Abstract
Summary
To compare the absolute risk of fracture to the risk of other conditions by race/ethnicity, we studied 83,724 women, aged 70–79. The projected number of fractures was similar to or exceeded the combined number of cardiovascular events and breast cancers. Osteoporosis prevention efforts should target women of all ethnicities.
Introduction
The relative risk of fracture is lower in non-white compared to white women but the absolute risk of fracture in comparison to other common chronic conditions is uncertain.
Methods
We performed a prospective cohort study of 83,724 women, age 50–79 years. Cardiovascular disease (CVD), invasive breast cancer and all fractures were identified over an average of 7.7±2.6 years.
Results
The incidence of fracture, breast cancer, stroke and CVD varied across ethnicity. The annualized (%) incidence of fracture was greatest in whites (2.4%) and American Indians (2.8%) and lowest among blacks (1.3%). The majority of hip fractures occurred in white women. The projected number of women who will experience a fracture in one year exceeded the combined number of women who would experience invasive breast cancer or a broad category of CVD events in all ethnic groups except blacks. In 10,000 black women, an estimated 153 women would experience CVD, and 35 women, breast cancer compared to 126 women expected to fracture in one year.
Conclusion
The annual risk of suffering a fracture is substantial in women of all ethnicities. Osteoporosis prevention efforts should target all women irrespective of their race/ethnic backgrounds.
Keywords: Breast cancer, Cardiovascular disease, Fracture, Osteoporosis, Race/ethnicity, Women’s Health Initiative
Introduction
Although the lifetime risk of fractures is lower in non-white women [1–3], the number of fractures is expected to increase largely reflecting the greater improvements in overall life expectancy in minority women [4, 5]. It is not known, however, how the incidence of fracture in minority women compares to the incidence of other chronic conditions that affect women. This information is important for projecting the overall health needs of older women. The objective of the current analysis was to compare the absolute risk of fracture to the absolute risk of cardiovascular disease, stroke and breast cancer in a multiethnic group of women enrolled in the Women’s Health Initiative Observational Study (WHI-OS). We hypothesized that although the relative risk of fracture is lower in minority women, the absolute risk of fracture will be higher than the absolute risk of developing other major common conditions within each ethnic group.
Materials and methods
Study population
The study population consisted of 92,368 women enrolled in the WHI-OS, a multi-center cohort of women who were 50–79 years. Details of the scientific rationale, design, eligibility requirements, and baseline characteristics of the cohort have been published elsewhere [6]. Women were recruited from 1993 to 1998 either directly or by virtue of ineligibility or unwillingness to participate in the clinical trial component of WHI. Eligibility criteria included being age 50–79, postmenopausal, planning to live in the clinical center area for at least three years, cognitively able to participate and free from serious conditions such as class IV congestive heart failure, or severe chronic liver, kidney or lung disease. All participants signed informed consent forms that were approved by institutional review boards.
For this analysis, we excluded women who reported at study entry that they had a history of a hip fracture, myocardial infarction, stroke or breast cancer leaving a total of 83,724 women. Information on race/ethnicity was obtained by self report.
Other measures
Baseline questionnaires ascertained information on educational level, smoking, history of hip fracture, breast cancer, myocardial infarction, stroke and general health status. Current alcohol consumption was estimated from a personal habits questionnaire and expressed as servings per week. Physical activity was assessed by questionnaire on the frequency and duration of walking and other types of activities (strenuous, moderate and mild). Using standardized classification of the energy expenditures associated with physical activities, we calculated a weekly energy expenditure in metabolic equivalents (MET score) for total physical activity. Current and previous use of menopausal hormone therapy (HT) was ascertained by interview. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The Gail five-year breast cancer risk was calculated as previously described [7].
Ascertainment of endpoints
At study end, 5.7% of participants had withdrawn or were lost to follow-up. Procedures for follow-up and outcome ascertainment involved annual mailed follow-up forms to collect updated information on select risk factors and for initial reporting of clinical outcome events (response rates have been > 95%). Follow-up extended through March, 2005. The average follow-up time ranged from 7.7 years in white women to 7.0 years in Hispanic women.
