Abstract
The initiation, progression, and natural variation of autumn senescence in European aspen (Populus tremula) was investigated by monitoring chlorophyll degradation in (1) trees growing in natural stands and (2) cloned trees growing in a greenhouse under various light regimes. The main trigger for the initiation of autumn senescence in aspen is the shortening photoperiod, but there was a large degree of variation in the onset of senescence, both within local populations and among trees originating from different populations, where it correlated with the latitude of their respective origins. The variation for onset of senescence with a population was much larger than the variation of bud set. Once started, autumn senescence was accelerated by low temperature and longer nights, and clones that started to senescence late had a faster senescence. Bud set and autumn senescence appeared to be under the control of two independent critical photoperiods, but senescence could not be initiated until a certain time after bud set, suggesting that bud set and growth arrest are important for the trees to acquire competence to respond to the photoperiodic trigger to undergo autumn senescence. A timetable of events related to bud set and autumn senescence is presented.
Leaf senescence is a highly regulated process that involves the sequential degradation of macromolecules and extensive salvage of nutrients (Gan and Amasino, 1997; Noodén et al., 1997; Lim et al., 2003). The nitrogen from chlorophyll seems to remain in the vacuole and is lost with the abscission of leaves (Hörstensteiner, 2006). In contrast, during autumn senescence in deciduous trees like European aspen (Populus tremula), the nitrogen from leaf proteins is transferred to bark storage proteins, stored over the winter, and then remobilized and used for growth in the next spring (Black et al., 2001; Cooke and Weih, 2005). About 90% of the leaf nitrogen is remobilized during autumn senescence in aspen (Keskitalo et al., 2005). The adaptive value of timing leaf senescence appropriately in northern latitudes is obvious: if leaf senescence occurs too early in the season, the growing period will be shortened and the photosynthetic carbon gain reduced, while if senescence occurs too late, green leaves will be killed by frost and leaf nitrogen will be lost, with a negative impact on growth, since nitrogen availability typically limits tree growth in boreal forests (Näsholm et al., 1998). The optimal timing of autumn senescence, therefore, represents a trade-off between conflicting optima for carbon and nitrogen metabolism and is likely to show adaptation to the local environment, like other developmental processes such as bud set (Ingvarsson et al., 2006). However, despite its importance, very little is known about the factors that trigger and modulate autumn senescence, although changes in gene expression (Bhalerao et al., 2003; Andersson et al., 2004) and the main physiological and biochemical changes (Keskitalo et al., 2005) that occur during autumn senescence in aspen have been characterized. The cited studies established (among other things) that autumn senescence starts in a given aspen tree every year at around the same date, suggesting that the main trigger is the reduction in the photoperiod (Keskitalo et al., 2005). Interestingly, the expression of bark storage protein genes during autumn is also regulated by the photoperiod, through the action of phytochrome (Zhu and Coleman, 2001). These findings provide strong support for the hypothesis that the start of autumn senescence in aspen is controlled by photoperiod. The length of the dark period may be the critical factor that controls senescence, although this has not, to our knowledge, been formally proved. However, other factors might have interactive effects on its timing, since stresses like low temperature, shortage of water or nutrients, and pathogen infection can also lead to leaf senescence, even in the absence of photoperiodic cues.
Ultimately, we would like to identify the genes that regulate autumn senescence in aspen and the alleles that are responsible for their adaptation to northern climates. To achieve this goal, we need better understanding of the mechanisms that initiate senescence in aspen. In particular, we need to elucidate (1) the interactive effects of photoperiod and temperature changes on the induction and progression of senescence and (2) the relationship between the timing of bud set/growth cessation and the initiation of autumn senescence. Useful materials to meet these objectives include appropriate germplasm resources for phenotypic evaluations of the trait(s) of interest and a reliable set of candidate genes, since we have shown that association mapping is a powerful technique for identifying the genetic bases, down to single nucleotides, that may be responsible for phenotypic variations in aspen (Ingvarsson et al., 2008). We present here an analysis of such material, in which we examined the genetic and environmental control of autumn senescence in aspen. The plant materials used included a free-growing aspen tree at the Umeå University campus described in several previous works (Bhalerao et al., 2003; Andersson et al., 2004; Keskitalo et al., 2005), the Swedish aspen (SwAsp) collection of genotypes sampled throughout Sweden (Ingvarsson et al., 2006; Hall et al., 2007; Luquez et al., 2008), and a local population of aspen from the Umeå region (the UmAsp collection). The plants were examined in a series of experiments under both controlled and natural conditions.
RESULTS
The Onset of Senescence Is Controlled by Photoperiod, But Low Temperatures Accelerate Chlorophyll Degradation
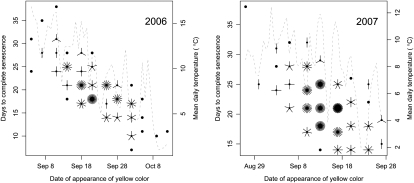
Common gardens have been established at Ekebo and Sävar in southern and northern Sweden, respectively, to examine the performance of members of the SwAsp collection in contrasting environments. However, some of the aspen genotypes from northern Sweden grow poorly in the common garden in southern Sweden, since the maximum daylength at this latitude is below their critical value for bud set (Luquez et al., 2008). On the other hand, in the northern common garden, the critical photoperiod for bud set of southern genotypes is typically reached after the first frosts have come, leading to premature bud set and senescence. Therefore, the number of genotypes that could be reliably scored for senescence in each garden was significantly less than the total size of the collection (116 clones), reducing the value of these common gardens for accurate scoring of senescence. However, since senescence can be easily visually scored, we decided to study it also in trees growing around Umeå (the UmAsp collection). During the autumns of 2006 and 2007, we scored senescence twice per week in trees of the UmAsp collection in their natural stands using a scorecard. In the UmAsp collection, there was clearly a large variation in the timing of the appearance of yellow color, here defined as score 3. The earliest trees reached score 3 on September 4 in 2006 and completed senescence by September 28 in 2006, 2 weeks before the first symptoms of autumn senescence (score 3) became apparent in the latest tree (on October 12). The time to complete senescence (defined as the time between scores 3 and 7) also varied greatly between trees, and there was a correlation between these two parameters: trees that started to senesce early progressed more slowly than trees that started later (Fig. 1).
