Abstract
Background
Past studies have clearly established that matrix metalloproteinases (MMPs) contribute to adverse myocardial remodeling with ischemia and reperfusion. However, these studies measured MMP levels in extracted samples, and therefore whether and to what degree actual changes in interstitial MMP activity occur within the human myocardium in the context of ischemia/reperfusion remained unknown.
Methods
The present study directly quantified MMP interstitial activity (MMPact) within the myocardium of patients (n=14) undergoing elective cardiac surgery during steady-state conditions, as well as during and following an obligatory period of myocardial arrest and reperfusion achieved by cardiopulmonary bypass (CPB). Interstitial MMP activity was continuously monitored using a validated MMP fluorogenic substrate, a microdialysis system placed within the myocardium, and in-line fluorescent detection system.
Results
MMP activity, as measured by fluorescent emission, reached a stable steady state level by 10 minutes following deployment of the microdialysis system. During initiation of CPB, MMP activity increased by 20% from baseline values (p<0.05), and then rapidly fell with cardiac arrest and longer periods of CPB. However, with restoration of myocardial blood flow and separation from CPB, MMP interstitial activity increased by over 30% from baseline (p<0.05).
Conclusions
The present study directly demonstrated that MMP proteolytic activity exists within the human myocardial interstitium and is a dynamic process under conditions such as myocardial arrest and reperfusion.
Keywords: microdialysis, cardiac surgery, fluorogenic substrates, extracellular matrix
INTRODUCTION
The matrix metalloproteinases (MMPs) are a family of enzymes that are involved in proteolytic processing of interstitial structural proteins, signaling molecules and growth factors.1–4 Through altering interstitial tissue structure and function, expression of MMPs have been shown to be an important proteolytic event in tumor progression, inflammation, and cardiovascular disease.4–6 For example, increased MMP levels within the myocardium have been demonstrated in animal models of ischemia and reperfusion and heart failure.7–14 Using transgenic and pharmacological approaches, modifying MMP expression and activity has been shown to directly effect myocardial structure and function.15–18 Moreover, clinical studies have documented important relationships between blood levels of MMPs and the progression of heart failure.19–21 While clinical studies have shown an association between MMP levels and disease progression, there have been no studies which have directly demonstrated and quantified interstitial MMP activity within the human myocardium and to what extent interstitial MMP activity is altered as a function of interruption of myocardial blood flow and with reperfusion.
Past studies have quantified relative MMP levels in tissue or blood samples utilizing an ex-vivo approach.15–21 While changes in the relative ex-vivo abundance of MMP types is an important consideration with respect to in-vivo proteolytic activity, this approach can be problematic for several reasons. First, measuring MMP levels in tissue samples requires extraction techniques which separate the enzyme substrate binding domains and alters interactions with interstitial proteins. Second, MMPs are synthesized in a pro-enzyme form and require a localized and highly orchestrated set of biochemical events for full activation.1,3,4 Third, MMP activity is also determined by the relative amount, location and interaction of a family of proteins, the tissue inhibitors of MMPs (TIMPs).1,3,4 Tissue/blood extraction techniques will often result in disassociation of MMP-TIMP complexes that would be present in-vivo. In order to avoid the inherent limitations to these ex-vivo approaches, the current study utilized an in-situ approach which allowed for measurement of total interstitial MMP activity. This laboratory has reported previously that the placement of a microdialysis membrane within the myocardial allows for continuous sampling of the myocardial interstitial fluid and measurement of local bioactive peptides.22 In addition, it has been demonstrated that small peptides can be utilized which change fluorescent characteristics when specifically cleaved by active MMPs.23,24 Moreover, using in-line micro-fluorimetry, and using calibrated algorithms, this laboratory has demonstrated that the fluorescent signal reflects actual MMP activity.12,25,26 Using this microdialysis-fluorimetry system, myocardial interstitial MMP activity was continuously recorded within the human myocardium under steady state conditions and following myocardial arrest and reperfusion.
METHODS
Patients
This protocol was approved by the Human Subjects Review Committee of the Medical University of South Carolina (HR#9435) and by the affiliated Ralph H. Johnson Veterans Affairs Medical Center Research and Development Committee. Patients undergoing elective coronary artery bypass surgery requiring cardiopulmonary bypass (CPB) provided informed consent to participate in the study (n=14). The study was conducted at the Ralph H. Johnson Veterans Affairs Medical Center, and because the patient population at this institution is primarily male, this study was conducted in male patients only. The inclusion criteria included: ≥18 and ≤80 years of age; body mass index <40 kg/m2; left ventricular ejection fraction >35%; no heart failure symptoms (NYHA Class I), if diabetic, be under proper control (fasting glucose <350 mg/dL or recent hemoglobin A1c [HgbA1c] <9%); if hypertensive, be on a stable medical regimen with no significant changes over the past 30 days. The mean age for the 14 male subjects enrolled in this study was 65±3 years with a left ventricular ejection fraction of 63±2%.