The primary cardiovascular end points for this analysis were newly diagnosed coronary heart disease (CHD) (non-fatal myocardial infarction (MI) or death from coronary causes), expanded CHD (non-fatal MI, fatal CHD, hospitalized angina, coronary revascularization, congestive heart failure (CHF), and deep vein thrombosis (DVT)), stroke and transient ischemic attacks (TIA) and total cardiovascular events (MI, fatal CHD, coronary revascularization, angina, CHF, stroke, TIA and DVT).
The diagnosis of nonfatal MI was confirmed if data in the hospital record met standardized criteria of diagnostic electrocardiographic changes, elevated cardiac-enzyme levels or both [8]. Treatment for coronary revascularization was confirmed by documentation of the procedure in the medical record. The presence of angina was confirmed by hospitalization and confirmatory evidence on angiography, diagnostic stress test, or diagnosis by a physician and medical treatment. The presence of congestive heart failure was confirmed by hospitalization and diagnostic confirmatory tests.
The occurrence of stroke was confirmed by documentation in the medical record of the rapid onset of a neurologic deficit consistent with stroke and lasting at least 24 hours or until death [9]. Transient ischemic attacks requiring and/or occurring during hospitalization were defined as: one or more episodes of a focal neurologic deficit lasting more than 30 seconds and no longer than 24 hours, rapid evolution of the symptoms to the maximal deficit in less than five minutes, with subsequent complete resolution and no head trauma occurring immediately before the onset of the neurological event.
Fatal coronary disease was considered confirmed if there was documentation in the hospital or autopsy records or if coronary disease was listed as the cause of death on the death certificate and evidence of previous coronary disease was available [10]. DVTs were self reported and not adjudicated.
Validation of breast cancer diagnoses were based on pathology reports, discharge summaries, operative reports and radiology reports for biopsies and surgeries. Central adjudication by physicians and cancer coders classified cases according to the National Cancer Institute Surveillance Epidemiology and End Results guidelines.
The “total” fracture outcome included all clinical fractures that occurred after study entry except for those of the fingers, toes, face, skull or sternum. Local and central reviews of radiology reports were carried out for hip fractures and for all fractures in women enrolled at the three bone density centers. For all others, we relied on self-report of non-hip fractures. In the WHI, 80% of self-reported non-hip fractures were confirmed by physician review of medical records, suggesting that self-report of fractures is reasonably accurate [11].
Statistical analysis
All analyses were performed using SAS 9.1 (Cary, NC). The baseline characteristics of the women were compared across ethnicity using ANOVA and Chi-squared tests. We calculated age-adjusted (weighted to the overall sample age distribution) annualized incidence rates (%) of total fracture, hip fracture, lower extremity fracture, upper extremity fracture, CHD, expanded CHD, stroke, TIA, total CVD and breast cancer in each ethnic group. P-values testing for differences by ethnicity were computed from likelihood ratio tests derived using generalized linear models with Poisson distribution and log link function, adjusted for age. In secondary analyses, we excluded women who reported HT use at baseline because HT has been linked to a number of these conditions. We estimated the projected number of cases of each outcome that would be observed in one year in 10,000 women using these ethnic specific annual incidence rates.
Results
The mean age of the cohort ranged from 60.4 years in Hispanic women to 63.6 years in Asian and white women, Table 1 (all p-values <0.0001). The mean BMI was greatest in black women and lowest among Asian women. Physical activity was highest in white women and Asian women and lowest in black women. Alcohol consumption was relatively low in all groups ranging from 0.7 in Asians to 2.8 servings per week in whites. The Gail five-year breast cancer risk score was greatest in white women and Asian women, exceeding the 1.67% used to describe high risk women [7]. The percent with a college degree or higher was greatest among Asian women and white women and lowest among Hispanic women and American Indian women. Less than 11% reported current smoking, but history of past smoking was relatively common. Use of postmenopausal hormones was relatively high at entry into WHI, ≥40% of all women except blacks. The percent of women who reported fair or poor health ranged from a low 6.8% among white women to about 20% among black, Hispanic and American Indian women.
Table 1.
