Abstract
Emerging evidence suggests that the rewarding, abuse-related effects of nicotine are modulated by the endocannabinoid system of the brain. For example, pharmacological blockade or genetic deletion of cannabinoid CB1 receptors can reduce or eliminate many abuse-related behavioral and neurochemical effects of nicotine. Furthermore, doses of Δ9-tetrahydrocannabinol (THC) and nicotine that are ineffective when given alone can induce conditioned place preference when given together. These previous studies have used systemically-administered CB1-receptor agonists and antagonists and gene deletion techniques, which affect cannabinoid CB1 receptors throughout the brain. A more functionally selective way to alter endocannabinoid activity is to inhibit fatty acid amide hydrolase (FAAH), thereby magnifying and prolonging the effects of only the endocannabinoid anandamide (AEA) when and where it is synthesized and released on demand. Here we combined behavioral and neurochemical approaches to evaluate whether the FAAH inhibitor cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester (URB597) could alter the abuse-related effects of nicotine in rats. We found that URB597, at a dose (0.3 mg/kg) that had no behavioral effects by itself, prevented development of nicotine-induced conditioned place preference (CPP) and acquisition of nicotine self-administration. URB597 also reduced nicotine-induced reinstatement in both CPP and self-administration models of relapse. Furthermore, in vivo microdialysis showed that URB597 reduced nicotine-induced dopamine elevations in the nucleus accumbens shell, the terminal area of the brain’s mesolimbic reward system. These findings suggest that FAAH inhibition can counteract the addictive properties of nicotine and that FAAH may serve as a new target for development of medications for treatment of tobacco dependence.
Introduction
Nicotine, the main psychoactive component of tobacco, plays a major role in tobacco dependence by acting directly as a reinforcer of drug-seeking and drug-taking behavior (Le Foll and Goldberg, 2006). In rats, nicotine can reinforce drug self-administration behavior (Corrigal and Coen, 1989) and induce conditioned place preference (CPP) (Le Foll and Goldberg, 2005), and it can trigger relapse to previously acquired drug-seeking behavior (Shaham et al., 1997). Nicotine's rewarding effects are believed to stem from its ability to activate the mesolimbic dopaminergic system by enhancing firing rate and burst firing of dopaminergic neurons in the ventral tegmental area (VTA) (Mereu et al., 1987) and increasing dopamine release in terminal areas, especially in the nucleus accumbens shell (Pontieri et al., 1996).
Recent findings suggest that behavioral and motivational effects of nicotine are modulated by the endocannabinoid system (Castané et al., 2005) and that cannabinoid CB1 receptors play a key role in this interaction. For example, pharmacological blockade or genetic ablation of CB1 cannabinoid receptors can decrease nicotine self-administration (Cohen et al., 2002; Shoaib, 2008), prevent development and expression of nicotine-induced CPP (Castané et al., 2002; Le Foll and Goldberg, 2004; Forget et al., 2005, Merritt et al., 2008), prevent relapse to nicotine-seeking behavior in rats (Shoaib, 2008), and prevent nicotine-induced dopamine elevations in the nucleus accumbens shell (Cohen et al., 2002). Furthermore, doses of Δ9-tetrahydrocannabinol (THC) and nicotine that are ineffective when administered alone can induce significant CPP in mice when given in combination (Valjent et al., 2002). The endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are natural ligands for CB1 receptors, and animals chronically treated with nicotine show increased AEA content in the limbic forebrain, a key brain region for reward (Gonzalez et al., 2002). Thus, it is possible that nicotine regulates endocannabinoid signaling at CB1 receptors by triggering the formation and release of endogenous AEA.
These previous studies of cannabinoid system modulation of the behavioral and motivational effects of nicotine used systemically-administered cannabinoid CB1-receptor agonists and antagonists, which affect signalling at cannabinoid CB1 receptors globally, wherever they occur in the brain. A more selective way to alter activity of this system is by inhibiting fatty acid amide hydrolase (FAAH), the main enzyme responsible for AEA degradation. FAAH is abundantly expressed throughout the central nervous system and many FAAH-positive neurons in the brain are found in the proximity of nerve terminals that contain cannabinoid CB1 receptors, supporting a role of FAAH in AEA deactivation and in the cannabinoid signaling mechanism of the brain (Piomelli et al., 2006). FAAH inhibition magnifies and prolongs the actions of AEA only in brain areas where AEA is synthesized and released (Kathuria et al., 2003; Fegley et al., 2005). The selectivity of URB597 vis-à-vis cannabinoid agonists lies primarily in the fact that it enhances only one of the endocannabinoid signals, the AEA signal, and, thus, it is not strictly speaking a regional selectivity, but rather a functional selectivity. Of course, such functional selectivity might also result in regional differences, with certain regions of the brain accumulating more anandamide than others (Bortolato et al., 2007). Also, AEA is synthesized on demand and affects only a specific subgroup of cannabinoid CB1 receptors, i.e. those located in activated synapses (Di Marzo and Petrosino, 2007; Marsicano et al., 2003; Pertwee, 2005). Thus, unlike direct cannabinoid agonists, the effects of FAAH inhibition are functionally selective, increasing the actions of AEA only, when and where there is a demand for AEA (Piomelli et al., 2006; Pertwee, 2005).