Figure 1.
Relationships between mean temperatures during scores 3 to 7, date of appearance of yellow color (score 3), and time to complete senescence (score 7) in aspens growing naturally. The number of petals on the flower plots indicates the number of overlapping points at the indicated positions.
We wanted to see if the late-senescing genotypes had a more rapid senescence only as a consequence of the lower temperature during the later part of the scoring period. Overall, the mean temperature gradually decreased during the whole scoring period from approximately 16°C to 4°C in 2006 and from 12°C to 2°C in 2007 (Fig. 1). Therefore, we examined the possibility that the rate of senescence progression was directly correlated to temperature by subjecting the phenological and environmental data to ANOVA. However, the results indicated that the time to complete senescence was more strongly correlated with the date of appearance of first yellow leaves (Supplemental Table S1, model 1) than with the mean temperature during the time to complete senescence (Supplemental Table S1, model 2). Furthermore, model 3, based on both parameters, gave a considerably better fit, with the combined effects of date of first yellow leaves and temperature explaining 85% and 67% of the variation in the time to complete senescence in 2006 and 2007, respectively. Both the date of appearance of yellow color and the time to complete senescence were significantly correlated between the 2 years, with correlation coefficients of 0.636 and 0.406, respectively (data not shown).
Further support for the increased speed of senescence in lower temperatures came from our measurements of the onset and rate of senescence in tree 201, which we have followed from 1999 to 2007 by monitoring its chlorophyll contents. Here, starting date and rate of progression of autumn senescence in relation to temperature have been quantified more accurately and quantitatively since chlorophyll levels have been measured directly, not just estimated by visual scoring. The start of autumn senescence, as we have shown before, was remarkably constant in the monitored years (Table I), occurring within a 4-d period (September 9–12) in 7 of the 8 years studied. The date for start of senescence did not correlate to prior temperature conditions (data not shown). In contrast, the rate of subsequent chlorophyll degradation varied substantially among years. The possibility that variations in temperature may have been responsible for some of the variation in the rate of chlorophyll degradation was tested by examining the strength of linear relationships between the chlorophyll estimates and both calendar date and temperature (Supplemental Table S2). Calendar date alone explained approximately 82% of the variation in chlorophyll content over all 7 years (model 1). However, chlorophyll degradation was slowest in the warmest year, 2006, and two to three times faster in the coldest years (2003, 2004, and 2007). Consequently, including cumulated mean daily temperatures in the statistical model resulted in a significant improvement (Supplemental Table S2, model 2), with cumulated temperatures showing a positive correlation with chlorophyll content. This shows that chlorophyll degradation occurred more slowly in warm years than in cold years and explains why visual scoring detected the appearance of yellow color on this tree already on September 13 in the cold year 2007 but not until September 28 in the warm year 2006, despite the fact that onset of chlorophyll degradation occurred almost the same day. Taken together, onset of senescence was temperature independent and speed of senescence was temperature dependent.
Table I.
Mean temperatures between August 16 and October 10, estimated onset of senescence dates, and degradation rates of chlorophyll in tree 201 growing on the university campus
| Year | Temperature | Start Day | Year Day | Degradation Rate |
|---|---|---|---|---|
| °C | % d−1 | |||
| 1999 | 11.1 | September 9 | 251.2 | 4.48 |
| 2001 | 11.6 | September 9 | 250.9 | 3.06 |
| 2002 | 11.1 | September 10 | 252.5 | 3.51 |
| 2003 | 10.6 | September 9 | 251.0 | 4.34 |
| 2004 | 10.8 | September 12 | 254.7 | 6.14 |
| 2005 | 11.7 | September 5 | 247.1 | 3.00 |
| 2006 | 13.3 | September 11 | 253.2 | 2.01 |
| 2007 | 10.2 | September 10 | 252.4 | 4.24 |
| Mean | 11.3 | September 9 | 251.6 | 3.85 |
| sd | 0.93 | 2.2 | 1.25 |
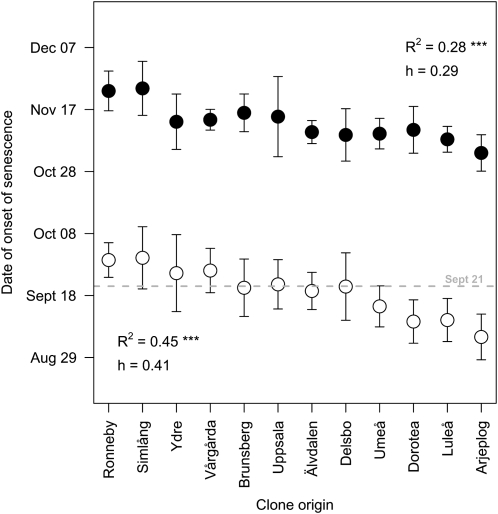
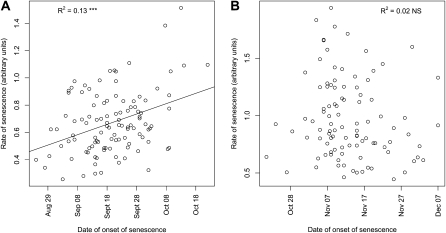
However, since temperatures decreased during the scoring, it was not possible to study in the field if late-senescing trees were fast only due to the lower temperature during their senescence or if they had a faster senescence due to some genotypic property. To study this, we analyzed data from direct measurements of onset and speed of senescence in the trees of the SwAsp collection in the greenhouse in Umeå. These measurements were performed in 2006 under natural photoperiods but in controlled temperature. Since the changes in photoperiod in this experiment were identical to those experienced by the trees of the UmAsp collection, this allowed the effects of low temperature and photoperiod on the initiation of senescence to be distinguished. All clones started to senesce as the photoperiod gradually decreased (Fig. 2), demonstrating that photoperiodic cues were sufficient to induce autumn senescence. Moreover, the mean date of the onset of senescence in the greenhouse for the SwAsp clones sampled from the Umeå region was September 15, 2006, close to the date at which senescence of the model tree began (September 11, 2006). This also correlated well with the mean date on which yellow color appeared (score 3, corresponding to approximately 40% chlorophyll loss) on the trees from the UmAsp collection (September 21 in 2006 and September 14 in 2007). These observations strongly suggest that the senescence was induced under natural photoperiods in the greenhouse in the same way as in the field. Also in the greenhouse, the rate of senescence was weakly but significantly correlated with the starting date of senescence under natural photoperiods (Fig. 3A), suggesting that late-senescing trees also were fast-senescing trees.
