Abstract
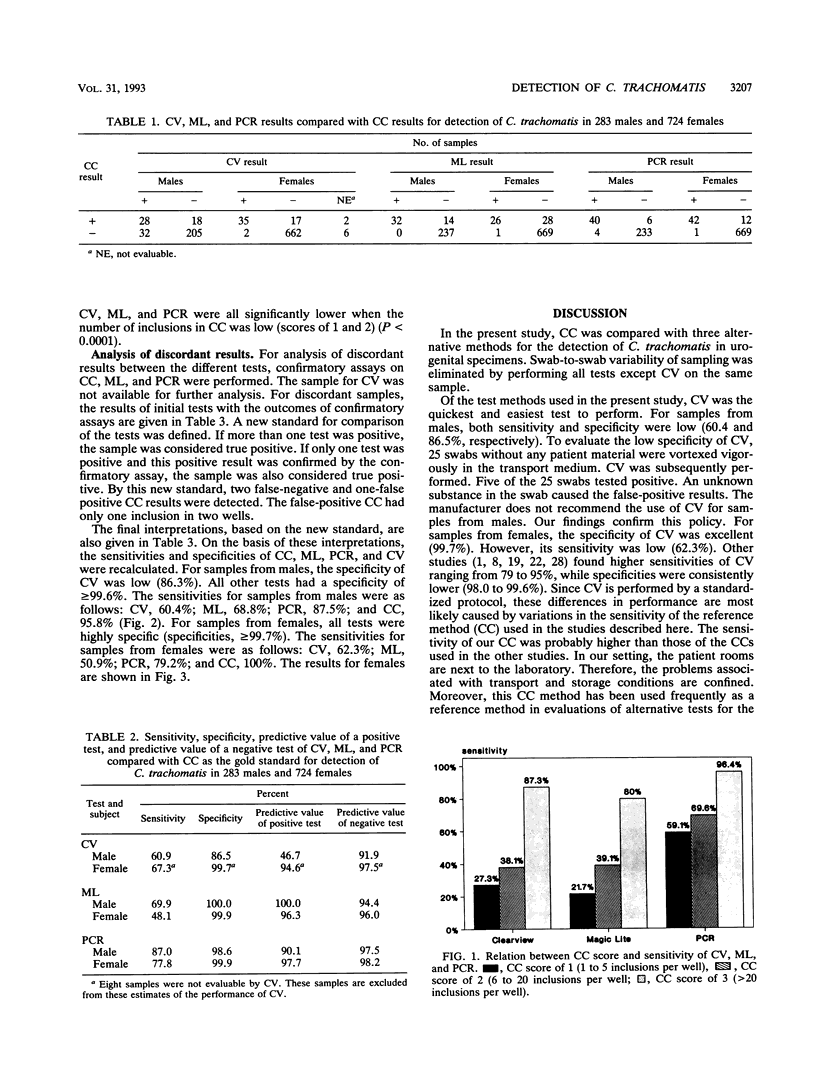
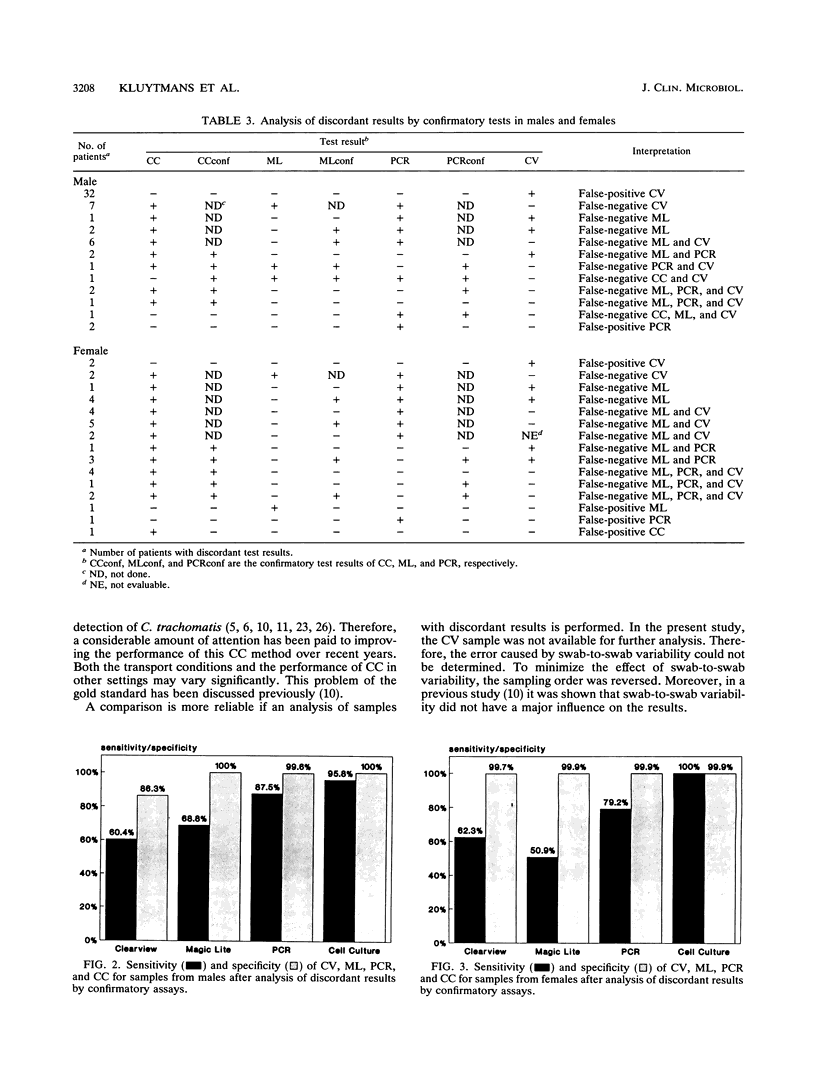
The Clearview Chlamydia test (CV; Unipath Ltd., Bedford, United Kingdom), the Magic Lite Chlamydia test (ML; CIBA Corning, Medfield, Mass.), a polymerase chain reaction (PCR), and cell culture (CC) were evaluated for detection of Chlamydia trachomatis in urogenital specimens. Specimens were collected from 283 men and 724 women visiting the outpatient clinic for Sexually Transmitted Diseases at the University Hospital Rotterdam, Rotterdam, The Netherlands. ML, PCR, and CC were all performed on the same sample to prevent swab-to-swab variability. CV was performed on a separate sample. Analysis of discordant results was performed by application of the following confirmatory assays: first, PCR on the CC, second, ML was repeated, and third, PCR was repeated by using a different DNA extraction protocol. If more than one test was positive, the sample was considered true positive. If only one test was positive, which was confirmed by the confirmatory assay, the sample was also considered true positive. By using these interpretations, the following results were obtained. The sensitivity and specificity of CV for samples from men were 60.4 and 86.3%, respectively. For samples from women, these values were 62.3 and 99.7%, respectively. The low specificity for samples from men was caused by unidentified substances in the swab that was used. The use of CV on samples from men is not recommended by the manufacturer. For samples from women, the specificity of CV was high, but the low sensitivity of CV limits its use for diagnostic purposes. The sensitivities of ML were low for samples from both men and women (68.8% and 50.9% respectively), while specificities were excellent for samples from both groups (100 and 99.9%, respectively). The low sensitivity of ML limits its diagnostic value. The PCR technique was highly specific for samples from both men (99.6%) and women (99.9%). The sensitivity of PCR, however, was unexpectedly low for samples from both groups (men, 87.5%; women, 79.2%), most likely because of the sample treatment method used. The sensitivity and specificity values of CC for samples from men were 95.8 and 100%, respectively. For samples from women, these values were 100 and 99.9%, respectively. In the present study, CC was the most reliable technique for the detection of C. trachomatis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arumainayagam J. T., Matthews R. S., Uthayakumar S., Clay J. C. Evaluation of a novel solid-phase immunoassay, Clearview Chlamydia, for the rapid detection of Chlamydia trachomatis. J Clin Microbiol. 