Abstract
Arabidopsis (Arabidopsis thaliana) roots perceive gravity and reorient their growth accordingly. Starch-dense amyloplasts within the columella cells of the root cap are important for gravitropism, and starchless mutants such as pgm1 display an attenuated response to gravistimulation. The altered response to gravity1 (arg1) mutant is known to be involved with the early phases of gravity signal transduction. arg1 responds slowly to gravistimulation and is in a genetically distinct pathway from pgm1, as pgm1 mutants enhance the gravitropic defect of arg1. arg1 seeds were mutagenized with ethylmethane sulfonate to identify new mutants that enhance the gravitropic defect of arg1. Two modifier of arg1 mutants (mar1 and mar2) grow in random directions only when arg1 is present, do not affect phototropism, and respond like the wild type to application of phytohormones. Both have mutations affecting different components of the Translocon of Outer Membrane of Chloroplasts (TOC) complex. mar1 possesses a mutation in the TOC75-III gene; mar2 possesses a mutation in the TOC132 gene. Overexpression of TOC132 rescues the random growth phenotype of mar2 arg1 roots. Root cap amyloplasts in mar2 arg1 appear ultrastructurally normal. They saltate like the wild type and sediment at wild-type rates upon gravistimulation. These data point to a role for the plastidic TOC complex in gravity signal transduction within the statocytes.
Auxin and its asymmetrical redistribution across organs following gravistimulation are essential for normal gravitropic curvature responses (Chen et al., 2002). Yet the mechanisms that allow plant organs to perceive their orientation within the gravity field remain poorly understood (for review, see Harrison and Masson, 2008). The starch-statolith hypothesis posits that the sedimentation of starch-filled amyloplasts in root cap columella cells or endodermis cells provides directional cues to the root or hypocotyl and shoot, respectively (for reviews, see Boonsirichai et al., 2002; Morita and Tasaka, 2004). Importantly, starch-deficient pgm1 mutants lack a normal response to gravistimulation (Caspar and Pickard, 1989; Kiss et al., 1989), while the starch excess mutant sex1 displays an increased sensitivity to gravistimulation (Vitha et al., 2007). Likewise, mutants containing intermediate levels of starch display gravitropic sensitivity corresponding to the relative amount of starch present in either the roots or hypocotyls (Kiss et al., 1997). In the root cap in particular, laser ablation studies have demonstrated that the second story (S2) central columella cells contribute most to gravitropism (Blancaflor et al., 1998). Within these cells, the sedimentation of the mass of amyloplasts or a subset thereof is critical for gravity perception.
The sedimentation of the amyloplasts is relayed through an unknown mechanism, although several models have been proposed. The actin tensegrity model posits that sedimenting amyloplasts disrupt a dense actin network that is tethered to the plasma membrane. This disruption alters the tension applied to mechanosensitive ion channels, thereby activating them (Yoder et al., 2001). Alternatively, close associations between sedimenting amyloplasts and the endoplasmic reticulum in root statocytes may activate mechanosensitive ion channels (Sack, 1991). Yet another model proposes that the cell can sense the pressure exerted by the entire protoplast. In this model, amyloplasts function primarily as dense ballast to augment the total mass of the protoplast (Staves, 1997). Although the importance of statoliths within the statocytes has been well elaborated, they may not account for the entirety of the gravitropic response. Even the starchless pgm1 mutant, whose amyloplasts do not sediment relative to the direction of gravity, still responds to gravity, albeit poorly (Caspar and Pickard, 1989; Kiss et al., 1989). Furthermore, experiments using a rotating stage (ROTATO) showed that a region of the distal elongation zone in maize (Zea mays) is capable of generating a curvature even when the root cap is vertically oriented (Wolverton et al., 2002).
Forward genetic screens have been utilized to help unravel these complexities. We previously reported on ARG1, a J-domain protein that participates in early gravity signal transduction in statocytes, along with its paralog ARL2 (Sedbrook et al., 1999; Boonsirichai et al., 2003; Guan et al., 2003; Harrison and Masson, 2008). arg1 and arl2 mutants affect gravitropism without altering starch accumulation, root growth response to phytohormones or polar auxin transport inhibitors, or phototropism (Sedbrook et al., 1999; Boonsirichai et al., 2003; Guan et al., 2003). Whereas ARL2 is expressed specifically in the statocytes (Harrison and Masson, 2008), ARG1 is expressed ubiquitously (Sedbrook et al., 1999; Boonsirichai et al., 2003). However, the root and hypocotyl gravitropic defects of arg1-2 can be rescued with expression of ARG1 using root cap- or endodermis-specific promoters. ARG1 is associated with the secretory pathway, and its role in vesicular trafficking affects normal auxin transport through the root cap, at least in part by regulating the localization and/or activity of PIN3 (Boonsirichai et al., 2003; Harrison and Masson, 2008). Importantly, like pgm1, arg1-2 roots that have been reoriented 90° display only an attenuated, yet distinctly positive, gravitropic response. However, pgm1 arg1 and pgm1 arl2 double mutants have a more severe gravitropic phenotype than any of the single mutants (Guan et al., 2003). Therefore, arg1-2 and arl2-3 are ideally sensitized backgrounds that could be used in a genetic modifier screen to identify new gravity signal transducers functioning in the PGM1 pathway.
We mutagenized arg1-2 seeds of Arabidopsis (Arabidopsis thaliana) with ethylmethane sulfonate (EMS) to identify mutants with enhanced root gravitropic defects. In particular, we hoped to find mutants that exacerbate the root phenotypes of arg1-2 while retaining wild-type amyloplast morphology and behavior as well as normal responses to phytohormones and polar auxin transport inhibitors. Here, we report the identification of two recessive mutants, modifier of arg1 (mar1) and mar2, both of which affect proteins in the Translocon of Outer Membrane of Chloroplasts (TOC) complex.
Most plastid-targeted proteins are encoded in the nuclear genome and translated in the cytosol (for review, see Jarvis, 2008). These proteins require a transport apparatus to translocate across the two plastidic membranes. This apparatus consists of two complexes, the TOC complex and the Translocon of the Inner Membrane of Chloroplasts complex. The TOC complex consists of three primary components: a central pore and two receptors (Jarvis, 2008). Each component is a member of a small gene family. The receptor families are TOC33/TOC34 (Jelic et al., 2003) and TOC159/TOC132/TOC120/TOC90 (Kubis et al., 2004). The primary central pore is TOC75-III, and its paralog is TOC75-I (Baldwin et al., 2005). The receptors are thought to recognize preproteins and feed them through the central pore in a GTP-dependent process (Kessler and Schnell, 2004). In addition to serving as the central pore for plastid-targeted preproteins, TOC75 also mediates the insertion of proteins into the outer envelope of the plastid (Tu et al., 2004).