Figure 2.
Relationships between origins of the clones (ranked from south to north) and date of initiation of autumn senescence in the SwAsp collection grown in greenhouses under natural (white circles) and controlled (black circles) photoperiods. The dotted line indicates when the additional light was turned off in the controlled-photoperiod experiment (on September 21). Values are means ± sd of six to 10 clones per location. h, Repeatability within populations; R2, squared Pearson correlation coefficients for the relationship between trait and latitude of origin. ***, Statistically significant at P < 0.001.
Figure 3.
Relationships between the date of onset and the rate of senescence in greenhouses under natural (A) and controlled (B) photoperiods. NS, Not significant; R2, squared Pearson correlation coefficients for the relationship between trait and latitude of origin. ***, Statistically significant at P < 0.001.
We also performed another greenhouse experiment in 2005, when the trees were first grown under a controlled photoperiod of 23 h, sufficient to keep all clones actively growing, then on September 21 the photoperiod was suddenly changed to natural photoperiod (12 h) and clones subsequently experienced natural decline of photoperiod, while the other environmental conditions were very similar to those experienced by the trees in 2006. The two light regimes are compared in Supplemental Figure S1. This experiment will be further denoted “sudden shift.” Under natural photoperiods, the earliest tree started to senesce on August 24, when the daylength was 15.1 h, while the latest tree started on October 22, when the daylength was only 8.6 h. After the sudden shift, senescence of the trees occurred under shorter and less variable photoperiods (4.1–8.7 h of light at the starting date of senescence). Onset of senescence in the sudden-shift experiment has a complex control (see below); therefore, late-senescing trees in this experiment do not correlate well with those that senesce late under natural photoperiod (data not shown). In the sudden-shift experiment, there was no significant correlation between the starting date and the rate of senescence (Fig. 3B). Taken together, the greenhouse experiments suggested that clones that were late in onset of senescence had a tendency to senesce faster. It also appeared that photoperiod during senescence influenced the rate: on average, the rate of chlorophyll degradation was greater after the sudden shift (0.97 relative chlorophyll content index [CCI] per day), when the nights were longer, than under natural photoperiods (0.67 CCI per day).
Therefore, we conclude that (1) onset of autumn senescence at a specific photoperiod is a genotype property that is not significantly influenced by temperature and (2) low temperature accelerated the rate of autumn senescence, after it had been initiated by photoperiodic cue. The data also suggest that (3) clones that have a late onset of senescence tended to senescence faster and (4) the rate of senescence was also influenced by the photoperiod, longer nights leading to faster senescence.
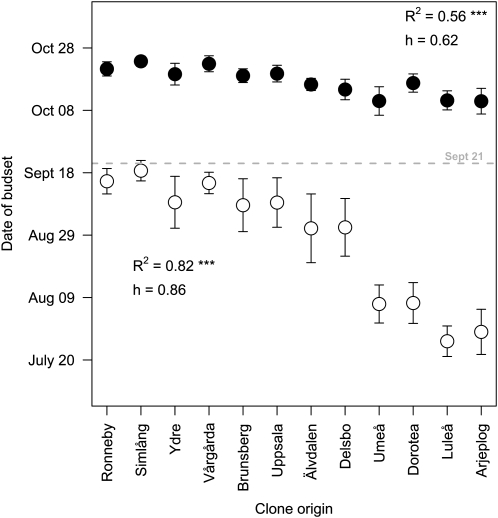
Within-Population Differences Are More Variable for the Onset of Senescence Than for Bud Set
During the two greenhouse experiments with the SwAsp collection (the natural-photoperiod and the sudden-shift experiments), we also scored bud set (Fig. 4). When the bud set and senescence data under natural photoperiod were compared (Figs. 2 and 4), the within-population variation in onset of senescence was much greater than the within-population variation in bud set. The repeatability within populations for the starting date of senescence under natural photoperiods was 0.41, implying that only 41% of the variation was attributed to differences between populations. This is significantly lower than the within-population repeatability for bud set (0.86). Second, the correlation with geographical origin was substantially lower for the start date of senescence (r2 = 0.45) than for the date of bud set (r2 = 0.82). The extreme populations differed by 50 d in bud set (Fig. 4) but only by 20 d in onset of senescence (Fig. 2), and there was an overlap in the timing of autumn senescence between clones from the far north and the far south, whereas for bud set all clones obtained from southern populations differed from northern clones.
Figure 4.
