Abstract
Plants modify growth in response to the proximity of neighbors. Among these growth adjustments are shade avoidance responses, such as enhanced elongation of stems and petioles, that help plants to reach the light and outgrow their competitors. Neighbor detection occurs through photoreceptor-mediated detection of light spectral changes (i.e. reduced red:far-red ratio [R:FR] and reduced blue light intensity). We recently showed that physiological regulation of these responses occurs through light-mediated degradation of nuclear, growth-inhibiting DELLA proteins, but this appeared to be only part of the full mechanism. Here, we present how two hormones, auxin and ethylene, coregulate DELLAs but regulate shade avoidance responses through DELLA-independent mechanisms in Arabidopsis (Arabidopsis thaliana). Auxin appears to be required for both seedling and mature plant shoot elongation responses to low blue light and low R:FR, respectively. Auxin action is increased upon exposure to low R:FR and low blue light, and auxin inhibition abolishes the elongation responses to these light cues. Ethylene action is increased during the mature plant response to low R:FR, and this growth response is abolished by ethylene insensitivity. However, ethylene is also a direct volatile neighbor detection signal that induces strong elongation in seedlings, possibly in an auxin-dependent manner. We propose that this novel ethylene and auxin control of shade avoidance interacts with DELLA abundance but also controls independent targets to regulate adaptive growth responses to surrounding vegetation.
Plants respond to competing neighbors in a variety of ways. Among these are an upward leaf movement and enhanced shoot elongation to consolidate light capture in dense stands (Aphalo et al., 1999; Ballaré, 1999; Vandenbussche et al., 2005; Franklin, 2008). These so-called shade avoidance responses can be initiated early on in canopy development upon sensing the reduced red:far-red ratio (R:FR) in the canopy light by the phytochrome family of photoreceptors (Morgan and Smith, 1976; Ballaré et al., 1990; Franklin et al., 2003). Plant neighbor detection also involves blue light (Aphalo et al., 1999; Ballaré, 1999; Vandenbussche et al., 2005), which, like red light, is strongly reduced in well-developed canopies as a result of absorption by chlorophyll. When applied individually, both light signals can induce functional shade avoidance responses, such as hypocotyl, stem, and petiole elongation and upward leaf movement (Ballaré et al., 1991; Casal and Sánchez, 1994; Pierik et al., 2004b; Franklin and Whitelam, 2005; Franklin, 2008).
Many downstream signal transduction components involving several plant hormones operate to induce the growth responses upon detection of canopy signals. A reduction of the R:FR can either sensitize plants to GA (Weller et al., 1994; López-Juez et al., 1995) or enhance the production of bioactive GAs (Beall et al., 1996). Absence of GA or proper GA signaling consequently results in strongly attenuated elongation responses to low R:FR (Reid et al., 1990; López-Juez et al., 1995; Pierik et al., 2004a). Recent work on Arabidopsis (Arabidopsis thaliana) has greatly enhanced our understanding of the mechanisms underpinning this regulation mechanism. Key factors in GA responses are DELLA proteins. The Arabidopsis genome encodes five DELLAs (GA-INSENSITIVE [GAI], REPRESSOR OF GA1-3 [RGA], RGA-LIKE1 [RGL1], RGL2, and RGL3). DELLAs are negative regulators of GA responses such as elongation growth (primarily GAI and RGA; Dill and Sun, 2001; King et al., 2001), are targeted for degradation by GA (Schwechheimer, 2008), and are degraded upon low R:FR detection through phytochromes (Djakovic-Petrovic et al., 2007). This may in part be through enhanced GA biosynthesis, as suggested by the enhanced expression of the GA biosynthesis gene GA 20-OXIDASE (Hisamatsu et al., 2005) but can also follow from more direct interactions between phytochromes and DELLA proteins. Phytochromes act in part through their direct interaction with the bHLH family of phytochrome-interacting factors (PIFs), and one of these (PIF3-LIKE5) has been shown to control the expression of DELLA genes (Oh et al., 2007). Furthermore, DELLA proteins can bind to PIF4, thus preventing PIF4-induced transcriptional regulation of target genes associated with cell elongation (de Lucas et al., 2008). Interestingly, shade avoidance responses can occur normally in the absence of GA if DELLA proteins are not present, such as in DELLA knockouts (Djakovic-Petrovic et al., 2007). Furthermore, DELLA absence alone (e.g. through GA application or DELLA gene knockouts) does not suffice to induce the full shade avoidance response in Arabidopsis.
Thus, although GA is required to degrade DELLAs, this is not the only route that is engaged to regulate shade avoidance. Therefore, we are investigating alternative mechanisms that regulate shade avoidance responses. The plant hormone auxin has been suggested to be important for shade avoidance (Morelli and Ruberti, 2000), although rigorous experimental evidence is still limited. Transcript levels of several auxin-related genes are regulated by reduced R:FR, such as various AUX/IAA genes, auxin efflux-associated PIN genes (Devlin et al., 2003), and SMALL AUXIN UPREGULATED15 (SAUR15) and SAUR68 (Roig-Villanova et al., 2007). A newly characterized route for auxin biosynthesis from l-Trp to indole-3-pyruvic acid using a Trp aminotransferase (TAA1; Stepanova et al., 2008; Tao et al., 2008) is also rapidly enhanced upon far-red enrichment in a low-light background (Tao et al., 2008). Furthermore, the auxin-resistant axr1-12 mutant displays an attenuated hypocotyl elongation response to low R:FR (Steindler et al., 1999).
In addition, the volatile hormone ethylene has been associated with shade avoidance, both as a primary neighbor detection signal (through atmospheric accumulation) and as a downstream target for photoreceptor signaling (Pierik et al., 2004b, 2007). Therefore, ethylene is another pathway involved in the regulation of shade avoidance responses. Both auxin and ethylene, however, have been suggested to affect DELLA stability, resulting in DELLA regulation by GA, auxin, and ethylene (Achard et al., 2003; Fu and Harberd, 2003). Furthermore, ethylene can also stimulate auxin biosynthesis through the TAA1 route (Stepanova et al., 2008) that is also enhanced during shade treatment, and ethylene can affect auxin responses, as was shown for AUXIN REPONSE FACTOR2 expression during apical hook formation (Li et al., 2004). Ethylene-auxin interactions are also known for root growth control, where ethylene appears to stimulate auxin production and transport, thus controlling root growth (Ruzicka et al., 2007; Stepanova et al., 2007; Swarup et al., 2007).
Rigorous studies are now required to shed light on the roles and interactions of these two hormones during the control of shade avoidance responses to neighbor-derived light signals.
Here, we investigated how interactions between auxin, ethylene, and DELLA proteins regulate shade avoidance responses induced by reduced R:FR and reduced blue light photon fluence rates. We show that ethylene and auxin are important regulators of shade avoidance in Arabidopsis, where ethylene at least partly acts through auxin action. This pathway affects DELLA abundance, but this interaction appears to have only limited functionality during shade avoidance. We conclude that the ethylene-auxin pathway is an obligatory signaling route that is functionally parallel to the earlier identified GA-DELLA signaling system controlling shade avoidance responses.