Here we combined behavioral and neurochemical approaches to evaluate whether the abuse-related effects of nicotine would be altered by the FAAH inhibitor cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester (URB597) in rats. URB597 is a highly selective inhibitor of FAAH with no significant affinity for nicotinic receptors or any other known G-protein coupled receptors (Piomelli et al., 2006) and no intrinsic rewarding or aversive effects in when given over a wide range of doses in rats (Gobbi et al., 2005; Scherma et al., 2008), mice (Merritt et al., 2008) and monkeys (Justinova et al., 2008). The effects studied in the present experiments with rats were: (1) nicotine's rewarding effects in CPP and drug self-administration models of drug abuse; (2) nicotine's ability to induce reinstatement in the CPP and drug self-administration models of relapse to nicotine-seeking after a period of abstinence; and (3) nicotine's ability to induce elevations of dopamine levels in a rewardrelated area of the brain, the nucleus accumbens shell.
Methods
Animals
Subjects were male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA, or Harlan-Nossan, Milan, Italy) or male Long Evans rats (Charles River Laboratories, Inc., Lachine, Quebec, Canada), weighing 250–325g at the beginning of experiments, and housed in temperature-and humidity-controlled rooms on a 12-h light/dark cycle with ad libitum access to food and water. For self-administration experiments, after surgery, food intake was limited to 20 g a day and animals were housed individually. All experiments were conducted in accordance with the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse (NIDA) and European Commission regulations for animal use in research (86/609/EEC).
Drugs
Nicotine [(−)-nicotine hydrogen tartrate salt] (Sigma Chemical Company, St. Louis, Mo., USA or Sigma, Italy) was diluted in saline (pH: 7.0) and injected subcutaneously (s.c.) or intraperitoneally (i.p.). URB597 (synthesized at the Department of Pharmacology, University of California, Irvine, CA, USA (Mor et al., 2004)) was dissolved in 20% dimethylsulfoxide (DMSO) in saline and injected i.p. URB597 doses of 0.1 to 0.3 mg kg, i.p. were used in these experiments, since (1) the ID50 for FAAH inhibition by URB597 in rats ex vivo is 0.15 mg kg, i.p., and (2) doses of 0.1 to 0.3 mg kg, i.p. achieve maximal efficacy in most tests of stress-coping behavior, while (3) higher doses tend to lose efficacy (Kathuria et al., 2003; Fegley et al., 2005; Gobbi et al., 2005; Bortolato et al., 2007). All drugs were injected in a volume of 1.0 ml/kg.
Development of nicotine-induced CPP
Apparatus and procedure were as described previously (Le Foll and Goldberg, 2004). A two-compartment place-conditioning apparatus was used, in which the compartments were differentiated by floor type (mesh vs bar) and a small light was added on the wall of the sound-attenuation chambers on the side of the mesh floor, to increase the difference between the two compartments. Sprague-Dawley rats were used. The first session was a 15-min pre-test, during which the rat could move freely between compartments in the place-conditioning apparatus and time spent in each compartment was recorded. Then, two 20-min conditioning sessions/day were conducted for three days. On these conditioning days, in the morning rats received saline immediately before being placed into one compartment; four hours later, they received either nicotine (0.05, 0.1, 0.4 or 1.0 mg/kg, s.c.) or saline immediately before being placed in the other compartment. Forty min before the second session on each conditioning day, half the rats received URB597 (0.3 mg/kg, i.p.), and the other half received vehicle. On the day after the last conditioning day, a test session was conducted using the same 15-min procedure as the pre-test session.
Nicotine-induced reinstatement of extinguished CPP
After development of CPP using a nicotine dose of 0.4 mg/kg, s.c. with the same procedure in Sprague-Dawley rats as above, 20-min extinction sessions were conducted twice a day for eight days, using the same procedure as in conditioning sessions, except that saline was administered instead of nicotine. A 15-min extinction test with no injection was then conducted in the same manner as the earlier test session. Animals that did not meet the extinction criterion (preference for compartment previously associated with nicotine reduced by 80% or more), were removed from the study. One day after the extinction test, a 15-min reinstatement test was then conducted. Rats were randomly divided into groups and were given an i.p. priming injection of either, nicotine (0.4 mg/kg, s.c., immediately before session), URB597 (0.3 mg/kg, i.p., 40 min before session) or URB597 plus nicotine (40 min and immediately before the session, respectively) and were allowed to explore both compartments; time spent in each compartment was recorded.
Acquisition of nicotine self-administration behavior
Apparatus and procedure were as described previously (Shoaib et al., 1997). Sprague-Dawley rats prepared with chronic venous catheters were randomly divided into two groups: 30 min before each 2-h self-administration session, one group was injected i.p. with 0.3 mg/kg of URB597 while the control group was injected i.p. with vehicle. Under a fixed-ratio 1 (FR1) schedule, each nose-poke in an active hole produced a 1-s intravenous nicotine injection (30 µg/kg) followed by a 20-s signaled timeout during which responding had no programmed consequences. Nose pokes in an adjacent inactive hole had no programmed consequences. Development of self-administration responding was evaluated over 19 consecutive sessions. The criterion for self-administration was met if a rat obtained at least 10 nicotine injections per session for three consecutive sessions.