Operative Procedure
The coronary revascularization procedure utilizing CPB followed standard operative protocols and has been described in greater detail previously.10,22 Standard induction and maintenance of anesthesia was accomplished with a combination of sufentanyl, midazolam and isoflurane. CPB was maintained at a cardiac index of 2.0 to 2.4 l/min/m2 and initial cardioplegic arrest was accomplished with antegrade normothermic administration of a 200 mL of a solution of D5/0.2 NaCL containing 29 ml of tromethamine buffer, 34 mL of adenosine citrate phosphate dextrose and 60 meq of KCL (120 meq/L) in a 4:1 blood:crystalloid mixture to produce cardiac arrest. This was followed immediately with antegrade administration of 1000 ml of hypothermic cardioplegic solution. At the termination of CPB, heparin was neutralized with protamine in a 1:1 ratio. The microdialysis probe was placed as described below, immediately following the sternotomy and pericardial incision allowing for full visualization of the left ventricular free wall. The microdialysis probe was removed following separation from CPB, removal of all CPB cannulae, and prior to sternotomy closure.
Microdialysis Instrumentation and MMP Activity Measurements
A sterile microdialysis probe containing a 4 mm long membrane (20 kDA, outer diameter of probe shaft 0.77mm; CMA/Microdialysis, North Chelmsford, MA) was placed into the mid-myocardium of the beating left ventricle and directed toward the left anterior descending coronary artery (LAD) parallel to the diagonal branches of the LAD. The probe was positioned using needle guidance and maintained in place utilizing a 4-0 Prolene suture. The probe was placed in a normally perfused region of the myocardium (based upon angiographic analysis) devoid of large coronary vessels. We have placed this microdialysis probe in patients undergoing cardiac surgery previously in order to measure local endothelin concentrations.22 Moreover, we have demonstrated that this surgical approach for placement of the microdialysis probe was not associated with alterations in blood flow or extravasation of red blood cells, did not change local bioactive peptide levels, and was not associated with an acute inflammatory response.12,25,26 Both the inflow and outflow ports of the microdialysis probe were connected to sterile tubing (PEEK, 0.12 mm ID) and then passed from the operating field. Maintaining sterile technique at all times, the inflow of the microdialysis probe was connected to a computer controlled micro-infusion syringe pump (Bioanalytical Systems, West Layfayette, IN) containing a sterile MMP fluorogenic substrate solution as described in the following paragraph. The microdialysis inflow rate was 6 uL/min, previously established to provide optimal steady-state flow characteristics within the myocardial interstitium.12,22,25,26 The outflow tube was connected to an in-line fluorescence detector system (FIAlab PMT-FL Detector, FIAlab Instruments, Inc, Bellevue, WA). Using this approach, the returning microdialysate traveled through a UV detection cell, (Model D-1000CE, Analytical Instruments Systems, Inc, Flemington, NJ) which was set was at 280nm. Subsequent UV emission was collected at 360 nm by an integrated photomultiplier tube and then converted to a digital signal (FIAlab Software, ver. 5). The entire closed volume from the microdialysis probe to the detection cell was 28 uL and the digitized fluorescence signal was collected at 4 second intervals. Thus, the response time of this system, as defined as the time delay for a change in fluorescence occurring at the site of the microdialysis probe to be detected as a change in fluorescence emission, was approximately 4 minutes.