RESULTS
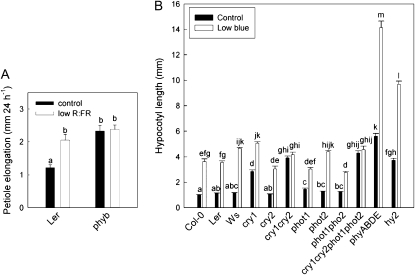
Low R:FR-Induced Petiole Elongation Depends on Phytochrome B and Low Blue Light-Induced Hypocotyl Elongation Depends on Cryptochromes
The R:FR and the blue light photon fluence rate are reduced in plant canopies, and individually both signals can induce shade avoidance responses. Reduced R:FR led to a fast increase of petiole elongation. This response was abolished in the phyB mutant (Fig. 1), confirming that R:FR-mediated shade avoidance occurs primarily through PhyB signaling. Arabidopsis petioles respond only weakly to reduced blue light photon fluence rates (data not shown), but hypocotyls of light-grown seedlings show a very dramatic elongation response to this signal. As shade avoidance responses to reduced levels of blue light have hardly been studied in Arabidopsis, we first tested which photoreceptors contribute to this response. Our data (Fig. 1) indicate that the two cryptochromes CRY1 and CRY2 are redundantly involved in the induction of hypocotyl elongation upon low blue treatment. Single mutants for these two photoreceptors displayed no severe reduction of the response, whereas the double mutant cry1 cry2 was essentially unresponsive to a reduction of blue light. Even double phototropin mutants were hardly disturbed for this response, suggesting that this family of blue light photoreceptors does not play an important role in the hypocotyl elongation response studied here. Phytochromes also do not seem to affect the cryptochrome-mediated responses to low blue light, as even the quadruple phytochrome mutant phya phyb phyd phye and the chromophore mutant hy2 displayed clear low blue light responses, despite constitutively elongated phenotypes.
Figure 1.
Photoreceptor involvement in shade avoidance responses of petioles to low R:FR (A) and hypocotyl responses to low blue light photon fluence rates (B). Data are means ± se (n = 10 for petioles, n = 30–50 for hypocotyls). Different letters indicate significant differences.
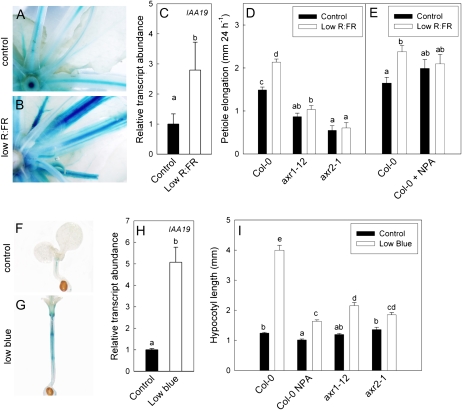
Low R:FR and Low Blue Light Signaling Result in Enhanced Auxin Activity
In order to establish the involvement of auxin in growth responses to low R:FR and low blue light, auxin action was visualized in pIAA19∷GUS-expressing lines (Tatematsu et al., 2004) and quantified with quantitative reverse transcription (qRT)-PCR for this IAA19 auxin reporter gene. These data confirm that low R:FR leads to increased auxin action in petioles (almost 3-fold increase of IAA19 expression quantified with qRT-PCR), especially in the more lateral tissues (Fig. 2). Low blue light gave only a slight increase in auxin activity in petioles (data not shown), which is consistent with the very weak petiole elongation response upon this light cue (data not shown). However, low blue light led to strongly enhanced GUS staining of pIAA19∷GUS, corresponding to a 5-fold increase of this gene compared with control light measured with qRT-PCR in hypocotyls, where also the elongation response was very pronounced. Similar data were obtained with another auxin reporter, DR5∷GFP (data not shown). Low R:FR, which can also stimulate hypocotyl elongation, also led to enhanced pIAA19∷GUS activity in hypocotyls (data not shown). Taken together, these data confirm the occurrence of increased auxin action during shade avoidance, and our next step was to establish whether this is functionally relevant to shade avoidance.
Figure 2.
Auxin involvement in shade avoidance responses of petioles to low R:FR and hypocotyl responses to low blue light photon fluence rates. A to E, Petioles in control and low R:FR light. F to I, Hypocotyls in control and low blue light. A to C and F to H, Auxin activity, shown with the auxin-responsive pIAA19∷GUS reporter (A, B, F, and G) and IAA19 gene expression (C and H), is increased upon low R:FR and low blue exposure. D, E, and I, Undisturbed auxin signaling and transport are required for shade avoidance responses to low R:FR (D and E) and low blue light photon fluence rates (I). Data are means ± se (n = 8–12 for petioles, n = 30–50 for hypocotyls). Different letters indicate significant differences (P < 0.05). [See online article for color version of this figure.]
The petiole elongation response to low R:FR was inhibited by treatment with the auxin transport inhibitor naphthylphthalamic acid (NPA; Fig. 2). In accordance with this, low blue light-induced hypocotyl elongation was much reduced upon NPA treatment as well (Fig. 2). As could be expected, NPA led to a reduction of the increased pIAA19∷GUS activity of low blue light-exposed hypocotyls and restricted the GUS staining in low blue light to a faint staining in the central cylinder of the upper 30% of the hypocotyl (Supplemental Fig. S1), consistent with the fact that NPA disturbs auxin transport (Petrasek et al., 2003). Auxin involvement was further suggested by the lack of low R:FR-induced petiole elongation in the axr1-12 and axr2-1 auxin signaling mutants (Fig. 2). Likewise, these mutants displayed a much reduced hypocotyl elongation response to low blue light (Fig. 2). We also tested the iaa19/msg2-3 mutant, which showed a somewhat reduced elongation response to low blue light and low R:FR (data not shown), but this effect was much less severe than shown for axr1-12 and axr2-1. Hypocotyl lengths for axr1-12 and axr2-1 under control light conditions were not notably different from those for wild-type accession Columbia (Col-0). This is in agreement with some other reports (Steindler et al., 1999) showing similar hypocotyl lengths for axr1-12 and Col-0 (around 1 mm) but in contrast to others. For example, Collett et al. (2000) report a somewhat reduced hypocotyl length for axr1-12 (measured under low-light conditions, which probably induce low blue light-mediated shade avoidance) and a more strongly reduced length for axr1-3 in higher light. Timpte et al. (1994) report reduced hypocotyl length for axr2-1 relative to Col-0 under continuous light. In those two studies, Col-0 hypocotyls were much longer than those under control light conditions in our experiments, whereas mutant hypocotyl lengths were very similar to what we found. Tentatively, the very different light conditions (both photoperiod and intensity), in combination with the addition of sugars to their medium (which were not added in these experiments), may explain why the constitutive lengths differ between our experiments and those by Collett et al. (2000) and Timpte et al. (1994).
We conclude that auxin action is enhanced during light-mediated shade avoidance responses in petioles and hypocotyls, particularly in the more lateral regions of these organs. Auxin action appears to be important for petiole and hypocotyl elongation in response to low R:FR and reduced blue light fluence rates, as these responses are diminished when auxin transport or signaling is disrupted.
