Abstract
The goal of this study was to determine the morphological and sub-cellular mechanical effects of Rac activation on fibroblasts within 3-D collagen matrices. Corneal fibroblasts were plated at low density inside 100 μm thick fibrillar collagen matrices and cultured for 1 to 2 days in serum-free media. Time-lapse imaging was then performed using Nomarski DIC. After an acclimation period, perfusion was switched to media containing PDGF. In some experiments, Y-27632 or blebbistatin were used to inhibit Rho-kinase (ROCK) or myosin II, respectively. PDGF activated Rac and induced cell spreading, which resulted in an increase in cell length, cell area, and the number of pseudopodial processes. Tractional forces were generated by extending pseudopodia, as indicated by centripetal displacement and realignment of collagen fibrils. Interestingly, the pattern of pseudopodial extension and local collagen fibril realignment was highly dependent upon the initial orientation of fibrils at the leading edge. Following ROCK or myosin II inhibition, significant ECM relaxation was observed, but small displacements of collagen fibrils continued to be detected at the tips of pseudopodia. Taken together, the data suggests that during Rac-induced cell spreading within 3-D matrices, there is a shift in the distribution of forces from the center to the periphery of corneal fibroblasts. ROCK mediates the generation of large myosin II-based tractional forces during cell spreading within 3-D collagen matrices, however residual forces can be generated at the tips of extending pseudopodia that are both ROCK and myosin II-independent.
Keywords: Rac, Rho Kinase, PDGF, Collagen Matrices, Wound Healing, Cornea
INTRODUCTION
The organization of extracellular matrices by cells through the exertion of mechanical forces drives fundamental processes such as developmental morphogenesis, wound healing, and the organization of bioengineered tissues. Previous studies have established that the Rho-family small GTPases such as Rho, Rac, and Cdc42 play a central role in regulating the cytoskeletal changes associated with cell mechanical activity (Hall, 2005; Jaffe and Hall, 2005). These GTP binding proteins function as molecular switches; alternating between the active GTP-bound state and the inactive GDP-bound state. In Swiss 3T3 fibroblasts on rigid substrates, activated Rho stimulates the formation of stress fibers, the development of large focal adhesions (focal contacts) and increased cell contractility via activation of Rho kinase (ROCK) (Kaibuchi et al., 1999; Kawano et al., 1999; Parizi et al., 2000; Rottner et al., 1999; Totsukawa et al., 2000). In contrast to Rho, Rac and Cdc42 induce cell spreading via the creation of smaller focal complexes and actin polymerization (Demali and Burridge, 2003; Rottner et al., 1999; Sander et al., 1999; Svitkina and Borisy, 1999; Totsukawa et al., 2000). While significant progress has been made in our understanding of how Rac regulates actin polymerization during cell spreading (Kraemer et al., 2007; Pollard and Borisy, 2003; Suetsugu et al., 2006; Ten Klooster et al., 2006), much less is known on how the sub-cellular pattern of force generation is altered by Rac activation.
Most previous studies investigating the mechanics of Rac-induced cell spreading have been performed using 2-D substrates (Galbraith et al., 2002; Raucher and Sheetz, 2000). However, cells generally reside within 3-D extracellular matrices in vivo, and significant differences in morphology, adhesion organization, and mechanical behavior have been identified between 2-D and 3-D culture models (Abbott, 2003; Bard and Hay, 1975; Cukierman et al., 2001; Cukierman et al., 2002; Doane and Birk, 1991; Friedl and Brocker, 2000; Grinnell et al., 2003; Jiang and Grinnell, 2005; Larsen et al., 2006; Rhee et al., 2007; Tomasek et al., 1982). Platelet derived growth factor (PDGF), which activates Rac (Grinnell, 2000; Sander et al., 1999), has been shown to enhance spreading and migration of fibroblasts within 3-D collagen matrices and to stimulate global matrix contraction (Grinnell et al., 2006; Jester and Chang, 2003; Shreiber et al., 2001; Tamariz and Grinnel, 2002). However, direct assessment of the dynamic mechanical interactions between cells and collagen fibrils induced by Rac activation in 3-D culture has not been reported.
We previously developed an experimental model for investigation of cell mechanical behavior inside fibrillar collagen matrices by using high magnification time-lapse DIC and fluorescent imaging (Petroll and Ma, 2003; Petroll et al., 2003). Using this system, temporal changes in cell morphology and collagen fibril organization can be directly visualized and correlated, and changes in cell behavior in response to mechanical or biochemical stimuli can be assessed in real-time (Petroll et al., 2004; Vishwanath et al., 2003). In this study, we use this model to investigate the effects of PDGF on the dynamic sub-cellular mechanical activity of corneal fibroblasts, and the role of ROCK and myosin II in mediating these effects. The data demonstrate that during Rac-induced spreading in 3-D matrices, there is a shift in the distribution of forces from the center to the periphery of corneal fibroblasts. The pattern of pseudopodial extension and cell-induced local collagen fibril realignment during spreading is highly dependent upon the initial orientation of the collagen fibrils at the leading edge, and can result in either alignment of fibrils parallel with the pseudopodia, or compaction of collagen fibrils in a direction perpendicular to the extending process. Following ROCK or myosin II inhibition, there is a significant reduction of cell-induced matrix deformation during cell spreading; however, transient displacements of collagen fibrils at the ends of extending pseudopodia are still observed. Overall, the data suggest that ROCK mediates the generation of myosin II based tractional forces during cell spreading within 3-D collagen matrices, however residual forces can be generated at the tips of extending pseudopodia that are both ROCK and myosin II-independent.