Baseline demographic characteristics by race/ethnicity
| Ethnicity |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
Hispanic |
American Indian |
Asian/Pacific Islander |
|||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Age at screening (years) | 70,769 | 63.6 | 7.3 | 6693 | 61.8 | 7.3 | 3369 | 60.4 | 7.1 | 370 | 61.4 | 7.9 | 2523 | 63.6 | 7.5 |
| Body mass index (BMI, km2) | 69,962 | 26.9 | 5.6 | 6605 | 30.6 | 6.8 | 3320 | 28.6 | 5.9 | 358 | 29.3 | 6.4 | 2507 | 24.2 | 4.2 |
| BL total expend from phys act (MET-hrs/week) | 70,002 | 14.2 | 14.4 | 6627 | 10.7 | 13.7 | 3241 | 11.6 | 14.7 | 365 | 13.1 | 17.3 | 2505 | 14.0 | 14.4 |
| Alcohol (servings/week) | 70,671 | 2.8 | 5.3 | 6675 | 1.3 | 5.4 | 3351 | 1.3 | 3.6 | 369 | 1.4 | 4.0 | 2520 | 0.7 | 2.8 |
| Gail 5-year risk (%) | 70,769 | 1.9 | 1.0 | 6693 | 1.0 | 0.6 | 3369 | 1.0 | 0.6 | 370 | 1.6 | 1.0 | 2523 | 1.9 | 0.9 |
| N | % | N | % | N | % | N | % | N | % | ||||||
| Education | |||||||||||||||
| High school diploma/GED or less | 13,784 | 19.5 | 1713 | 25.7 | 1414 | 42.0 | 137 | 37.1 | 541 | 21.5 | |||||
| School after high school | 25,582 | 36.1 | 2444 | 36.5 | 1154 | 34.3 | 146 | 39.5 | 842 | 33.4 | |||||
| College degree or higher | 30,890 | 43.6 | 2434 | 36.4 | 740 | 22.0 | 83 | 22.4 | 1123 | 44.5 | |||||
| Smoking | |||||||||||||||
| Never smoked | 34,808 | 49.2 | 3330 | 49.8 | 2074 | 61.6 | 185 | 50.0 | 1857 | 73.6 | |||||
| Past smoker | 30,955 | 43.7 | 2513 | 37.5 | 982 | 29.1 | 138 | 37.3 | 564 | 22.4 | |||||
| Current smoker | 4088 | 5.8 | 702 | 10.5 | 225 | 6.7 | 39 | 10.5 | 87 | 3.4 | |||||
| HT usage status | |||||||||||||||
| Never used | 26,269 | 37.1 | 3773 | 56.4 | 1578 | 46.8 | 174 | 47.0 | 859 | 34.0 | |||||
| Past user | 9855 | 13.9 | 868 | 13.0 | 386 | 11.5 | 45 | 12.2 | 326 | 12.9 | |||||
| Current user | 34,587 | 48.9 | 2041 | 30.5 | 1399 | 41.5 | 151 | 40.8 | 1336 | 53.0 | |||||
| Health status | |||||||||||||||
| Excellent/Very Good | 44,884 | 63.4 | 2445 | 36.5 | 1378 | 40.9 | 166 | 44.8 | 1345 | 53.3 | |||||
| Good | 20,657 | 29.2 | 2873 | 42.9 | 1148 | 34.1 | 127 | 34.3 | 938 | 37.2 | |||||
| Fair/Poor | 4841 | 6.8 | 1292 | 19.3 | 746 | 22.2 | 75 | 20.2 | 233 | 9.3 | |||||
The age-adjusted annualized incidence rates of total fractures, hip fracture, upper and lower extremity fractures, breast cancer, stroke, CHD, expanded CHD and total CVD are summarized in Table 2. Fracture rates were highest among white and American Indian women, and lowest among black women. A similar pattern was observed for hip fractures but 95% of all hip fractures occurred in white women. Upper and lower extremity fractures were also more common in white women. Lower extremity fracture rates were similar in black, Hispanic and Asian women. Breast cancer rates were highest in white women with little difference across other ethnicities. The incidence of stroke was greatest in black women and lowest in Hispanic women. The annualized incidence of total CVD events was highest among black and American Indian women. Similar patterns of disease incidence by ethnicity were observed after we excluded women who reported current use of hormones at baseline.