Reinstatement of nicotine-seeking behavior under a FR1 schedule
Apparatus and procedure were as described previously (Fattore et al., 2003). Sprague-Dawley rats were allowed to intravenously self-administer nicotine (30 µg/kg/injection) by lever pressing during 1-h daily sessions under a FR1 schedule with a 20-s timeout. Sessions continued until responding was stable (20% or less variation from day to day) for at least five sessions. Then, extinction sessions started, with saline substituted for nicotine while other parameters remained unchanged. A “between-session” reinstatement model was used. Once extinction criteria were reached (less than eight active-lever presses/session), rats were randomly divided into groups and were given an i.p. priming injection of either saline, nicotine (0.15 mg/kg, i.p., 10 min before session), URB597 (0.1 and 0.3 mg/kg, i.p., 40 min before session) or URB597 plus nicotine (40 min and 10 min before the session, respectively) in a counterbalanced within-subject design. Lever pressing was then monitored during a 1-h reinstatement test session in which responding resulted in intravenous injections of saline, as before.
Reinstatement of nicotine-seeking behavior under a FR5 schedule
Findings in Sprague-Dawley rats were replicated in another laboratory with a different rat strain (Long Evans) and with a different schedule of nicotine self-administration (FR5). Apparatus and procedure were adapted from Corrigal and Coen (1989). Long Evans rats were allowed to intravenously self-administer nicotine (30 µg/kg/injection) by lever pressing under a FR1 schedule with a 60-s timeout during 1-h daily sessions for five days, followed by a FR2 schedule for three days, and a FR5 schedule for seven days. When responding was stable (at least 10 nicotine injections per session for five consecutive sessions), extinction training started, with saline substituted for nicotine and no nicotine-associated stimuli. Extinction criteria were reached in one week (mean number of active-lever presses decreased by 85% or more for at least five sessions). Reinstatement tests were then conducted with WIN 55,212-2 and rimonabant (data not shown), followed by additional extinction sessions. After one week rats again reached the extinction criteria stated above and were then given a priming injection of 0.15 mg/kg nicotine (s.c.) immediately before the session, with and without URB597 (0.1 and 0.3 mg/kg, i.p., 30 min before the session), in a counterbalanced within-subject design. Lever pressing was monitored during the 1-h reinstatement test sessions in which responding resulted in intravenous injections of saline. Additional extinction sessions were then conducted until extinction criteria were again reached and reinstatement tests were conducted, as before, with a higher dose of 0.4 mg/kg nicotine (s.c.), with and without URB597 (0.1 and 0.3 mg/kg, i.p.).
In vivo microdialysis
Apparatus and procedure were the same as described previously (Fadda et al., 2003). Sprague Dawley rats were surgically implanted with a concentric dialysis probe aimed at the shell of the nucleus accumbens [anterior +2.0 and lateral 1.1 from bregma, vertical −7.9 from dura, according to the atlas by Paxinos and Watson (1998)] and dialysate samples were collected every 20 min and immediately analyzed by an HPLC system coupled to electrochemical detection. Rats were treated only after dopamine values (<10% variability) were stable for at least three consecutive samples. URB597 (0.3 mg/kg, i.p.) or its vehicle were injected 40 min before saline or nicotine (0.05 and 0.4 mg/kg, s.c.). Only rats with correct probe placement were included in the study.
Food-reinforced responding
Apparatus and procedure were as described previously (Le Foll and Goldberg, 2004). Sprague-Dawley rats learned to press a lever for 45-mg food pellets under a FR10 schedule, with a 45-sec timeout after each food reinforcement. Each session ended after 20 reinforcements or 30 min. URB597 (0.3 mg/kg, i.p.) was given alone or with various doses of nicotine (0.01 to 0.4 mg/kg, s.c.) 40 min before the session.
Locomotor activity
Apparatus and procedure were as described previously (Scherma et al., 2008). Motor activity was measured during 1-h sessions in male Sprague Dawley rats not habituated to the activity boxes. Nicotine (0.05 to 1.0 mg/kg, s.c.) or saline was administered immediately before placing the animal in the activity box. URB597 (0.3 mg/kg, i.p.) was injected in the home cage, 40 min before nicotine or saline injection. Distance traveled was recorded.
Statistical analysis
All results are presented as group means (± SEM). Place-conditioning and self-administration data were analyzed by Proc Mixed (SAS Institute, Cary NC). Microdialysis data were analyzed using two-way analysis of variance (ANOVA). Post-hoc comparisons, when appropriate, were performed by Student-Newman-Keuls or Tukey-Kramer multiple comparisons tests. In all cases, differences with a P<0.05 were considered significant.
Results
Effects of URB597 on development of nicotine-induced CPP
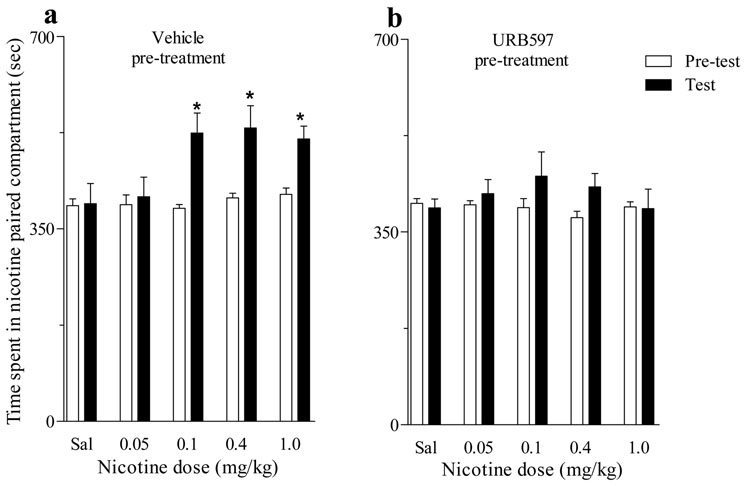
Consistent with previous findings by Le Foll and Goldberg (2004), using a place-conditioning paradigm we found that in Sprague-Dawley rats, nicotine doses of 0.1, 0.4 and 1.0 mg/kg (s.c.) induced a significant CPP (Figure 1a). However, when rats were pre-treated with URB597 at a dose of 0.3 mg/kg (i.p.) before each nicotine conditioning session, regardless of the dose of nicotine (0.05 to 1 mg/kg), CPP did not develop (Figure 1b). Importantly, URB597 did not potentiate the effects of a subthreshold dose of nicotine (0.05 mg/kg), which alone did not induce CPP. For the data in Figure 1, there was a significant interaction of URB and nicotine [Proc Mixed F4,82=3.21, P<0.02].
