Abstract
Serotonergic involvement has been implicated in preferential consumption of treat foods. We tested the effect of acute tryptophan depletion (ATD) on food consumption by overweight and lean adults with and without a history of recurrent major depressive disorder (MDD). ATD and taste-matched placebo challenges were administered double-blind in counter-balanced order. Participants were classified as lean (n = 36) or overweight (n=19) on the basis of body mass index (BMI). Total calorie, carbohydrate, protein, and sweet food consumption were assessed via a test meal 8-hours following ATD. Four food items of comparable palatability were offered as a part of the test: two sweet (one carbohydrate-rich, and one protein-rich) and two non-sweet (one carbohydrate-rich, and one protein-rich). As compared to the placebo challenge, ATD significantly increased sweet calorie intake among overweight participants and increased their propensity to consume sweet food first before any other type of food. Lean participants’ sweet calorie intake and food preference were unaffected by ATD. Findings suggest serotonergic involvement in the sweet food consumption by overweight individuals.
Keywords: acute tryptophan depletion, sweet food, overweight, serotonin, obesity
1. Introduction
Obesity, defined as a body mass index of 30 or greater, is a significant public health threat in the US, with 31% of the population affected (Flegal, Carroll, Ogden, & Johnson, 2002; Hedley et al., 2004). The growing obesity epidemic has been attributed to an environment where high-calorie, palatable foods are increasingly plentiful and easily accessible (Wadden, Brownell, & Foster, 2002). However, not all humans who are exposed to an abundance of highly palatable foods overeat or become overweight. Neurobiological variations may influence vulnerability towards overconsumption and weight dysregulation (Levitan et al., 2004).
Variation in brain serotonergic activity (5-hydroxytryptophan, 5-HT) has been implicated in appetite regulation and impulse control. Serotonin inhibits neuropeptide Y, resulting in suppression of hunger and food intake (Halford & Blundell, 2000). A chronic hyposerotonergic state could result in a prolonged pattern of compulsive overeating, ultimately contributing to obesity. Supporting the role of 5-HT in weight regulation are data showing that stimulation of postsynaptic 5-HT receptors inhibits carbohydrate consumption in rats (Leibowitz, Weiss, & Shor-Posner, 1987; Shor-Posner, Grinker, Marinescu, Brown, & Leibowitz, 1986), with more vigorous stimulation reducing total calorie intake. Conversely, a low brain 5-HT level is associated with enhanced appetite (Leibowitz et al., 1987) and impaired impulse control in rats (Bizot, Le Bihan, Puech, Hamon, & Thiebot, 1999) and in humans at risk for alcoholism (Crean, Richards, & de Wit, 2002; LeMarquand, Benkelfat, Pihl, Palmour, & Young, 1999). Among obese humans, many medications that enhance serotonergic function induce weight loss (Hanotin, Thomas, Jones, Leutenegger, & Drouin, 1998; Pijl et al., 1991; Strain, Strain, & Zumoff, 1985; Toornvliet, Pijl, Hopman, Westendorp, & Meinders, 1997; Wadden et al., 2005), and reduce caloric intake (McTavish & Heel, 1992; Pijl et al., 1991; Wurtman et al., 1981) (although it should be noted that some serotonergic agents with prominent anticholinergic and antihistaminic action cause weight gain) (Ruetsch, Viala, Bardou, Martin, & Vacheron, 2005). Whether 5-HT plays a role in inhibitory control of eating in overweight individuals, specifically, has not been well studied.
ATD is a procedure that acutely and transiently reduces 5-HT synthesis. By 5–7 hours following depletion, brain 5HT synthesis is reduced by 85–90%, and depressive symptoms increase in those vulnerable to MDD (Hood, Bell, & Nutt, 2005). Most research using ATD methodology has examined affective changes among those vulnerable to affect dysregulation (Delgado et al., 1994; Leyton et al., 1997; Neumeister et al., 2004; Smith, Fairburn, & Cowen, 1997; Spring et al., 2007). The effect of lowering brain 5-HT synthesis on eating behavior has been much less well characterized, and one possibility is that ATD-induced negative mood is a predisposing factor for emotionally-driven eating.
Most existing research has been confined to examining ATD effects on disordered eating and cognition among bulimics. In bulimia, ATD has been found to engender increased desire to binge (Kaye et al., 2000), fear of losing control over eating (Smith, Fairburn, & Cowen, 1999; Weltzin, Fernstrom, Fernstrom, Neuberger, & Kaye, 1995), and weight concern (Smith et al., 1999). Findings are mixed, however, on the effect of ATD on caloric intake and binge eating (Kaye et al., 2000; Oldman, Walsh, Salkovskis, Fairburn, & Cowen, 1995; Weltzin et al., 1995). In some studies bulimics have increased palatable food intake following ATD (Smith et al., 1999; Weltzin et al., 1995), whereas in others no change in intake was observed (Kaye et al., 2000; Oldman et al., 1995). Small samples and varying methodology may account for mixed findings (Kaye et al., 2000). Because most of these studies recruited lean participants, few data characterize 5-HT effects on the food consumption of overweight people who do not have an eating disorder.