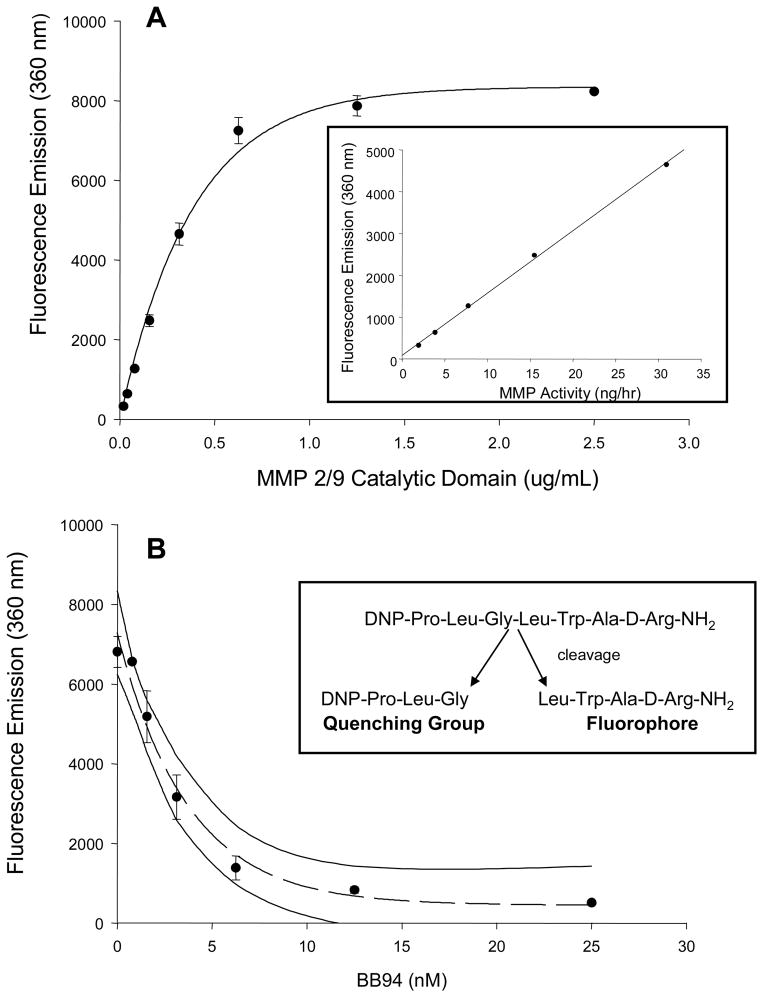
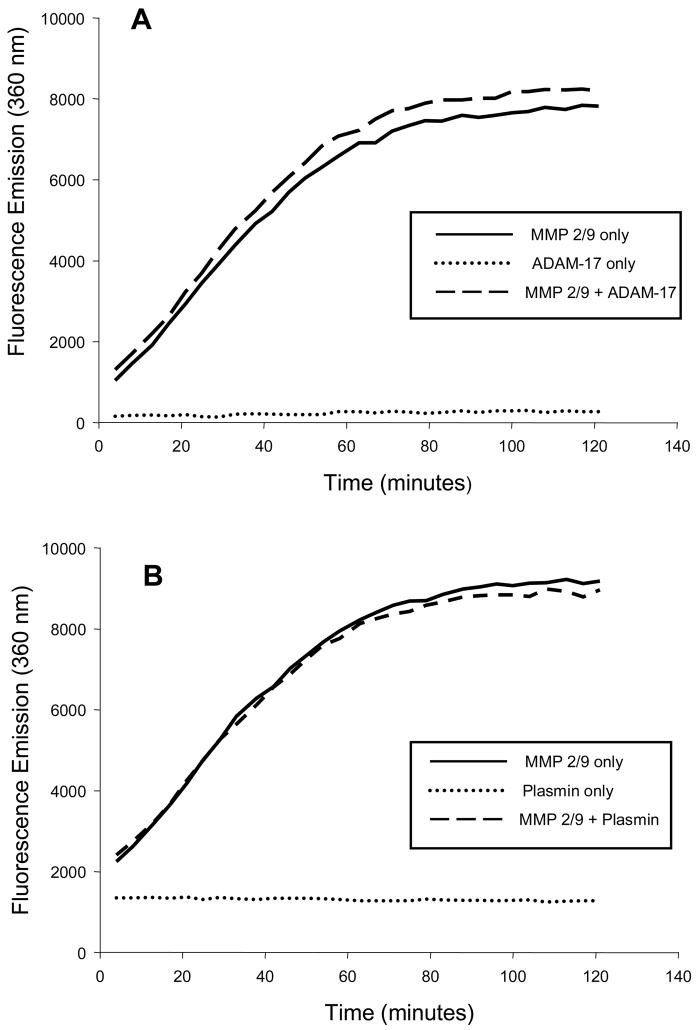
The infusion solution used in the microdialysis system contained an MMP fluorogenic substrate (Anaspec #27074) that is cleaved by all active MMPs with equivalent kinetics.23,24 This MMP fluorogenic peptide will only yield a detectable UV emission when proteolytically processed at a specific amino acid sequence (Figure 1).23,24 Since we have demonstrated that the interstitial space surrounding the microdialysis probe is a closed compartment system,12,22,25 this quenched MMP fluorogenic substrate will equilibrate with the interstitial space, and therefore the microdialysate fluorescence will reflect MMP activity.23,24 We have demonstrated that steady-state interstitial fluorescence of this MMP substrate can be achieved within approximately 5 minutes.12,25 Prior to the patient microdialysis studies, a series of critical validation procedures were performed for the combined use of the MMP substrate and the microdialysis system. We first used an in-vitro microdialysis model,12,22 where a recombinant active MMP construct (MMP-2/9 BIOMOL, SE-237, SE-244) was present in the surrounding fluid compartment. The entire microdialysis system was protected from ambient light and the dialysate collected into chilled amber tubes and immediately processed for fluorimetry (280/360 nm). A step-wise increase in MMP activation of the fluorogenic substrate was observed and this calibration curve could be linearized using regression analysis (Figure 1). When the MMP substrate/MMP catalytic reaction was recorded in the presence of increasing concentrations of an MMP inhibitor (BB94, British Biotech),25 fluorescence emission was eliminated (Figure 1). The specificity of this MMP fluorogenic substrate was further validated through incubation with other proteases such as a disintegrin and metalloprotease-17 (ADAM-17) and the serine protease, plasmin (Figure 2). Fluorescent emission was absent when incubated with these other proteases, but could be quickly restored with co-incubation of the MMP catalytic domain. In previously performed in-vivo animal experiments, we determined that optimal response was achieved with a 60 uM MMP substrate concentration25 and this was utilized in the present study.
Figure 1.
A) Validation of the MMP fluorogenic substrate (0.03 mM, Anaspec, # 27074) was performed by using increasing concentrations of an MMP 2/9 recombinant catalytic domain (BIOMOL, SE-237,SE-244). Following an incubation period of 2 hrs at 37°C, fluorescence emission was determined and yielded a classic enzymatic activity curve. Inset: Using the linear portion of the fluorescence emission-concentration curve, MMP activity (ng of MMP catalytic domain with respect to time) was calculated (y = 148.98x + 87.8; r2 = 0.9989; p > 0.0001) B) The specificity of the fluorescence emission was next established by incubating the MMP substrate with the MMP catalytic domain (1.25 ug/mL) in the presence of increasing concentrations of a broad spectrum MMP-inhibitor (BB94, British Biotech). Fluorescence emission was reduced in an exponential decay fashion with the MMP inhibitor (y = 446.6 + 6841e−0.2696x), indicative of quenching proteolytic processing of the MMP substrate. The 95% confidence interval of this inhibition curve is shown by the two solid lines. Inset: The structure of the global MMP fluorogenic substrate utilized in this study is shown with arrows indicating the site of specific MMP proteolysis.