Relationships between clone origins (ranked from south to north) and date of bud set in the SwAsp collection grown in greenhouses under natural (white circles) and controlled (black circles) photoperiods. The dotted line indicates when the additional light was turned off in the controlled-photoperiod experiment (on September 21). Values are means ± sd of six to 10 clones per location. h, Repeatability within populations; R2, squared Pearson correlation coefficients for the relationship between trait and latitude of origin. ***, Statistically significant at P < 0.001.
Senescence Is Triggered by a Different Critical Photoperiod Than Bud Set, But Trees Have to Set Buds before They Can Undergo Autumn Senescence
Since growth arrest and bud set are under strong photoperiodic control and sometimes occur well before autumn senescence, there are three possible explanations for the temporal relationship between these two events. First, it is possible that they are under independent photoperiodic control. Second, it is possible that only growth arrest and bud set are under photoperiodic control and autumn senescence is initiated after a certain lag phase, which may differ between genotypes. A third possibility is that after induction of growth arrest and bud set by short days the tree has to acquire competence to senesce and then senescence occurs when a second critical photoperiod is reached. In order to assess which of these possibilities is most likely to be correct, we compared the relationship between bud set and senescence under different conditions.
We calculated the time lag between bud set and onset of senescence when the SwAsp collection was grown under natural photoperiods in the greenhouse in 2006 (Fig. 5A). The exact relationship between growth arrest in the cambium and in the apical meristems and bud set in aspen is not known, but since bud set is easy to score it can be used as a good proxy for growth arrest in general. It has to be kept in mind that bud set, measured as development of bud scales, happens rather late in the process, when the trees have already stopped growing both apically and laterally. When scored in the greenhouse under natural photoperiod, some trees from southern populations started to senesce shortly after bud set: the average lag phase between bud set and senescence was about 10 d for the southernmost populations, while the time lag for trees from northern populations was much longer, up to about 40 d (the most extreme, clone 105, set buds on July 15 and started to senesce 61 d later, on September 14). In the sudden-shift experiment, photoperiod was switched from 23 to 12 h, a value below the critical limits for bud set of almost all clones (Fig. 4), and buds were set on average after 26.5 d (Fig. 5B), consistent with reports regarding the lag between perception of critical photoperiods and bud set (Ruttink et al., 2007). Furthermore, although there were large differences between the genotypes (from clone 76 setting buds 5 d after the photoperiod switch to clone 58 setting buds after 47 d), there was no significant relationship between the duration of the lag phase and the latitude of origin, unlike the situation under natural photoperiod. Therefore, the results are incompatible with senescence simply being initiated a certain number of days after bud set. Comparisons of bud set and onset of senescence in the southern common garden also corroborated this finding. In fact, the daylength there is always below the critical value for bud set of northern clones, which therefore set buds very shortly after bud burst, in reality in late June when days are longest (Luquez et al., 2008). However, some of these trees still had green leaves well into September (data not shown), almost 3 months after bud set. Therefore, we concluded that senescence could not simply be initiated a certain number of days after bud set, implying that perception of a second critical photoperiod is necessary for the initiation of autumn senescence. However, our data are also incompatible with senescence being induced by a critical photoperiod but independently of bud set. If so, all southern clones with a critical photoperiod for senescence induction after September 21 under natural photoperiods would have started to senesce at about the same date in the experiment in which the photoperiod was switched from 23 h to natural lengths on September 21. However, all clones started to senesce much later in the sudden-shift experiment (Fig. 2). In other words, trees must have set buds to be able to undergo autumn senescence. Taken together, the only mechanistic model that is compatible with our data is that the trees can acquire competence to senesce only after growth arrest, and then they initiate senescence when the critical photoperiod for senescence is reached.
Figure 5.
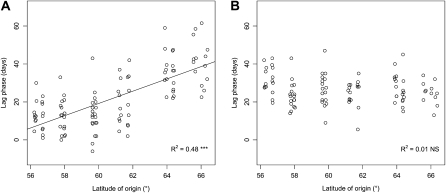
Relationship between latitude of origin and lag phase between bud set and start of senescence in the SwAsp collection grown in greenhouses under natural (A) and controlled (B) photoperiods. NS, Not significant; R2, squared Pearson correlation coefficients for the relationship between trait and latitude of origin. ***, Statistically significant at P < 0.001.
DISCUSSION
The Scottish surgeon John Hunter (1728–1793) wrote, “When I was a boy, I wanted to know all about the clouds and grasses, and why leaves changed color in the Autumn; … I pestered people with questions about what nobody knew or cared anything about” (cited by Ellis, 2001). Since then, science has made immense progress, and we now have abundant information about clouds and grasses. Nevertheless, research about changes in leaf color during the autumn is still in its infancy; very little is known, for example, about the genes that regulate the process. However, with the recent development of Populus as a model system for tree genetics and genomics, better tools are now available to address the question pondered by Hunter more than 200 years ago.
There is enormous within-species variation in Populus due to their very large populations, dioecious nature, wind pollination, and wind-dispersed seeds. For instance, the average nucleotide diversity within genes in aspen is approximately 1% (Ingvarsson, 2005) higher than that of other species studied to date. Consequently, phenotypic variation is also extensive, although selection has often created strong clines for certain phenological traits (Hall et al., 2007; Luquez et al., 2008). In this contribution, we study autumn senescence in a single selected individual, in cloned trees in greenhouses, and in a large natural population in the field. We think that there is strong justification for such a multiple approach. The analysis of a single genotype facilitates the acquisition of detailed information regarding, for instance, sequences of events such as the “cellular timetable of autumn senescence” (Keskitalo et al., 2005) and the recently described “molecular time timetable of apical bud formation” (Ruttink et al., 2007). However, analysis of a single individual clearly provides no indications of the genetic variation in these timetables within or among populations. Similarly, analysis of clones in controlled conditions can provide valuable indications of the effects of specific variables on such timetables, but plants should also be evaluated in the field to observe the full spectrum of natural variation. We show that the speed of autumn senescence varies considerably between genotypes, and the same holds true for bud formation: the time from perception of the inducing photoperiod to bud set varies among our clones from 13 to 34 d, compared with 4 to 5 weeks for the genotype studied by Ruttink et al. (2007). Nevertheless, cloned trees like those used by Ruttink et al. (2007) and those of the SwAsp collection cannot be used for all kinds of studies; young aspens cannot always be compared with large mature trees, exemplified by the study of Hoenicka et al. (2008). By combining data from three levels, ranging from a single individual to large populations, we have been able to draw conclusions about autumn senescence in aspen, and we believe that at least some of them will also hold true for other deciduous trees.