The present study is the first to test the hypothesis that greater body mass index (BMI) is associated with disproportionately increased food consumption following ATD. Debate exists about whether brain 5-HT has a generic influence on overall intake, or whether it selectively influences intake of carbohydrates, sweets, or palatable “comfort” foods (Asin, Davis, & Bednarz, 1992; Spring, Chiodo, & Bowen, 1987; Wogar, Bradshaw, & Szabadi, 1991). To evaluate that question, total energy consumption, carbohydrate-rich food consumption, and sweet food consumption were each assessed. The carbohydrate hypothesis predicted that ATD, relative to placebo, would increase consumption of carbohydrate-rich foods. The sweetness hypothesis, predicted that ATD, relative to placebo, would increase consumption of sweet tasting foods. We also tested the prediction that each hypothesized effect would occur disproportionately among overweight participants.
2. Methods and Materials
2.1 Participants and Study Design
Participants (N=55) in the current study were part of a larger study that examined the effects of ATD on dysphoric mood in smokers and never-smokers with and without a personal and family history of DSM-IV major depression (Spring et al., 2007). A complete description of study design and methods are reported elsewhere (Spring et al., 2007). Candidates were excluded from participation if they were pregnant or lactating, had food allergies, were receiving smoking treatment including nicotine replacement, had received substance abuse treatment within the past year, or were taking psychotropic medications or medications known to influence mood or appetite. Participants with eating disorders, current depression, or other psychiatric illness also were excluded. Finally, participants were excluded for BMI ≥ 35 (Class II or higher obesity) or unstable medical condition (e.g., diabetes). The study was approved by local institutional review boards. Participants were compensated $100.00.
2.2 Measures
Body Mass Index (BMI)
Body mass index was measured by weight and height using self report. BMI was calculated by (weight in pounds/(height in inches)2) × 704.5. Participants with BMI of 19–24.9 or less were classified as lean, and those 25 to 34.9 were classified as overweight.
Palatability Scale
The palatability scale assessed liking for 70 total food items. Food items consisted of sweet and non-sweet foods presented in random order. Items were drawn from a large pool of foods previously rated as palatable by a normative sample (Spring, Pagoto, McChargue, Hedeker, & Werth, 2003). Participants were asked to rate how much they liked each food on a 1–10 point scale. Higher responses indicated greater palatability.
2.3 Procedures
Experimental session
Participants were given the ATD and placebo challenge drinks on different test days separated by one week. Mixtures were administered double-blind and in counterbalanced order. Female participants completed the study between days 7 and 21 of their menstrual cycle. Smokers within each BMI group (58% of each) smoked at hourly intervals during the test sessions to prevent nicotine withdrawal. On each test day, participants arrived by 8:00 a.m. after a 12-hour fast. Ad libitum intake preceded the 12-hour fast because dietary restrictions for more than 12 hours preceding ATD do not appear to be necessary to produce significant reduction in plasma tryptophan.(Spring et al., 2007) Participants underwent baseline testing of mood (Hamilton Depression Rating Scale (Hamilton, 1960) and blood collection through venipuncture to assess plasma amino acid levels (see Table 1 for experimental session timeline). At 9:30 a.m., participants ingested one of two calorie-matched beverages. The ATD mixture consisted of the following 15 amino acids (102.5 total g): l-alanine (5.5g), l-arginine (4.9g), l-cystine (2.7g), glycine (3.2g) l-histidine (3.2g), l-isoleucine (8.0g), l-leucine (13.5g), l-lysine monohydrochloride (11.0g), l-methionine (3.0g), l-phenylalanine (5.7g), l-proline (12.2g), l-serine (6.9g), l-threonine (6.9g), l-tyrosine (6.9g), and l-valine (8.9g). Three amino acids, methionine, cystine, and arginine, were encapsulated because their unpalatable taste could not be masked by chocolate and peppermint. The remaining amino acids were mixed with 3 oz. of tonic water and blended with crushed ice, chocolate syrup, and peppermint extract. The capsules were ingested approximately 15 minutes prior to the drink. In the placebo condition, participants ingested capsules containing confectioner’s sugar, followed 15 minutes later by a mixture consisting of 3 oz. of tonic water blended with crushed ice, chocolate syrup, and peppermint extract. Baking soda and psyllium were also added to the placebo beverage in order to mimic the granular, salty taste of the amino acid mixture.
Table 1.