Figure 2.
In order to further examine the specificity of the MMP substrate (0.03 mM) was incubated in the presence of (A) a disintegrin and metalloprotease, ADAM-17 (1.25 ug/mL, R&D Systems, #930-ADB), or (B) with the serine protease plasmin (1.25 ug/mL, Sigma, #P1867) and fluorescence emission continuously monitored at 37degC. There was no fluorescence emission detected with either of these non-MMP proteases (dotted line). However, with co-incubation of the protease and the MMP 2/9 catalytic domain (1.25 ug/mL), fluorescence emission consistent with cleavage of the MMP substrate was achieved (dashed line).
In the present study, following placement of the microdialysis probe and making all connections, the MMP fluorogenic substrate was infused through the entire microdialysis system for a 20 minute equilibration period prior to data collection. Following this equilibration period, fluorescent emission was continuously recorded under steady-state conditions, during cardioplegic arrest and CPB, and for up to 20 minutes following separation from CPB. The interstitial fluid, following passage through the detection cell, was collected in chilled 0.5 mL tubes and subjected to cytokine measurements.
Plasma MMP Profiling and Interstitial Cytokine Assay
Blood samples (5 mL) were obtained immediately following placement of the microdialysis probe and again immediately following removal of the microdialysis probe. Plasma from these samples was then subjected to enzyme linked multiplex suspension array (Human Fluorokine MAP MMP Kit, R&D Systems, LMP000) in order to measure representative subtypes from each class of MMPs which included the gelatinases (MMP-2 and MMP-9), the stromelysins/matrilysins (MMP-3/MMP-7) and the collagenases (MMP-8). The relative fluorescence obtained for each distinct MMP (Bio-Plex 200, BioRad Laboratories) was converted to an absolute concentration using standards that were included in each assay. Differences in hemodilution effects were taken into account by normalizing the computed MMP values by hematocrit.10,22 The interstitial fluid (20 uL) collected during the study intervals was subjected to enzyme linked multiplex suspension array (Human Fluorokine MAP Cytokine Kit, R&D Systems, LMH000) in order to measure interleukin-6 (IL-5), tumor necrosis factor (TNF) concentrations (sensitivity of assay <1 pg/mL). These cytokines were chosen for several reasons. First, the molecular weight of these cytokines would allow for rapid traversal and equilibration with respect to the microdialysis membrane.27,28 Second, these cytokines have been measured using a microdialysis approach and demonstrated a high fidelity.27,28 Third, these cytokines would be indicative of an acute inflammatory response.
Data Analysis
The continuous fluorescence emission values were digitized and a running real-time average was obtained coincident with clinically relevant time points. The baseline, normalized fluorescence emission was subjected to analysis of variance (ANOVA) followed by pairwise tests of individual group means using Bonferroni adjusted probabilities. Potential relationships between changes in blood glucose and white blood cell counts were performed using a correlation analysis. For the MMP plasma profiles, pair-wise comparisons were performed using a t-statistic. All statistical procedures were performed using STATA statistical software (STATA Intercooled V 8.0. College Station, TX). Results are presented as mean ± standard error of the mean (SEM). Values of p<0.05 were considered to be statistically significant.
RESULTS
The total duration of cardiopulmonary bypass (CPB) was 73±4 minutes, the duration of myocardial arrest (aortic cross clamp time) was 61±4 minutes, and an average of 3 coronary grafts were placed. Cardiac output was 5.2±0.5 L/min at baseline and was similar immediately following separation from CPB (5.9±0.4 L/min). The placement of the microdialysis probe was not associated with any adverse events, and all patients were successfully extubated within 12 hours and discharged from the intensive care unit within 24 hours following CPB. Baseline blood glucose levels were 150±22 mg/dL and were not significantly different at 12 hours post-CPB (124±10 mg/dL). The total white blood cell count was 7.4±0.6 K/mm2 at baseline and was significantly increased at 12 hours post-CPB (10.3±0.7 K/mm3, p<0.05).