RESULTS
Mutagenesis of arg1-2 Identifies Two Genetic Enhancers
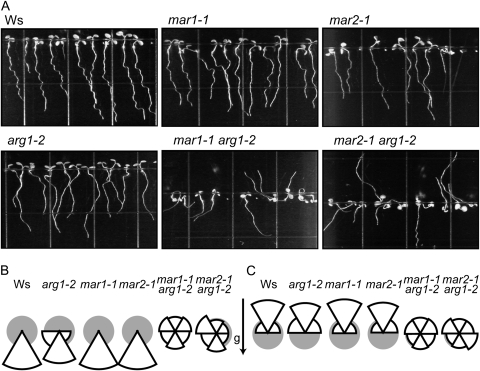
arg1-2 seeds were mutagenized with EMS to identify novel extragenic mutations that modify the phenotype of arg1-2. Plants were screened at the M2 generation to isolate mutants with extreme root phenotypes. Several individual lines were then transformed with Agrobacterium tumefaciens with genomic ARG1 DNA (Sedbrook et al., 1999) to test if they were true genetic modifiers of arg1-2. Additionally, these lines were crossed with arg1-2, and their segregating F2 progeny were observed to identify lines that behave as expected assuming Mendelian ratios of a single-locus recessive extragenic modifier. Two such recessive mar mutants were isolated: mar1-1 and mar2-1. Under all conditions tested, the roots and hypocotyls of arg1-2 mar1-1 and arg1-2 mar2-1 double mutants grow in random directions (Fig. 1; Supplemental Fig. S1).
Figure 1.
arg1-2 mar1-1 and arg1-2 mar2-1 roots and hypocotyls grow in random directions. A, Light-grown seedlings (n = 31–39) grown on the surface of inclined 1.5% agar GM plates. B and C, Root (B) and hypocotyl (C) angles relative to the gravity vector were measured and placed into one of six bins. The area of each wedge is proportional to the number of plants observed in each 60° bin; the total area for each pie chart is 1 (gray circle). For both roots and hypocotyls, mar1-1, mar2-1, and Ws grow most frequently within 30° of the vertical, while a higher proportion of arg1-2 roots and hypocotyls deviates from this range. Double mutants grow randomly.
TOC Complex Genes Are Mutated in mar1-1 and mar2-1
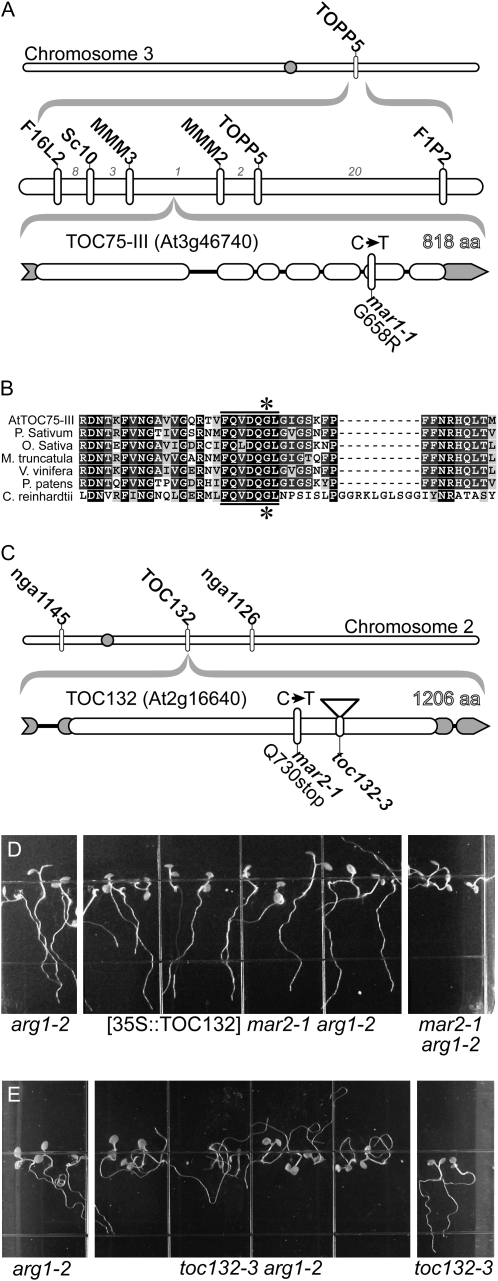
Mapping populations for both mar1-1 and mar2-1 were created by crossing the mutant (Wassilewskija [Ws]) with Landsberg erecta (Ler). Recombinant breakpoint analysis of 1,113 segregating F2 or F3 plants using existing and newly derived PCR-based markers demonstrated that mar1-1 maps to a region on chromosome III containing 24 predicted genes (Fig. 2), whereas mar2-1 maps to chromosome II between nga1145 and nga1126 (Fig. 2). Sequence analysis of candidate genes in mar1-1 revealed a C-T transition mutation in At3g46740, resulting in a G658R change. Amino acid 658 is a highly conserved amino acid in a pore-forming β-sheet of TOC75-III (Schleiff et al., 2003; Fig. 2). To the best of our knowledge, this allele is the first reported hypomorphic allele of TOC75-III; null alleles of TOC75-III bearing a T-DNA insertion are embryo lethal (Baldwin et al., 2005). Members of the TOC complex that mapped to the appropriate region of chromosome II were then sequenced in mar2-1 plants. The mar2-1 mutant is characterized by a C-T transition in TOC132 (At2g16640), resulting in a premature stop codon (Q730Stop). The TOC132 protein is characterized by an N-terminal acidic domain, a central GTPase domain, and a C-terminal membrane anchor domain (Ivanova et al., 2004). The stop codon occurs 64 amino acids from the interface between the GTPase domain and the membrane anchor domain.
Figure 2.
Molecular mapping of mar1-1 and mar2-1. A, mar1-1 is linked to TOPP5. Recombinant breakpoint analysis narrowed the region to between MMM3 and MMM2. The number of recombinants between marker pairs is indicated by gray numbers. B, Sequencing revealed a C-T transition in TOC75-III. The region of TOC75-III that encompasses the mutation (star) is highly conserved throughout plants. It lies in a conserved seven-amino acid (aa) motif (bar) predicted to form a β-barrel. C, mar2-1 maps between nga1145 and nga1126. mar2-1 has a C-T mutation in TOC132 that creates a premature stop codon. D, 35S-driven TOC132 cDNA expression restores the Arg-1 phenotype to arg1-2 mar2-1 plants. Shown are T3 plants homozygous for the transgene compared with arg1-2 and untransformed control plants. The growth conditions were the same as those described in the legend to Figure 1. E, The toc132-3 allele also displays random root growth only when in the arg1-2 background.