MATERIALS AND METHODS
Cells
Studies were performed using a previously characterized telomerase-infected, extended lifespan human corneal fibroblast cell line, HTK (Jester and Chang, 2003; Jester et al., 2003; Vishwanath et al., 2003). HTK cells were maintained in “complete media” consisting of Dulbecco’s modified Eagle’s medium (DMEM, GIBCO Invitrogen Cell Culture, Carlsbad, CA) supplemented with 1% Penicillin, 1% Streptomycin and 1% Fungisome (Biowhitaker, Inc., Wakersville, MD) and 10% fetal bovine serum (FBS, Sigma Chemical Corp., St. Louis, MO). For some experiments, corneal fibroblasts were transfected to express GFP-zyxin as previously described (Petroll and Ma, 2003; Petroll et al., 2003).
Collagen Matrices
Hydrated collagen matrices were prepared as described previously (Petroll et al., 2003). Briefly, neutralized bovine dermal collagen (Vitrogen 100; Collagen Corporation, Palo Alto, CA) was mixed with 10X DMEM to achieve a final collagen concentration of 2.48 mg/ml. For plating inside the matrix, a 50 μl suspension of fibroblasts was mixed with 500 μl of collagen solution. The cell/collagen mixture was pre-incubated at 37°C for 5 minutes, and 30 μl aliquots (containing approximately 1,000 cells) were then poured onto Delta T culture dishes (Bioptechs, Inc., Butler, PA), placed in a humidified incubator (37°C, 5% CO2) for 60 minutes for polymerization, and overlaid with 2 ml of serum-free media consisting of DMEM supplemented with 1% RPMI 1640 Vitamin and Glutathione mix, 1% ascorbic acid, and 1% MEM non-essential amino acids.
Time-lapse Digital Imaging
Collagen matrices were incubated for 1 to 2 days prior to each time-lapse imaging experiment. Microscopy was performed using a Nikon TE300 inverted microscope (Nikon Inc., Melville, NY) equipped for time-lapse fluorescence and DIC imaging (Petroll and Ma, 2003; Vishwanath et al., 2003). The hardware was controlled using a PC running MetaMorph (Molecular Devices, Sunnyvale, CA). To maintain cell viability during imaging, a Bioptechs microincubation system and objective heater was used (Delta T; Bioptechs, Inc., Butler, PA). Cells were continuously perfused while on the microscope stage with serum-free media containing 50 mM HEPES buffer at a rate of 6 ml/hour.
Cells were allowed to acclimate to the microscope microincubation system for 1 hour prior to imaging. Time-lapse imaging of a single, isolated cell was then performed at 1–2 minute intervals using a 40X oil immersion objective (1.2 NA, 220 μm free working distance) with Nomarski DIC. 3-D datasets were obtained at each time point by repeating the acquisition at 5–6 sequential focal planes in z steps of 1–2 microns. After a minimum of 30 minutes of time-lapse imaging in basal media, 50 μl of a PDGF BB stock solution (10 μg/ml, Upstate USA Inc., Charlottesville, VA) was added to the culture dish to achieve a final concentration of 50 ng/ml, and the perfusion was simultaneously switched to basal media containing 50 ng/ml PDGF. Control experiments were performed using vehicle alone. In other experiments (n=10 cells), after 1–2 hours of time-lapse imaging with PDGF, the Rho-kinase inhibitor Y-27632 (Cal Biochem, La Jolla, CA) was added to the culture dish to achieve a final concentration of 10–100 μM. The perfusion media was simultaneously switched to media containing 50 ng/ml PDGF and Y-27632, and time-lapse imaging was continued for another 1–2 hours. In other experiments (n = 7 cells), the myosin II ATPase inhibitor blebbistatin (100 μM, Toronto Research Chemicals, Downsview, Canada) was used to assess the role of myosin II in mediating the cellular response to PDGF. This concentration provides maximal inhibition of non muscle myosin IIs without affecting unconventional myosins (Allingham et al., 2005; Straight et al., 2003). Because blebbistatin has a phototoxic effect with concomitant loss of pharmacological activity at wavelengths below 500 nm, a 570 nm long pass filter was inserted into the light path (E570LP, Chroma Technology Corp, Rockingham, VT).
For experiments using GFP-zyxin expressing cells, time-lapse imaging was performed using a 60X oil immersion objective (1.3 NA, 220 μm free working distance). At each time point separate FITC (for EGFP) and DIC images were acquired in rapid succession as described previously (Petroll and Ma, 2003). To minimize phototoxicity and photobleaching, neutral density filters were used and exposure times were kept to a minimum by using 2×2 on-chip camera binning.
Assessment of Matrix Deformation
In order to quantify the ECM deformation, the x,y coordinates of landmarks in DIC images were determined using the “track points” feature in MetaMorph (Petroll and Ma, 2003). In order to display the ECM and adhesion displacements, we used a custom program using Visual Basic (Microsoft, Redmond, WA). This program draws the path of each tracked object onto a sequence of images. The tracks can then be overlaid onto other image sequences as needed using MetaMorph (Vishwanath et al., 2003).
Biochemistry
Cells were plated within collagen matrices (0.2 ml volume and 2 × 105 cells each), and cultured for 24 hours in basal media. PDGF was added for 0 (control), 10 or 30 minutes, and cell lysates were collected as previously described (Grinnell et al., 2003). To measure Rac activation, the G-LISA™ activation kit (Kit #BK126, Cytoskeleton, Inc., Denver, CO) was used according to the manufacturer’s instructions. This assay uses a Rac-GTP-binding protein linked to the wells of a 96 well plate. Active, GTP bound Rac in cell lysates binds to the wells while inactive, GDP-bound Rho is removed during wash steps. Bound GTP Rac1 is detected by incubation with a Rac1 specific antibody followed by a secondary antibody conjugated to HRP and a detection reagent. The signal was read by measuring luminescence with a spectrophotometer (Beckman Coulter, Model DU800, Fullerton, CA). For each experiment, cell lysates were collected and pooled from four collagen matrices for each of the three treatment groups (0 min, 10 min and 30 min of PDGF treatment). The entire experiment was repeated three times (n=4).