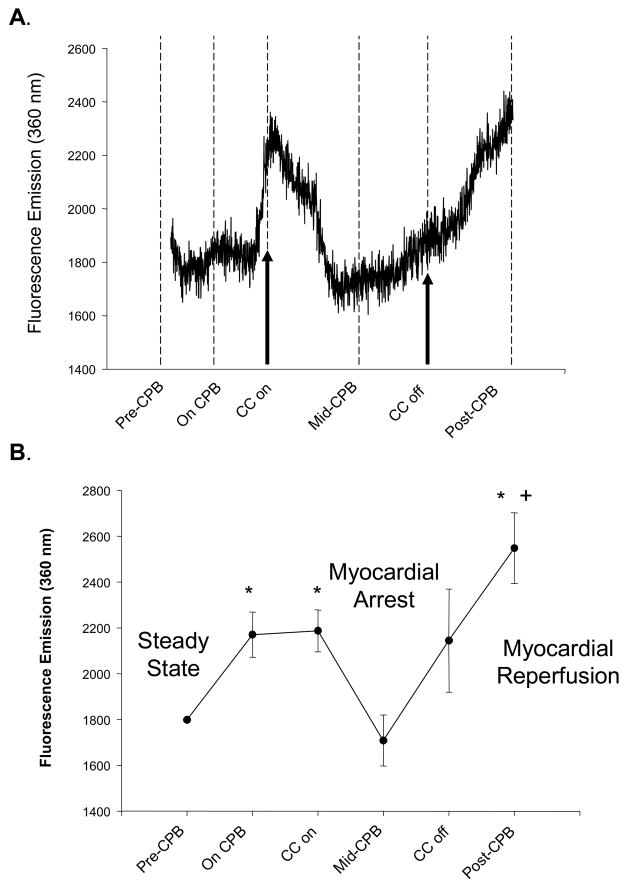
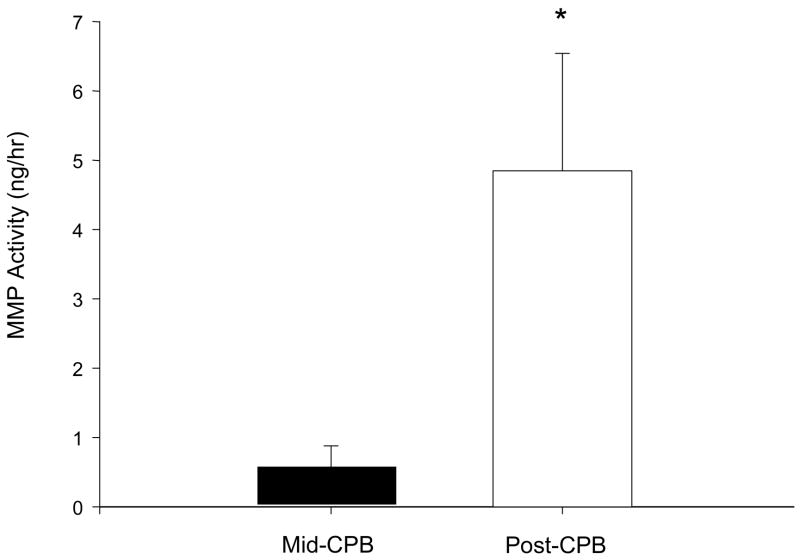
A representative continuous readout of interstitial fluorescence emission, reflective of interstitial MMP activity, is shown for a single patient in Figure 3A. The summary of these fluorescent measurements for all patients, coincident with specific time points of this study, is shown in Figure 3B. Fluorescence emission within the myocardial interstitial samples increased from steady-state, baseline values with the institution of CPB and interruption of myocardial blood flow (aortic-cross-clamp placement), but fell to within baseline values with longer periods of myocardial arrest. With myocardial reperfusion and separation from CPB, fluorescence emission increased from myocardial arrest and from baseline. Using the in-vitro calibration curve (Figure 1) and the running average fluorescence emission values obtained both at the mid-point of the CPB interval and at the final post-CPB time point (just prior to probe removal), actual myocardial interstitial MMP activity was computed and is summarized in Figure 4. MMP activity increased by approximately 4-fold from the period of myocardial arrest to reperfusion. There were no significant relationships observed between absolute values or relative changes in blood glucose (r=0.19, p=0.51) or white blood cell counts (r=−0.35, p=0.25) to interstitial MMP activity.
Figure 3.
(A) A representative continuous fluorescence recording of myocardial interstitial fluid from a patient undergoing coronary bypass surgery requiring cardiopulmonary bypass (CPB) and myocardial arrest. After collection of baseline recordings (Steady-State), the patient was placed on CPB and a cross-clamp placed across the ascending aorta interrupting blood flow to the myocardium (Myocardial Arrest). Cardioplegia solution was administered at pre-specified intervals during CPB and this myocardial arrest period. Restoration of myocardial blood flow was achieved by release of the aortic cross-clamp (Myocardial Reperfusion) and following restoration of sinus rhythm and ventricular pump function, the patient was separated from CPB and the probe removed. In this particular example, the baseline, steady-state collection period was 40 minutes, the cross-clamp time was 35 minutes (Myocardial Arrest time), total CPB time was 55 minutes and the probe was removed at 20 minutes following separation from CPB. The initiation of CPB caused a small increase in fluorescent emission, reflective of increased interstitial activity. With myocardial arrest, fluorescent emission fell to within steady-state levels. However, with myocardial reperfusion, there was a surge in fluorescent emission, indicative of heightened MMP activity within the myocardial interstitium. (B) An average of fluorescent emission was computed for all patients with complete recordings (n=14) at critical time points. Fluorescent emission increased with the onset of CPB, significantly fell with myocardial arrest, and increased significantly with myocardial reperfusion. (*p<0.05 vs normalized steady-state values, +p<0.05 vs CPB values prior to myocardial arrest).
Figure 4.
Using the in-vitro calibration curve generated from known MMP catalytic activity as shown in Figure 1, interstitial MMP activity was computed at the midpoint of the myocardial arrest period and at the myocardial reperfusion period, prior to microdialysis probe removal. Interstitial MMP activity increased by over 6-fold with myocardial reperfusion.