TOC132 Rescues the Gravitropic Defect of mar2-1 arg1-2
arg1-2 mar2-1 plants expressing TOC132 cDNA under control of the cauliflower mosaic virus 35S promoter (Kubis et al., 2004) display arg1-like phenotypes (Fig. 2D). Furthermore, in the arg1-2 background, a null T-DNA allele of TOC132, toc132-3 (Kubis et al., 2004), produced a phenotype indistinguishable from arg1-2 mar2-1 (Fig. 2E). Taken together, these data demonstrate that the mutated form of TOC132 is causative of the phenotypes seen in arg1-2 mar2-1 populations.
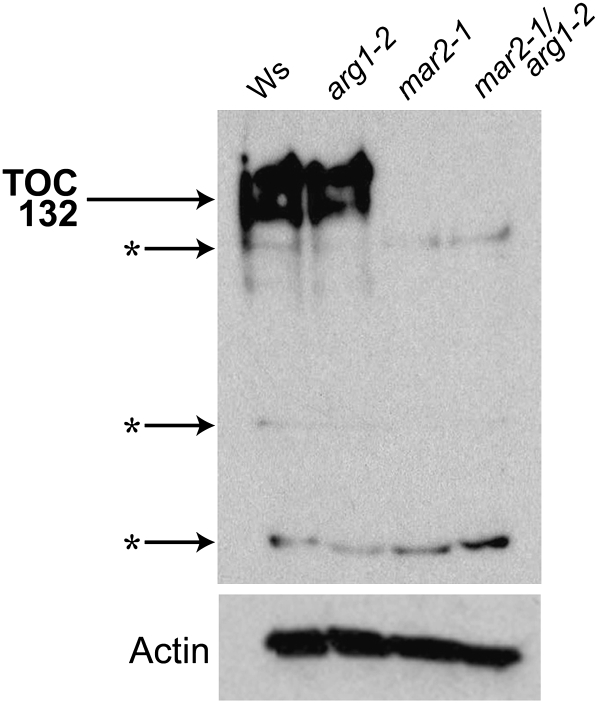
To determine if mar2-1 is also a null allele of TOC132, we analyzed its expression. Reverse transcription-PCR analysis of TOC132 expression using RNA derived from 10-d-old seedlings indicates that mar2 plants still produce TOC132 RNA. However, when proteins extracted from whole 10-d-old seedlings were probed by western blot using an antibody raised against the N-terminal 431 amino acids of TOC132 (Ivanova et al., 2004), no protein was detected in mar2-1 plant extract, suggesting that mar2-1 is also protein null (Fig. 3).
Figure 3.
mar2-1 is a protein-null allele of TOC132. Anti-TOC132 antibody recognizes TOC132 in protein extracts from Ws and arg1-2 but not in mar2-1 or mar2-1 arg1-2 extracts. Stars represent nonspecific binding. The anti-actin positive control is shown below.
mar2-1 Resembles the Wild Type Gravitropically
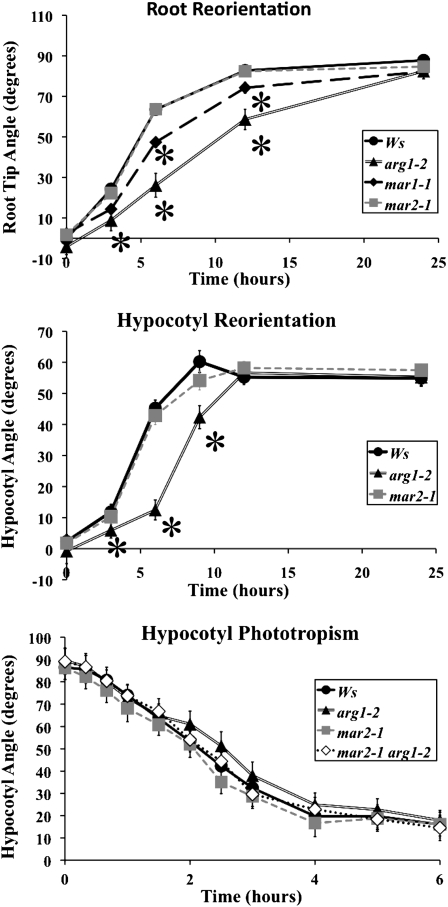
mar2-1 and mar1-1 produce randomly oriented roots and hypocotyls only when paired with the arg1-2 mutation. When mar2-1 single mutant seedlings are grown in the light embedded in 0.8% agar growth medium, they show no gravitropic defects following 90° reorientation (Fig. 4A). Unlike mar2-1, the reorientation kinetics of the mar1-1 single mutant are slightly delayed following 90° reorientation (Fig. 4A). Furthermore, the mar1-1 single mutant plants are noticeably pale and smaller than wild-type plants. The paleness seen represents a 30% decrease in chlorophyll content (Supplemental Fig. S2). Although mar2-1 plants appear to accumulate slightly less chlorophyll than Ws plants, in agreement with previous findings, the difference is not statistically significant (P < 0.05) in our experiment (Kubis et al., 2004). Because mar2-1 has fewer pleiotropic phenotypes than mar1-1, it is a more appropriate mutant to use for further investigation of gravity signaling; therefore, it is the primary focus of this study.
Figure 4.
mar2-1 resembles Ws, and mar1-1 has pleiotropic defects. A, mar2-1 roots respond like Ws to 90° gravistimulation, whereas mar1-1 roots reorient more slowly toward the gravity vector compared with Ws but not as slowly as arg1-2. B, Dark-grown mar2-1 hypocotyls also respond to gravistimulation like Ws. C, mar2-1 and mar2-1 arg1-2 hypocotyls (n = 38–45) grown on the surface of 0.8% agar GM plates both develop wild-type phototropism. Error bars, which are sometimes masked by the symbols, represent se. Stars represent significant t-test values (P < 0.05) compared with Ws.
arg1-2 mar2-1 Is Affected in Gravity Signal Transduction
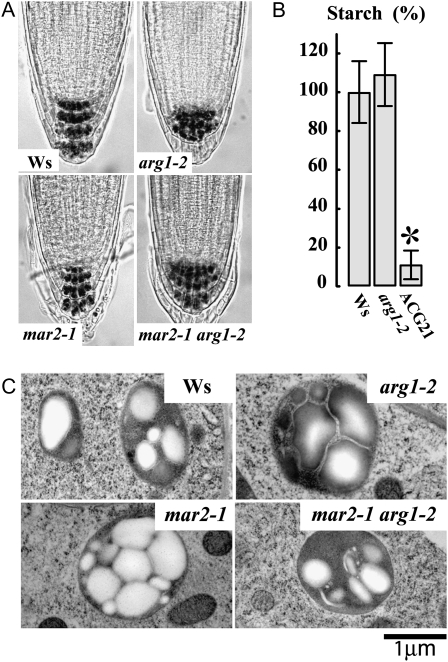
To test the possibility that mar2-1 might affect the differential growth response to lateral stimulation in an arg1-2 mutant background, we investigated the phototropic response of wild-type and mutant seedlings. Although the main focus of this project is on root gravitropism, we chose to analyze phototropism in the hypocotyl because this organ develops a stronger curvature response to lateral light stimulation than roots do under our growth conditions. We found that all single and double mutants have a normal phototropic response (Fig. 4C). Although these mutants retain apparently normal, starch-filled amyloplasts in the root cap (Fig. 5), they are likely to be deficient in the early phases of gravity signaling, because arg1-2 mar2-1 responds like the wild type (Ws) to exogenous application of brassinolide, cytokinin, auxin, the polar auxin transport inhibitor 1-N-naphthylphthalamic acid, and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (Supplemental Fig. S3).