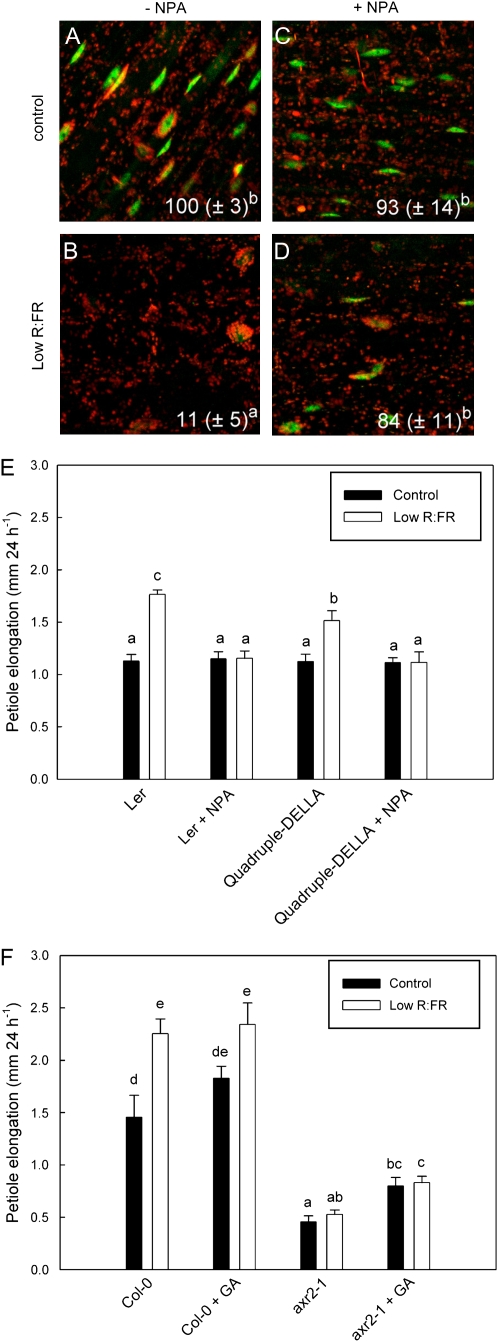
Auxin-Mediated Shade Avoidance Is DELLA Independent
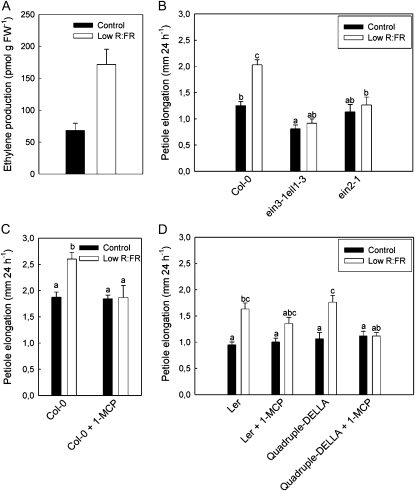
As auxin can affect GA action by reducing DELLA stability (Fu and Harberd, 2003), we tested if auxin involvement in shade avoidance is through interaction with DELLA proteins. We showed recently that the DELLA protein RGA is degraded upon exposure to low R:FR in petioles and low blue light in hypocotyls (Djakovic-Petrovic et al., 2007). Figure 3 shows that inhibition of auxin transport by NPA leads to higher abundance of this protein (visualized through confocal microscopy of the GFP-RGA fusion protein) in low R:FR-treated petioles as compared with non-NPA-treated plants. These data indicate that auxin can interact with GA signaling during shade avoidance and suggest that auxin facilitates the degradation of DELLA proteins, subsequently allowing growth. We tested this hypothesis by studying whether low R:FR-induced petiole elongation is still inhibited by NPA in the quadruple DELLA knockout mutant rga24 gait6 rgl1 rgl2, where DELLA accumulation can obviously not occur. Figure 3E shows that NPA strongly inhibited low R:FR-induced petiole elongation in the wild type. However, the quadruple DELLA knockout mutant also showed a complete inhibition of shade avoidance by NPA. These data suggest that auxin acts functionally independent of DELLA proteins. In accordance with this, GA addition to the auxin-resistant mutant axr2-1 could not rescue low R:FR-induced petiole elongation of this mutant (Fig. 3F). Very similar interactions were found for low blue light-induced hypocotyl elongation (Fig. 4), where NPA partly prevented low blue light-induced degradation of the DELLA protein RGA (Fig. 4, compare B and D). However, NPA very strongly inhibited low blue light-induced hypocotyl elongation in the quadruple DELLA knockout, similar to what was observed for low R:FR-induced petiole elongation. Interestingly, and different from petioles, NPA reduced hypocotyl length in control light, but this was not observed in plants with low DELLA abundance: quadruple DELLA knockouts and GA-treated seedlings (Fig. 4G). As shown previously, enhanced DELLA stability in the gai gain-of-function mutant inhibits shade avoidance, and the reduction of hypocotyl length was even more severe in the presence of NPA (Fig. 4G). In conclusion, auxin-mediated shade avoidance likely represents an alternative route toward shade avoidance, functionally parallel to the earlier described GA-DELLA route.
Figure 3.
Interactions between auxin action and DELLA abundance during low R:FR-induced petiole elongation. A to D, The pRGA∷GFP:RGA reporter shows the abundance of the DELLA protein RGA (green speckles on a background of red-fluorescing chloroplasts). Note that RGA abundance is enhanced upon auxin transport inhibition with the NPA. E, Petiole elongation responses to low R:FR are inhibited by NPA treatment, also in the quadruple DELLA knockout mutant. F, GA addition cannot rescue the lack of shade avoidance in auxin-resistant mutants. Data are means ± se (n = 8–12). Different letters indicate significant differences.
Figure 4.
Auxin-DELLA interaction during low blue light-induced hypocotyl elongation. A to F, Inhibition of auxin transport (NPA) prevents low blue light-induced DELLA degradation, and this is overcome by the addition of GA, as evidenced by the pRGA∷GFP:RGA reporter. G, NPA effects under standard and low blue light conditions on hypocotyl length in the quadruple DELLA knockout mutant and the DELLA gain-of-function mutant gai. Data are means ± se (n = 30–50). Different letters indicate significant differences.