Figure 1. Effects of URB597 on development of nicotine-induced CPP.
(a) Nicotine doses ranging from 0.1 mg/kg to 1.0 mg/kg produced significant CPP (*P<0.05 vs corresponding pre-test; n=8–10). (b) When rats were pre-treated with URB597 (0.3 mg/kg) before each nicotine conditioning session, CPP did not develop (P=ns; n=8–10) and URB597 did not potentiate effects of a low 0.05 mg/kg ineffective threshold dose of nicotine. All data are expressed as time spent in sec (mean ± SEM) in the nicotine-paired compartment during 15-min Pre-test (open bars), Test (filled black bars),
Effects of URB597 on nicotine-induced reinstatement of extinguished CPP
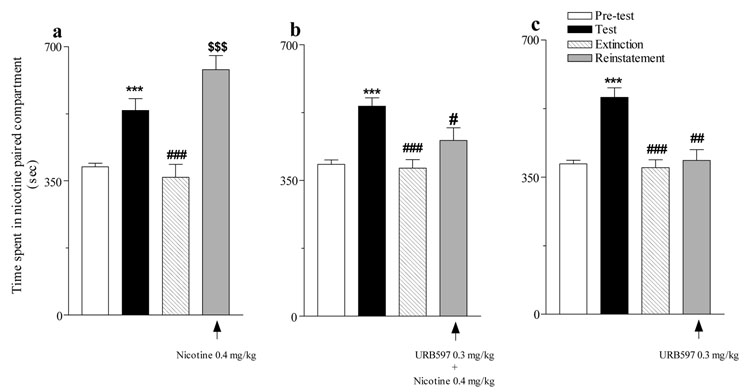
After CPP had been extinguished by substituting saline for nicotine, a priming injection of nicotine (0.4 mg/kg, s.c.) completely reinstated the extinguished CPP (Figure 2a). Pre-treatment with URB597 (0.3 mg/kg, i.p.) prevented this nicotine-induced reinstatement (Figure 2b). URB597 (0.3 mg/kg) by itself did not reinstate the extinguished CPP (Figure 2c). For the data in Figure 1, there was a significant interaction of URB and session [Proc Mixed F6,108= 3.67, P<0.003].
Figure 2. Effects of URB597 on nicotine-induced reinstatement of extinguished CPP.
(a) A priming injection of nicotine (0.4 mg/kg) completely reinstated the extinguished CPP developed by a nicotine dose of 0.4 mg/kg (***P<0.0001 Test vs Pre-test; ###P<0.001 Extinction vs Test; $$$P<0.0001 Reinstatement vs Extinction; n=7). (b) URB597 (0.3 mg/kg) pre-treatment prevented nicotine-induced reinstatement of CPP (***P<0.0001 Test vs Pre-test; ###P<0.0001 Extinction vs Test; n=9; #P>0.05 Reinstatement vs. Test). (c) URB597 failed to reinstate the extinguished CPP (***P<0.0001 Test vs Pre-test; ###P<0.0001 Extinction vs Test; n=9; ##P<0.0007 Reinstatement vs. Test). All data are expressed as time spent in sec (mean ± SEM) in the nicotine-paired compartment during 15-min Pre-test (open bars), Test (filled black bars), Extinction (striped bars) and Reinstatement (filled gray bars) sessions.
Effects of URB597 on acquisition of nicotine self-administration behavior
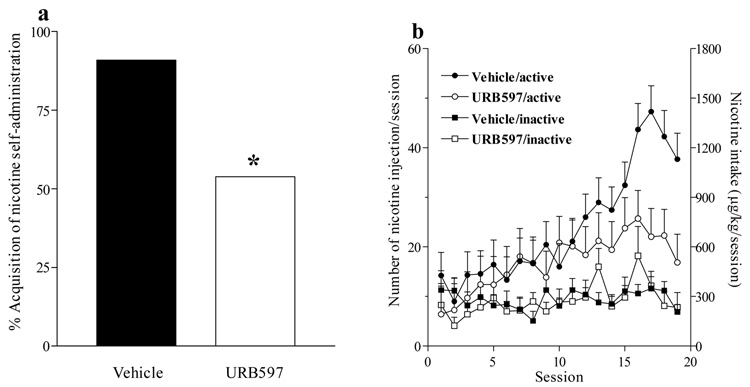
Acquisition of nicotine (30 µg/kg/injection) self-administration behavior was studied over 19 sessions in independent groups of Sprague-Dawley rats treated with URB597 (0.3 mg/kg, i.p.) or URB597 vehicle before each daily session. Treatment with URB597 significantly decreased the likelihood of rats developing nicotine self-administration behavior [χ2 1=3.96, P<0.05], with 90% of URB597 vehicle-treated rats, but only 50% of URB597-treated rats, meeting the acquisition criterion of self-administering at least 10 injections per session for 3 consecutive days (Figure 3a). Among rats that met the acquisition criterion, URB597 prevented the increase in nicotine intake that occurred in control rats around day 12 (Figure 3b); over the 19-day period, these URB597-treated rats self-administered significantly fewer nicotine injections than control rats [Proc Mixed, Main effect of treatment F1,16=6.65, P<0.02]. On the final day of acquisition, nicotine intake in URB597-treated rats was 62% lower than in vehicle-treated rats (Figure 3b)
Figure 3. Effects of URB597 on acquisition of nicotine self-administration behavior.