Figure 5.
Starch content is similar across genotypes. A, Iodine staining of approximately 20 roots per genotype shows similar starch accumulation in all roots, while arg1-2 and arg1-2 mar2-1 have expanded domains of starch accumulation in the root cap, as expected (Harrison and Masson, 2008). B, Total starch of etiolated seedlings was measured and standardized to Ws = 100%. The t-test values show that Ws and arg1-2 are not statistically different (P > 0.05) from each other, while ACG21 accumulates significantly less starch (star). Error bars represent se. C, Transmission electron microscopy images demonstrate the presence of large starch granules in all amyloplasts. Shown are representatives of 10 images per genotype. No obvious structural defects were detected; the variation in plastid starch content shown between genotypes is similar to that detected between plastids within genotypes. Bar = 1 μm.
arg1-2 mar2-1 Root Cap Amyloplast Morphology and Sedimentation Resemble Those of the Wild Type
Because mutations in the TOC protein import machinery may affect plastid morphology (notably starch content) and/or behavior, we investigated the morphology and behavior of columella amyloplasts. Although iodine staining of the root statocytes showed starch accumulation in Ws, mar2-1, mar2-1 arg1-2, and arg1-2 seedlings (Fig. 5A), it was still possible that the overall size or morphology of the amyloplasts might be substantially different between mutant and the wild type. However, this was not the case, as transmission electron microscopy revealed similarly shaped plastids in Ws, arg1-2, mar2-1, and arg1-2 mar2-1 root tips (Fig. 5C).
Next, we wanted to test the possibility that although the mar2-1 arg1-2 and Ws amyloplasts appeared indistinguishable, they might be sedimenting differently. Because the sedimentation of plastids after gravistimulation is a complex and dynamic process, we opted to observe it in living roots. Not only does this approach allow for careful observation of plastids over a time course, it also allows for the same root to be monitored over time. Therefore, we analyzed the behavior of amyloplasts in S2 columella cells in growing roots after 90° reorientation. Bright-field images of mar2-1 arg1-2 and Ws amyloplasts were collected every 10 s for about 12 min after gravistimulation. In these studies, mar2-1 arg1-2 amyloplasts were found to saltate like the wild type in columella cells of the root cap (Supplemental Movies S1 and S2; data not shown).
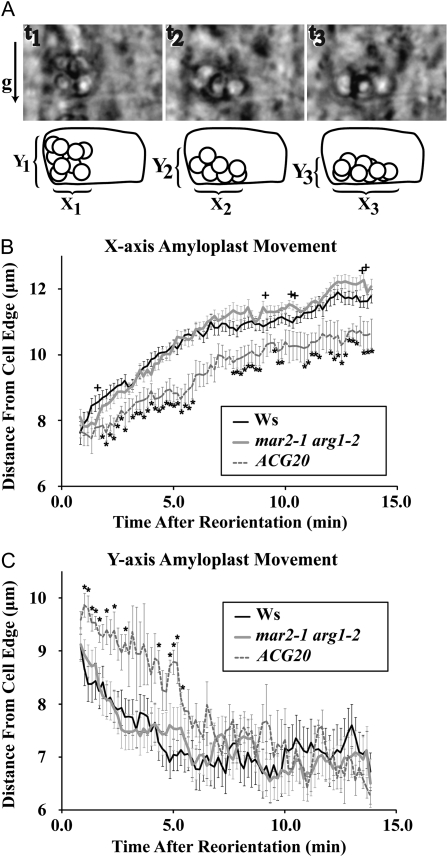
Preliminary examination of amyloplast movement upon gravistimulation also indicated that columella plastids sediment as a group. Therefore, the distances along both the x and y axes from the distal lower cell corner to the upper and proximal edges of the upper and leading amyloplast, respectively, were measured for every frame (Fig. 6A). These measurements were chosen because it can be difficult to discriminate individual plastids in every frame, as they often move behind other plastids. A t test at each time point using a 95% probability threshold showed only five of 79 time comparisons as significantly different between Ws and arg1-2 mar2-1 for the x axis measurement (Fig. 6B) and zero of 79 for the y axis measurement (Fig. 6C), well within the range of expected type I error. As a control, we also included a mutant with intermediate levels of starch in its roots, ACG20. Under our experimental conditions, ACG20 roots do not respond to 90° gravistimulation slower than Ws roots (Supplemental Fig. S4). However, previous experiments detected slightly slower root reorientation and demonstrated a slower rate of amyloplast sedimentation in ACG20 (Kiss et al., 1997). In this experiment, we reaffirm that ACG20 amyloplasts sediment slower than the wild type, as 51 of 79 of the ACG20 time points are statistically significant compared with Ws for the x axis measurement and 13 of 79 for the y axis, all among the first 28 measurements. Therefore, the similarities of the sedimentation behaviors of the arg1-2 mar2-1 double mutant and Ws indicate that TOC132 likely contributes to gravitropic signaling in a specific manner, not as a result of altering the sedimenting behavior of columella amyloplasts.
Figure 6.
mar2-1 arg1-2 plastid movement following 90° reorientation is like that of the wild type. A, Amyloplasts within central S2 columella cells were analyzed by placing live, growing roots (Ws, n = 7; arg1-2, n = 9; ACG20, n = 5) on a vertically oriented rotatable stage. Images were collected in 10-s intervals following 90° reorientation. B, The distance from the former cell bottom to the leading plastid was measured for each time point on the x axis. C, The distance from the new cell bottom to the top-most plastid was measured for each time point on the y axis. Error bars represent the se, and stars and crosses represent significant t-test values (P < 0.05) compared with Ws at each time point.