Ethylene-Induced Shade Avoidance Does Not Act through DELLA Regulation
The gaseous hormone ethylene is known to play an important role in the regulation of shade avoidance in addition to auxin (Pierik et al., 2004b). Furthermore, interactions between ethylene and GA, and more specifically DELLA abundance, and between ethylene and auxin are well known. Low R:FR treatment gave the classic stimulation of ethylene production (Fig. 5A) and a consistent up-regulation of ERS2 expression (data not shown), an ethylene receptor-encoding gene that is a marker for ethylene signaling (Millenaar et al., 2005). Low R:FR-induced petiole elongation was absent in the ethylene-insensitive mutants ein2-1 and ein3-1 eil1-3 (ein3 ein3-like1) and in plants pretreated with the ethylene action inhibitor 1-methylcyclopropane (1-MCP; Fig. 5, B and C), confirming that ethylene is a positive regulator of shade avoidance. Low R:FR-induced petiole elongation was also inhibited by 1-MCP in quadruple DELLA knockout plants, suggesting that ethylene-mediated petiole elongation in low R:FR does not require DELLA proteins (Fig. 5D). Notably, the inhibitory effect of 1-MCP on low R:FR-induced petiole elongation was stronger in the Col-0 accession as compared with Landsberg erecta (Ler; Fig. 5), suggesting that there could be genetic variation for ethylene involvement in these responses. Despite the importance of endogenous ethylene signaling for low R:FR-induced petiole elongation, exogenous ethylene application has only little effect on petiole elongation in Arabidopsis (data not shown; Millenaar et al., 2005). Hypocotyl elongation of light-grown seedlings, on the other hand, is stimulated by ethylene (Smalle et al., 1997). Consistently, application of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), known to yield high ethylene levels, also stimulates hypocotyl length (Fig. 6B, compare control Col-0 with control Col-0 + ACC). Low blue light exposure, however, did not affect ethylene production (Fig. 6A), nor did it affect the expression of the ethylene marker gene ERS2 (data not shown). Accordingly, low blue light-induced hypocotyl elongation is only partly dependent upon endogenous ethylene levels, as evidenced by the reduced but still very clearly present response of ethylene-insensitive mutants (Fig. 6C). An additional experiment on the hypocotyl elongation response to low R:FR also indicated no involvement of ethylene (data not shown), suggesting that ethylene involvement in light-mediated elongation growth is more prominent in petioles than in hypocotyls.
Figure 5.
Ethylene involvement in low R:FR-induced petiole elongation. A, Low R:FR stimulates ethylene production. FW, Fresh weight. B and C, Petiole elongation under standard and low R:FR light conditions in ethylene-insensitive mutants (B) or upon exposure to the ethylene action inhibitor 1-MCP (C). D, The effect of inhibition of ethylene action does not depend on DELLAs. Data are means ± se (n = 8–12). Different letters indicate significant differences.
Figure 6.
Ethylene regulation of hypocotyl elongation in control and low blue light. A, Ethylene evolution from control light-exposed and low blue light-exposed seedlings is similar. FW, Fresh weight. B, Exogenous application of the ethylene precursor ACC stimulates hypocotyl elongation. C, Ethylene-insensitive mutants have a slightly reduced hypocotyl elongation response to low blue light conditions. Data are means ± se (n = 3 for A, n = 30–50 for B and C). Different letters indicate significant differences.
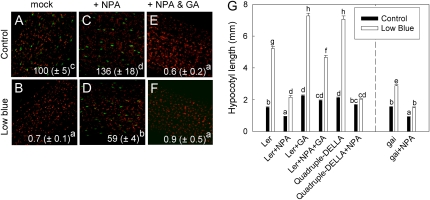
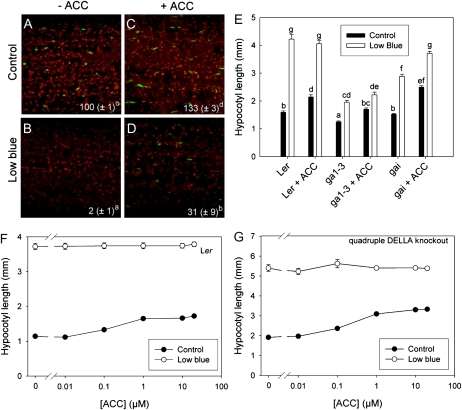
Ethylene, however, is not only a putative downstream component in light-mediated shade avoidance but can also act as a primary volatile neighbor detection signal that can induce shade avoidance even in the absence of light quality changes (Pierik et al., 2004b). As this may occur through an interaction with GA, we tested whether ACC application affects DELLA abundance in light-grown hypocotyls. Figure 7 shows that under control light conditions a small (30%) ACC-induced stimulation of the GFP-RGA signal was found, whereas ACC under these light conditions gave a pronounced stimulation of hypocotyl length. ACC, on the other hand, gave a pronounced (more than 10-fold) increase of RGA-GFP abundance under low blue light conditions compared with non-ACC-exposed seedlings under low blue light (Fig. 7, compare B and D). However, under these low blue light conditions, no effect of ACC on the hypocotyl length of Ler seedlings was found (Fig. 7E), whereas in Col-0 there was even a slight stimulation under these conditions (Fig. 6B). To further understand if interactions of ethylene with DELLAs are relevant to the effects of ethylene on hypocotyl length, a range of ACC concentrations were tested on wild-type Ler as well as on the quadruple DELLA knockout in which DELLA stabilization by ACC cannot occur. These knockouts appeared to respond similarly to wild-type Ler, although DELLA knockout hypocotyls were constitutively elongated due to the absence of the DELLA growth repressors. Further evidence for the hypothesis that ethylene-induced hypocotyl elongation does not act through DELLA regulation came from experiments on the severe GA-deficient ga1-3 mutant and the DELLA gain-of-function mutant gai that expresses a mutant DELLA protein, gai, that is irresponsive to GA. Although ga1-3 is constitutively dwarfed, it still showed an approximately 60% stimulation of hypocotyl length by ACC, which is only slightly less than the 80% increase in wild-type Ler under control light conditions (Fig. 7E). The gai mutant showed no disturbance of the ethylene response at all under control light conditions and under low blue light conditions; this mutant even gained a response where there was none in Ler. These data together indicate that stimulation of hypocotyl elongation by ethylene can occur independently of GA in Arabidopsis, despite the finding that ACC seems to enhance DELLA abundance under low blue light photon fluence rates.
Figure 7.
Ethylene-DELLA interactions during hypocotyl elongation. A to D, The pRGA∷GFP:RGA reporter shows the abundance of the DELLA protein RGA (green speckles on a background of red-fluorescing chloroplasts). Note that RGA abundance is enhanced by the ethylene precursor ACC under low blue light conditions. E and F, ACC dose-response curves in wild-type Ler (E) and the quadruple DELLA knockout mutant (F) under normal and low blue light conditions. G, ACC-induced stimulation of hypocotyl length does not depend on GA, as evidenced by the GA-deficient ga1-3 and the GA-insensitive gai (DELLA gain-of-function) mutants. Data are means ± se (n = 30–50). Different letters indicate significant differences.
Ethylene-Induced Hypocotyl Elongation Is Reduced in Two Auxin-Resistant Mutants
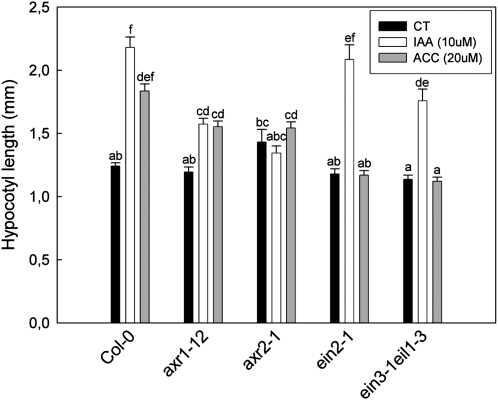
The final step to be elucidated is if auxin and ethylene regulate hypocotyl elongation separately or if these two hormones are in fact part of one pathway. To address this question, we studied the importance of ethylene for auxin-induced hypocotyl elongation and vice versa. To this end, ACC was applied to the auxin-resistant axr1-12 and axr2-1 mutants and indole-3-acetic acid (IAA; a plant-produced auxin) was applied to the ethylene-insensitive ein2-1 and double ein3-1 eil1-3 mutants under control light conditions (Fig. 8). We found that axr1-12 still showed a weak response, albeit much reduced compared with Col-0, to ACC, but this mutant also appeared to still respond weakly to auxin. The more severely auxin-resistant axr2-1 mutant, on the other hand, showed virtually no response to ACC, suggesting that intact auxin signaling could be required for ethylene to stimulate hypocotyl elongation. The ein2-1 and ein3-1 eil1-3 mutants were entirely unresponsive to ACC, confirming their ethylene insensitivity, but still responded properly to exogenous auxin. These data suggest that auxin signaling can be required for ethylene effects on hypocotyl elongation. It thus appears that auxin controls hypocotyl elongation in a pathway that is parallel to GA and that ethylene might stimulate elongation growth through this auxin pathway.
