Heo WD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, Lee KJ, Park CY, Park HC, Choi JY, et al (1999) Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc Natl Acad Sci USA 96 766–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübel A, Schöffl F (1994) Arabidopsis heat shock factor: isolation and characterization of the gene and the recombinant protein. Plant Mol Biol 26 353–362 [DOI] [PubMed] [Google Scholar]
- Jang HJ, Pih KT, Kang SG, Lim JH, Jin JB, Piao HL, Hwang I (1998) Molecular cloning of a novel Ca2+-binding protein that is induced by NaCl stress. Plant Mol Biol 37 839–847 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5 387–405 [Google Scholar]
- Kotak S, Larkindale J, Lee U, van Koskull-Döring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10 310–316 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161 405–413 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Liu HT, Sun DY, Zhou RG (2004) Ca2+ and calmodulin modulate DNA-binding activity of maize heat shock transcription factor in vitro. Plant Cell Physiol 45 627–634 [DOI] [PubMed] [Google Scholar]
- Liu HT, Gao F, Cui SJ, Han JL, Sun DY, Zhou RG (2006) Primary evidence for involvement of IP3 in heat-shock signal transduction in Arabidopsis. Cell Res 16 394–400 [DOI] [PubMed] [Google Scholar]
- Liu HT, Gao F, Han JL, Li GL, Liu DL, Sun DY, Zhou RG (2008) The calmodulin-binding protein kinase 3 is part of heat shock signal transduction in Arabidopsis thaliana. Plant J 5 760–773 [DOI] [PubMed] [Google Scholar]
- Liu HT, Li B, Shang ZL, Li XZ, Mu RL, Sun DY, Zhou RG (2003) Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol 132 1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Sun DY, Zhou RG (2005) Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant Cell Environ 28 1276–1284 [Google Scholar]
- Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W (2002) Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell (Suppl) 14 S389–S400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack E, Tsai YC, Braam J (2005) Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci 10 383–389 [DOI] [PubMed] [Google Scholar]
- Miernyk JA (1999) Protein folding in the plant cell. Plant Physiol 121 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD (2002) In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev 16 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Kotzbauer PT, Sarge KD, Morimoto RI (1990) In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci USA 87 3748–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Sotelo J, Martinez LM, Ponce G, Cassab GI, Alagón A, Meeley RB, Ribaut JM, Yang R (2002) Maize HSP101 plays important roles in both induced and basal thermotolarance and primary root growth. Plant Cell 14 1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S (2006) Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J 48 535–547 [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI (2002) Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci 115 2809–2816 [DOI] [PubMed] [Google Scholar]
- Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf KD (2001) Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones 6 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Sundberg H, Vierling E (1993) Poly(A) tail length of a heat shock protein RNA is increased by severe heat stress, but intron splicing is unaffected. Mol Gen Genet 239 323–333 [DOI] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquest S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ASN (2001) Calcium: silver bullet in signaling. Plant Sci 160 381–404 [DOI] [PubMed] [Google Scholar]
- Reddy VS, Ali GS, Reddy ASN (2000) Genes encoding calmodulin-binding proteins in the Arabidopsis genome. J Biol Chem 275 35457–35470 [DOI] [PubMed] [Google Scholar]
- Sangwan V, Örvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31 629–638 [DOI] [PubMed] [Google Scholar]
- Sanmiya K, Suzuki K, Egawa Y, Shono M (2004) Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS Lett 557 265–268 [DOI] [PubMed] [Google Scholar]
- Scharf KD, Rose S, Zott W, Schöffl F, Nover L (1990) Three tomato genes code for heat stress transcription factors with a region of remarkable homology to the DNA-binding domain of the yeast HSF. EMBO J 9 4495–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F, Prändl R, Reindl A (1998) Regulation of the heat shock response. Plant Physiol 117 1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden WA, Fromm H (1998) Calmodulin, calmodulin-related proteins and plant responses to the environment. Trends Plant Sci 3 299–304 [Google Scholar]
- Snedden WA, Fromm H (2001) Calmodulin as a versatile calcium signal transducer in plants. New Phytol 151 35–66 [DOI] [PubMed] [Google Scholar]
- Sung DY, Kaplan F, Lee KJ, Guy CL (2003) Acquired tolerance to temperature extremes. Trends Plant Sci 8 179–187 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Komeda Y (1989) Characterization of two genes encoding small heat-shock proteins in Arabidopsis thaliana. Mol Gen Genet 219 365–372 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Naito S, Komeda Y (1992) Isolation and analysis of the expression of two genes for the 81-kilodalton heat-shock proteins from Arabidopsis. Plant Physiol 99 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa D, Liu ZH, An G, Poovaiah BW (1995) Calmodulin gene family in potato: developmental and touch-induced expression of the mRNA encoding a novel isoform. Plant Mol Biol 27 693–703 [DOI] [PubMed] [Google Scholar]
- Townley HE, Knight MR (2002) Calmodulin as a potential negative regulator of Arabidopsis COR gene expression. Plant Physiol 128 1168–1172 [DOI] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, DeGara DL (2004) Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco bright-yellow 2 cells. Plant Physiol 134 1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42 579–620 [Google Scholar]
- Volkov RA, Panchuk II, Mullineaux PM, Schöffl F (2006) Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61 733–746 [DOI] [PubMed] [Google Scholar]
- Wang XZ, Khaleque MA, Zhao MJ, Zhong R, Gaestel M, Calderwood SK (2006) Phosphorylation of HSF1 by MAPK-activated protein kinase 2 on serine 121 inhibits transcriptional activity and promotes HSP90 binding. J Biol Chem 281 782–791 [DOI] [PubMed] [Google Scholar]
- Wang Y, Liang SP, Xie QG, Lu YT (2004) Characterization of a calmodulin-regulated CDPK-related protein kinase, AtCRK1 from Arabidopsis. Biochem J 383 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E (1996) Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot 47 325–338 [Google Scholar]
- Wunderlich M, Werr W, Schoffl F (2003) Generation of dominant-negative effects on the heat shock response in Arabidopsis thaliana by transgenic expression of a chimaeric HSF1 protein fusion construct. Plant J 35 442–451 [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Mitsuhara I, Ito N, Seo S, Kamada H, Ohashi Y (2001) Transcriptionally and post-transcriptionally regulated response of 13 calmodulin genes to tobacco mosaic virus-induced cell death and wounding in tobacco plant. Eur J Biochem 268 3916–3929 [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Mizukami Y, Sakurai H (2005) Identification of a novel class of target genes and a novel type of binding sequence of heat shock transcription factor in Saccharomyces cerevisiae. J Biol Chem 280 11911–11919 [DOI] [PubMed] [Google Scholar]
- Yang JY, Sun Y, Sun AQ, Yi SY, Qin J, Li MH, Liu J (2006) The involvement of chloroplast HSP100/ClpB in the acquired thermotolerance in tomato. Plant Mol Biol 62 385–395 [DOI] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW (2003) Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 8 505–512 [DOI] [PubMed] [Google Scholar]
- Zielinski RE (1998) Calmodulin and calmodulin-binding proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 49 697–725 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.