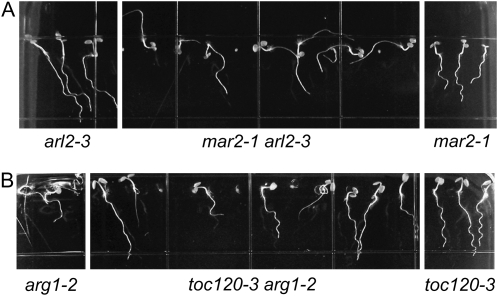
mar2-1 Enhances the Phenotype of arl2-3; toc120-3 Does Not Enhance the Phenotype of arg1-2
By double mutant analyses, both the ARG1 and TOC132 genes have been shown to share some functional redundancy with their respective paralogs, ARL2 and TOC120 (Kubis et al., 2004; Harrison and Masson, 2008). To test if the genetic interaction observed here extends to these paralogs, we created the arl2-3 mar2-1 and the toc120-3 arg1-2 double mutants. The gravitropic response of arl2-3 mar2-1 mutant roots appears severely defective (Fig. 7A), consistent with the hypothesis that ARG1 and ARL2 function in the same genetic pathway (Harrison, 2007), distinct from that of mar2-1. However, the toc120-3 arg1-2 double mutant roots behave like arg1-2 single mutant roots (Fig. 7B), indicating that the functional redundancy previously reported for TOC132 and TOC120 does not extend to the role of TOC132 in gravitropism.
Figure 7.
A, mar2-1 also enhances the root defect of arl2-3. B, toc120-3 does not enhance the root defect of arg1-2. Seedlings were grown on the surface of inclined GM growth media plates containing 1.5% agar.
DISCUSSION
In this study, we took advantage of the relatively subtle gravitropic phenotype of arg1-2 roots in a genetic screen to identify mutants that have more severe root phenotypes. We were able to successfully identify two independent mutations in different members of the same protein complex. Interestingly, these mutants display a nearly identical random growth phenotype in the arg1-2 mutant background despite the toc75 mutant also having pleiotropic growth defects. These additional defects are likely due to the difference in ascribed functions of each component: TOC75 is the central pore in the outer membrane of plastids through which vital preproteins must pass in order to perform their functions. As such, insertional alleles at this locus are lethal (Baldwin et al., 2005). Unlike previously described toc75 lethal mutants, the toc75 mutation that we describe, mar1-1, is sufficient to attenuate, but not to eliminate, the function of TOC75. In contrast, TOC132 is one of a four-member family of receptors (TOC90, TOC120, TOC132, and TOC159) that displays functional specialization. Although specialized, these receptors also demonstrate functional redundancy. In particular, toc120 toc132 double mutants are more abnormal than either single mutant (Kubis et al., 2003). However, the toc120 mutant does not enhance the root phenotype of arg1-2, suggesting that TOC132 and TOC120 retain functional specialization despite sharing some functional redundancy.
The TOC complex likely participates in early gravity signaling, as the mutants respond like the wild type to phytohormones and drugs that affect the auxin-transport phase of the gravity response as well as to exogenously applied brassinolide, cytokinin, and an ethylene precursor. These hormones have all been implicated in various aspects of gravitropism (Li et al., 2005; Aloni et al., 2006; Buer et al., 2006).
As a plastid-based protein complex, there is a compelling possibility that mutants of the TOC complex may produce amyloplasts that are aberrant in their morphology or sedimentation. However, when we examined amyloplasts of the root columella cells by iodine staining and electron microscopy, we were unable to detect significant differences in starch accumulation or amyloplast morphology between mutant and the wild type. Furthermore, our in vivo analysis of plastid movements in central S2 columella cells revealed no behavioral differences: arg1-2 mar2-1 mutant amyloplasts saltate like the wild type, and they sediment at wild-type rates upon gravistimulation (Fig. 6; Supplemental Movies S1 and S2). Therefore, it is unlikely that the TOC complex participates in gravity signal transduction by altering the structure or density of amyloplasts. Likewise, the Ws-like sedimentation and saltation behaviors of the mutant plastids suggest that the actin cytoskeleton is not structurally altered.
Within the cell, the ARG1 and ARL2 proteins were shown to be associated with both the plasma membrane and components of the vesicle trafficking pathway, suggesting a role in mediating the targeting or activity of gravity signal transducers at the plasma membrane or at organelles of the secretory pathway (Boonsirichai et al., 2003). Importantly, ARG1 and ARL2 are conspicuously absent from plastids, suggesting that the genetic interactions between arg1 and toc132, or between arl2 and toc132, do not represent a continuous physical interaction between the corresponding proteins. It is possible, however, that both ARG1/ARL2 and the TOC complex mediate the proper localization and/or activity of gravity signal transducers at specific compartments within the cell (plasma membrane or endoplasmic reticulum for ARG1, and plastid envelope for the TOC complex). In this context, the genetic interaction we observed between arg1-2 or arl2-3 on the one hand and between mar2-1, toc132-3, or mar1-1 on the other hand would reflect a decreased ability for these putative transducers to interact and promote gravity signal transduction when sedimenting plastids hit the peripheral endoplasmic reticulum or plasma membrane of the mutant statocytes upon gravistimulation (Fig. 8A). Accordingly, removal of TOC132 would reduce the amount of plastid-associated transducer to a level that remains sufficient to trigger a normal gravitropic response in ARG1 ARL2 plants (probably due to functional redundancy with other members of the TOC complex) but is insufficient in the context of the attenuated pool of functional interacting ligand in arg1-2 or arl2-3 membranes. Similarly, removal of ARG1 or ARL2 may reduce the amount of peripheral membrane-associated signal transducer to a level that still permits a significant, although partially altered, gravitropic response in a wild-type TOC132 background, but it becomes insufficient for significant pathway activation in a TOC132-deficient background.
Figure 8.
Possible models of TOC132 action in gravitropism. A, TOC132 may mediate the insertion into the outer membrane of amyloplasts of a protein (Y) that, upon gravity-induced amyloplast sedimentation to the lateral side of the statocytes, may interact with a plasma membrane- or endoplasmic reticulum-associated transducer (X), regulating its signal-transducing activity. B, Alternatively, TOC132 may interact directly with X upon gravity-induced amyloplast sedimentation, thereby triggering signal transduction. In both models, ARG1 and ARL2 (not shown here) would modulate the location and/or activity of transducer X at the sensitive membrane.
In the context of the ligand-interaction model of gravity sensing discussed above, it is important to note, once again, that TOC75 also contributes to the proper targeting of plastid outer envelope proteins, including members of the TOC159 family (Tu et al., 2004). Therefore, the missense mutation in TOC75 associated with mar1-1 may affect the proper targeting of the proposed plastid-associated transducer, which, in view of the latter observation, could conceivably be TOC132 itself, as illustrated in Figure 8B.