Figure 8.
Hypocotyl elongation responses to applied auxin (IAA) or ethylene (applied as the ethylene precursor ACC) of wild-type (Col-0) plants and auxin-resistant (axr1-12 and axr2-1) and ethylene-insensitive (ein2-1 and ein3-1 eil3-1) mutants. Seedlings were in control light conditions. Data are means ± se (n = 30–50). Different letters indicate significant differences. CT, Control.
DISCUSSION
Reaching out for light is essential to plant survival in dense stands. Shade avoidance responses are induced upon neighbor detection through various signals, among which are a reduced R:FR and a low blue light photon fluence rate. It is well known that low R:FR is primarily signaled by phytochrome B (Franklin, 2008). Here, we show that hypocotyl elongation in response to reduced blue light is mediated by the blue light receptors cryptochromes 1 and 2 (Fig. 1B). This indicates that cryptochrome photoreceptors are involved in plant neighbor detection in addition to phytochromes. Although much is known about photoreceptor signaling itself, little is known about how these signals are translated into an adaptive growth response.
We showed recently that GA regulation of shade avoidance acts through DELLA proteins. It was shown that DELLA degradation is essential to allow for shade avoidance responses in hypocotyls and petioles in response to low blue light and low R:FR, respectively (Djakovic-Petrovic et al., 2007). However, it was also noted that DELLA degradation alone is not sufficient to induce these responses, as multiple DELLA knockouts showed normal shade avoidance responses in both the presence and absence of GA. This suggests that additional signal transduction pathways have to be engaged to induce light-mediated shade avoidance responses in Arabidopsis. Here, we investigated if auxin and ethylene could be involved in those alternative pathways, as these hormones have been implicated in shade avoidance previously (Morelli and Ruberti, 2000; Pierik et al., 2004b).
Using an auxin-responsive promoter-GUS fusion (pIAA19∷GUS) reporter and qRT-PCR for this auxin-responsive IAA19 gene, we show that low R:FR and low blue light conditions lead to enhanced auxin action in Arabidopsis petioles and hypocotyls (Fig. 2). This is in agreement with a model for auxin action in shade avoidance that was posed a number of years ago, where enhanced lateral auxin distribution in stems or hypocotyls was suggested to regulate cell elongation during shade avoidance (Morelli and Ruberti, 2000). The patterns we found for this auxin action reporter are not only consistent with the predicted auxin distribution pattern but are also disrupted when the auxin transport inhibitor NPA is applied (Supplemental Fig. S1). The enhanced auxin action, therefore, is likely brought about by regulated auxin transport toward the lateral regions of the hypocotyl and petiole. In addition, auxin biosynthesis is also likely to be enhanced under these shade-avoiding conditions, as was recently shown for Arabidopsis seedlings (Tao et al., 2008). As this additional auxin would also be transported toward the more lateral regions of the elongating petioles and hypocotyls, this would further contribute to the observed auxin-reporter staining patterns.
Next, we tested the importance of auxin for shade avoidance responses. The disrupted auxin transport caused by NPA is associated with strongly reduced shade avoidance responses in both hypocotyls and petioles. Furthermore, genetic evidence confirms that auxin signaling is important for shade avoidance, as both of the auxin-resistant mutants, axr1-12 and axr2-1, show much reduced responses. This appears to apply to elongation responses in both petioles and hypocotyls to low R:FR and low blue light, respectively (Fig. 2). In addition, stimulation of hypocotyl elongation upon low R:FR has also been shown to be impaired in the axr1-12 mutant (Steindler et al., 1999). Therefore, we conclude that enhanced auxin action, indicated by the pIAA19∷GUS reporter, is required for shade avoidance, which had been suggested before (Morelli and Ruberti, 2000) but for which little causal evidence existed so far.
Auxin is well known to affect GA biosynthesis (Ross et al., 2000) and DELLA protein stability (Fu and Harberd, 2003). Therefore, we investigated whether auxin accumulation during shade avoidance requires GA signaling. First, we confirmed that auxin affects GA signaling by studying DELLA protein abundance in NPA-treated and non-NPA-treated plants (Figs. 3 and 4). NPA inhibits shade avoidance to low blue light and low R:FR in hypocotyls and petioles, respectively; accordingly, it leads to enhanced DELLA abundance under these light conditions. This would be an indication that NPA-induced inhibition of shade avoidance may be related to an enhanced abundance of growth-inhibiting DELLA proteins under these conditions. This would be in agreement with the earlier observed stabilizing effects of auxin transport inhibition on DELLA proteins in Arabidopsis roots (Fu and Harberd, 2003). These data on Arabidopsis roots have led to the idea that DELLA protein stability can be affected by several signals, among which is auxin, and that these proteins can thus be seen as a molecular mechanism for cross talk. As enhanced DELLA stability (e.g. in the gai mutant) leads to reduced shade avoidance (Djakovic-Petrovic et al., 2007; Fig. 4G), it would have seemed a likely option that NPA-mediated DELLA stabilization would explain the reduced shade avoidance upon NPA treatment.
However, although we show here that this auxin-DELLA cross talk may occur during shade avoidance, we also show that this is not fundamental for shade avoidance to occur. This is most clearly indicated by the novel finding that low R:FR-induced elongation is abolished by NPA treatment in the quadruple DELLA knockout gait6 rga24 rgl1 rgl2 to the same extent as in wild-type plants (Fig. 4E). In other words, DELLA proteins are most likely not essential for the reduction of shade avoidance during auxin inhibition. Although DELLA proteins are more abundant during NPA treatment, this does not explain the inhibition of shade avoidance under these conditions, as the same growth inhibition occurs when these DELLAs are not present (Figs. 3 and 4). These data indicate that the shade avoidance response mediated by auxin does not require GA signaling but rather constitutes a separate hormonal pathway regulating shade avoidance. This is further confirmed by the fact that the addition of GA does not rescue shade avoidance responses in the auxin-resistant axr2-1 mutant.