Timeline of experimental session
| Time | Procedure |
|---|---|
| 8:00 a.m. | Baseline testing of depressive symptoms (M-HAMD) and plasma amino acid levels |
| 8:30 a.m. | Standardized breakfast (puffed rice, peaches, nondairy creamer, gelatin, and sugar) |
| 9:30 a.m. | Consumption of amino acid or placebo mixture |
| 10:30 a.m. | Four low-protein cookies and Kool-aid |
| 12:30 p.m. | Lunch (graham crackers, cream cheese, boiled potatoes and butter, green beans, gelatin) |
| 1:30 p.m. | All reading materials removed from observation room |
| 2:30 p.m. | Following the guided imagery procedure, the M-HAMD assessment and blood sampling for amino acid levels was repeated |
| 3:00 p.m. | Snack (4 low-protein cookies and Kool-aid) |
| 5:30 p.m. | Dinner test meal |
All breakfast, lunch and snack foods were provided to participants on test days. The foods served comprised the low-tryptophan diet used elsewhere (i.e.,(Delgado et al., 1990)). Breakfast consisted of puffed rice, peaches, nondairy creamer, Knox gelatin and sugar. Lunch was graham crackers, cream cheese, boiled potatoes and butter, green beans and Knox gelatin. The mid-day snack consisted of four low-protein cookies and Kool-Aid. When not participating in experimental procedures, each participant sat alone in a private room in which he or she could watch videos or read magazines of emotionally neutral content.
At 5-hours post-challenge (2:30 p.m.), which marked the estimated onset of maximal tryptophan depletion (range 5–7 hours;(Delgado et al., 1990)), participants underwent a 3-minute guided imagery negative mood induction procedure. Seated in a reclining chair in a soundproof room, each participant listened to a tape of a negative event script. Scripts were equated between conditions on vividness and emotional valence (anxious, angry, sadness). Participants were encouraged to sit back, close their eyes, and visualize the event as vividly as possible. Negative autobiographical imagery was used to establish a depression-related cognitive set that would allow the biological state produced by ATD to be experienced and interpreted as genuine dysphoria. Blood sampling was then repeated to allow an assessment of change in amino acid levels.
After the 8-hour assessment period, participants were served the dinner test meal. Based on each participant’s palatability ratings, four food items of comparable palatability were offered: two sweet (one carbohydrate-rich, and one protein-rich) and two non-sweet (one carbohydrate-rich, and one protein-rich).1 A carbohydrate-rich food was defined as having a carbohydrate to protein ratio of 6:1 or greater. A protein-rich food was defined as having a carbohydrate to protein ratio less than 6:1. To ensure that all food items were comparable on palatability, only those with palatability of 9 or 10 were selected (see Table 2 for sample foods and classifications). Most items were prepackaged foods (i.e., frozen pizza, French fries, string cheese, candy bars, fruit ice cups). Other items such as baked chicken, nuts, gummy bears, and chocolate pudding were pre-portioned by the study dietician. Two servings of each food item were pre-weighed and served. The order in which participants consumed each item was recorded by a research assistant. Leftovers were weighed, from which total grams consumed was calculated. Consumption in grams was later converted to calories for the analyses. Twenty participants from the parent study sample of 75 were not included in this study because, due to a miscoded food item, they inadvertently received food from only 3 of the 4 food categories. At the end of the session, participants were given a high protein drink (Sustical) and instructed to consume it the following morning.
Table 2.
Sample Foods Classified as Carbohydrate- or Protein-Rich and Sweet or Non-Sweet
| Carbohydrate | Protein | |
|---|---|---|
| Sweet | M&Ms | Cheesecake |
| Applesauce | Sweet and sour chicken | |
| Crumb cake | Choc. covered peanuts | |
| Gummy bears | Honey glazed ham | |
| Chocolate chip cookies | Chocolate pudding | |
| Non-sweet | Mashed potatoes | Peanuts |
| Popcorn | Cheese pizza | |
| French fries | String cheese | |
| Stir fried vegetables | Ham & cheese sandwich | |
| Spaghetti w/marinara | Spaghetti w/meatballs |
2.4 Statistical Analysis
Preliminary analyses compared lean and overweight participants on demographic characteristics, nausea in response to amino acid and placebo challenges, and changes in plasma amino acids from baseline to 5 hours. The dependent variable for the primary analysis of consumption was operationalized in two ways (calorie intake and first choice food).
Calorie intake model
The first model evaluated total calorie intake. Square root transformations were performed to normalize calorie data, which was positively skewed. A multivariate analysis of variance (MANOVA) for repeated measures was implemented with depression history (positive, negative) and BMI (lean, overweight) as between-subjects factors and condition (placebo, ATD), sweetness (sweet, non-sweet), and food type (carbohydrate-rich/protein-poor versus protein-rich/carbohydrate poor) as within-subjects factors. Gender, smoking status (non-smoker, smoker), depressive symptom (HAMD) response to ATD challenge, and condition order (ATD challenge first, placebo first) were entered as covariates.