To our knowledge, while fully compatible with our genetic studies of the interaction between arg1, arl2, and mar mutations, the interaction model of gravity sensing discussed above has not been considered as a possible mechanism for gravity sensing in higher plants. It should be noted, however, that a ligand-receptor model of gravity sensing has been proposed to function in Chara rhizoids based on careful evaluations of gravity sensing in the context of parabolic-flight microgravity environments (Limbach et al., 2005). Future work should evaluate its significance in higher plants.
While the ligand interaction model of gravity sensing is quite attractive, we cannot rule out the possibility that the TOC complex affects gravitropism through an unidentified regulatory molecule that contributes to gravity signal transduction. Indeed, plastids are known to play a central role in the manufacture and/or storage of many diverse and important biological products (Neuhaus and Emes, 2000). However, the strong genetic interaction observed between toc132 and arg1-2 or arl2-3 would require that the corresponding regulatory molecule be needed only in the absence of ARG1 or ARL2. Alternatively, internal plastid proteins may act in a signal transduction pathway without altering the structural or behavioral characteristics of amyloplasts. For example, recent evidence implicates a thylakoid-localized calcium-binding protein in the transduction of stomata-closing signals (Nomura et al., 2008). However, here again, such internal plastid protein would be needed for gravity signal transduction only in an arg1-2 or arl2-3 background. These possibilities cannot be fully addressed without future metabolic and/or proteomic profiles of the mar2-1 arg1-2 or mar2-1 arl2-3 plants and their amyloplasts or until new participants in this genetic pathway are identified.
Finally, our observations are in stark contrast to a recent report that details the plastid sedimentation of arg1-2 amyloplasts in endodermal cells of hypocotyls (Kumar et al., 2008). Unlike our results in roots, their findings show that arg1-2 plastids sediment more slowly than wild-type amyloplasts in hypocotyl statocytes. As a result, they conclude that ARG1 affects gravitropism by contributing to both gravity signal transduction and plastid movements, possibly through interactions with the actin cytoskeleton. However, in this report, we show that mar2-1 arg1-2 plastids do not sediment more slowly than Ws plastids in root statocytes, even though mar2-1 arg1-2 seedlings are far more severely defective in their gravitropic response than arg1-2 single mutants. The discrepancy between these two studies may reflect different contributions of ARG1 in hypocotyl and root gravitropism, as documented previously (Harrison and Masson, 2008). Alternatively, it may be the consequence of the different experimental approaches used in these studies. Indeed, Kumar et al. (2008) observe hypocotyl gravitropism in seedlings grown on media of different agar and sugar contents overlaid with a cellophane film. The film modifies the physical properties of the surface and may affect water and nutrient availability, thereby inhibiting root waving, skewing, and organ growth rates (Kumar et al., 2008; data not shown). Therefore, we speculate that seedlings grown on such a film might be responding to mechanical and nutrient/water stresses. Interestingly, while ARG1 expression limited to the statocytes is sufficient to rescue arg1-2 gravitropic defects, ARG1 is expressed throughout the plant and is likely functioning in multiple cellular processes (Boonsirichai et al., 2003). Therefore, the additional phenotypes reported by Kumar et al. (2008) may reflect the involvement of ARG1 in nongravitropic processes and provide a basis for investigating additional roles for ARG1.
This report details, to our knowledge, an exciting new component of gravity signal transduction. Our current experiments are directed at uncovering the mechanism of TOC132 action in gravity signal transduction and its relation to the ARG1/ARL2 signaling pathway.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were sterilized in 20% bleach solution containing 0.01% SDS for 10 min followed by four rinses with distilled water, then plated with the appropriate medium. Plants were grown as described previously (Rutherford and Masson, 1996) on growth medium (GM; half-strength Linsmeier and Skoog salts, pH 5.7, and 1.5% Suc) containing varying concentrations (0.8%–1.5%) of type E agar (Sigma). Plates were wrapped in aluminum foil and kept in the dark at 4°C for 24 to 48 h before germinating in a Conviron TC16 growth chamber set to our standard conditions (16-h-light/8-h-dark photoperiod at approximately 75 μmol m−2 s−1 under cool-white fluorescent bulbs, 22°C, 75% relative humidity).
Procedures used to test plant growth responses to phytohormones and polar auxin transport inhibitor are described in Supplemental Materials and Methods S1.
Statistical Analysis
Unless otherwise indicated in the text, statistical comparisons were performed using unpaired two-tailed Student's t tests assuming equal variance where P < 0.05 was deemed significant.
Mutagenesis
arg1-2 seeds (Ws ecotype) were mutagenized (Joy, 2001) with EMS (Sigma), and 1,250 mutagenized seeds (M1) were planted into individual pots and allowed to self-fertilize. Approximately 40 M2 seeds from each plant were grown on the surface of tilted hard-agar (1.5%) plates and screened for altered root growth phenotypes. Approximately 1,500 M2 individuals, some of which were siblings, were selected for enhanced or suppressed root gravitropism relative to arg1-2, grown, and allowed to self-fertilize, and the resulting M3 seeds were rescreened on tilted hard-agar plates. Several individual lines showing strongly enhanced gravitropic defects were then transformed with Agrobacterium tumefaciens (Clough and Bent, 1998) with genomic ARG1 DNA in the pBIN19 vector (Sedbrook et al., 1999); progeny of kanamycin-resistant transformants were grown on the surface of 1.5% agar-containing plates to identify lines with suppressed mutant phenotypes. mar1-1 and mar2-1 were backcrossed to Ws six and four times, respectively.