Table 2.
Age-adjusted annualized rates (%) of fracture, breast cancer, stroke and CHD: number with outcome (annualized rates (%))
| white | black | Hispanic | American Indian | Asian/Pacific Islander | P-value* | |
|---|---|---|---|---|---|---|
| All OS participants† | ||||||
| Total fractures | 11,855 (2.36%) | 586 (1.26%) | 331 (1.59%) | 66 (2.79%) | 245 (1.36%) | <.0001 |
| Hip fractures | 881 (0.16%) | 18 (0.04%) | 9 (0.05%) | 3 (0.12%) | 11 (0.06%) | <.0001 |
| Lower extremity fractures‡ | 5823 (1.11%) | 318 (0.65%) | 151 (0.65%) | 41 (1.69%) | 110 (0.59%) | <.0001 |
| Upper extremity fractures§ | 4400 (0.83%) | 159 (0.34%) | 134 (0.70%) | 16 (0.70%) | 91 (0.49%) | <.0001 |
| Invasive breast cancer | 2542 (0.48%) | 167 (0.35%) | 68 (0.28%) | 11 (0.47%) | 65 (0.36%) | <.0001 |
| Stroke | 1394 (0.26%) | 155 (0.36%) | 39 (0.24%) | 7 (0.30%) | 43 (0.23%) | .001 |
| CHD | 1640 (0.30%) | 163 (0.39%) | 32 (0.16%) | 12 (0.53%) | 30 (0.16%) | <.0001 |
| Expanded CHD** | 4564 (0.87%) | 470 (1.10%) | 130 (0.67%) | 32 (1.50%) | 76 (0.41%) | <.0001 |
| Stroke and TIA | 2077 (0.39%) | 220 (0.50%) | 56 (0.32%) | 9 (0.39%) | 60 (0.32%) | .0003 |
| CVD†† | 6231 (1.20%) | 646 (1.53%) | 177 (0.95%) | 37 (1.72%) | 133 (0.73%) | <.0001 |
| Excluding HT users | ||||||
| Total fractures | 6847 (2.71%) | 406 (1.28%) | 207 (1.79%) | 44 (3.65%) | 131 (1.62%) | <.0001 |
| Hip fractures | 566 (0.20%) | 15 (0.06%) | 6 (0.05%) | 3 (0.24%) | 7 (0.08%) | <.0001 |
| Lower extremity fractures‡ | 3207 (1.20%) | 215 (0.64%) | 93 (0.73%) | 29 (2.45%) | 58 (0.69%) | <.0001 |
| Upper extremity fractures§ | 2801 (1.05%) | 108 (0.34%) | 91 (0.83%) | 11 (0.96%) | 56 (0.67%) | <.0001 |
| Invasive breast cancer | 1123 (0.41%) | 116 (0.35%) | 38 (0.27%) | 4 (0.32%) | 29 (0.35%) | .07 |
| Stroke | 822 (0.30%) | 118 (0.39%) | 27 (0.28%) | 5 (0.35%) | 24 (0.29%) | .03 |
| CHD | 1022 (0.37%) | 125 (0.43%) | 27 (0.22%) | 7 (0.54%) | 17 (0.20%) | .01 |
| Expanded CHD** | 2768 (1.03%) | 356 (1.22%) | 98 (0.85%) | 17 (1.40%) | 40 (0.48%) | <.0001 |
| Stroke and TIA | 1226 (0.45%) | 163 (0.54%) | 35 (0.34%) | 6 (0.43%) | 35 (0.42%) | .03 |
| CVD†† | 3728 (1.41%) | 485 (1.67%) | 124 (1.12%) | 21 (1.70%) | 74 (0.91%) | <.0001 |
P-value is computed from a likelihood ratio test derived from a generalized linear model with Poisson distribution and log link function, adjusted for age
Excluded WHI OS participants with previous history of hip fractures, breast cancer, stroke or CHD
Lower extremity fractures include hip, upper leg (not hip), pelvis, knee (patella), lower leg or ankle, foot (not toe) and tailbone (coccyx) fractures