(a) The proportion of Sprague Dawley rats that developed nicotine self-administration behavior under a FR1 schedule over 19 sessions was reduced in rats treated with URB597 (0.3 mg/kg) prior to each session (P<0.05; n=11−13). (b) The number of injections and total amount of nicotine self-administered per session were significantly reduced by URB597 treatment. Note that responding in the active nose-poke hole, which delivered nicotine injections, gradually increased after the fourth session while responses in the inactive hole remained low, demonstrating reinforcing effects of nicotine.
Effects of URB597 on nicotine-induced reinstatement of nicotine-seeking behavior
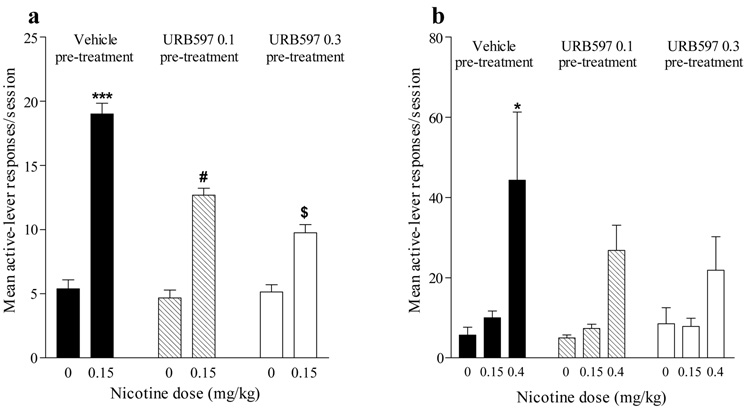
The ability of URB597 to reduce nicotine-induced reinstatement of nicotine-seeking behavior was demonstrated in two separate experiments, one using Sprague-Dawley rats and an FR 1 schedule, and the other using Long-Evans rats and an FR 5 schedule. In both experiments, after nicotine-seeking responses were extinguished by discontinuing nicotine delivery, non-contingent priming injections of nicotine (0.15 and 0.4 mg/kg) reinstated high rates of active-lever responding (Figure 4a,b). In both experiments, URB597 (0.1 and 0.3 mg/kg) significantly reduced this nicotine-induced relapse [Proc Mixed, interaction of nicotine and URB597 F2,6= 21.01, P<0.002 (Figure 4a); main effect of nicotine F2,10=13.44, P<0.0015 (Figure 4b)]. URB597 by itself did not reinstate nicotine seeking (Figure 4a, b).
Figure 4. Effects of URB597 on nicotine-induced reinstatement of nicotine-seeking behavior.
(a) After Sprague Dawley rats acquired nicotine self-administration behavior under a FR1 schedule, behavior was extinguished by substituting saline vehicle for nicotine. Nicotine (0.15 mg/kg) priming before the session produced significant reinstatement of responding (***P<0.0001; n=8). URB597 (0.1 and 0.3 mg/kg) pre-treatment significantly reduced reinstatement of drug-seeking behavior by nicotine (#P<0.016 and $P<0.0008 respectively; n=6–8). URB597 alone did not reinstate responding. (b) After Long Evans rats acquired nicotine self-administration behavior under a FR5 schedule, behavior was extinguished by substituting saline vehicle for nicotine. Nicotine (0.4 mg/kg) priming before the session produced significant reinstatement of responding (*P<0.05; n=6). Responding was not increased by nicotine (compared to vehicle control) after pre-treatment with URB597 (0.1 and 0.3 mg/kg) (P=ns; n=6). URB597 alone did not reinstate extinguished nicotine-seeking behavior and did not potentiate effects of a lower 0.15 mg/kg dose of nicotine, which was ineffective in reinstating extinguished nicotine-seeking behavior under the FR5 schedule.