First, we have shown that although the timing of onset of autumn senescence is a genotypically governed property, the natural variation in onset of senescence among aspen trees growing at a given site is larger than the variation in bud set, and the effect of latitude of origin is weaker. The reasons for this are not obvious. However, it is possible that the timing of growth arrest and bud set is more closely related to winter survival, so it is under more uniform selection pressure at a given latitude. Alternatively, since the timing of autumn senescence involves a trade-off between carbon acquisition and nitrogen loss, local variations in microclimate and soil nutrient levels may enhance natural variation in senescence but not in bud set. A third possible explanation is linked to biotic interactions. Since autumn senescence phenology is likely to be important for many pathogens and herbivores, a tree that differs phenologically from the others in its local environment is more likely to escape attack by pathogens and herbivores, which develop local adaptation. It is also possible that all of these selective pressures have interactive effects.
Second, we have shown that the speed of senescence varies between genotypes, with those starting senescence later senescing more rapidly than earlier starters. In addition, low temperature accelerates senescence. Senescence is probably accompanied by oxidative stress due to imbalances between light capture and carbon assimilation (Kar et al., 1993), due to the presence of chlorophyll catabolites capable of generating oxygen singlets (Matile et al., 1999) and, perhaps, the inactivation of certain antioxidative defenses (Kukavica and Jovanovic, 2004). This stress may be exacerbated at low temperature, resulting in faster progression of autumn senescence. Why the senescence process is most rapid in trees that start senescing late is less obvious. However, one possibility is that autumn senescence, once initiated, is accelerated or occurs mainly during the night, perhaps through a process similar to dark-induced, starvation-mediated senescence, which has been studied extensively in annual plants (Buchanan-Wollaston et al., 2003, 2005). In our model tree, a shift of energy source from chloroplasts to mitochondria was observed in 2003 around September 19, 10 d after the initiation of senescence when most chloroplasts still appeared normal, and some active PSII centers were observed until October (Keskitalo et al., 2005). It is likely, therefore, that photosynthesis is still an appreciable source of energy during the early stages of senescence and that longer daylengths can significantly delay the starvation involved in the senescence process. This is also consistent with the acceleration of senescence at low temperature discussed above, since low temperature inhibits photosynthesis. Another obvious possibility is that a signaling substance, putatively related to phytochrome action, accumulates during the night and accelerates senescence. Regardless of the mechanism involved, it would appear beneficial for trees to senesce as rapidly as possible to both maximize carbon gain through photosynthesis and minimize nitrogen losses in years when severe frost would kill leaf cells before complete nutrient remobilization had occurred. A testable hypothesis is that trees that senesce fast have a less efficient nitrogen remobilization.
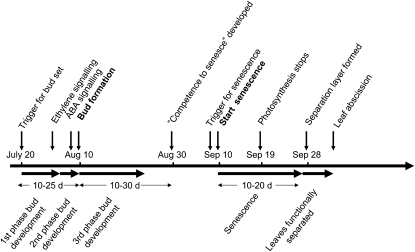
Third, our data on the relationship between bud set and the onset of senescence allow a model of the events that occur during autumn in aspen to be formulated, integrating data from the recently published molecular timetable of apical bud set (Ruttink et al., 2007) and the cellular timetable of autumn senescence (Keskitalo et al., 2005). An emerging aspect in our model is that leaves need to be competent to respond to the critical photoperiod inducing autumn senescence. Competence or ripeness to senesce has been genetically dissected by Jing et al. (2003, 2005), who demonstrated that Arabidopsis (Arabidopsis thaliana) leaves have to reach a certain age before they can respond to an ethylene treatment by senescence. This “ripening” process is analogous to the old “ripeness to flower” concept in plant developmental biology (Bopp, 1996). Another ripening phenomenon is the development of endodormancy in the cambium of trees, including Populus (Rohde and Bhalerao, 2007). The sequence of events occurring in buds and leaves of aspen during autumn, typical dates for each event for a tree in Umeå, and the natural variation in the different phases are all indicated in Figure 6. Our measurement of bud set corresponds to the transition from phase 2 to phase 3, as defined by Ruttink et al. (2007). Among our clones, the time from triggering of bud set by shortening of the photoperiod to bud set varied between 13 and 34 d. During the third phase of bud development, when dormancy is developed, competence to senesce is also developed. Metabolism in the apical bud during this period is very active and is likely to produce sinks for carbohydrates. In parallel, the lateral cambium has shifted from growth to growth arrest. The precise relationship between the timing of growth arrest in apical buds and the cambium has not been established in Populus, but 4 weeks of short-day treatment is sufficient to induce ecodormancy in hybrid aspen cambium and 6 weeks suffices to induce endodormancy (Espinosa-Ruiz et al., 2004). This means that strong carbohydrate sinks are present during this period and that high photosynthetic capacity of the leaves is beneficial to complete this step. About 20 d after bud set, but with significant variation between clones, competence to senesce has developed. It is tempting to speculate that this transition is linked to the carbohydrate status of the tree and/or the transition to ecodormancy in the cambium. As long as the sinks are sufficiently strong, it will be highly adaptive for a tree to maintain leaves in photosynthetic mode, but as the sink strength gradually decreases during this period, a point will be reached at which saving nitrogen becomes more important. This may set the leaves in a state in which they are “ripe to senesce,” and the process may start once the critical photoperiod has been perceived.