First choice
The sequence in which the participant chose to consume foods was also measured. The item that a participant elected to consume first was conceptualized as the most immediately desired food. Two mixed effects logistic regression models estimated the frequencies of first choices under placebo and ATD conditions as a function of BMI group (overweight, lean). The outcome variable was dichotomous. First choice was equal to 0 for protein and 1 for carbohydrate in the first model, and then first choice was equal to 0 for non-sweet and 1 for sweet in the second model.
3. Results
3.1 Participant Characteristics
Participants (N=55) were 55% female and had a mean age of 33.19 years (SD=10.85). The sample was 62% Caucasian, 24% African-American, 12% other, and 2% unknown. BMI ranged from 18.64 to 32.39. Thirty-six participants were lean (BMI between 18.5 and 24.9) and 19 were overweight (BMI between 30 and 34.9). Mean BMI was 21.82 (sd = 1.67) for participants in the lean group (BMI < 25) and 27.75 (sd=1.95) for participants in the overweight group. A total of 30 participants were smokers (21.8 ± 10.4 cigarettes per day, 18.4 ± 11.2 years). Almost half of participants (n=25; 45%) had a history of MDD, because individuals with a history of MDD were selectively recruited for the parent study where history of MDD was an independent variable.
Table 3 displays demographic characteristics of lean and overweight participants. No differences were observed between lean and overweight participants on demographic variables, MDD history, or smoking status. The ATD mixture did not appear to cause significant nausea, as evidenced by the lack of difference in Visual Analogue Scale scores of nausea between baseline and 5 hours (p = .50). Scores were 6.9 ± 13.4 at baseline and 10.2±20.0 at 5-hours. The possible range of values was 0–100. No differences were observed between low and high BMI groups in either condition in Visual Analogue Scale scores of hunger between baseline and 5 hours [PBO: t(48)=.108, p=.914; AA: t(49)=−.323, p=.748]. Further, no differences were observed between low and high BMI groups in either condition on change in tryptophan [PBO: t(32)=−1.148, p=.260; AA: t(32)=.344, p=.733] or the ratio of tryptophan to other LNAAs [PBO: t(32)=1.492, p=.145; AA: t(32)=.055, p=.956].
Table 3.
Demographic characteristics of participants.
| Lean (n=36) | Overweight (n=19) | |
|---|---|---|
| Age | 32.51 (11.52) | 34.21 (9.39) |
| Gender | ||
| (% female) | 64% | 42% |
| Ethnicity (%) | ||
| Caucasian | 61% | 58% |
| African American | 22% | 32% |
| Hispanic | 6% | 0% |
| Asian | 9% | 5% |
| Missing | 2% | 0% |
| Smokers | 58% | 58% |
| Depression History | 44% | 47% |
Notes. Values in parentheses are standard deviations.
3.2 Caloric Intake
The repeated measures MANOVA for total calorie intake revealed no significant interaction of condition by BMI, F(1,46)=.40, p =.52, indicating that the effect of ATD on caloric consumption did not differ between lean and overweight participants. The carbohydrate hypothesis, which suggested that ATD would affect carbohydrate consumption, was not supported, F(1,46) = .32, p =.86. The three-way interaction of condition by food type by BMI was also not significant, F(1,46) = .41, p =.52.
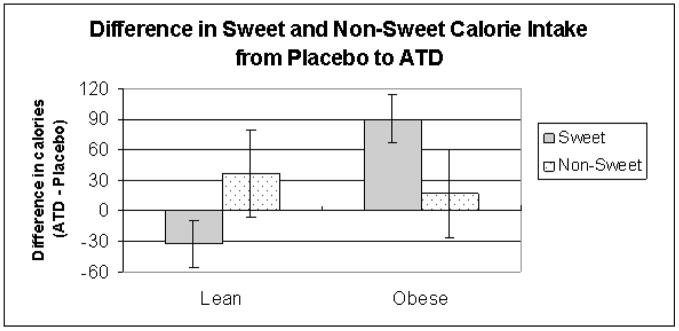
The sweetness hypothesis, which suggested that ATD would affect sweet calorie consumption was supported by a significant condition by sweetness interaction, F(1,46) = 6.90, p =.01. Participants consumed on average 10.78 calories more of sweets during ATD compared to placebo. They also consumed 28.71 calories more of non-sweets during ATD compared to placebo. BMI moderated this effect, as revealed by a significant interaction between BMI and condition and sweetness, F(1,46) = 7.23, p =.01. This significant interaction was followed by post-hoc paired comparisons, which were not significant for lean participants, t(33) = .99, p = .32, but significant for overweight participants, t(18) = −2.37, p = .02. Overweight participants increased their sweet intake in ATD compared to placebo by an average of 91 calories (see Figure 1). Although statistically non-significant, lean participants decreased their sweet intake by an average of 32 calories. Non-sweet intake increased slightly for both overweight and lean participants by an average of 16.97 and 36.82 calories, respectively, in ATD. The significant interaction between BMI, ATD, and sweetness persisted when gender and MDD history were included in the model. Table 4 shows the mean consumption of total calories, carbohydrate/protein calories and sweet/non-sweet calories across the two conditions.