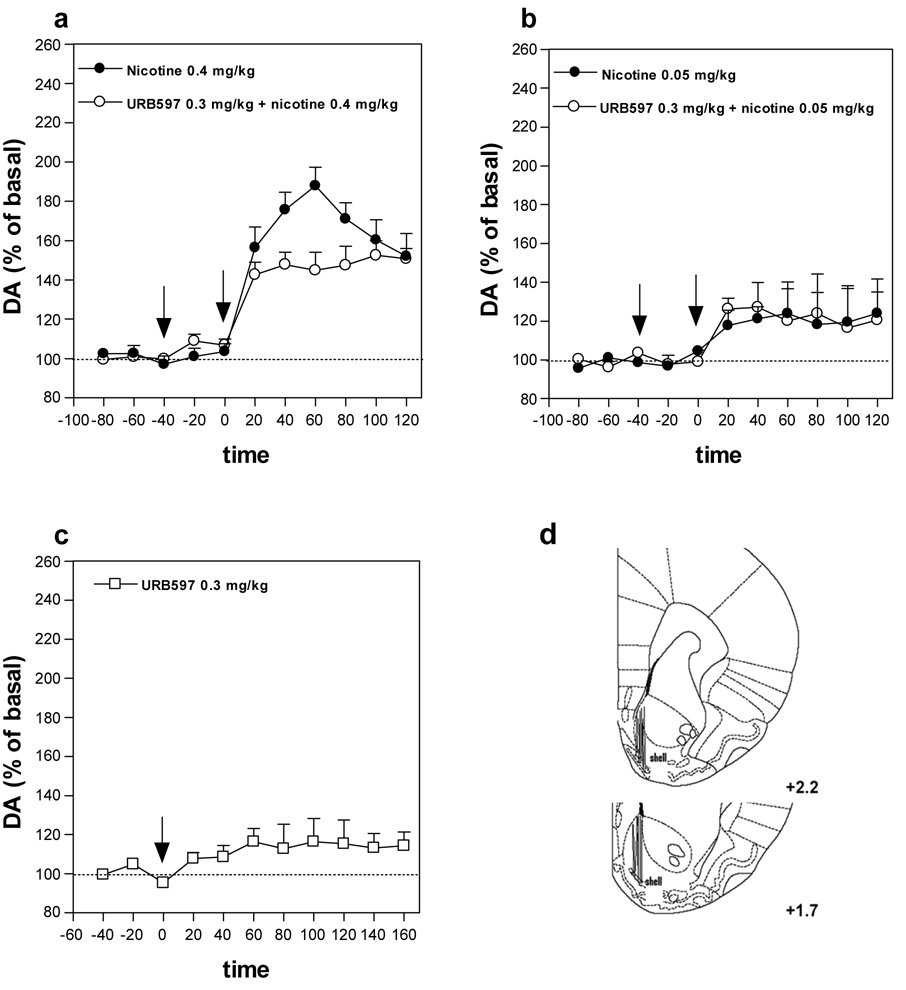
Effects of URB597 on nicotine-induced elevation in dopamine levels in the nucleus accumbens shell
In vivo microdialysis experiments showed that nicotine (0.4 mg/kg s.c.) increased extracellular levels of dopamine in the nucleus accumbens shell by about 80%, compared with basal levels [one-way ANOVA F10,55=21.44, P<0.0001] (Figure 5a). Administration of URB597 (0.3 mg/kg) reduced nicotine-induced elevations in dopamine levels (Figure 5a). Two-way ANOVA showed a significant effect of URB597 treatment (F1,60=14.97, P=0.0003). Importantly, URB597 (0.3 mg/kg) did not potentiate the effects of a low threshold dose of nicotine (0.05 mg/kg), that by itself had no significant effect on dopamine levels (Figure 5b), and URB597 (0.3 mg/kg) alone did not alter dopamine levels (Figure 5c) in the nucleus accumbens shell.
Figure 5. Effects of URB597 on nicotine-induced elevations in dopamine levels in the nucleus accumbens shell.
(a) Pre-treatment with URB597 (0.3 mg/kg), but not its vehicle, given before nicotine (0.4 mg/kg), significantly reduced the increase in extracellular dopamine levels produced by nicotine (two-way ANOVA P=0.0003, n=6). (b) Pre-treatment with URB597 (0.3 mg/kg) did not potentiate the effects of a low threshold dose of nicotine (0.05 mg/kg), that by itself had no significant effect on dopamine levels and alone (c) did not alter dopamine levels. Arrows represent time of injection of each drug. Results are means, with vertical bars representing SEM, of dopamine levels in 20-min dialysate samples, expressed as a percentage of basal values. (d) Verification of microdialysis probe location by histology. Vertical lines indicate the placement of microdialysis probes within brain sections (as modified from Paxinos & Watson, 1998) in the shell of the nucleus accumbens. Numbers beside each plate represent distance from bregma.
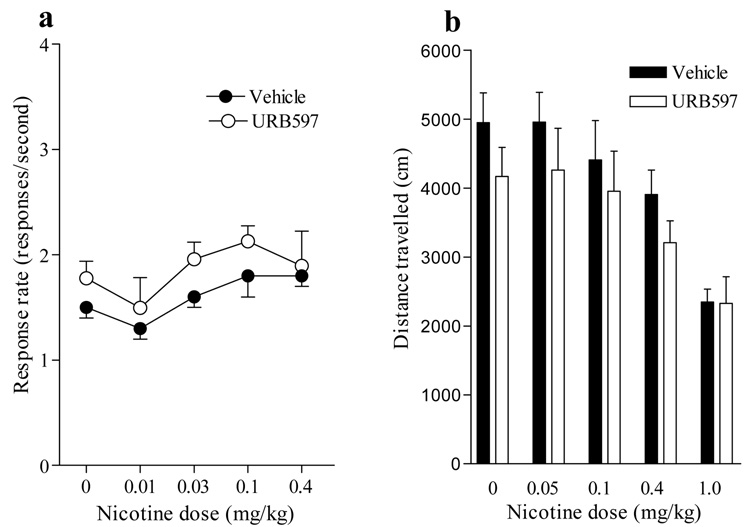
Effects of URB597 and nicotine on food-reinforced responding and locomotor activity
Nicotine doses as high as 0.4 mg/kg s.c. had no significant effect on food-reinforced responding, either when given alone or after FAAH inhibition by 0.3 mg/kg URB597 (Figure 6a). The effects of nicotine (0.05–1.0 mg/kg s.c.) on locomotor activity were not altered by treatment with 0.3 mg/kg URB597 (Figure 6b).