Figure 6.
Timetable of autumn senescence-related events in aspen based on data presented by Keskitalo et al. (2005), Ruttink et al. (2007), and this study. Dates above the line indicate typical dates for the event in trees naturally grown in Umeå, and numbers below the line indicate the variation (days; bottom horizontal arrows) in the length of certain processes. ABA, Abscisic acid.
The time between bud set and the date when the critical photoperiod for senescence is reached is longer for the northern clones than for the southern clones (Fig. 5A). This may reflect adaptation to northern latitudes, where ensuring winter survival and safeguarding carbon gains may be more important, relative to maximizing nitrogen saving, than it is farther south due to the short growing seasons and highly fluctuating temperatures. It has not been established if there is a lag phase between perception of the critical photoperiod and onset of senescence, but it is likely that this would be shorter than the lag between the critical photoperiod for bud set and bud set, since other factors that induce leaf senescence, such as ethylene treatment, pathogen infection, and drought, do so very rapidly, most likely because the catabolic machinery seems to be, at least in part, already present in green leaves (Zelisko et al., 2005; Garcia-Lorenzo et al., 2006). We believe that this is also true for autumn senescence, and we have a model to test this prediction. Our data show that nitrogen remobilization typically takes 2 weeks but is highly dependent on temperature. It is likely that the timing of the other senescence-related events is also temperature dependent, but this has not been tested, to our knowledge. Once the remobilization process is complete, abscission occurs. Our mechanistic model and collection of genotypes allow experiments to be designed to test whether gene expression is important in the induction of autumn senescence. If trees of different genotypes are exposed to sufficiently short days to induce bud set, but not senescence, then left until competence to senesce has developed and subsequently exposed to an even shorter photoperiod that induces senescence in some genotypes but not others, it may be possible to determine whether and which transcriptional changes are specifically involved in the transition to autumn senescence, which has not been possible so far (Jansson and Thomas, 2008).
The strict photoperiodic control of the onset of autumn senescence in aspen might be rather unusual. In sugar maple (Acer saccharum), American beech (Fagus grandifolia), and yellow birch (Betula alleghaniensis), 90% of the variation in autumn canopy senescence has been attributed to variations in temperature (Richardson et al., 2006). Furthermore, delays in the development of autumn colors have been attributed to global warming in a meta-analysis covering several hundred plant species (Menzel and Fabian, 1999; Menzel et al., 2006), suggesting that photoperiod is not the most common trigger of autumn senescence. It may be much more important at high latitudes than elsewhere, because the rapid changes in daylength that occur at such latitudes in spring and autumn provide powerful, high-resolution cues regarding seasonal changes that trees must respond to if they are to survive. Since cold acclimation can be induced by low temperature (several weeks at 0.5°C) independently of short days in hybrid aspen (Welling et al., 2002), it is likely that senescence can be induced in our trees by low temperature too. Temperature control of senescence under natural conditions might be more important for adaptation to environments at different altitudes at a given latitude, as found by Richardson et al. (2006) in other species. Leaf senescence can be caused by many different factors, raising questions about whether senescence induced by short photoperiods in aspen differs from senescence induced by other factors. We believe that, once started, senescence in most (or all) leaf systems is very similar (Keskitalo et al., 2005). However, tree species that have successfully colonized boreal habitats may have acquired a novel trait that would be highly adaptive in this climate: the capacity to initiate senescence in response to changes in photoperiod alone. This hypothesis needs further attention because if it is true it may be possible to transfer the trait to other genotypes, which are not naturally well adapted to boreal climates.
CONCLUSION
We have found that the initiation of autumn senescence in European aspen is under photoperiodic control but senescence is not provoked until the leaves are competent to senesce, a process that may be related to the carbohydrate status of the tree and influenced by growth arrest and dormancy. With our germplasm resources (the SwAsp and UmAsp collections) and a set of candidate genes, we can now attempt to correlate nucleotide polymorphisms in these genes to the autumn-related traits dissected in this contribution.
MATERIALS AND METHODS
The UmAsp Collection
A set of 180 European aspen (Populus tremula) trees was selected during the summer of 2006 along roads in the region of Umeå, Sweden (20° 15′ E, 63° 50′ N). The single tree we have studied previously (Bhalerao et al., 2003; Andersson et al., 2004; Keskitalo et al., 2005) was included in this collection (tree 201). Another tree in this collection (tree 202) has also been used earlier for global expression profiling (Sjödin et al., 2008). All of these trees are likely to have established naturally, since aspen is not, and has never been, planted for commercial or ornamental purposes in Sweden, except the easily recognizable erecta mutant, which was not included in this study. Each tree in the collection was photographed and marked to facilitate further scoring, its height and diameter were measured using standard techniques, and its location was determined using a global positioning system unit (Garmin 60CSx). Since aspen has a tendency to reproduce clonally, neighboring trees (within 50 m of each other) were avoided, unless their visual appearance indicated that they were different clones. The collection was expanded to 315 trees during the summer of 2007. The height of the trees in the collection varied between 8.2 and 26.5 m. A data file in which the trees are marked in Google Earth (315trees.kml) is available as Supplemental Data File S1.
Senescence Scoring
All trees in the UmAsp collection were visually scored for autumn senescence using an autumn senescence score card (Supplemental Fig. S2) on August 23, September 4, 7, 11, 14, 18, 21, 25, and 28, and October 2, 5, 9, 12, 16, 19, and 23 in 2006 and August 20, 23, 27, and 30, September 3, 6, 10, 13, 17, 20, 24, and 27, and October 1, 4, 8, 12, 16, and 19 in 2007. The start of autumn senescence was defined as the date when yellow color appeared (score 3), and senescence was considered complete when more than 90% of the leaves had fallen (score 7). The duration of senescence was determined as the number of days between these two dates. Three (2006) and two (2007) trees that already had yellow leaves in mid-August were excluded from the analysis because senescence in these cases was probably induced by other factors (e.g. water stress during the unusually dry summer of 2006).