RESULTS
PDGF induces Rac activation in corneal fibroblasts
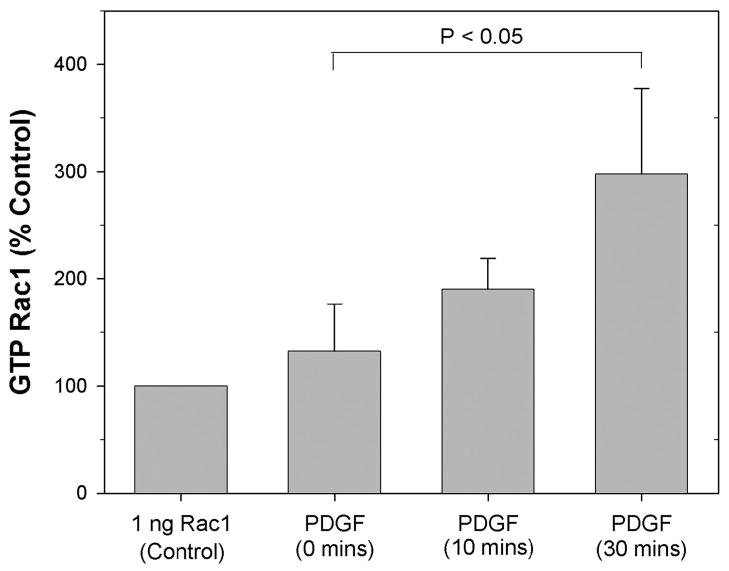
Previous studies have demonstrated that PDGF induces Rac activation in dermal fibroblasts (Grinnell, 2000; Sander et al., 1999). To assess whether similar changes occur in corneal fibroblasts, cells were plated in collagen matrices, cultured for 24 hours, and exposed to PDGF for 0 minutes (control), 10 minutes or 30 minutes. The level of Rac activation was then measured using the G-LISA method. Figure 1 shows the mean and standard deviation of four independent experiments for each treatment group, normalized to the positive control. In all four experiments, both 10 and 30 minutes exposure to PDGF increased the level of Rac activation as compared to untreated cells; this increase reached statistical significance at 30 minutes.
Figure 1.
Rac activation data. Graph shows the mean and standard deviation of four independent experiments. Data shown is luminescence over background signal (wells incubated with lysis buffer alone instead of cell lysates), normalized to 1 ng Rac1 protein controls. In all four experiments, both 10 and 30 minutes exposure to PDGF increased the level of Rac activation as compared to untreated cells; this increase reached statistical significance at 30 minutes (P < 0.05, Repeated Measures ANOVA, with Holm-Sidak method for multiple comparisons).
PDGF induces spreading of corneal fibroblasts in 3-D culture
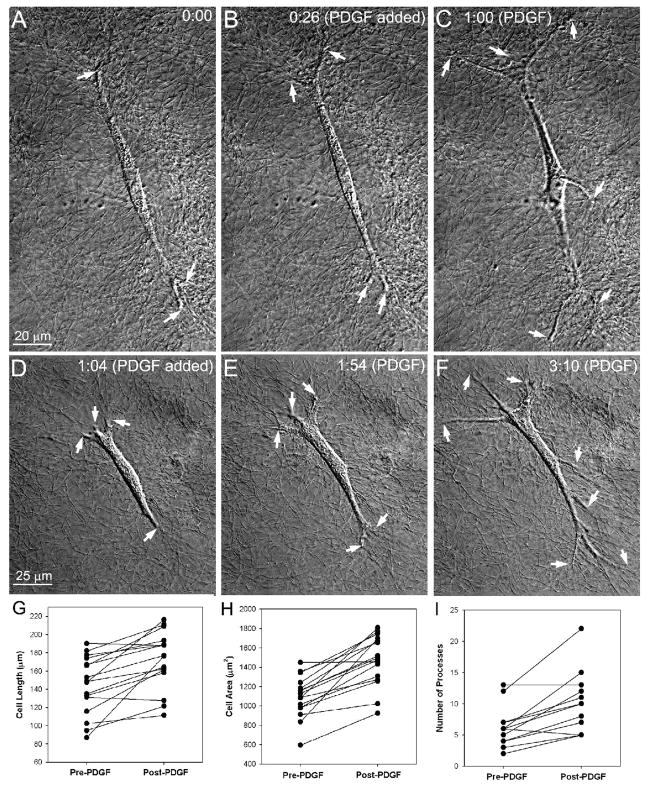
HTK cells plated inside 3-D collagen matrices cultured in serum-free media generally had a bipolar morphology with thin pseudopodial processes. Cells were always aligned nearly parallel to the dish on which the collagen matrix was plated. DIC imaging allowed detailed visualization of the cells and the individual collagen fibrils surrounding them (Fig. 2). Pseudopodia underwent small extensions and retractions, but significant changes in cell morphology were not observed (Fig. 2A, B). Addition of PDGF induced cell spreading via the extension of existing pseudopodial processes (arrows) and the formation of new processes (Figure 2C, D–F). Quantitative analysis (Fig. 2G–H, n = 16 cells) demonstrated an increase in projected cell length (22.4 ± 25.2%, P < 0.01), projected cell area (36.4 ± 24.0%, P<0.01), and the number of pseudopodial processes (9.6 ± 4.5 vs. 5.6 ± 3.1 processes per cell, P<0.01) following PDGF induced spreading.
Figure 2.