The volatile hormone ethylene can also be a player in shade avoidance, both as a hormone required to regulate petiole elongation responses to low R:FR and as a direct neighbor detection signal (Pierik et al., 2004b). We demonstrate that ethylene production increases upon low R:FR signaling. Furthermore, we show that an intact ethylene signaling pathway is required for low R:FR-induced petiole elongation in Arabidopsis (Fig. 5). Unlike ethylene involvement in petiole elongation, low blue light-induced hypocotyl elongation in Arabidopsis appeared not to rely heavily on intact ethylene signaling. However, ethylene application to light-grown Arabidopsis seedlings does induce strong hypocotyl elongation under control light conditions (Figs. 6 and 7, E–G; Smalle et al., 1997; Vandenbussche et al., 2003; Pierik et al., 2006). We show that this hypocotyl elongation response to ethylene does not occur in the axr2-1 mutant, which is also not responsive to IAA (Fig. 8). The axr1-12 mutant, however, does still show a weak but significant response to IAA and accordingly also shows a weak response to ACC. In line with these observations, ACC-induced hypocotyl elongation is also abolished upon treatment with the polar auxin transport inhibitor NPA (Vandenbussche et al., 2003). Therefore, we hypothesize that auxin may be a downstream regulator of ethylene-induced hypocotyl elongation. This would be consistent with the fact that in order to control root growth, ethylene also acts through auxin by enhancing auxin production and transport in roots (Ruzicka et al., 2007; Stepanova et al., 2007; Swarup et al., 2007). Similar to what was shown for auxin, the interaction of ethylene with GA and DELLA proteins does not seem to explain the involvement of ethylene in shoot elongation growth. In fact, ethylene-mediated hypocotyl elongation in Arabidopsis seems to be independent of GA altogether, since the GA-insensitive gai1 and GA-deficient ga1-3 mutants both retain a substantial hypocotyl elongation response to ACC (Fig. 7E). This is consistent with findings by De Grauwe et al. (2007), who also showed that gai is still ACC responsive. Those authors also show that GA-induced hypocotyl elongation does not require ethylene, despite the fact that their transcript-profiling experiments suggested that some interactions were present (De Grauwe et al., 2007). The lack of GA involvement in ethylene-induced hypocotyl elongation in Arabidopsis, however, is not general for all species. For example, tobacco (Nicotiana tabacum) stem elongation to low blue light photon fluence rates requires ethylene, which in turn can stimulate stem elongation only if sufficient GA is present (Pierik et al., 2004a). Furthermore, ethylene-induced elongation of internodes in rice (Oryza sativa) and petioles in Rumex palustris, two flooding-tolerant species, is entirely diminished by inhibition of GA production (for review, see Bailey-Serres and Voesenek, 2008; Jackson, 2008). Ethylene itself can already induce very different, and sometimes opposite, growth responses in different species (Pierik et al., 2006), and even simple responses, like stimulation of elongation growth, can be regulated in different ways among different plant species.
In summary, we propose that enhanced lateral distribution of auxin activity in elongating shoot organs constitutes an essential regulatory mechanism to adaptively modulate elongation growth upon light-mediated neighbor detection. The volatile hormone ethylene may exert its effects in the shade avoidance response by acting through the auxin pathway in Arabidopsis. While this novel route can affect the stability of the nuclear growth-suppressing DELLA proteins, ethylene- and auxin-mediated regulation of shade avoidance appears to act predominantly through DELLA-independent mechanisms.
MATERIALS AND METHODS
Plant Growth
For experiments on petioles of full-grown plants, Arabidopsis (Arabidopsis thaliana) plants were grown essentially as described (Millenaar et al., 2005; Djakovic-Petrovic et al., 2007). In short, seeds were put on moist filter paper, stratified at 4°C in the dark, germinated in 200 μmol m−2 s−1 photosynthetically active radiation (PAR; 9 h of light, 15 h of dark) for 4 d, transferred to pots after germination, and put at 200 μmol m−2 s−1 PAR (9 h of light, 15 h of dark, 21°C, 70% relative humidity). The petioles of the third youngest leaves of plants at 36 to 38 d after sowing were used for experiments and measured at the start of the experiment (time 0) and after 24 h of treatment.
For hypocotyl experiments, seeds were surface sterilized in hypoclorite (0.4%) for 10 min and rinsed three times with ethanol and then two times with sterile demineralized water. Seeds were then transferred to sterile low-nutrient (0.4% Murashige and Skoog medium) agar (0.8%, w/v) plates and stratified for 4 d in the dark (4°C). Thereafter, plates were placed in the light for 2 h and then kept in the dark for 24 h to synchronize germination. After this period, the seeds were placed under standard light conditions (described in the next section) or in light conditions with the same total photon fluence rate but depleted in the blue light region. Seedlings were allowed to grow for 7 d in the low blue light treatment before photographs were taken through a stereo microscope. From these photographs, hypocotyl lengths were determined digitally with ImageJ software (http://rsb.info.nih.gov/ij/).
Involvement of auxin in shade avoidance responses was tested using the auxin-resistant axr1-12 (Lincoln et al., 1990) and axr2-1 (Wilson et al., 1990) gain-of-function mutants and the pIAA19∷GUS auxin reporter (Tatematsu et al., 2004). Ethylene involvement was tested using the ethylene-insensitive mutants ein2-1 (Guzman and Ecker, 1990) and ein3-1 eil1-3 (Alonso et al., 2003). As these mutants are in a Col-0 background, this accession served as the wild-type control. Interactions between auxin and GA signaling were tested with a quadruple DELLA knockout mutant (rga24 gait6 rgl1-1 rgl2-1; Achard et al., 2007), the GA-insensitive gai gain-of-function mutant (Koornneef et al., 1985), and the pRGA∷GFP:RGA reporter, all in the Ler background and with Ler as the wild-type control. The involvement of photoreceptors in the induction of shade avoidance was tested with the following photoreceptor mutants (background in parentheses): cry1-304 (Col-0), cry2-1 (Col-0; Guo et al., 1998); cry1 cry2 (= hy4-2 fha-1; Ler), hy2-1 (Ler), phyb-1 (Ler; Koornneef et al., 1980); phya-201 phyb-1 phyd-1 phye-1 (Ler; Franklin et al., 2003); phot1-101 (Ler; Liscum and Briggs, 1995); phot2-5 (Wassilewskija [Ws]), phot1-101 phot2-5 (Ws/Ler; Sakai et al., 2001); and phot1-101 phot2-5 cry1 cry2 (Ws/Ler; Ohgishi et al., 2004).
Light Treatments
Control light conditions were obtained by filtering standard growth chamber light (Philips HPI 400 W + Philips Halogen 150 W) through spectrally neutral shading cloth, achieving a total light intensity of 147 μmol m−2 s−1 PAR (400–700 nm), which contained 25 μmol m−2 s−1 blue light (400–500 nm) and had a R:FR (655–665 nm:725–735 nm) of 1.1. Low blue light conditions were obtained by filtering the standard growth chamber light through a double layer of blue light-absorbing filter paper (Medium Yellow 010; Lee Filters), yielding 0.7 μmol m−2 s−1 blue light, R:FR of 1.1, and 147 μmol m−2 s−1 PAR. The R:FR was lowered in the low R:FR treatment by adding far-red light (730 nm far-red light-emitting diodes; Shinkoh Electronics) to a control light background. As a result, R:FR was lowered to 0.28 in the low R:FR treatment, whereas PAR was 140 μmol m−2 s−1 and blue light photon fluence rate was 24 μmol m−2 s−1. Full spectra are available in Supplemental Figure S2.