Figure 1.
Difference in sweet and non-sweet calorie intake between placebo and ATD
Table 4.
Total, carbohydrate, protein, sweet and non-sweet caloric intake of participants in each condition.
| Lean (n=36) | Overweight (n=19) | |
|---|---|---|
| Total | ||
| Placebo | 882.38 (492.24) | 1041.43 (681.57) |
| ATD | 886.26 (328.83) | 1148.99 (738.54) |
| Carbohydrate | ||
| Placebo | 290.68 (214.18) | 353.94 (245.92) |
| ATD | 307.98 (203.16) | 371.28 (277.20) |
| Protein | ||
| Placebo | 591.41 (448.35) | 687.49 (611.02) |
| ATD | 578.27 (305.13) | 777.71 (773.82) |
| Sweet | ||
| Placebo | 266.76 (202.85) | 254.74 (256.19) |
| ATD | 234.10 (133.97) | 345.33 (286.95) |
| Non-sweet | ||
| Placebo | 615.33 (395.77) | 786.69 (563.64) |
| ATD | 652.15 (301.87) | 803.66 (561.62) |
We also evaluated whether the effect of ATD on sweet food consumption persisted after adjusting for ATD associated differences in depressive symptoms. This was defined as the HAMD score at 7 hours on the ATD day subtracted from the HDRS score at 7 hours on the placebo day. This variable was entered into the calorie intake model as a covariate. Results showed that the difference between conditions in depressive symptom score at 7 hours had no effect on calorie intake and was thus removed from the model.
First Choice
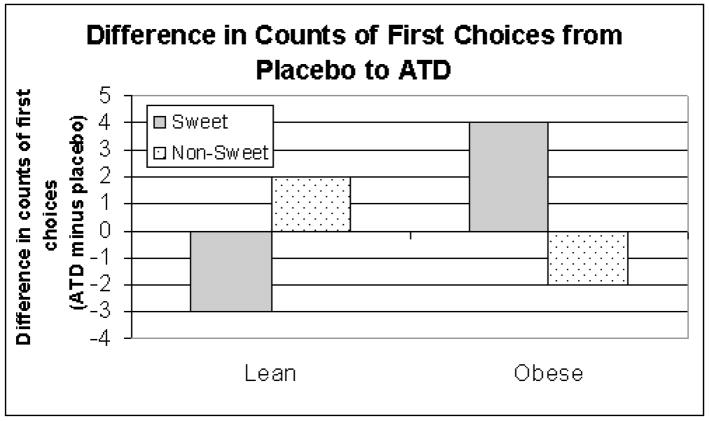
In the carbohydrate model, condition did not significantly interact with body mass index (z =.60, p = .54). In the sweet model, BMI and condition significantly interacted (z = 2.15, p = 01). Among lean participants, ATD diminished the odds of sweet being selected as first choice. Among overweight participants, ATD increased the odds of a sweet being selected as first choice (See Figure 2).
Figure 2.
Change in number of participants making sweet and non-sweet first choices from placebo to ATD2
4. Discussion
Our study is the first to reveal a differential intake among overweight as compared to lean individuals in response to ATD. Overweight participants increased their sweet calorie intake in response to ATD relative to placebo. Lean individuals actually tended to decrease their sweet calorie intake in response to ATD. On average, overweight participants consumed 123 more calories of sweet-tasting food than their lean counterparts. We also found that overweight participants responded to ATD by choosing to eat sweet foods first, before consuming non-sweet foods. In contrast, lean participants responded to ATD in the opposite manner, that is, they choose to eat non-sweet foods first. These effects on sweet food calorie intake and preference persisted after adjusted for gender, MDD history, and any ATD associated depressive symptoms.
Results of the present study suggest that acutely lowering 5-HT has a selective influence on sweet food consumption. The present findings are consistent with previous research that links sweet, palatable food intake to 5-HT deficiency (Asin et al., 1992; Wogar et al., 1991). At first glance, results appear to be at variance with prior studies showing that indirect serotonergic agonists selectively reduce elevated intake of carbohydrates, independent of sweetness (Wurtman et al., 1993; Wurtman et al., 1981). However, since the high-carbohydrate snack foods often overconsumed by overweight dysphoric adults also tend to be sweet (Drewnowski, Kurth, Holden-Wiltse, & Saari, 1992), the discrepancy may be more apparent than real.