PDGF-induced cell spreading. A–C. HTK cell plated inside a 3-D collagen matrix, cultured for 24 hours in serum-free media, and transferred to the microscope stage. DIC imaging allowed detailed visualization of pseudopodial processes (arrows) and the individual collagen fibrils surrounding them. No significant changes in cell morphology were observed in basal media (compare A and B). However, addition of PDGF induced rapid cell spreading via the extension of existing pseudopodial processes and the formation of new processes (C). Time shown is hours:minutes since the start of time-lapse imaging. D–F. A second HTK cell showed similar spreading over a longer time course. G–H. Quantitative analysis (n = 16 cells) demonstrated an increase in projected cell length, projected cell area, and the number of pseudopodial processes following PDGF treatment.
PDGF induces tractional force generation and local collagen reorganization
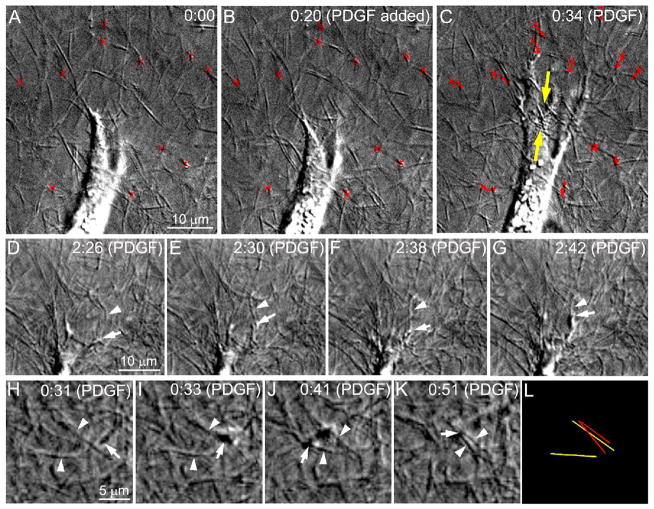
In addition to the morphological changes, cell-induced displacement and realignment of collagen fibrils was also observed during PDGF-induced spreading. In general, relaxation (decompression) of the ECM was observed along the cell body, whereas tractional forces were generated by extending cell processes, as indicated by centripetal displacement of collagen fibrils (Movie 1). More detailed assessment of the dynamic cell-matrix mechanical interactions by extending pseudopodia is shown in Figure 3. In the top panel, tracking of the ECM displacements showed minimal collagen displacement prior to the addition of PDGF (3A and B, red tracks, crosses mark positions at time 0:00). However, following addition of PDGF, the matrix in front of the cell was pulled inward by the extending pseudopodial processes (Figure 3C, Movie 2), resulting in compression of the ECM (yellow arrows). Tractional forces generated by an extending cell processes were often transient, and sometimes dissipated as processes continued to branch and spread (Movie 3, note lower left process). While there was significant variation in the timing and amount of cell-induced ECM deformation following PDGF treatment, the pattern of matrix decompression along the cell body (decreased tractional force), and matrix compression in front of extending pseudopodia (increased tractional force) was observed in all 16 cells.
Figure 3.
Cell-matrix mechanical interactions in response to PDGF. A–C. Cell-induced displacement and realignment of collagen fibrils was observed during PDGF-induced spreading. Tracking of the ECM displacements showed minimal collagen displacement prior to the addition of PDGF (B, red tracks, crosses mark position at time 0:00). However, following addition of PDGF, the matrix in front of the cell was pulled inward by the extending pseudopodial processes (C), resulting in compression of the ECM (yellow arrows). D–G. Higher magnification assessment of cellular interactions with individual collagen fibrils. PDGF was added at 1:04. In this example, a collagen fibril (arrowheads) in front of an extending process (arrows) is aligned somewhat parallel to the direction of spreading (D). The extending process engages the fibril (E), pulls it into alignment (F), then continues to spread along it (G). This results in alignment of the collagen fibril parallel with the pseudopodia. H–L. Cellular interactions with two collagen fibrils (arrowheads) aligned somewhat perpendicular to the direction of spreading. PDGF was added at 0:26 minutes. The extending process (arrows) engages the first fibril (I), pushes past it to engage the second fibril (J), then pulls the fibrils together (K). This results in compaction of the collagen fibrils in a direction perpendicular to the extending process (L; yellow lines indicate initial position of collagen fibrils, red lines indicate final position of collagen fibrils).
To further investigate the mechanics of PDGF-induced spreading, cellular interactions with individual collagen fibrils were studied. In general, two types of collagen displacement were observed at the leading edge during PDGF induced spreading. First, when a collagen fibril in front of an extending process was aligned somewhat parallel to the direction of spreading (Figure 3D), the extending process often engaged the fibril (Figure 3E), pulled it into alignment (Figure 3F), then continued to spread along it (Figure 3G). Second, when collagen fibrils in front of an extending process were aligned more perpendicular to the direction of spreading (Figure 3H), the extending process would often engage the first fibril (Figure 3I), push past it to engage the second fibril (Figure 3J), then pull the fibrils together (Figure 3K,L). The first pattern of interaction tended to pull collagen fibrils at the ends of cells into an alignment parallel with the pseudopodia (Movie 4); whereas, the second pattern resulted in compaction of the collagen fibrils in a direction perpendicular to the extending process (Movie 5).