Mapping and Molecular Marker Development
mar1-1 arg1-2 and mar2-1 arg1-2 plants were crossed with Ler to create a mapping population. DNA was extracted from leaves of F2 seedlings and used with PCR to genotype both the ARG1 locus and mapping markers throughout the genome. The MAR genotype could be inferred from the F3 generation root phenotypes if the arg1-2 allele was also present. Initial mapping linked mar1-1 to TOPP5 (chromosome III), while mar2-1 was linked to nga1145 and nga1126 (chromosome II). Additional molecular markers linked to mar1-1 include the simple sequence length polymorphism markers F1P2 (5′-AAGTGGTGGTTGGTTTTGTC-3′ and 5′-ACCCCACTCTTCATTATTGTTAC-3′) and F16L2 (5′-CATTAGTAACCAAAGACCAAAGAGACAC-3′ and 5′-TTTTTGCAGGTACATAGAGC-3′) and the cleaved-amplified polymorphic sequence (CAPS) markers SC10 (5′-CTTGCCACTCAGGTAGACGA-3′ and 5′-ACTGAGGGAAGACTGGCGTA-3′; Sau3a), MMM2 (5′-GCTCGTATTGACAAAGCTAACG-3′ and 5′-CATACGAACACCACCGTTCA-3′; RsaI), and MMM3 (5′-TGGAAAATCGTAATCTTGTGGA-3′ and 5′-GCGTGCATGTATGGATTAGG-3′; DdeI). CAPS markers were developed by sequencing noncoding regions of Ws and Ler to identify polymorphisms and then selecting appropriate restriction enzymes using New England Biolabs NEBcutter version 2.0 (http://www.neb.com). Using these molecular markers, recombinant breakpoint analysis mapped mar1-1 between At3g46590 and At3g46820. Candidate genes were then sequenced and compared with Ws sequence to identify a C-T transition mutation in TOC75-III (At3g46740). To facilitate genotyping of mar1-1 plants, this polymorphism was used with derived CAPS (dCAPS) finder (Neff et al., 2002; http://helix.wustl.edu/dcaps/dcaps.html) to develop two molecular markers: dCAPSMAR1 (5′-GCAAATCACAGGTGGACCAG-3′ and 5′-TGCCCATGGAGGACTAGAAC-3′; ScrFI cuts Ws) and dCAPSmar1-1 (5′-GCAAATCACAGGTGGATCTG-3′ and 5′-TGCCCATGGAGGACTAGAAC-3′; DdeI cuts mar1-1). Members of the TOC complex that mapped to the appropriate region of chromosome II were then sequenced in mar2 plants to identify a C-T transition mutation in TOC132 (At2g16640). dCAPS markers were developed in the same manner for mar2-1 as for mar1-1: dCAPSMAR2 (5′-AGCTGCTTGGCGAATGGCCA-3′ and 5′-CCCAAATGGCACTGCTTCTA-3; PvuII cuts Ws) and dCAPSmar2-1 (5′-AGCTGCTTGGCGAATGGCGT-3′ and 5′-CCCAAATGGCACTGCTTCTA-3′; RsaI cuts mar2-1).
The transgenic rescue experiment aimed at verifying the molecular identity of mar2-1 is described in Supplemental Materials and Methods S1.
Root and Hypocotyl Gravitropism
For the root gravitropism assay, seedlings were grown for 4 d embedded in 0.8% agar-containing GM in vertically oriented square petri dishes incubated within a Conviron TC16 growth chamber under our standard conditions. Plates were rotated 90°, and images were collected with a digital camera at various times over a 24-h period. For the hypocotyl gravitropism assay, seeds were sown on the surfaces of 0.8% agar-containing GM plates, wrapped in aluminum foil, and allowed to grow in a Conviron TC16 growth chamber under our standard conditions for 4 d. All plates were rotated 90° simultaneously and then unwrapped, and digital photographs were taken at various times over a 24-h period. Using NIH Image J, root and hypocotyl angles were then measured as described previously (Rutherford and Masson, 1996).
Hypocotyl Phototropism
The kinetics of hypocotyl phototropism were determined by growing Ws and mutant seedlings on vertically oriented 0.8% agar-containing GM plates wrapped in black paper and aluminum foil. After 3 d of growth, the plates were unwrapped on the top side only, and a 75-W incandescent lamp was shone on the exposed side for 18 h to allow all of the hypocotyls to orient upward. Next, the plates were unwrapped in the dark and mounted on an infrared backlight (Advanced Illumination). A 75-W incandescent lamp was positioned horizontally 12 inches away. Images of the hypocotyls were collected using an infrared-sensitive Marlin F146B CCD camera (Allied Vision Technologies) controlled by AVT SmartView software. Images were collected every 20 to 60 min over the course of 6 h. Using NIH Image J or Photoshop CS3 (Adobe), the angles between planes tangential to the hypocotyl tips and vertical planes were measured.
Starch Staining
Five-day-old seedlings were stained in 10%/5% KI/I solution (Lugol). Stained roots were cleared with chloral hydrate prior to observation under bright-field microscopy using a Leica DM LB2 microscope and a 40× objective.
Starch and Chlorophyll Quantification
Three independent replicates of Ws, arg1-2, and ACG20 seeds (n = approximately 250–400) were plated on the surface of 0.8% agar GM plates. Plates were wrapped in foil and placed at 4°C in the dark for 48 h before moving to a Conviron TC16 growth chamber set to our standard conditions. Seeds were dark grown vertically for 4 d before collecting, weighing, and freezing the etiolated hypocotyls. Frozen samples were ground and used with the Megazyme Amyloglucosidase/α-Amylase Total Starch Kit (Megazyme; catalog code K-TSTA) following the dimethyl sulfoxide-modified procedure for samples containing resistant starch.
Procedures used to quantify chlorophyll levels in wild-type and mutant plants are described in Supplemental Materials and Methods S1.
High-Pressure Freezing and Electron Microscopy
Six-day-old seedlings grown on 1.5% agar-containing GM were loaded into sample holders filled with 0.1 m Suc and then frozen in a Baltec HPM 010 high-pressure freezer (Technotrade). Cryosubstitution and sample embedding were performed as described by Otegui and Staehelin (2004). Longitudinal sections of root tips were cut with a microtome and stained with uranyl acetate and lead citrate, and then images were collected using a Philips CM120 scanning transmission electron microscope.
Plastid Sedimentation
Following 2 to 3 d of stratification at 4°C in the dark, seeds (Ws, ACG20, or mar2-1 arg1-2) were germinated on a glass microscope slide containing approximately 500 μL of GM with 0.75% agar and grown in the light in a petri dish sealed with Parafilm. The slides were then mounted and sealed onto a custom-made chamber that allows the live, growing roots to be mounted on a microscope stage. Roots that were growing along the slide at 4 to 7 d after germination were analyzed with bright-field microscopy using a Nikon microscope placed on its side equipped with a rotatable stage. Roots were oriented in the vertical position for at least 20 min prior to gravistimulation. At time 0, the stage was rotated 90° and images were collected with a 40× objective as soon as possible following gravistimulation, typically 40 to 70 s. Images were collected every 10 s for approximately 13 min using a CCD camera and software (Spot RT-slider; Diagnostic Instruments). Sequential images were combined into a stack using NIH Image J. Movement of plastids in central S2 columella cells was then analyzed. For each image, plastid movement was measured relative to projections through a cell corner along either the old bottom of the cell or the new bottom of the cell. Two values were measured: X-movement, which is the distance in micrometers of the leading edge of the plastid located farthest from the cell edge (former cell bottom) at any given time point; and Y-movement, which is the distance in micrometers of the top-most edge of the plastid located farthest from the new cell bottom at any given time point. Measurements from seven individual Ws roots, nine individual mar2 arg1 roots, and five ACG20 roots were collected and grouped into 10-s bins.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. arg1-2 mar1-1 and arg1-2 mar2-1 roots and hypocotyls grow in random directions.
Supplemental Figure S2. mar1 plants accumulate less chlorophyll than the wild type.
Supplemental Figure S3. Root growth sensitivity to phytohormones and a polar auxin transport inhibitor (1-N-naphthylphthalamic acid).