Upper extremity fractures include lower arm or wrist, hand (not finger), elbow, and upper arm or shoulder fractures
Including hospitalized angina, revascularization, non-fatal and fatal CHD, CHF, and DVT
Including hospitalized angina, revascularization, non-fatal and fatal CHD, CHF, DVT, stroke and TIA
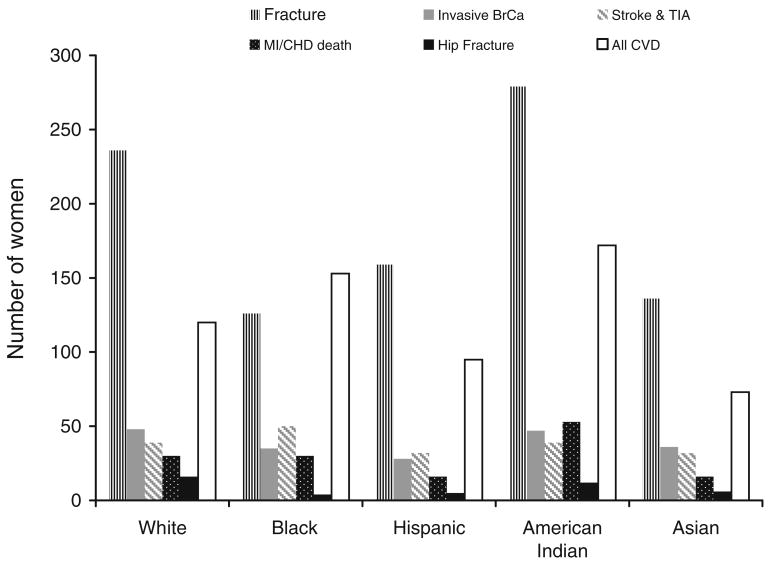
The projected number of 10,000 women who would experience a fracture in one year exceeded the combined number of women expected to develop invasive breast cancer, stroke or total CVD, Fig. 1. This was observed in every ethnic group except blacks. In 10,000 black women, an estimated 153 would experience a CVD event, 35 women, breast cancer, compared to 126 women expected to fracture.
Fig. 1.
The estimated number of cases that will occur in 10,000 women in one year: total fractures, hip fractures, invasive breast cancer, stroke and TIA, MI, CHD death and all CVD (including MI, CHD death, angina, CHF, DVT, coronary revascularization, TIA and stroke)
Discussion
The overarching goals of Healthy People 2010 are to increase quality and years of healthy life and to eliminate health disparities [12]. One of the specific targets is to prevent illness and disability related to osteoporosis. However, achievement of this goal depends a large part on recognizing who is at risk and who should be targeted for screening and intervention. CVD, cancer and stroke are the three major causes of death in older women, and account for a large proportion of the overall morbidity in older women with major public health impact [13]. In the current analysis, there were substantial ethnic differences in the incidence of these diseases. However, we showed that the number of fractures that will occur in one year exceeded the combined number of women who will develop CVD, stroke or breast cancer in all groups except black women. Yet, even in black women, the incidence of fractures was similar to the incidence of a broad category of CVD that included MIs, unstable angina, CHF, revascularization, DVT, strokes and TIA. Thus, even though fracture rates are lower in minority groups, most women in this age group are more likely to experience a fracture than a cardiovascular disease event or breast cancer.
Hip fractures are the most serious consequence of osteoporosis and there were few hip fractures in minority women. Nevertheless, resource utilization attributable to other osteoporotic fractures is substantial [14]. For example, of the more than 2.6 million physician visits for osteoporosis, 86% of these visits were for other fractures; 40% of all home health care visits and 77% of all physical therapy sessions were for non-hip fractures.