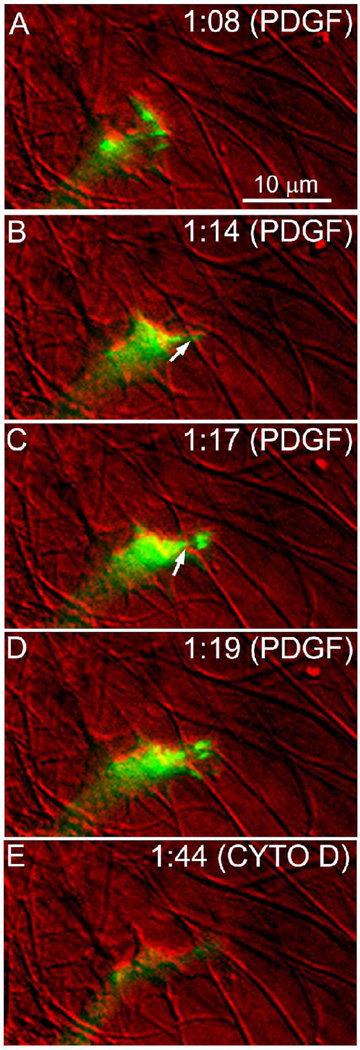
In order to investigate the role of cell-matrix adhesions on Rac-induced spreading mechanics, cell were transfected to express GFP-zyxin and treated with PDGF (Fig. 4, Movie 6). Formation of adhesions at the leading edge of extending pseudopodia was associated with centripetal displacement and/or bending of the collagen fibrils with which they interacted (Fig. 4B&C, arrows). Cytochalasain D induced disassembly of adhesions and matrix relaxation (Fig. 4E). It should be noted that zyxin is a component of both focal complexes (point contacts) and focal adhesions (focal contacts) (Rottner et al., 2001). While we did not attempt to distinguish between these adhesive structures, in general, focal complexes have been associated with lamellipodia in 2-D culture and are thought to play a key role in protrusion and traction at the leading edge of migrating cells (Beningo et al., 2001; Kaverina et al., 2002; Rottner et al., 1999).
Figure 4.
Color overlays of GFP-zyxin (green) and DIC (red). PDGF was added at 0:40 minutes. Formation of cell-matrix adhesions at the leading edge of an extending pseudopodia was associated with centripetal displacement and/or bending of the collagen fibrils with which it interacted (B and C, arrows). Cytochalasain D induced disassembly of adhesions and matrix relaxation (E).
Tractional forces are more localized when Rho-kinase or myosin II is inhibited
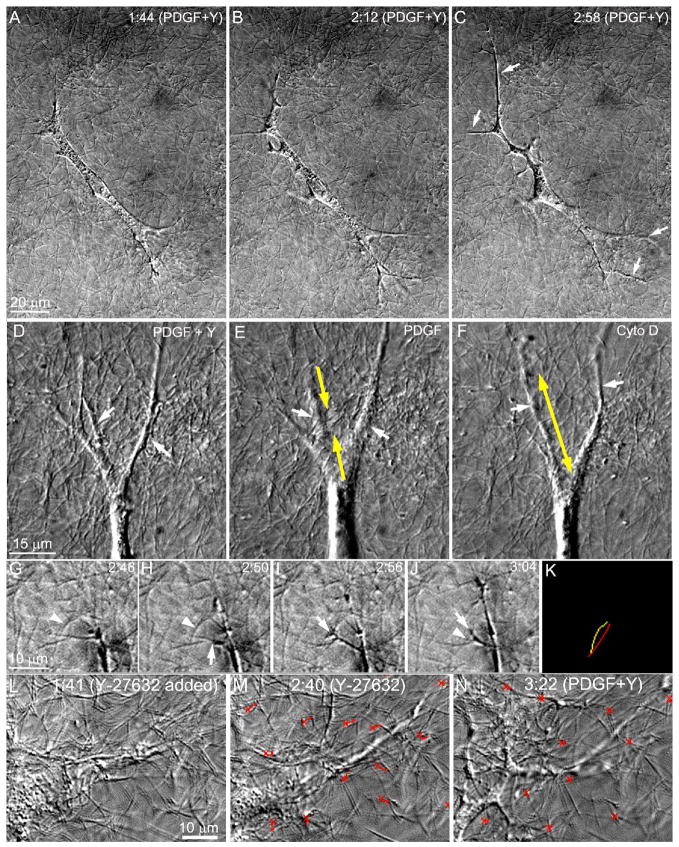
Studies by Grinnell and coworkers have demonstrated that PDGF-induced reorganization of attached collagen matrices is partially dependent on ROCK (Tamariz and Grinnel, 2002). To investigate the role of ROCK on the subcellular pattern of force generation and cell-matrix interactions in response to PDGF, the specific ROCK inhibitor Y-27632 was used. Addition of Y-27632 to PDGF treated cells induced additional cell spreading and elongation (Fig. 5A–C). Cells also assumed a more convoluted shape with thinner cell processes (arrows), suggesting a reduction of cellular tension. Significant relaxation of cell-induced tractional forces was also observed following ROCK inhibition (Movie 7). When Y-27632 was washed out by switching the perfusion back to PDGF alone (Movie 8), cell processes became thicker (compare Fig 5D and E), and increased tractional forces were observed, particularly at the base of pseudopodial processes (Fig. 5E, yellow arrows). Treatment with cytochalasin D resulted in elongation and thinning of cellular processes, and ECM decompression (Fig. 5F, yellow arrow).
Figure 5.