1990 Dec;28(12):2813–2814. doi: 10.1128/jcm.28.12.2813-2814.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes R. C. Laboratory diagnosis of human chlamydial infections. Clin Microbiol Rev. 1989 Apr;2(2):119–136. doi: 10.1128/cmr.2.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo L., Coutlee F., Yolken R. H., Quinn T., Viscidi R. P. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with an enzyme immunoassay. J Clin Microbiol. 1990 Sep;28(9):1968–1973. doi: 10.1128/jcm.28.9.1968-1973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas H. C., Melchers W. J., de Bruijn I. H., de Graaf M., van Dijk W. C., Lindeman J., Quint W. G. Detection of Chlamydia trachomatis in clinical specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1990 Dec;9(12):864–868. doi: 10.1007/BF01967500. [DOI] [PubMed] [Google Scholar]
- Claas H. C., Wagenvoort J. H., Niesters H. G., Tio T. T., Van Rijsoort-Vos J. H., Quint W. G. Diagnostic value of the polymerase chain reaction for Chlamydia detection as determined in a follow-up study. J Clin Microbiol. 1991 Jan;29(1):42–45. doi: 10.1128/jcm.29.1.42-45.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret J. M., Judson F. N. Genital Chlamydia infections. Clin Lab Med. 1989 Sep;9(3):481–500. [PubMed] [Google Scholar]
- Ferris D. G., Martin W. H. A comparison of three rapid chlamydial tests in pregnant and nonpregnant women. J Fam Pract. 1992 May;34(5):593–597. [PubMed] [Google Scholar]
- Iwen P. C., Blair T. M., Woods G. L. Comparison of the Gen-Probe PACE 2 system, direct fluorescent-antibody, and cell culture for detecting Chlamydia trachomatis in cervical specimens. Am J Clin Pathol. 1991 Apr;95(4):578–582. doi: 10.1093/ajcp/95.4.578. [DOI] [PubMed] [Google Scholar]
- Kluytmans J. A., Niesters H. G., Mouton J. W., Quint W. G., Ijpelaar J. A., Van Rijsoort-Vos J. H., Habbema L., Stolz E., Michel M. F., Wagenvoort J. H. Performance of a nonisotopic DNA probe for detection of Chlamydia trachomatis in urogenital specimens. J Clin Microbiol. 1991 Dec;29(12):2685–2689. doi: 10.1128/jcm.29.12.2685-2689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans J. A., van der Willigen A. H., van Heyst B. Y., van der Meyden W. I., Stolz E., Wagenvoort J. H. Evaluation of an enzyme immunoassay for detection of Chlamydia trachomatis in urogenital specimens. Int J STD AIDS. 1990 Jan;1(1):49–52. doi: 10.1177/095646249000100112. [DOI] [PubMed] [Google Scholar]