Senescence and Bud Set in the SwAsp Collection Growing in a Greenhouse
The SwAsp collection, comprising 116 clones, was obtained by sampling 12 populations across Sweden (from 55° to 67° N) as described in detail by Luquez et al. (2008). A data file showing the positions of the cloned trees in Google Earth (116trees.kml) is available as Supplemental Data File S2. Trees representing 106 of the 116 clones, and all of the populations, were planted in pots in 2004 and kept in a greenhouse and growth chambers at Umeå University as described by Ingvarsson et al. (2006). In most cases, there were two repetitions of each clone, but for some clones only one tree was present. In an experiment in 2005, 2-year-old trees in 3-L pots with soil were placed in a cold chamber at 5°C for 25 d and −4°C for 14 d. On August 2, 2005, the trees were transferred to a greenhouse with a 23-h photoperiod (maximum irradiance, 700 μmol photons m−2 s−1) and a temperature of 20°C. Before the trees started to sprout, they were transferred to 7-L pots, and they were watered daily and fertilized twice with a commercial NPK fertilizer before bud set started. A short-day treatment was started on September 21, 2005, by exposing the trees to natural photoperiod (Supplemental Fig. S1). After the 2005 experiment, the plants were kept in the greenhouse at 6°C from January 3 until May 19, 2006, when the temperature was raised to 16°C. Before the trees started to sprout, they were pruned, leaving only two main branches. The plants grew under natural photoperiods, hence the photoperiod started to decrease after June 21. Beginning on August 22, 2006, the temperature was changed to 15°C during the day (8 am to 8 pm) and 10°C during the night (8 pm to 8 am). The trees were fertilized every second week during the growing season and watered daily.
In both the 2005 and 2006 experiments, the trees were sprayed with a commercial fungicide and insecticide (Baymat; Bayer Crop Science) to avoid rust infection and insect damage. Spider mite infections were treated by biological control using spider mite predators (Ambluseius swiskii; provided by Lindesro). The temperature in the greenhouse could briefly rise on sunny days until the ventilators opened.
The trees' chlorophyll contents were estimated twice per week by measuring the relative CCI of three (2005) or five (2006) leaves per tree using a CCM-200 chlorophyll meter (Opti-Sciences). The average values of the three or five measurements were used to calculate the senescence parameters. Chlorophyll degradation occurred in two almost linear phases: a first phase with no or slow chlorophyll degradation and a second phase with rapid chlorophyll decay (Supplemental Fig. S3). Data from both phases were fitted to linear models, and the intercept of the two lines was defined as the starting date of senescence. The negative value of the slope of the second regression was used as an estimate of the rate of senescence. Bud set was scored according to Luquez et al. (2008).
Senescence in a Free-Growing Aspen (Tree 201)
Chlorophyll content was measured during each of seven autumns in a tree growing on the university campus. From 1999 to 2003, chlorophyll was determined from leaf extracts as described by Keskitalo et al. (2005), then from 2004 to 2007, chlorophyll was estimated from daily measurements with a CCM-200 chlorophyll meter on five (2004–2006) or 20 (2007) randomly chosen leaves, as described above for the SwAsp collection in greenhouses. To combine the different data sets, relative values were used, the highest value for each year (obtained in mid-August, near the beginning of the measuring period) being defined as 100%. The starting date of senescence and the rate of chlorophyll degradation were determined by two linear regressions as described above for the SwAsp collection. The weather data were recorded by a weather station located on the campus approximately 100 m from the model tree. Mean daily temperatures were computed from the data recorded at hourly intervals.
Statistical Analysis and Figures
Statistical analyses were performed using functions of the “base” and the “stat” packages of the Windows version of R software (R Development Core Team, 2006). The figures were prepared using the standard plotting functions of the R for Windows software (R Development Core Team, 2006).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Natural (continuous line, 2006) and controlled (dotted line, 2005) photoperiods in greenhouse experiments.
Supplemental Figure S2. Senescence score card based on pictures from tree 201 shot in autumn 2005.
Supplemental Figure S3. Illustrative measurements of chlorophyll degradation in a tree in the greenhouse in autumn 2006, where the regression lines were used to determine the onset and rate of senescence.
Supplemental Table S1. Linear models relating the number of days between scores 3 and 7 (first yellow leaves to complete senescence) to date of first yellow leaves (score 3) and mean temperature during scores 3 to 7.
Supplemental Table S2. Linear models for the relationships between chlorophyll content and date and cumulated mean daily temperature in tree 201.
Supplemental Data File S1. A Google Earth map with the 315 trees in the UmAsp collection.
Supplemental Data File S2. A Google Earth map with the 116 trees in the SwAsp collection.
Supplementary Material
Acknowledgments
Lena Wallheim and Frank Klimmek are acknowledged for participation in the creation of the UmAsp collection.