The role of ROCK on the subcellular pattern of force generation and cell-matrix interactions in response to PDGF. A–C. Perfusion was changed to PDGF at 0:40 (hours:minutes), and PDGF + Y-27632 at 1:40. Addition of the ROCK inhibitor Y-27632 (10μM) to PDGF treated cells induced additional cell spreading and elongation. Cells also assumed a more convoluted shape with thinner cell processes (arrows), suggesting a reduction of cellular tension. D–F. Following 30 minutes of ROCK inhibition, Y-27632 was washed out by switching the perfusion back to PDGF alone. Cell processes (arrows) became thicker (compare D and E), and increased cellular forces were observed, particularly at the base of pseudopodial processes (E, yellow arrows). Subsequent treatment with cytochalasin D resulted in elongation and thinning of cellular processes, and ECM decompression (F, yellow arrow). G–K. Cell matrix interactions at the end of a pseudopodia following ROCK inhibition. A thin dendritic process (arrows) branches off from another process. The branching process extends along a collagen fibril, engages a second fibril which is oriented perpendicular (arrowhead), and displaces this fibril inward (K, yellow line indicates initial fibril position, red line indicates final fibril position). L–N. The effect of PDGF following pre-incubation with Y-27632. Addition of Y-27632 to basal media induced cell elongation and relaxation of tractional forces (M, red tracks, crosses mark position at time 1:41), indicating that there is a basal level of ROCK activity in serum-starved corneal fibroblasts. Subsequent addition of PDGF induced additional elongation, and significant pseudopodial ruffling and branching of cell processes (N), resulting in a more dendritic appearance. Large tractional forces were not observed (M, red tracks, crosses mark position at time 2:42). Perfusion with Y-27632 (100 μM) began at 1:41, and perfusion with Y-27632 plus PDGF at 2:42.
Although tractional forces were dramatically reduced following ROCK inhibition, upon closer inspection, transient inward displacements of collagen fibrils at the ends of extending pseudopodia were often still observed. An example is shown in Fig. 5G–K, in which a thin dendritic processes branches off from another process. The branching process (arrows) extends along a collagen fibril (Fig. 5H), engages a second fibril (arrowheads) which is oriented perpendicular (Fig. 5I), and displaces this fibril inward (Fig. 5J, K). These small displacements of collagen fibrils were observed even at high concentrations (100 μm) of Y-27632 (Movie 9).
We also investigated the effect of PDGF following pre-incubation with Y-27632. Addition of Y-27632 to basal media induced cell elongation and relaxation of tractional forces (Fig. 5 L, M), indicating that there is a basal level of ROCK activity in serum-starved corneal fibroblasts. Subsequent addition of PDGF induced additional elongation, and significant pseudopodial ruffling and branching of cell processes (Fig. 5N), resulting in a more dendritic appearance. Tractional force generation was again limited to the tips of extending pseudopodia.
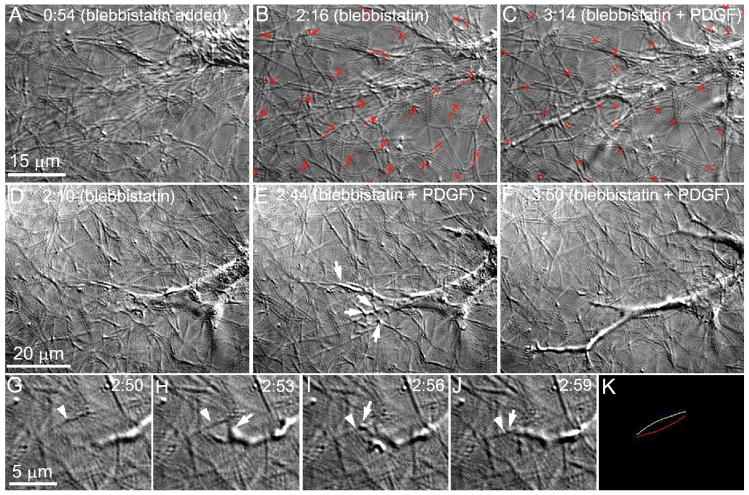
Together these data suggest that there are both ROCK -dependent and -independent contributions to tractional force generation during Rac induced cell spreading in 3-D matrices. To investigate whether the ROCK-independent forces at the pseudopodial tips were mediated by myosin II, we used the specific non muscle myosin II inhibitor blebbistatin. Treatment of cells with blebbistatin induced cell elongation and relaxation of tractional forces (Fig. 6A,B). Subsequent addition of PDGF stimulated rapid cell spreading, with extensive branching and pseudopodial ruffling (Fig. 6C, D–F). Interestingly, small inward displacements of collagen fibrils were still generated at the tips of extending pseudopodia in the presence of blebbistatin (Fig. 6G–K), similar to those observed following treatment with Y-27632. Localized displacement of collagen fibrils was also observed when PDGF was added in the presence of both blebbistatin and Y-27632 (Movie 10). Overall, the data suggest that ROCK mediates the generation of myosin II based tractional forces during spreading; however, small, residual forces can be generated at the tips of extending pseudopodia that are both ROCK and myosin II-independent.
Figure 6.
The role of myosin II on the subcellular pattern of force generation and cell-matrix interactions in response to PDGF. A–C. Perfusion was changed to the non muscle myosin II inhibitor blebbistatin (100 μM) at 0:54 (hours:minutes), and PDGF + blebbistatin at 2:18. Treatment of cells with blebbistatin resulting in significant relaxation of tractional forces (B, red tracks, crosses mark position at time 0:54). Subsequent addition of PDGF stimulated additional cell spreading, without the generation of large tractional forces (C, red tracks, crosses mark position at time 2:18). D–F. Perfusion was changed to the non muscle myosin II inhibitor blebbistatin at 1:00 (hours:minutes), and PDGF + blebbistatin at 2:10. Addition of PDGF in the presence of blebbistatin also induced extensive branching and pseudopodial ruffling (E, arrows), resulting in a dendritic cell morphology (F). G–K. Cell matrix interactions at the end of a pseudopodia during cell spreading following myosin II inhibition. A thin dendritic process (arrow) branches off from another process. The branching process engages fibril (arrowhead), and displaces this fibril inward (K, yellow line indicates initial fibril position, red line indicates final fibril position). Perfusion with blebbistatin began at 0:55, and perfusion with blebbistatin plus PDGF at 2:10.