- Limberger R. J., Biega R., Evancoe A., McCarthy L., Slivienski L., Kirkwood M. Evaluation of culture and the Gen-Probe PACE 2 assay for detection of Neisseria gonorrhoeae and Chlamydia trachomatis in endocervical specimens transported to a state health laboratory. J Clin Microbiol. 1992 May;30(5):1162–1166. doi: 10.1128/jcm.30.5.1162-1166.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J. B., Chernesky M. A. Effect of swab type and storage temperature on the isolation of Chlamydia trachomatis from clinical specimens. J Clin Microbiol. 1985 Nov;22(5):865–867. doi: 10.1128/jcm.22.5.865-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer L. J., Robinson D. C., Sahm D. F., Lawrie M. J., Hajj S. N. Comparison of chemiluminescent DNA probe to cell culture for the screening of Chlamydia trachomatis in a gynecology clinic population. Obstet Gynecol. 1990 Jul;76(1):114–117. [PubMed] [Google Scholar]
- Neman-Simha V., Delmas-Beauvieux M. C., Geniaux M., Bébéar C. Evaluation of a chemiluminometric immunoassay and a direct immunofluorescence test for detecting Chlamydia trachomatis in urogenital specimens. Eur J Clin Microbiol Infect Dis. 1991 Aug;10(8):662–665. doi: 10.1007/BF01975822. [DOI] [PubMed] [Google Scholar]
- Ossewaarde J. M., Rieffe M., Rozenberg-Arska M., Ossenkoppele P. M., Nawrocki R. P., van Loon A. M. Development and clinical evaluation of a polymerase chain reaction test for detection of Chlamydia trachomatis. J Clin Microbiol. 1992 Aug;30(8):2122–2128. doi: 10.1128/jcm.30.8.2122-2128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L., Birkelund S., Christiansen G. Use of polymerase chain reaction for detection of Chlamydia trachomatis. J Clin Microbiol. 1990 Jun;28(6):1254–1260. doi: 10.1128/jcm.28.6.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scieux C., Bianchi A., Vassias I., Meouchy R., Felten A., Morel P., Perol Y. Evaluation of a new chemiluminometric immunoassay, Magic Lite Chlamydia, for detecting Chlamydia trachomatis antigen from urogenital specimens. Sex Transm Dis. 1992 May-Jun;19(3):161–164. [PubMed] [Google Scholar]