The behavioral pattern of reacting to 5-HT deficiency and emotional distress by increasing sweets intake resembles the clinical profile in atypical depression, which is often accompanied by overweight (Paykel, Parker, Rowan, Rao, & Taylor, 1983). Binge eating is at the extreme end on a continuum of emotionally-triggered eating that can vary considerably in frequency and energy content. In some overweight and obese individuals the 5-HT neuronal system may be dysregulated, similar to what is observed in bulimics (Kaye et al., 2000). Jimerson and colleagues propose that binge eating increases the plasma tryptophan to large neutral amino acid ratio, increasing brain tryptophan availability and enhancing 5-HT synthesis (Jimerson, Lesem, Kaye, & Brewerton, 1992). The current findings raise the prospect that serotonin plays a role in the overeating patterns of overweight and obese individuals similar to its involvement in bulimic binge eating.
Intake of sweets has also been shown to release mesolimbic dopamine (DA) in nonhumans (Avena & Hoebel, 2003; Salamone, Cousins, McCullough, Carriero, & Berkowitz, 1994), not unlike drugs of abuse. Serotonin neurons, in turn, inhibit mesolimbic DA neurons (Alex & Pehek, 2007; Rothman & Baumann, 2006). Reduced 5-HT synthesis via ATD has been shown to diminish serotonergic inhibitory control of the dopaminergic system which stimulates DA release, craving, and compulsive drug-seeking (Cox et al., 2006). Possibly, via an indirect effect on DA release, ATD may both heighten the incentive salience of sweet foods (Robinson & Berridge, 1993, 2000; Roiser et al., 2006) and undermine control of the impulse to respond to rewarding treats (Olausson, Engel, & Soderpalm, 2002).
The ATD challenge we used in the current study (neurotransmitter challenge combined with psychological negative mood induction) may also have acted as a stressor by perturbing brain neuroregulatory and psychological coping systems thereby provoking emotional discomfort, particularly in populations prone to depression (Spring et al., 2007). Data from human experiments show that undergoing a stressor increases the reward value of palatable treat foods (Spring et al., 2003). Dallman and colleagues (M. F. Dallman et al., 2004; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004) posit that preference for high energy “comfort foods” following stress has evolved to replenish the energy stores depleted by physiological and behavioral coping responses. Nonhuman data show increased intake of sweet and fatty foods after various stressors (M. F. Dallman et al., 2004; Dess, Choe, & Minor, 1998), as well as an association with abdominal obesity (M.F. Dallman, Pecoraro, & la Fleur, 2005). Our finding that ATD increased overweight participants’ consumption of calories from sweets suggests a need to elucidate common neurobiological mechanisms whereby ATD and other stressors promote increased consumption of sweets leading to overweight and obesity.
This study has several limitations. First, the range of BMI was restricted by exclusion criteria. Differences between the lean and overweight groups might have been greater if more obese individuals (BMI ≥ 35) had participated in the study. Those with class II or greater obesity were excluded because it remains unclear whether the amino acid dosages used in the present ATD protocol are effective in more obese individuals. In addition, self-reported measures of height and weight were used instead of more objective measures. Second, carbohydrate-rich foods were not purely carbohydrate in composition and protein-rich foods were not purely protein, because single nutrient foods rare in nature or in a typical diet. Carbohydrate-rich foods were chosen to be have at least a >6:1 carbohydrate to protein because such foods have insufficient protein to block the insulin-mediated rise in plasma tryptophan after carbohydrate intake (Yokogoshi & Wurtman, 1986). Illustrativecarbohydrate-rich menu items included potato chips, spaghetti, and M&M candies. Illustrative protein-rich foods were baked chicken, cheese, and roasted nuts. A third limitation is that the first food consumed might not have necessarily been the most immediately desired food. This assumption was based on findings that palatable food cues trigger activation of brain regions that enhance preferences for immediate over delayed rewards (Hariri et al., 2006; Kelley, Schlitz, & Landry, 2005; McClure, Ericson, Laibson, Lowenstein, & Cohen, 2007; McClure, Laibson, Loewenstein, & Cohen, 2004; Tanaka et al., 2004), however other factors could have guided the first food consumed. Finally, the sample size was estimated based on the hypotheses of the parent study. The number of participants tested may, therefore, may have been insufficient to detect differences in food consumption across conditions, given that previously observed effects of ATD on food intake have been modest (Oldman et al., 1995). On the other hand, however, some studies using much smaller sample sizes (N=20) did detect effects of ATD on food intake in recovered bulimics (e.g., (Weltzin et al., 1995)). The present study was an initial exploration into the effect of ATD on food intake in a non-eating disordered population, larger studies are merited to further explore this effect which will help elucidate the role of serotonin in “comfort eating.”