DISCUSSION
The process of corneal wound repair following lacerating injury or refractive surgery generally depends upon mechanical processes such as the migration of activated keratocytes (corneal fibroblasts) into the wound from the surrounding stroma, apposition of the wound edges (wound contraction), and extracellular matrix reorganization (remodeling) (Netto et al., 2005). These biomechanical mechanisms ultimately control corneal clarity and refractive (visual) outcome (Jester et al., 1999; Moller-Pedersen et al., 1998a; Moller-Pedersen et al., 1998b; Moller-Pedersen et al., 1997; Petroll et al., 1992). The small GTPases Rho and Rac are prime candidates for regulating the cytoskeletal and mechanical phenotype of fibroblasts during various phases of wound healing. Activation of Rho by lysophosphatidic acid (LPA) increases contractility on both silicon substrates (Chrzanowska-Wodnicka and Burridge, 1994; Craig and Johnson, 1996) and within collagen lattices (Kolodney and Elson, 1993; Tomasek et al., 1992), and these responses appear to be mediated by ROCK (Grinnell, 2000; Parizi et al., 2000; Tamariz and Grinnel, 2002). PDGF activates Rac in dermal fibroblasts (Grinnell, 2000; Sander et al., 1999), and enhances both cell spreading and migration within 3-D collagen matrices (Andresen et al., 1997; Grinnell et al., 2006). PDGF has also been shown to induce local matrix deformation during cell spreading in 3-D culture, as indicated by the inward displacement of microspheres embedded in the ECM (Tamariz and Grinnel, 2002). In general, the low density and random distribution of embedded beads does not provide a detailed mapping of ECM deformation at the sub-cellular level. Furthermore, cell-induced changes in collagen fibril organization cannot be visualized using this indirect approach.
In this study, we used high resolution time-lapse DIC imaging to directly investigate the dynamic effects of PDGF on the sub-cellular mechanical behavior and local collagen matrix interactions of corneal fibroblasts within 3-D collagen matrices. Addition of PDGF activated Rac and induced dramatic cell spreading, via both the extension of existing pseudopodial processes and the formation of new processes. Cell-induced displacement and realignment of collagen fibrils was also observed during PDGF-induced spreading. In general, relaxation (decompression) of the ECM was observed along the cell body, whereas tractional forces were generated by extending cell processes, as indicated by centripetal displacement of collagen fibrils at the ends of cells. Thus overall, there was a shift in the tractional force distribution from the center to the periphery of corneal fibroblasts in response to Rac activation.
Previous studies have demonstrated that PDGF-induced global reorganization of attached collagen matrices is partially dependent on ROCK (Rhee and Grinnell, 2006; Tamariz and Grinnel, 2002). To investigate the role of ROCK on the subcellular pattern of force generation in response to PDGF, we tested the effects of the specific Rho-kinase inhibitor Y-27632. Addition of Y-27632 following PDGF stimulation induced additional cell spreading and elongation. Interestingly, cells also assumed a more convoluted shape with dendritic cell processes, suggestive of a reduction of cellular tension. Overall, there was dramatic relaxation of cell-induced tractional forces following ROCK inhibition. When Y-27632 was washed out by switching the perfusion back to PDGF alone, cell processes again became thicker, and increased tractional forces were observed, particularly at the base of pseudopodial processes. Thus a large portion of the tractional forces observed during Rac-induced spreading were ROCK-dependent.
Various forms of crosstalk between Rho and Rac signaling pathways have been identified, but these can vary substantially depending on cell type and culture conditions (Brzeska et al., 2004; Burridge and Wennerberg, 2004; Jilkine et al., 2007; Pestenjamasp et al., 2006; Romano et al., 2006). We did not assess the effect of PDGF on Rho or ROCK activation in the current study. However, a substantial reduction of cellular forces was observed when Y-27632 was added in the absence of PDGF, indicating a significant basal level of ROCK activity under the culture conditions used in this study. Subsequent addition of PDGF induced extensive branching and ruffling of pseudopodia, indicating that induction of cell spreading by PDGF does not require Rho Kinase activation.
Importantly, despite the overall decrease in cellular tractional forces in the presence of Y-27632, small inward displacements of collagen fibrils at the ends of extending pseudopodia were still observed during spreading. To determine whether these small tractional forces were generated through a myosin II dependent mechanism, we used the specific non muscle myosin II inhibitor blebbistatin. A recent study using tractional force microscopy demonstrated that ROCK plays a central role in producing myosin II-based tractional forces in NIH 3T3 fibroblasts (Beningo et al., 2006). Consistent with these results, we found that ROCK and myosin II had similar effects on corneal fibroblast contractility in 3-D culture. However, despite an overall relaxation of cellular forces, small inward displacements of collagen fibrils continued to be generated at the tips of extending pseudopodia in the presence of blebbistatin, similar to those observed following treatment with Y-27632. Thus a novel finding in our study is that a component of tractional force generation by extending pseudopodia in 3-D matrices appears to be independent of both ROCK or myosin II. It should be noted that our collagen matrices are much more compliant than the planar elastic substrates used in 2-D tractional force microscopy, and therefore may allow detection of smaller forces on the ECM (such as those required to displace collagen fibrils at the pseudopidal tips). Since PDGF-induced elongation, ruffling and branching of pseudopodia still occurs in the presence of Y-27632 and blebbistatin, it is tempting to speculate that this residual traction may be the result of protrusive forces associated with actin polymerization (Abraham et al., 1999; Bohnet et al., 2006; Marcy et al., 2004; Pollard and Borisy, 2003; Prass et al., 2006).