- Skulnick M., Small G. W., Simor A. E., Low D. E., Khosid H., Fraser S., Chua R. Comparison of the Clearview Chlamydia test, Chlamydiazyme, and cell culture for detection of Chlamydia trachomatis in women with a low prevalence of infection. J Clin Microbiol. 1991 Sep;29(9):2086–2088. doi: 10.1128/jcm.29.9.2086-2088.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Stamm W. E. Diagnosis of Chlamydia trachomatis genitourinary infections. Ann Intern Med. 1988 May;108(5):710–717. doi: 10.7326/0003-4819-108-5-710. [DOI] [PubMed] [Google Scholar]
- Stratton N. J., Hirsch L., Harris F., de la Maza L. M., Peterson E. M. Evaluation of the rapid CLEARVIEW Chlamydia test for direct detection of chlamydiae from cervical specimens. J Clin Microbiol. 1991 Jul;29(7):1551–1553. doi: 10.1128/jcm.29.7.1551-1553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewessen E. A., Freundt I., van Rijsoort-Vos J. H., Stolz E., Michel M. F., Wagenvoort J. H. Comparison of HeLa 229 and McCoy cell cultures for detection of Chlamydia trachomatis in clinical specimens. J Clin Microbiol. 1989 Jun;27(6):1399–1400. doi: 10.1128/jcm.27.6.1399-1400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjiam K. H., van Heijst B. Y., de Roo J. C., de Beer A., van Joost T., Michel M. F., Stolz E. Survival of Chlamydia trachomatis in different transport media and at different temperatures: diagnostic implications. Br J Vener Dis. 1984 Apr;60(2):92–94. doi: 10.1136/sti.60.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenvoort J. H., Suchland R. J., Stamm W. E. Serovar distribution of urogenital Chlamydia trachomatis strains in The Netherlands. Genitourin Med. 1988 Jun;64(3):159–161. doi: 10.1136/sti.64.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenvoort J. T., van Rijsoort-Vos T., Overkleeft-van de Ree A., Stolz E. Enhancement of yield of Chlamydia trachomatis Hela 229 cell culture. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):822–822. doi: 10.1007/BF01975064. [DOI] [PubMed] [Google Scholar]
- Woods G. L., Young A., Scott J. C., Jr, Blair T. M., Johnson A. M. Evaluation of a nonisotopic probe for detection of Chlamydia trachomatis in endocervical specimens. J Clin Microbiol. 1990 Feb;28(2):370–372. doi: 10.1128/jcm.28.2.370-372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H., Moyes A., Lough H., Smith I. W., McKenna J. G., Thompson C. Preliminary evaluation of "Clearview Chlamydia" for the rapid detection of chlamydial antigen in cervical secretions. Genitourin Med. 1991 Apr;67(2):120–123. doi: 10.1136/sti.67.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]