A summary of baseline plasma MMP levels for all patients are shown in Table 1 and are consistent with reference control values reported previously.10,21 Following separation from CPB and microdialysis probe removal, plasma MMP-9 and MMP-7 levels increased significantly from baseline values. Baseline interstitial fluid concentrations of IL-6 were 1.81±0.66 pg/mL and remained unchanged at the mid point of CPB 2.54±1.05 pg/mL, as well as at separation from CPB 2.34±0.47 pg/mL (all p>0.50). TNF values within the interstitial fluid samples were below detection limits (lowest standard value) at all time points.
Table 1.
MMP Plasma Profiles at Baseline, Steady-State Conditions and Following Separation from Cardiopulmonary Bypass (CPB)
| Baseline | Post –CPB | |
|---|---|---|
| MMP-2 (ng/mL) | 667.4 ± 54.9 | 756.6 ± 83.8 |
| MMP-9 (ng/mL) | 151.3 ± 16.1 | 647.5 ± 81.4 * |
| MMP-3 (ng/mL) | 7.4 ± 0.8 | 9.1 ± 1.0 * |
| MMP-7 (ng/mL) | 1.9 ± 0.4 | 3.3 ± 0.7 * |
| MMP-8 (ng/mL) | 4.6 ± 1.6 | 4.7 ± 0.8 |
n=14;
p <0.05 vs. Baseline
Values presented as Mean ± SEM
DISCUSSION
Clinical and basic studies have implicated changes in matrix metalloproteinase (MMP) activity in myocardial remodeling and dysfunction which can occur following periods of ischemia and reperfusion.4,7–12 However, MMP activity within the myocardial interstitium is regulated by a complex number of factors such as abundance, activation state, and the presence of endogenous inhibitors.1–4,14 Thus, whether and to what degree MMP activity is directly altered within the human myocardial interstitium during a period of myocardial arrest, with a cessation of blood flow, followed by reperfusion, remained unknown. Accordingly, the present study developed, validated and then utilized a method to directly measure total MMP activity within the human myocardial interstitium during and following myocardial arrest and reperfusion attendant to cardiac surgery. Through the combined use of a fluorogenic substrate and microdialysis techniques, the present study demonstrated that a continuous fluorescent signal could be detected from interstitial fluid samples under hemodynamically stable, steady-state conditions. These results indicate that an ambient level of MMP proteolytic activity exists within the human myocardial interstitium. With the cessation of coronary blood flow and myocardial arrest, interstitial MMP activity transiently increased and then fell to within steady-state levels. However with reperfusion, interstitial MMP activity surged above ambient, baseline levels. Thus, this study for the first time demonstrated that MMP activity exists within the human myocardial interstitium and changes in a dynamic fashion with changes in myocardial perfusion.
In general terms, MMPs are synthesized as inactive zymogens and are secreted into the extracellular space as pro-enzymes (proMMPs), or are inserted into the membrane as fully active enzymes. The secreted proMMPs remain quiescent until the pro-peptide domain is cleaved yielding a proteolytically competent enzyme. The cleavage of the pro-peptide domain can be accomplished through several diverse routes which include serine proteases, other activated soluble MMPs, or the membrane bound MMPs.4,28 Following proteolyisis of targeted substrates, active MMPs can undergo autocatalytic degradation to an inactive form as well as form complexes with tissue inhibitors of MMPs (TIMPs).1–4 TIMPs will bind to critical domains on MMPs and therefore constitute an important determinant of overall MMP activity. Accordingly, net MMP proteolytic activity within the interstitium is dependent upon the summation of a number of transcriptional, translational and post-translational events. The majority of past studies that have examined MMPs within the myocardium have utilized detergent extraction and electrophoretic separation techniques on harvested myocardial samples.9,13–18 Using this in-vitro approach, MMP interactions with substrates and TIMPs, MMP activational states, as well as spatial location within the myocardium, are destroyed and as a consequence, any conclusions regarding MMP activity within the intact myocardial interstitium based on these studies remained speculative. Thus, whether and to what degree any actual MMP proteolytic activity existed within the human myocardial interstitium under ambient physiological conditions remained unknown. Moreover, whether the changes in MMP transcriptional/translational/post-translational events which have been identified in animal models of ischemia-reperfusion,12,15–18,25 could be translated into in-vivo interstitial MMP activity, particularly within the human myocardium, also remained to be established. The present study demonstrated that dynamic changes in MMP interstitial activity occurred within the human myocardium. However, the underlying mechanistic cause(s) for these changes are likely to be multifactorial. For example, interstitial MMP activity initially increased from baseline with the onset of CPB, which may have been due to changes in mechanical factors such as systemic hemodynamics and coronary flow patterns as well as biological factors such as hemodilution and the induction of a systemic inflammatory response. With cardioplegic arrest, interstitial MMP activity sharply fell which was likely due to hypothermia and hyperkalemia, both of which would directly affect enzyme kinetics. With myocardial reperfusion of warm, oxygenated blood and induction of myocardial metabolism, a significant increase in MMP activity occurred- over and above that observed prior to myocardial arrest. Contributory factors for this rapid surge in interstitial MMP activity likely included the localized effects of ischemia-reperfusion as well as the abrupt introduction of warm blood perfusate which contained MMPs. Future studies which examine MMP interstitial activity within the myocardial compartment as well as in other tissue compartments, such as peripheral muscle, during and following CPB may provide some insight into complex systems responsible for evoking the changes in myocardial MMP activity. The present study provides the foundation for these future studies, as well as directly addressed a critical gap in our current understanding of MMP biology within the human myocardium by establishing that MMP proteolytic activity is a continuous process within the interstitium and rapidly changed as a function of myocardial arrest followed by reperfusion.