Figure 6. Effects of URB597 and nicotine on food-reinforced responding and locomotor activity.
(a) URB597 (0.3 mg/kg i.p.) given acutely before the session did not disrupt food-maintained responding either alone or in combination with different doses of nicotine (0.01 to 0.4 mg/kg s.c.). Rates of responding (responses/second) on the active lever associated with food reinforcement are shown as a function of nicotine dose (mg/kg, log scale). Data are expressed as responses per sec (mean ± SEM; n=16). (b) URB597 (0.3 mg/kg i.p.) also failed to modify the effects of any dose of nicotine (0.05 to 1.0 mg/kg s.c.) on locomotor activity. Data are expressed as distance traveled in cm (mean ± SEM; n=7) during a single daily session.
Discussion
Previous studies investigating interactions between the endocannabinoid system and nicotine reward have focused on systemically administered cannabinoid agonists and antagonists, which affect cannabinoid receptors globally regardless of demand and their effects are ubiquitous. In contrast, in this study we used a selective inhibitor of FAAH, URB597, to magnify and prolong the effects of only one of the endocannabinoid signals, the AEA signal, when and where AEA is synthesized and released on demand in activated synapses (Kathuria et al., 2003; Fegley et al., 2005; Pertwee, 2005; Piomelli et al., 2006). URB597 decreased nicotine's rewarding effects, preventing the acquisition of nicotine self-administration behavior and the development of nicotine-induced CPP. URB597 also reduced nicotine's ability to trigger relapse in both of these animal models of drug abuse. Furthermore, systemic administration of URB597 significantly reduced nicotine's ability to increase dopamine levels in the nucleus accumbens shell, an effect considered central for the reinforcing effects of many abused drugs, including nicotine (Pontieri et al., 1996). Notably, decreases in acquisition and relapse were not likely to be related to non-selective effects of URB597 on rates of operant responding or general activity, since combinations of URB597 and different doses of nicotine did not depress food-reinforced operant responding and did not alter nicotine's effects on locomotor activity. Also, decreases in acquisition and relapse were not likely to be related to the known detrimental effects of cannabinoids on memory acquisition or consolidation, since the doses of 0.1 and 0.3 mg/kg URB597 used in the present experiments markedly enhance memory acquisition and have no effect on memory consolidation using a passive-avoidance task in rats (Mazzola et al., paper submitted for publication), while systemically administered AEA or THC impair memory acquisition and consolidation under similar conditions (Castellano et al., 1997, 2003).
Nicotine has rewarding effects within the range of doses studied here, but at higher doses can have aversive effects (Le Foll and Goldberg, 2005). In addition, intravenously administered AEA has aversive effects when FAAH is inhibited by URB597 (Scherma et al., 2008). This suggests the possibility that the diminished reward we observed when nicotine was combined with URB597 in the self-administration and CPP procedures might actually have been due to an increased sensitivity to nicotine's effects (i.e., a shift to the left of the nicotine dose-effect function) or to additive effects of nicotine and elevated endogenous levels of AEA. However, these possibilities are not supported by the present results. That is, there was no evidence of place aversion when the high dose of nicotine (1.0 mg/kg) was combined with URB597, and a sub-threshold dose of nicotine (0.05 mg/kg) did not produce CPP when combined with URB597, indicating that FAAH inhibition did not enhance sensitivity to nicotine’s rewarding or aversive effects. In addition, a sub-threshold dose of nicotine (0.05 mg/kg), that by itself had no significant effects on dopamine levels in the nucleus accumbens shell, did not increase dopamine levels when combined with URB597. In mice, there is evidence that pharmacological inhibition or genetic ablation of FAAH can facilitate rewarding effects of a sub-threshold dose of nicotine (0.1 mg/kg) in a CPP procedure (Merritt et al., 2008) while reducing rewarding effects of higher nicotine doses, suggesting potentiation rather than suppression. This discrepancy between the present results and those of Merritt et al. might be due to species-related or procedural differences between the studies. In any case, the present study used multiple, independent behavioral procedures to assess the ability of URB597 to alter nicotine's rewarding and relapse-inducing effects in rats, and all evidence was consistent in showing that URB597 reduces the rewarding behavioral effects of nicotine as well as the reward-related dopaminergic effects in the shell of the nucleus accumbens.
The mesolimbic dopamine system plays a pivotal role in the development of nicotine addiction, and the VTA, in particular, is a critical brain area for nicotine’s rewarding effects (Corrigal et al., 1994). Nicotinic receptors in the VTA are located both on cell bodies of dopaminergic neurons and on presynaptic nerve endings of glutamatergic neurons which descend from the medial prefrontal cortex and impinge on these cell bodies (Mansvelder and McGehee, 2002). Nicotine can facilitate dopamine release in the nucleus accumbens shell directly through stimulation of nicotinic receptors on cell bodies of dopaminergic neurons and indirectly through stimulation of glutamate release. Activation of nicotinic receptors on dopaminergic neurons in the VTA by nicotine could result in depolarization, increased extracellular calcium, and release of AEA (Melis et al., 2004; Di Marzo et al., 1994). This AEA, protected from rapid degradation by URB597, could then activate CB1 receptors present on presynaptic nerve endings of glutamatergic neurons (Melis et al., 2004), reducing glutamate release from these neurons, and thereby reducing activation of dopaminergic neurons and release of dopamine from their terminals in the nucleus accumbens shell. This hypothesis is supported by the recent in vivo demonstration that in rats URB597 can reverse nicotine-induced excitation of dopamine-releasing neurons in the VTA that project to the accumbens shell (M. Pistis, personal communication).