We have shown that irrespective of ethnicity, women with multiple risk factors have a high risk of fracture [15]. The best approach to prevention of fractures is early identification of high risk women. Yet, preventive and therapeutic efforts focus primarily on white women. For example, black women were 60% less likely to be referred for bone mineral density measurements [16]. Only 9% of black women who presented with a low impact fracture received a diagnosis of osteoporosis and, of these, only 19% were discharged on osteoporosis medication [17]. Efforts are needed to identify ethnic women at high risk for fracture not only because fractures out number other events but also because the consequences of osteoporotic fractures may be greater among non-white women. Mortality following a hip fracture is higher among black than white women [18]. black women who suffer a hip fracture have longer hospitalization stays and are more likely to be non-ambulatory compared to white women [19]. Mexican Americans who reported a previous hip fracture were four times as likely to be disabled [20].
The WHI-OS is a large diverse population of older women. Longitudinal information was obtained on a number of important disease outcomes in a standard fashion, permitting us to compare rates across ethnicity. We considered several classifications of CVD and were broad in our inclusion of CVD events. There are, however, several limitations to our analysis. The women in the WHI were not enrolled to be representative of US women. We studied a small number of American Indian women recruited primarily from the southwest US, and their estimated incidence rates of disease are based on few events. In the US, the annualized incidence of invasive breast cancer was 0.45% and 0.37% in white women and black women age 65 or older, respectively [21], comparing closely to the incidence of breast cancer observed in WHI-OS women. The incidence of stroke and MI in WHI women was however, lower than in US women, especially for black women, but we considered an expanded list of CVD outcomes. Fracture rates were lower in WHI than in the Study of Osteoporotic Fractures (3.5%/yr), perhaps reflecting the younger age of the WHI cohort (Li-Yung Lui, personal communication).
In conclusion, improvements in life expectancy have been greater in minority women compared to white women resulting in rapid growth in the burden of osteoporotic fractures among non-white populations [4]. The absolute number of fractures is similar to the number of CVD events in black women but far exceeds the combined number of breast cancers and CVD events in other race/ethnic groups. More attention is needed for osteoporosis screening and prevention in minority women.
Acknowledgments
The National Heart Lung and Blood Institute has representation on the WHI Steering Committee which governed the design and conduct of the study, the interpretation of the data and preparation and approval of manuscripts. The National Heart Lung and Blood Institute Project office reviewed and approved the manuscript.
Funding The WHI program is funded by the National Heart, Lung and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services. The sponsor played a role in the design and analysis of the WHI. Additional support was obtained from NHLBI contract HHSN268200764318C.
Appendix
We wish to thank all WHI investigators and participants for their contributions. The following list is a short list of WHI investigators.
Program Office: Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD).
Clinical Coordinating Centers: Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan (Fred Hutchinson Cancer Research Center, Seattle, WA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); Evan Stein (Medical Research Labs, Highland Heights, KY); Steven Cummings (University of California at San Francisco, San Francisco, CA).
Clinical Centers: Sylvia Wassertheil-Smoller (Albert Einstein College of Medicine, Bronx, NY); Jennifer Hays (Baylor College of Medicine, Houston, TX); JoAnn Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Annlouise R. Assaf (Brown University, Providence, RI); Lawrence Phillips (Emory University, Atlanta, GA); Shirley Beresford (Fred Hutchinson Cancer Research Center, Seattle, WA); Judith Hsia (George Washington University Medical Center, Washington, DC); Rowan Chlebowski (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA); Evelyn Whitlock (Kaiser Permanente Center for Health Research, Portland, OR); Bette Caan (Kaiser Permanente Division of Research, Oakland, CA); Jane Morley Kotchen (Medical College of Wisconsin, Milwaukee, WI); Barbara V. Howard (MedStar Research Institute/Howard University, Washington, DC); Linda Van Horn (Northwestern University, Chicago/Evanston, IL); Henry black (Rush Medical Center, Chicago, IL); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Dorothy Lane (State University of New York at Stony Brook, Stony Brook, NY); Rebecca Jackson (The Ohio State University, Columbus, OH); Cora E. Lewis (University of Alabama at Birmingham, Birmingham, AL); Tamsen Bassford (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); John Robbins (University of California at Davis, Sacramento, CA); F. Allan Hubbell (University of California at Irvine, CA); Howard Judd (University of California at Los Angeles, Los Angeles, CA); Robert D. Langer (University of California at San Diego, LaJolla/Chula Vista, CA); Margery Gass (University of Cincinnati, Cincinnati, OH); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); David Curb (University of Hawaii, Honolulu, HI); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Judith Ockene (University of Massachusetts/Fallon Clinic, Worcester, MA); Norman Lasser (University of Medicine and Dentistry of New Jersey, Newark, NJ); Mary Jo O’Sullivan (University of Miami, Miami, FL); Karen Margolis (University of Minnesota, Minneapolis, MN); Robert Brunner (University of Nevada, Reno, NV); Gerardo Heiss (University of North Carolina, Chapel Hill, NC); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Karen C. Johnson (University of Tennessee, Memphis, TN); Robert Brzyski (University of Texas Health Science Center, San Antonio, TX); Gloria E. Sarto (University of Wisconsin, Madison, WI); Denise Bonds (Wake Forest University School of Medicine, Winston-Salem, NC); Susan Hendrix (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI).