Past clinical studies regarding MMP measurements have been performed directly on samples obtained from the remodeling/remodeled myocardium.13,14,29 For example, it has been reported previously that in patients with end-stage cardiomyopathy, certain MMP types increased significantly without a concomitant increase in TIMP levels; suggestive of increased MMP proteolytic potential.29 However, repeated myocardial sampling can be problematic and therefore constructing an MMP temporal profile within the myocardium has not been performed. Accordingly, high sensitivity assays have been developed in order to measure blood concentrations of MMPs in patients which would be amenable to repeated sampling.7–10,19–21 For example, this approach has been utilized in order to serially measure changes in plasma MMPs in patients following myocardial infarction/ischemia or cardiac surgery.10,21 However, blood samples are routinely obtained from the systemic vasculature and therefore MMP concentrations from these samples reflect spillover from multiple cellular sources and tissue compartments. In addition, whether the spillover of MMPs into the plasma is directly proportional to localized MMP content within the interstitium, and the kinetics of MMP egress into the vascular compartment remain unclear. Moreover, MMPs within the blood stream are tightly complexed to high molecular weight proteins as well as by TIMPs, and therefore direct measurement of MMP plasma activity is problematic. Thus, while relative levels of MMPs within the plasma reflect spillover from local tissue compartments and can provide insight into what MMP types are being induced, these plasma measurements may not be reflective of net MMP proteolytic activity. Nevertheless, the present study measured plasma concentrations of several MMP types before and immediately after myocardial arrest and reperfusion. Differential changes in MMP plasma levels were observed following myocardial reperfusion and separation from CPB. Specifically, the present study identified a robust increase in systemic plasma MMP-9 levels following separation from CPB, whereas plasma MMP-2 levels remained unchanged at this early time point. In addition, plasma MMP-7 and MMP-3 levels increased from baseline levels at this time point. An important source of MMP-9 as well as MMP-7 are inflammatory cells such as the neutrophil and macrophage.1–4,7–9 The emergence of these MMP types within the systemic vasculature is concordant with the increased myocardial interstitial MMP activity observed at this time point. However, since CPB causes a systemic inflammatory response, then it remains speculative as to what magnitude MMP-9 and MMP-7 contributed to the surge in myocardial interstitial MMP activation following myocardial arrest and reperfusion. This is further confounded by the fact that plasma MMP levels reflect spillover of soluble MMP types, and therefore to what degree membrane-type MMPs may have contributed to the local myocardial interstitial MMP proteolytic signal cannot be determined. This may be of particular relevance since in animal models of ischemia-reperfusion, changes in membrane-type MMPs, such as MT1-MMP, have been reported and likely contributed to overall MMP activity within the myocardial interstitium.12,26
The present study utilized a small peptide with a quenched fluorescent moiety which when cleaved at a specific site, would yield a detectable fluorescent signal.23–26 Previously performed experiments,25 as well as validation experiments performed in the present study, demonstrated that this quenched fluorescent peptide would specifically yield fluorescent emission when cleaved by MMPs. Thus, we infused this peptide into the interstitial space of the myocardium as “bait” for active MMPs. The active catalytic domain of all MMP types will interact with the amino acid sequence contained within this peptide and yield a fluorescent signal. Thus, changes in fluorescence emission obtained from samples collected from the interstitium would be directly reflective of changes in overall MMP activity. This laboratory has recently demonstrated that MMP substrates with a more specific amino acid sequence, and thereby imparting greater MMP specificity, can be successfully utilized with this microdialysis system.12,26 Thus, future studies which utilize different MMP substrates coupled to this microdialysis approach would allow for identification of specific MMP types activated within the human myocardium.
In addition to identifying the presence and activation of MMPs within the human myocardial interstitium, the results of the present study set the stage for future mechanistic studies. Specifically, in past in-vitro studies, it has been demonstrated that biologically active signaling molecules and oxidative stress can directly influence MMP induction/activation.4,30,31 The present study demonstrated the feasibility of directly measuring MMP activity within the interstitium as well as performing high sensitivity cytokine assays on the microdialysate. These initial measurements demonstrated that IL-6 levels could be detected within the myocardial interstitium, which remained unchanged during CPB and myocardial arrest with reperfusion. The significance of this observation is 2-fold. First, stable IL-6 concentrations within the interstitial fluid surrounding the microdialysis probe suggests that a localized inflammatory response was not evoked by probe placement in and of itself. Second, the stable interstitial IL-6 levels would suggest that this specific cytokine may not play a significant role in the early induction of myocardial interstitial MMP activation which occurred following myocardial arrest and reperfusion. It must also be recognized that other approaches are possible to provide an actual index of MMP interstitial activity. For example, non-invasive imaging methods which utilize a radiolabelled MMP substrate have been reported in animals.32 However, these imaging methods have not been applied to the human myocardium and would not provide for continuous monitoring of interstitial MMP activity. Finally, while the present study demonstrated that MMP activity can be continuously monitored during a period of myocardial arrest and reperfusion, the potential diagnostic/prognostic applications of this measurement approach are yet to be established. Nevertheless, through continuous interstitial interrogation using microdialysis, the present study demonstrated that MMP is a continuous and dynamic process within the human myocardium.