In conclusion, the present study provides evidence of serotonergic involvement in the food consumption of overweight individuals. Acutely lowering serotonin synthesis by tryptophan depletion heightened the intake of sweet-tasting foods by overweight individuals, regardless of gender, smoking status, history of MDD, or level of depressive symptoms. Whether tryptophan depletion enhanced the incentive salience of the rewarding food (Robinson & Berridge, 1993, 2000) or interfered with the ability to modulate food intake warrants further investigation.
Acknowledgments
This study was supported by a K23 HL073381 to Sherry Pagoto, PhD; an R01 HL59348, a VA Merit Review award to Bonnie Spring, PhD; a K08 DA00467 to Dennis McChargue; and a by K08 DA017145 to Brian Hitsman, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacology & Therapeutics. 2007;11:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin KE, Davis JD, Bednarz L. Differential effects of serotonergic and catecholaminergic drugs on ingestive behavior. Psychopharmacology (Berlin) 1992;109:415–421. doi: 10.1007/BF02247717. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacology, Biochemistry, and Behavior. 2003;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- Bizot J, Le Bihan C, Puech A, Hamon M, Thiebot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400–412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- Cox SM, Benkelfat C, Dagher A, Delaney JS, McKenzie SA, Kolivakis T, et al. Cocaine self-administration in humans: A PET study of serotonin-dopamine interactions. Neuropsychopharmacology. 2006;31:S144. [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behavioral Brain Research. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: Glucocortocoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain Behavior and Immunity. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Archives of Geeral Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Aghajanian GK, Heninger GR, et al. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Archives of General Psychiatry. 1994;51:865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- Dess NK, Choe S, Minor TR. The interaction of diet and stress in rats: High-energy food and sucrose treatment. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:60–71. doi: 10.1037//0097-7403.24.1.60. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: Carbohydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US Adults, 1999–2000. Journal of the American Medical Association. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. Separate systems for serotonin and leptin in appetite control. Annals of Medicine. 2000;32:222–232. doi: 10.3109/07853890008998829. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanotin C, Thomas F, Jones SP, Leutenegger E, Drouin P. A comparison of sibutramine and dexfenfluramine in the treatment of obesity. Obesity Researcj. 1998;6:285–291. doi: 10.1002/j.1550-8528.1998.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Hariri A, Brown S, Williamson D, Flory J, de Wit H, Manuck S. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Journal of Neuroscience. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Journal of the American Medical Association. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Hood SD, Bell CJ, Nutt DJ. Acute tryptophan depletion. Part I: Rationale and methodology. The Australian and New Zealand Journal of Psychiatry. 2005;39:558–564. doi: 10.1080/j.1440-1614.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Archives of General Psychiatry. 1992;49:132–138. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gendall KA, Fernstrom MH, Fernstrom JD, McConaha CW, Weltzin TE. Effects of acute tryptophan depletion on mood in bulimia nervosa. Biological Psychiatry. 2000;47:151–157. doi: 10.1016/s0006-3223(99)00108-0. [DOI] [PubMed] [Google Scholar]
- Kelley A, Schlitz C, Landry C. Neural systems recruited by drug- and food-related cues. Physiology and Behavior. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Weiss GF, Shor-Posner G. Medial hypothalamic serotonin in the control of eating behavior. International Journal of Obesity. 1987;11(Suppl 3):109–123. [PubMed] [Google Scholar]
- LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. American Journal of Psychiatry. 1999;156:1771–1779. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Masellis M, Lam RW, Muglia P, Basile VS, Jain U, et al. Childhood inattention and dysphoria and adult obesity associated wtih the dopamine D4 receptor gene in overeating women with seasonal affective disorder. Neuropsychopharmacology. 2004;29:179–186. doi: 10.1038/sj.npp.1300314. [DOI] [PubMed] [Google Scholar]
- Leyton M, Young SN, Blier P, Ellenbogen MA, Palmour RM, Ghadirian AM, et al. The effect of tryptophan depletion on mood in medication-free, former patients with major affective disorder. Neuropsychopharmacology. 1997;16:294–297. doi: 10.1016/S0893-133X(96)00262-X. [DOI] [PubMed] [Google Scholar]