This work was supported by grants from the Swedish Research Council, the Swedish Research Council for the Environment, Agricultural Sciences, and Spatial Planning, the Swedish Foundation for Strategic Research, and the Kempe Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Stefan Jansson (stefan.jansson@plantphys.umu.se).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Andersson A, Keskitalo J, Sjodin A, Bhalerao R, Sterky F, Wissel K, Tandre K, Aspeborg H, Moyle R, Ohmiya Y, et al (2004) A transcriptional timetable of autumn senescence. Genome Biol 5 R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao R, Keskitalo J, Sterky F, Erlandsson R, Bjorkbacka H, Birve SJ, Karlsson J, Gardestrom P, Gustafsson P, Lundeberg J, et al (2003) Gene expression in autumn leaves. Plant Physiol 131 430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BL, Parmentier Line C, Fuchigami LH, Coleman GD (2001) Ecotypic and genetic variation in poplar bark storage protein gene expression and accumulation. Tree Physiol 21 1289–1297 [DOI] [PubMed] [Google Scholar]
- Bopp M (1996) The origin of developmental physiology of plants in Germany. Int J Dev Biol 40 89–92 [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence: a genomics approach. Plant Biotechnol J 1 3–22 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, et al (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42 567–585 [DOI] [PubMed] [Google Scholar]
- Cooke JEK, Weih M (2005) Nitrogen storage and seasonal nitrogen cycling in Populus: bridging molecular physiology and ecophysiology. New Phytol 167 19–30 [DOI] [PubMed] [Google Scholar]
- Ellis H (2001) John Hunter's teachings on gunshot wounds. J R Soc Med 94 43–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Ruiz A, Saxena S, Schmidt J, Mellerowicz E, Miskolczi P, Bako L, Bhalerao RP (2004) Differential stage-specific regulation of cyclin-dependent kinases during cambial dormancy in hybrid aspen. Plant J 38 603–615 [DOI] [PubMed] [Google Scholar]
- Gan S, Amasino RM (1997) Making sense of senescence. Plant Physiol 113 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lorenzo M, Sjodin A, Jansson S, Funk C (2006) Protease gene families in Populus and Arabidopsis. BMC Plant Biol 6 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Luquez V, Garcia VM, St Onge KR, Jansson S, Ingvarsson PK (2007) Adaptive population differentiation in phenology across a latitudinal gradient in European aspen (Populus tremula, L.): a comparison of neutral markers, candidate genes and phenotypic traits. Evolution Int J Org Evolution 61 2849–2860 [DOI] [PubMed] [Google Scholar]
- Hoenicka H, Nowitzki O, Hanelt D, Fladung M (2008) Heterologous overexpression of the birch FRUITFULL-like MADS-box gene BpMADS4 prevents normal senescence and winter dormancy in Populus tremula L. Planta 227 1001–1011 [DOI] [PubMed] [Google Scholar]
- Hörstensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57 55–77 [DOI] [PubMed] [Google Scholar]
- Ingvarsson PK (2005) Nucleotide polymorphism and linkage disequilibrium within and among natural populations of European aspen (Populus tremula L., Salicaceae). Genetics 169 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson PK, Garcia MV, Hall D, Luquez V, Jansson S (2006) Clinal variation in phyB2, a candidate gene for day-length-induced growth cessation and bud set, across a latitudinal gradient in European aspen (Populus tremula). Genetics 172 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson PK, Garcia MV, Luquez V, Hall D, Jansson S (2008) Nucleotide polymorphism and phenotypic associations within and around the phytochrome B2 locus in European aspen (Populus tremula, Salicaceae). Genetics 178 2217–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson S, Thomas H (2008) Senescence: developmental program or timetable? New Phytol 179 575–579 [DOI] [PubMed] [Google Scholar]
- Jing HC, Hille J, Dijkwel RR (2003) Ageing in plants: conserved strategies and novel pathways. Plant Biol 5 455–464 [Google Scholar]
- Jing HC, Schippers JHM, Hille J, Dijkwei PP (2005) Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J Exp Bot 56 2915–2923 [DOI] [PubMed] [Google Scholar]
- Kar M, Streb P, Hertwig B, Feierabend J (1993) Sensitivity to photodamage increases during senescence in excised leaves. J Plant Physiol 141 538–544 [Google Scholar]
- Keskitalo J, Bergquist G, Gardström P, Jansson S (2005) A cellular time table of autumn senescence. Plant Physiol 139 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukavica B, Jovanovic SV (2004) Senescence-related changes in the antioxidant status of ginko and birch leaves during autumn yellowing. Physiol Plant 122 321–327 [Google Scholar]
- Lim PO, Woo HR, Nam HG (2003) Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci 8 272–278 [DOI] [PubMed] [Google Scholar]
- Luquez V, Hall D, Albrectsen B, Karlsson J, Ingvarsson PK, Jansson S (2008) Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genet Genomes 4 279–292 [Google Scholar]
- Matile P, Hörtensteiner S, Thomas H (1999) Chloropyll degradation. Annu Rev Plant Physiol 50 67–95 [DOI] [PubMed] [Google Scholar]
- Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397 659 [Google Scholar]
- Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kubler K, Bissolli P, Braslavska O, Briede A, et al (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12 1–8 [Google Scholar]
- Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392 914–916 [Google Scholar]
- Noodén LD, Guiamét JJ, John I (1997) Senescence mechanisms. Physiol Plant 101 746–753 [Google Scholar]
- R Development Core Team (2006) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org (May 1, 2008)
- Richardson AD, Bailey AS, Denny EG, Martin CW, O'Keefe J (2006) Phenology of a northern hardwood forest canopy. Glob Change Biol 12 1174–1188 [Google Scholar]
- Rohde A, Bhalerao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12 217–223 [DOI] [PubMed] [Google Scholar]
- Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19 2370–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Wissel K, Bylesjö M, Trygg J, Jansson S (2008) Global expression profiling in leaves of free-growing aspen. BMC Plant Biol 8 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling A, Moritz T, Palva ET, Junttila O (2002) Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol 129 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelisko A, Garcia-Lorenzo M, Jackowski G, Jansson S, Funk C (2005) AtFtsH6 is involved in the degradation of the light-harvesting complex II during high-light acclimation and senescence. Proc Natl Acad Sci USA 102 13699–13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Coleman GD (2001) Phytochrome-mediated photoperiod perception, shoot growth, glutamine, calcium, and protein phosphorylation influence the activity of the poplar bark storage protein gene promoter (bspA). Plant Physiol 126 342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.