Acknowledgments
This study was supported by NIH grants HL59165, PO1 HL48788-08 and a Merit Award from the Veterans’ Affairs Health Administration. Authors C.K. and A.D. contributed equally to this study.
References
- 1.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Woessner JF, Jr, Nagase H. Protein substrates of the MMPs. Matrix Metalloproteinases and TIMPs. 2000:87–97. [Google Scholar]
- 3.Woessner JF, Jr, Nagase H. Activation of the zymogen forms of MMPs. Matrix Metalloproteinases and TIMPs. 2000:72–86. [Google Scholar]
- 4.Spinale FG. Matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 5.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006 Jan 1;11:529–43. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 6.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006 Mar;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 7.Lalu MM, Pasini E, Schulze CJ, Ferrari-Vivaldi M, Ferrari-Vivaldi G, Bachetti T, Schulz R. Ischaemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J. 2005;26:27–35. doi: 10.1093/eurheartj/ehi007. [DOI] [PubMed] [Google Scholar]
- 8.Lin TC, Li CY, Tsai CS, Ku CH, Wu CT, Wong CS, Ho ST. Neutrophil-mediated secretion and activation of matrix metalloproteinase-9 during cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2005;100:1554–1560. doi: 10.1213/01.ANE.0000154307.92060.84. [DOI] [PubMed] [Google Scholar]
- 9.Mayers I, Hurst T, Puttagunta L, Radomski A, Mycyk T, Sawicki G, Johnson D, Radomski MW. Cardiac surgery increases the activity of matrix metalloproteinases and nitric oxide synthase in human hearts. J Thorac Cardiovasc Surg. 2001;122:746–752. doi: 10.1067/mtc.2001.116207. [DOI] [PubMed] [Google Scholar]
- 10.Joffs C, Gunasinghe HR, Multani MM, Dorman BH, Kratz JM, Crumbley AJ, 3rd, Crawford FA, Jr, Spinale FG. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001;71:1518–1523. doi: 10.1016/s0003-4975(01)02442-0. [DOI] [PubMed] [Google Scholar]
- 11.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 12.Deschamps AM, Yarbrough WM, Squires CE, Allen RA, McClister DM, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation. 2005;111:1166–1174. doi: 10.1161/01.CIR.0000157149.71297.3A. [DOI] [PubMed] [Google Scholar]
- 13.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 14.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O’Neill TP, Spinale FG. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation. 2003;108:1753–1759. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 17.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 18.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006 May 2;113(17):2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 20.Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PW, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004 Jun 15;109(23):2850–6. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 21.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006 Sep 5;114(10):1020–7. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 22.Multani M, Ikonomidis JS, Kim PY, Miller EA, Payne KJ, Mukherjee R, Dorman BH, Spinale FG. Dynamic and differential changes in myocardial and plasma endothelin in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005 Mar;129(3):584–90. doi: 10.1016/j.jtcvs.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Stack MS, Gray RD. Comparison of vertebrate collagenase and gelatinase using a new fluorogenic substrate peptide. J Biol Chem. 1989 Mar 15;264(8):4277–81. [PubMed] [Google Scholar]
- 24.Neumann U, Kubota H, Frei K, Ganu V, Leppert D. Characterization of Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, a fluorogenic substrate with increased specificity constants for collagenases and tumor necrosis factor converting enzyme. Anal Biochem. 2004 May 15;328(2):166–73. doi: 10.1016/j.ab.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Deschamps AM, Apple KA, Hardin AE, McLean JE, Yarbrough WM, Sample JA, Spinale FG. Myocardial interstitial matrix metalloproteinase activity is altered by mechanical changes in LV load: interaction with the angiotensin type 1 receptor. Circulation Research. 2005 May 27;96(10):1110–8. doi: 10.1161/01.RES.0000167830.12010.6b. [DOI] [PubMed] [Google Scholar]
- 26.Deschamps AM, Zavadzkas J, Murphy RL, McLean JE, Jeffords L, Saunders S, Sheats N, Stroud R, Beck C, Spinale FG. Interruption of endothelin signaling modifies membrane type-1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol. 2008 Feb;294(2):H875–83. doi: 10.1152/ajpheart.00918.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ao X, Wang X, Lennartz MR, Loegering DJ, Stenken JA. Multiple cytokine detection in microliter microdialysis samples obtained from activated cultured macrophages. J Pharm Biomed Anal. 2006 Mar 3;49(4):915–21. doi: 10.1016/j.jpba.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Lennartz MR, Loegering DJ, Stenken JA. Interleukin-6 collection through long term implanted microdialysis sampling probes in rat subcutaneous space. Anal Chem. 2007 Mar 1;79(5):1816–24. doi: 10.1021/ac061503b. [DOI] [PubMed] [Google Scholar]
- 29.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000 Oct 17;102(16):1944–9. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 30.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 31.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 32.Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, Dione DP, Cavaliere P, Chow C, Bourke BN, Hu XY, Azure M, Yalamanchili P, Liu R, Cheesman EH, Robinson S, Edwards DS, Sinusas AJ. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005 Nov 15;112(20):3157–67. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]