Pharmacological Experiments
The involvement of auxin was not only investigated genetically but also by the use of the auxin transport inhibitor NPA (Petrasek et al., 2003) and the auxin IAA. NPA was brushed onto the leaves (25 μm NPA, 0.1% ethanol, and 0.1% Tween) or added to the agar nutrient medium (25 μm). NPA concentrations were based on a dose-response curve for NPA in control and low blue light (Supplemental Fig. S3). For petioles of mature plants, this concentration was substantially lower than what has been used in other species (Cox et al., 2004). Controls received similar amounts and concentrations of dissolvent without NPA. IAA treatments occurred in a similar way, but with a concentration of 10 μm (this is the lowest concentration to saturate the hypocotyl elongation response to IAA in light-grown seedlings without having negative effects; Vandenbussche et al., 2003), and a similar 10 μm concentration was used for GA3 (Djakovic-Petrovic et al., 2007). The ethylene precursor ACC was tested at a range of concentrations (0, 0.01, 0.1, 1, 10, and 20 μm) and appeared to give an almost saturating effect at 1 μm, but its effect continued to increase, particularly in the quadruple DELLA knockout mutant, until 20 μm (Fig. 7, F and G). Therefore, in the other experiments, ACC was applied at a final concentration of 20 μm, a representative ACC concentration for studies on hypocotyl elongation that gives saturated responses without noticeable specific side effects (Smalle et al., 1997; Vandenbussche et al., 2003).
Ethylene perception was inhibited with 1-MCP gas (Sisler and Serek, 2003). 1-MCP was applied 3 h prior to the start of light treatment at a final concentration of 10 μL L−1, obtained from SmartFresh powder (Rohm and Haas). Prior to light treatment, the plants were incubated for 3 h with this concentration of 1-MCP, and this rendered the plants insensitive to ethylene during the experiment in a standard growth room environment (Sisler and Serek, 2003; Millenaar et al., 2005).
Ethylene Emission
The effect of light quality on ethylene production in mature plants (low R:FR) and seedlings (low blue light) was determined. Ethylene measurements were made in triplicate for the same treatment duration as for all growth and molecular reporter studies (i.e. 1 d of low R:FR treatment for mature plants and 7 d of low blue light exposure for seedlings). Measurements at earlier time points gave very similar data as those obtained from these final time points (data not shown). For mature plants, a 300-mg sample of shoot material was incubated in a small closed air volume for 20 min. This incubation time was found to be long enough for ethylene to accumulate to detectable levels but short enough to prevent wounding-derived ethylene production. Then, 1 mL of air sample was analyzed for ethylene with a gas chromatograph that was equipped with a Photo Ionization Detector (Syntech Spectras Analyzer GC955-100; Synspec). From these values, ethylene production was calculated in pmol g−1 fresh weight h−1. Ethylene release from seedlings was measured by growing 35 seedlings in a 10-mL cap flask that was filled with 5 mL of agar-containing (0.8%, v/v) low-nutrient growth medium (0.4% Murashige and Skoog medium). After 6 d, the cap flask was closed and ethylene was allowed to accumulate. After 24 h, the head space was sampled and analyzed for ethylene as described above for mature plants.
GUS Assay
In order to visualize auxin action in control and low R:FR-treated petioles and control and low blue light-treated hypocotyls, GUS abundance was studied in transgenic pIAA19∷GUS lines expressing the GUS enzyme driven by the IAA19 promoter (Tatematsu et al., 2004). This has been shown to be a good indicator of auxin action. The GUS assay for seedlings was performed by overnight incubation of freshly harvested material in the staining solution [1 mm 5-bromo-4-chloro-3-indolyl β-d-glucuronide in 100 mm NaPi buffer, pH 7, 0.1 mm EDTA, 0.1% Triton X-100, 1 mm K4Fe(CN)6, 1 mm K3Fe(CN)6, and 0.52 mg mL−1 dimethyl formamide].
The GUS assay for leaf rosettes was performed after a pretreatment of 20 s in acetone and a fixative treatment (0.3% formaldehyde, 10 mm MES, and 0.3 m mannitol) of 45 min. The rosettes were then washed with 100 mm NaPi (pH 7.0). The histochemical reaction was performed by incubating the rosette for 24 h with 1 mm 5-bromo-4-chloro-3-indolyl β-d-glucuronide in 100 mm NaPi buffer (pH 7.0) with 0.1 mm EDTA. The staining was followed by bleaching with an ethanol series from 50% to 90%, after which the material was photographed.
qRT-PCR
In order to give an independent quantitative estimation of the auxin-responsive IAA19 gene used in the GUS assay above, we analyzed its expression in low blue light-exposed hypocotyls (3 d of exposure) and low R:FR-exposed petioles (24 h of exposure). To this end, total RNA was extracted from petioles (two petioles from each of five plants were pooled per extraction, with three replicate extractions) and seedlings (up to 100 seedlings per extraction, with three replicate extractions) using the RNeasy Plant Mini Kit (Qiagen), including on-column DNase digestion to eliminate genomic DNA from the samples. RNA transcripts at 1 μg (hypocotyls) or 3 μg (petioles) were reverse transcribed to cDNA with the SuperScript III Reverse Transcriptase kit (Invitrogen) and random hexamer primers. qRT-PCR was performed using a Bio-Rad MyiQ single-color detection system on a 20-μL reaction mix containing 40 ng (hypocotyls) or 30 ng (petioles) of cDNA, 10 μL of SYBR Green Supermix (Bio-Rad), and gene-specific primers: IAA19-F (At3g15540), 5′-GGCTTGAGATAACGGAGCTG-3′; IAA19-R, 5′-ACCATCTTTCAAGGCCACAC-3′. 18S ribosomal RNA was used as an internal standard to normalize for differences in cDNA concentration between samples: 18S-F, 5′-CGTTGCTCTGATGATTCATGA-3′; 18S-R, 5′-GTTGATAGGGCAGAAATTTGAATGAT-3′. Threshold cycle values were obtained from PCR with an efficiency of approximately 2, and gene expression values were calculated according to Livak and Schmittgen (2001), with control light plants as the final reference with expression levels set at 1.
GFP Visualization and Quantification
To study DELLA protein abundance, GFP fluorescence was studied in pRGA∷GFP:RGA transgenic plants, as described (Djakovic-Petrovic et al., 2007). Essentially, DELLA-GFP fluorescence was visualized with confocal laser scanning microscopy (40× magnification) using a 488-nm excitation wavelength, a 505- to 530-nm band-path filter to separate GFP, and a 560-nm long-pass filter to determine chlorophyll fluorescence. Z-stacks were made for 149.5 μm tissue thickness from the basal end of petioles and hypocotyls. It has repeatedly been shown that the RGA-GFP signal shown through confocal imaging shows a good correspondence with the signal being studied through western blotting using an anti-GFP antibody (Achard et al., 2007; Navarro et al., 2008).
GFP fluorescence was quantified on at least three replicate images from independent specimens, with a macro developed in house using KS400 (version 3.0) software (Carl Zeiss Vision). Fluorescence values were calculated relative to control light conditions, which were set at 100%.
Statistical Analyses
Data were analyzed with one-way ANOVA and Tukey's post-hoc comparisons (SPSS version 14) to allow for comparisons among all means. When necessary, data were log transformed to meet the requirement of homogenic variances.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Inhibition of auxin transport with 25 μm NPA leads to reduced auxin activity in the hypocotyl, as shown with the auxin-responsive pIAA19∷GUS reporter.