- McClure S, Ericson K, Laibson D, Lowenstein G, Cohen J. Time discounting for primary rewards. Journal of Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S, Laibson D, Loewenstein G, Cohen J. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McTavish D, Heel RC. Dexfenfluramine. A review of its pharmacological properties and therapeutic potential in obesity. Drugs. 1992;43:713–733. doi: 10.2165/00003495-199243050-00007. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Archives of General Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Olausson P, Engel JA, Soderpalm B. Involvement of serotonin in nicotine dependence: Processes relevant to positive and negative regulation of drug intake. Pharmacology, Biochemistry, and Behavior. 2002;71:757–771. doi: 10.1016/s0091-3057(01)00673-6. [DOI] [PubMed] [Google Scholar]
- Oldman A, Walsh A, Salkovskis P, Fairburn CG, Cowen PJ. Biochemical and behavioural effects of acute tryptophan depletion in abstinent bulimic subjects: A pilot study. Psychological Medicine. 1995;25:995–1001. doi: 10.1017/s003329170003748x. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Parker RR, Rowan PR, Rao BM, Taylor CN. Nosology of atypical depression. Psychological Medicine. 1983;13:131–139. doi: 10.1017/s0033291700050133. [DOI] [PubMed] [Google Scholar]
- Pecoraro NC, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pijl H, Koppeschaar HP, Willekens FL, Op de Kamp I, Veldhuis HD, Meinders AE. Effect of serotonin re-uptake inhibition by fluoxetine on body weight and spontaneous food choice in obesity. International Journal of Obesity. 1991;15:237–242. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Researcj Review. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction, 95 Suppl. 2000;2:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW, et al. Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology. 2006;31:2264–2272. doi: 10.1038/sj.npp.1301055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Annals of the New York Academy of Sciences. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Ruetsch O, Viala A, Bardou H, Martin P, Vacheron M. Psychotropic drug induced weight gain: A review of the literature concerning epidemiological data, mechanisms, and management. L’Encephale. 2005;31(4 Pt 1):507–516. doi: 10.1016/s0013-7006(05)82412-1. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacology, Biochemistry, and Behavior. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Shor-Posner G, Grinker JA, Marinescu C, Brown O, Leibowitz SF. Hypothalamic serotonin in the control of meal patterns and macronutrient selection. Brain Research Bulletin. 1986;17:663–671. doi: 10.1016/0361-9230(86)90198-x. [DOI] [PubMed] [Google Scholar]
- Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- Smith KA, Fairburn CG, Cowen PJ. Symptomatic relapse in bulimia nervosa following acute tryptophan depletion. Archives of General Psychiatry. 1999;56:171–176. doi: 10.1001/archpsyc.56.2.171. [DOI] [PubMed] [Google Scholar]
- Spring B, Chiodo J, Bowen DJ. Carbohydrates, tryptophan, and behavior: a methodological review. Psychological Bulletin. 1987;102:234–256. [PubMed] [Google Scholar]
- Spring B, Hitsman B, Pingitore R, McChargue DE, Gunnarsdottir D, Corsica J, et al. Effect of tryptophan depletion on smokers and nonsmokers with and without a history of depression. Biological Psychiatry. 2007;61:70–77. doi: 10.1016/j.biopsych.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Spring B, Pagoto SL, McChargue D, Hedeker D, Werth JC. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacology, Biochemistry and Behavior. 2003;76:351–360. doi: 10.1016/j.pbb.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Strain GW, Strain JJ, Zumoff B. L-tryptophan does not increase weight loss in carbohydrate-craving obese subjects. International Jouranl of Obesity. 1985;9:375–380. [PubMed] [Google Scholar]
- Tanaka S, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neuroscience. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Toornvliet AC, Pijl H, Hopman E, Westendorp RG, Meinders AE. Predictors of weight loss during treatment with d-fenfluramine. Journal of Internal Medicine. 1997;241:401–406. doi: 10.1046/j.1365-2796.1997.131151000.x. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. New England Journal of Medicine. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Brownell KD, Foster GD. Obesity: Responding to the global epidemic. Journal of Consulting & Clinical Psychology. 2002;70:510–525. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- Weltzin TE, Fernstrom MH, Fernstrom JD, Neuberger SK, Kaye WH. Acute tryptophan depletion and increased food intake and irritability in bulimia nervosa. American Journal of Psychiatry. 1995;152:1668–1671. doi: 10.1176/ajp.152.11.1668. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Evidence for an involvement of 5-hydroxytryptaminergic neurones in the maintenance of operant behaviour by positive reinforcement. Psychopharmacology (Berlin) 1991;105:119–124. doi: 10.1007/BF02316873. [DOI] [PubMed] [Google Scholar]
- Wurtman J, Wurtman R, Berry E, Gleason R, Goldberg H, McDermott J, et al. Dexfenfluramine, fluoxetine, and weight loss among female carbohydrate cravers. Neuropsychopharmacology. 1993;9:201–210. doi: 10.1038/npp.1993.56. [DOI] [PubMed] [Google Scholar]
- Wurtman J, Wurtman R, Growdon J, Henry P, Lipscomb A, Zeisel S. Carbohydrate craving in obese people: Suppression by treatments affecting serotoninergic transmission. International Journal of Eating Disorders. 1981;1:1–15. [Google Scholar]
- Yokogoshi H, Wurtman RJ. Meal composition and plasma amino acid ratios: Effect of various proteins or carbohydrates, and of various protein concentrations. Metabolism. 1986;35:837–842. doi: 10.1016/0026-0495(86)90225-8. [DOI] [PubMed] [Google Scholar]