Supplemental Figure S4. Some intermediate starch mutant roots have a wild-type gravitropic reorientation.
Supplemental Materials and Methods S1. Procedures used to generate supplemental figures, including methods for exogenous drug application, chlorophyll measurement, and transgenic plant generation.
Supplemental Movie S1. Time course of Ws roots following 90° reorientation.
Supplemental Movie S2. Time course of mar2-1 arg1-2 roots following 90° reorientation.
Supplementary Material
Acknowledgments
We thank Paul Jarvis for providing us with the 35S∷TOC132 plasmid and toc132-3 and toc120-3 seeds. We thank Danny Schnell for providing us with anti-TOC132 antibody. We thank Chris Staiger for providing us with anti-actin antibody. We thank Carolyn Neal, John Kiss, and Katherine Baldwin for comments on the text and Katherine Baldwin for figure development.
This work was supported by the National Science Foundation (grant nos. MCB–0240084 and IOS–0642865 to P.H.M. and grant no. MCB–0619736 to M.S.O.), the National Aeronautics and Space Administration (grant no. NAG2–1602 to P.H.M.), and a fellowship to J.P.S. from the National Institutes of Health Training Grant in Genetics at the University of Wisconsin-Madison. This is manuscript 3642 of the Laboratory of Genetics.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Patrick H. Masson (phmasson@wisc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aloni R, Aloni E, Langhans M, Ullrich C (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot (Lond) 97 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Wardle A, Patel R, Dudley P, Park S, Twell D, Inoue K, Jarvis P (2005) A molecular-genetic study of the Arabidopsis Toc75 gene family. Plant Physiol 138 715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor E, Fasano J, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsirichai K, Guan C, Chen R, Masson P (2002) Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Biol 53 421–447 [DOI] [PubMed] [Google Scholar]
- Boonsirichai K, Sedbrook J, Chen R, Gilroy S, Masson P (2003) ARG1 is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell 15 2612–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer C, Sukumar P, Muday G (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Pickard B (1989) Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta 177 185–197 [PubMed] [Google Scholar]
- Chen R, Guan C, Boonsirichai K, Masson P (2002) Complex physiological and molecular processes underlying root gravitropism. Plant Mol Biol 49 305–317 [PubMed] [Google Scholar]
- Clough S, Bent A (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Guan C, Rosen E, Boonsirichai K, Poff K, Masson P (2003) The ARG1-LIKE2 (ARL2) gene of Arabidopsis thaliana functions in a gravity signal transduction pathway that is genetically distinct from the PGM pathway. Plant Physiol 133 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B (2007) Investigating the function of ARG1 and ARL2 as gravity signal transducers in Arabidopsis thaliana. PhD thesis. University of Wisconsin, Madison, WI
- Harrison B, Masson P (2008) ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J 53 380–392 [DOI] [PubMed] [Google Scholar]
- Ivanova Y, Smith M, Chen K, Schnell D (2004) Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol Biol Cell 15 3379–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P (2008) Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol 179 257–285 [DOI] [PubMed] [Google Scholar]
- Jelic M, Soll J, Schleiff E (2003) Two Toc34 homologues with different properties. Biochemistry 42 5906–5916 [DOI] [PubMed] [Google Scholar]
- Joy R (2001) Arabidopsis embryonic development: a screen for mutants disrupted in pattern formation, and analysis of BOBBER, a gene required for proper specification of the apical region of the Arabidopsis embryo. PhD thesis. University of Wisconsin, Madison, WI
- Kessler F, Schnell D (2004) Chloroplast protein import: solve the GTPase riddle for entry. Trends Cell Biol 14 334–338 [DOI] [PubMed] [Google Scholar]
- Kiss J, Guisinger M, Miller A, Stackhouse K (1997) Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol 38 518–525 [DOI] [PubMed] [Google Scholar]
- Kiss J, Hertel R, Sack F (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177 198–206 [PubMed] [Google Scholar]
- Kubis S, Baldwin A, Patel R, Razzaq A, Dupree P, Lilley K, Kurth J, Leister D, Jarvis P (2003) The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell 15 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis S, Patel R, Combe J, Bedard J, Kovacheva S, Lilley K, Biehl A, Leister D, Rios G, Koncz C, et al (2004) Functional specialization amongst the Arabidopsis Toc159 family of chloroplast protein import receptors. Plant Cell 16 2059–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Stevens M, Kiss J (2008) Plastid movement in statocytes of the ARG1 (ALTERED RESPONSE TO GRAVITY) mutant. Am J Bot 95 177–184 [DOI] [PubMed] [Google Scholar]
- Li L, Xu J, Xu Z, Xue H (2005) Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 17 2738–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach C, Hauslage J, Schäfer C, Braun M (2005) How to activate a plant gravireceptor: early mechanisms of gravity sensing studied in Characean rhizoids during parabolic flights. Plant Physiol 139 1030–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7 712–718 [DOI] [PubMed] [Google Scholar]
- Neff MM, Turk E, Kalishman M (2002) Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 18 613–615 [DOI] [PubMed] [Google Scholar]
- Neuhaus H, Emes M (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51 111–140 [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T (2008) Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J 53 988–998 [DOI] [PubMed] [Google Scholar]
- Otegui M, Staehelin L (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218 501–515 [DOI] [PubMed] [Google Scholar]
- Rutherford R, Masson P (1996) Arabidopsis thaliana sku mutant seedlings show exaggerated surface-dependent alteration in root growth vector. Plant Physiol 111 987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack F (1991) Plant gravity sensing. Int Rev Cytol 127 193–252 [DOI] [PubMed] [Google Scholar]
- Schleiff E, Eichacker L, Eckart K, Becker T, Mirus O, Stahl T, Soll J (2003) Prediction of the plant β-barrel proteome: a case study of the chloroplast outer envelope. Protein Sci 12 748–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook J, Chen R, Masson P (1999) ARG1 (Altered Response to Gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA 96 1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staves M (1997) Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta (Suppl) 203 S79–S84 [DOI] [PubMed] [Google Scholar]
- Tu S, Chen L, Smith M, Su Y, Schnell D, Li H (2004) Import pathways of chloroplast interior proteins and the outer-membrane protein OEP14 converge at Toc75. Plant Cell 16 2078–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Yang M, Sack F, Kiss J (2007) Gravitropism in starch-excess mutant of Arabidopsis thaliana. Am J Bot 94 590–598 [DOI] [PubMed] [Google Scholar]
- Wolverton C, Ishikawa H, Evans M (2002) The kinetics of root gravitropism: dual motor and sensors. J Plant Growth Regul 21 102–112 [DOI] [PubMed] [Google Scholar]
- Yoder T, Zheng H, Todd P, Staehelin L (2001) Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol 125 1045–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.