Footnotes
Conflicts of interest Dr. Cauley has received research support from Merck & Company, Eli Lilly & Company, Pfizer Pharmaceuticals and Novartis Pharmaceuticals. She has also received consulting fees from Eli Lilly & Company, and Novartis Pharmaceuticals. Dr. Robbins reports that he has worked on grants with industry support but that he has received no salary support. Dr. Jackson has received research support from and is on the speaker’s bureau for Procter & Gamble Pharmaceuticals, has received research and conference support from Novartis, and has received an honorarium as a Continuing Medical Education speaker for Aventis/Alliance for Better Bone Health. Drs. Wampler, Barnhart, Allison, Chen, Hendrix and Ms. Wu, have no conflicts to report.
References
- 1.Farmer ME, White LR, Brody JA, Bailey KR. Race and sex differences in hip fracture incidence. Am J Public Health. 1984;74(12):1374–1380. doi: 10.2105/ajph.74.12.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron JA, Karagas M, Barrett J, Kniffin W, Malenka D, Mayor M, Keller RB. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612–618. doi: 10.1097/00001648-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T. Racial differences in fracture risk. Epidemiology. 1994;5(1):42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 5.Zingmond DS, Melton LJ, 3rd, Silverman SL. Increasing hip fracture incidence in California Hispanics, 1983 to 2000. Osteoporos Int. 2004;15(8):603–610. doi: 10.1007/s00198-004-1592-7. [DOI] [PubMed] [Google Scholar]
- 6.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 7.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, Vogel V. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 8.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 9.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. Jama. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 12.http://www.healthypeople.gov/LHI/
- 13.Minino AM, Heron MP, Smith BL. Deaths: preliminary data for 2004. Natl Vital Stat Rep. 2006;54(19):1–49. [PubMed] [Google Scholar]
- 14.United States. Public Health Service. Office of the Surgeon General. 2004 Bone health and osteoporosis: a report of the Surgeon General. U.S. Dept. of Health and Human Services Public Health Service Office of the Surgeon General, Rockville, Md., pp xxxii, 404
- 15.Cauley JA, Wu L, Wampler NS, Barnhart JM, Allison M, Chen Z, Jackson R, Robbins J. Clinical risk factors for fractures in multi-ethnic women: The Women’s Health Initiative. J Bone Miner Res. 2007;22(11):1816–1826. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 16.Miller RG, Ashar BH, Cohen J, Camp M, Coombs C, Johnson E, Schneyer CR. Disparities in osteoporosis screening between at-risk African-American and white women. J Gen Intern Med. 2005;20(9):847–851. doi: 10.1111/j.1525-1497.2005.0157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam NM, Archer JA, Lee E. Osteoporotic fragility fractures in African Americans: under-recognized and under-treated. J Natl Med Assoc. 2004;96(12):1640–1645. [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147–1150. doi: 10.2105/ajph.82.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931–938. doi: 10.1016/0021-9681(87)90142-1. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Markides KS, Perkowski LP, Stroup-Benham CA, Lichtenstein M, Goodwin JS. Impact of selected medical conditions on self-reported lower-extremity function in Mexican-American elderly. Ethn Dis. 1998;8(1):52–59. [PubMed] [Google Scholar]
- 21.SEER Cancer Statistics 1975–2004 http://Seer.cancer.gov