In addition to this cannabinoid-mediated mechanism, there are several non-cannabinoid mechanisms that might contribute to URB597’s effects on nicotine reward. First, AEA, protected from degradation by URB597, may directly inhibit α4/β2 nicotinic acetylcholine receptors (Spivak et al., 2007; Butt et al., 2008), which are clearly implicated in the rewarding effects of nicotine (Picciotto et al., 1998; Maskos et al., 2005; Walters et al., 2006). Animals that lack expression of the β2 subunit do not readily self-administer nicotine, or express nicotine-induced CPP (Picciotto et al., 1999; Walters et al., 2006). Second, FAAH inhibition also increases brain levels and magnifies and prolongs the effects of the non-cannabinoid fatty acid ethanolamides oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) which are endogenous ligands for the peroxisome proliferator-activated nuclear receptor-alpha (PPAR-α) (Fegley et al., 2005; Astarita et al., 2006; Sun et al., 2007). OEA and PEA, protected from degradation by URB597, may modulate the responses of VTA dopaminergic neurons to nicotine by actions at PPAR-α nuclear receptors. This hypothesis is supported by the recent in-vivo finding that in rats OEA and PEA, but not the FAAH resistant analog of AEA, methandamide, can reverse nicotine-induced excitation of dopamine-releasing neurons in the VTA that project to the accumbens shell (M. Pistis, personal communication). Finally, AEA acts at TRPV1 (vanilloid) receptors (Ahern, 2003) and there is a recent report that AEA also acts at non-cannabinoid PPAR-α (Sun et al., 2007). It is possible that nicotine-induced release of AEA could regulate signaling at these non-cannabinoid receptors.
In conclusion, our findings demonstrate that nicotine's rewarding effects and its ability to trigger relapse to drug-seeking behavior after abstinence in rats are suppressed by inhibiting intracellular activity of FAAH, the enzyme that degrades the endocannabinoid AEA and the non-cannabinoid lipid amides OEA and PEA in the brain. These therapeutic behavioral effects of URB597 may be due to its indirect blockade of nicotine-induced increases in dopaminergic transmission in the nucleus accumbens. These findings point to FAAH as a novel molecular target for the development of medications for tobacco dependence.
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, by the Italian Ministry of University and Scientific Research and the Centre of Excellence on “Neurobiology of Dependence”, by the Department of Psychiatry, University of Maryland School of Medicine, by the Institute of Experimental Medicine, Hungarian Academy of Sciences, by the Centre for Addiction and Mental Health and University of Toronto, by the Division of Geriatric Medicine and Gerontology of Johns Hopkins University School of Medicine, and by the Department of Pharmacology, University of California, Irvine. The reinstatement of conditioned place preference experiments was supported in part by a grant from Philip Morris USA and Philip Morris International.
Abbreviations
- THC
Δ9-tetrahydrocannabinol
- FAAH
fatty acid amide hydrolase
- CPP
conditioned place preference
- VTA
ventral tegmental area
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- PPAR
peroxisome proliferator-activated nuclear receptor
- URB597
cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester
Footnotes
This work was presented at the 37th annual meeting of the Society for Neuroscience (Nov 3–7, 2007) in San Diego (Scherma et al., Program No. 843.7. 2007 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience. Online).
References
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR, Rivara S, Tarzia G, Mor M, Piomelli D. Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anoexiant properties. J Pharmacol Exp Ther. 2006;318:563–570. doi: 10.1124/jpet.106.105221. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Butt C, Alptekin A, Shippenberg T, Oz M. Endogenous cannabinoid anandamide inhibits nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem. 2008;105:1235–1243. doi: 10.1111/j.1471-4159.2008.05225.x. [DOI] [PubMed] [Google Scholar]
- Castané A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Castané A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cabib S, Palmisano A, Di Marzo V, Puglisi-Allegra S. The effects of anandamide on memory consolidation in mice involve both D1 and D2 dopamine receptors. Behav Pharmacol. 1997;8:707–712. doi: 10.1097/00008877-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie’ P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–140. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci. 2003;17:1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Forget B, Hamon M, Thiebot MH. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology. 2005;181:722–734. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernàndez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor M, Rivara S, Lodola A, Plazzi PV, Tarzia G, Duranti A, Tontini A, Piersanti G, Kathuria S, Piomelli D. Cyclohexylcarbamic acid 3'- or 4'-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies. J Med Chem. 2004;47:4998–5008. doi: 10.1021/jm031140x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res. 1999 Suppl 2:S121–S125. doi: 10.1080/14622299050011931. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makryiannis A, Pomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology. 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;54:438–444. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Spivak CE, Lupica CR, Oz M. The endocannabinoid anandamide inhibits the function of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2007;72:1024–1032. doi: 10.1124/mol.107.036939. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]