Supplemental Figure S2. Spectral composition of the different light conditions used throughout these studies.
Supplemental Figure S3. Dose-response relationship for hypocotyl length and applied NPA concentrations in control and low blue light-exposed seedlings.
Supplementary Material
Acknowledgments
We thank Diederik van Bentum and Rashmi Sasidharan for help with experiments and two anonymous reviewers for their very helpful comments on an earlier version of the manuscript. Seeds were obtained from the Nottingham Arabidopsis Stock Centre or provided by N.P. Harberd (gai, gait6 rga24 rgl1-1 rgl2-1, and pRGA∷GFP:RGA reporter), K.T. Yamamoto (pIAA19∷GUS), T. Sakai (phot1, phot2, phot1 phot2, and cry1 cry2 phot1 phot2), K.A. Franklin and G.C. Whitelam (phyABDE and cry2), M. Koornneef (hy2), C. Lin (cry1 cry2), and J.R. Ecker (ein3-1 eil1-3).
This work was supported by the Netherlands Organization for Scientific Research (VENI grant no. 86306001 to R.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ronald Pierik (r.pierik@uu.nl).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Achard P, Liao LL, Jiang CF, Desnos T, Bartlett J, Fu XD, Harberd NP (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiol 143 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard P, Vriezen WH, Van der Straeten D, Harberd N (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15 2816–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen of weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aphalo PJ, Ballaré CL, Scopel AL (1999) Plant-plant signalling, the shade avoidance response and competition. J Exp Bot 50 1629–1634 [Google Scholar]
- Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59 313–339 [DOI] [PubMed] [Google Scholar]
- Ballaré CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4 97–102 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Casal JJ, Kendrick RE (1991) Responses of light-grown wild-type and long-hypocotyl mutant cucumber seedlings to natural and simulated shade. Photochem Photobiol 54 819–826 [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247 329–331 [DOI] [PubMed] [Google Scholar]
- Beall FD, Yeung EC, Pharis RP (1996) Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can J Bot 74 743–752 [Google Scholar]
- Casal JJ, Sánchez RA (1994) Impaired stem-growth response to blue-light irradiance in light-grown transgenic tobacco seedlings overexpressing Avena phytochrome A. Physiol Plant 91 268–272 [Google Scholar]
- Collett CE, Harberd NP, Leyser O (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Benschop JJ, Vreeburg RAM, Wagemaker CAM, Moritz T, Peeters AJM, Voesenek LACJ (2004) The roles of ethylene, auxin, abscisic acid, and ethylene in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol 136 2948–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grauwe L, Vriezen WH, Bertrand S, Phillips A, Vidal AM, Hedden P, Van der Straeten D (2007) Reciprocal influence of ethylene and gibberellins on response-gene expression in Arabidopsis thaliana. Planta 226 485–498 [DOI] [PubMed] [Google Scholar]
- de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451 480–484 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Sun TP (2001) Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic-Petrovic T, de Wit M, Voesenek LACJ, Pierik R (2007) DELLA protein function in growth responses to canopy signals. Plant J 51 117–126 [DOI] [PubMed] [Google Scholar]
- Franklin KA (2008) Shade avoidance. New Phytol 179 930–944 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC (2003) Phytochromes B, D and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol 131 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Harberd N (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421 740–743 [DOI] [PubMed] [Google Scholar]
- Guo HW, Yang WY, Mockler TC, Lin CT (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M (2005) The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol 138 1106–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB (2008) Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot (Lond) 101 229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KE, Moritz T, Harberd NP (2001) Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen MEP, van Rijn L, Zeevaart JAD (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant 65 33–39 [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol 100 147–160 [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7 193–204 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Briffon JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCT method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- López-Juez E, Kobayashi M, Sakurai A, Kamiya Y, Kendrick RE (1995) Phytochrome, gibberellins, and hypocotyl growth. Plant Physiol 107 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Cox MCH, de Jong van Berkel YEM, Welschen RAM, Pierik R, Voesenek LACJ, Peeters AJM (2005) Ethylene-induced differential growth in petioles of Arabidopsis thaliana: analyzing natural variation, response kinetics and regulation. Plant Physiol 137 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G, Ruberti I (2000) Shade avoidance responses: driving auxin along lateral routes. Plant Physiol 122 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DC, Smith H (1976) Linear relationship between phytochrome photoequilibrium and growth in plants under simulated natural irradiation. Nature 262 210–212 [Google Scholar]
- Navarro L, Bari R, Achard P, Lison P, Nemri A, Harberd NP, Jones JDG (2008) DELLAs control plant immune responses by modulating the balance and salicylic acid signaling. Curr Biol 18 650–655 [DOI] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Hu JH, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgishi M, Saji K, Okada K, Sakai T (2004) Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc Natl Acad Sci USA 101 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Cerna A, Schwarzerova K, Elckner M, Morris DA, Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW (2004. a) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Sasidharan R, Voesenek LACJ (2007) Growth control by ethylene: adjusting phenotypes to the environment. J Plant Growth Regul 26 188–200 [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11 176–183 [DOI] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW (2004. b) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J 38 310–319 [DOI] [PubMed] [Google Scholar]
- Reid JB, Hasan O, Ross JJ (1990) Internode length in Pisum: gibberellins and the response to far-red-rich light. J Plant Physiol 137 46–52 [Google Scholar]
- Roig-Villanova I, Bou-Torrent J, Galstyan A, Carretero-Paulet L, Portoles S, Rodriguez-Conception M, Garcia JFM (2007) Interaction of shade avoidance and auxin responses: a role for two novel atypical bHLH proteins. EMBO J 26 4756–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ, O'Neill DP, Smith JJ, Kerckhoffs LHJ, Elliott RC (2000) Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J 21 547–552 [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C (2008) Understanding gibberellic acid signaling: are we there yet? Curr Opin Plant Biol 11 9–15 [DOI] [PubMed] [Google Scholar]
- Sisler EC, Serek M (2003) Compounds interacting with the ethylene receptor in plants. Plant Biol 5 473–480 [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu M, van der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I (1999) Shade avoidance responses are mediated by the ATHB-2 HD-Zip protein, a negative regulator of gene expression. Development 126 4235–4245 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133 177–191 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, van der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root elongation. Plant Cell 19 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong FX, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte C, Wilson AK, Estelle M (1994) The axr2-1 mutation of Arabidopsis thaliana is a gain-of-function mutation that disrupts an early step in auxin response. Genetics 138 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LACJ, Van der Straeten D (2005) Reaching out of the shade. Curr Opin Plant Biol 8 462–468 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Smalle J, Le J, Saibo NJM, de Paepe A, Chaerle L, Tietz O, Smets R, Laarhoven LJJ, Harren FJM, et al (2003) The Arabidopsis mutant alh1 illustrates a crosstalk between ethylene and auxin. Plant Physiol 131 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Ross JJ, Reid JB (1994) Gibberellins and phytochrome regulation of stem elongation in pea. Planta 192 489–496 [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M (1990) A dominant mutation in Arabidopsis confers resistance to auxin, ethylene, and abscisic acid. Mol Gen Genet 222 377–383 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.