Sheetz and coworkers recently demonstrated that fibroblasts plated on rigid substrates extend along isolated collagen fibrils placed on the upper surface of the cells, and retract them in a “hand over hand” cycle involving α2β1 integrin and myosin IIB (Meshel et al., 2005). Fibers remained stationary as lamellipodia extended, whereas centripetal fibril displacement was correlated with lamellipodial retraction. While many aspects of the cell/collagen interactions and GFP-zyxin dynamics observed during Rac-induced spreading in the current study are consistent with this mechanism, collagen fibril displacement was observed during both the extension and retraction phases of pseudopodial movement in our 3-D model. This may by due to the fact that in 2-D culture, the protrusive forces generated during lamellipodial extension can be transmitted to the rigid substrate underneath the cell, without affecting collagen fibrils on top. In contrast all of the forces associated with pseudopodial extension are transmitted to the extracellular collagen matrix in our 3-D model.
An important feature of our experimental model is the ability to directly assess the displacement and realignment of collagen fibrils surrounding cells at high magnification. Thus to further investigate the mechanics of Rac-induced spreading, cellular interactions with individual collagen fibrils at the leading edge were studied. Two main patterns of collagen displacement were observed following PDGF stimulation. First, when a collagen fibril in front of an extending process was aligned nearly parallel to the direction of spreading, the extending process generally engaged the fibril, pulled it into alignment, then continued to spread along it. Second, when collagen fibrils in front of an extending process were aligned more perpendicular to the direction of spreading, the extending process often engaged the first fibril, push past it to engage the second fibril, then pulled the fibrils together. The first pattern of interaction tended to pull collagen fibrils beyond the ends of cells into an alignment parallel with the pseudopodia; whereas, the second pattern resulted in compaction of the collagen fibrils in a direction perpendicular to the extending process. These interactions may underlie, in part, the pattern of collagen organization observed using static reflected light confocal imaging following cell spreading, which includes sprays of collagen aligned parallel to the long axis at the ends of cells, and fibrils aligned more perpendicular to the long axis at the base of pseudopodial processes (Friedl and Brocker, 2000; Kim et al., 2006). Overall, our observations indicate that the pattern of pseudopodial extension and of cell-induced fibril displacement and realignment are significantly influenced by the initial organization of the surrounding ECM.
Supplementary Material
PDGF-induced cell spreading of an HTK cell plated inside a 3-D collagen matrix, cultured for 24 hours in serum-free media, and transferred to the microscope stage. In general, relaxation (decompression) of the ECM was observed along the cell body (yellow arrows), whereas tractional forces were generated by extending cell processes, as indicated by centripetal displacement of collagen fibrils (red arrows).
Cell-matrix mechanical interactions in response to PDGF. Tracking of the ECM displacements showed minimal collagen displacement prior to the addition of PDGF (red tracks, crosses mark position at time 0:00). However, following addition of PDGF, the matrix in front of the cell was pulled inward by the extending pseudopodial processes, resulting in compression of the ECM.
Cell-matrix mechanical interactions during PDGF-induced spreading. Tractional forces generated by an extending cell processes were often transient, and sometimes dissipated as processes continued to branch and spread (note lower left process).
High magnification assessment of cellular interactions with individual collagen fibrils in response to PDGF. In this example, a collagen fibril (arrowhead) in front of an extending process is aligned somewhat parallel to the direction of spreading. The extending process engages the fibril, pulls it into alignment, then continues to spread along it. This results in a final alignment of the collagen fibril parallel with the pseudopodia.
Cellular interactions with two collagen fibrils (arrowheads) aligned somewhat perpendicular to the direction of spreading. Following PDGF treatment, the extending process (arrows) engages the first fibril, pushes past it to engage the second fibril, then pulls the fibrils together. This results in compaction of the collagen fibrils in a direction perpendicular to the extending process.
Time-lapse color overlays of GFP-zyxin (green) and DIC (red). Formation of focal adhesions at the leading edge of an extending pseudopodia was associated with centripetal displacement and/or bending of the collagen fibrils with which it interacted. Cytochalasain D induced disassembly of focal adhesions and matrix relaxation.
The role of ROCK on the subcellular pattern of force generation and cell-matrix interactions in response to PDGF. Addition of the ROCK inhibitor Y-27632 (10 μM) to a PDGF treated cell induced additional cell spreading and elongation. The cell also assumed a more convoluted shape with thinner cell processes, suggesting a reduction of cellular tension.
Following 30 minutes of ROCK inhibition, Y-27632 (10 μM) was washed out by switching the perfusion back to PDGF alone. Cell processes became thicker, and increased tractional forces were observed, particularly at the base of pseudopodial processes. Subsequent treatment with cytochalasin D resulted in elongation and thinning of cellular processes, and ECM decompression.
Cell matrix interactions at the end of a pseudopodia following ROCK inhibition. Movie begins after cell was treated with PDGF (50ng/ml) for 40 minutes, then with PDGF plus Y-27632 (100 μM) for 22 minutes. As the cell spreads, a pseudopodia branches off from another process and displaces a collagen fibril inward.
Cell matrix interactions at the end of a spreading pseudopodia following both ROCK and myosin II inhibition. Movie begins after cell was treated with Y-27632 and blebbistatin for 90 minutes, then Y-27632, blebbistatin and PDGF for 30 minutes.
Acknowledgments
Contract grant sponsor: NIH Contract grant number: R01 EY013322
Contract grant sponsor: NIH Contract grant number: R24 EY016664
The authors wish to thank Professor J. Wehland and coworkers (BGF, Braunschweig, Germany) for the generous gift of the EGFP-zyxin expression vector. This study was supported in part by NIH grants R01 EY13322 and R24 EY016664, and a Lew R. Wasserman Merit award (WMP) and unrestricted grant from Research to Prevent Blindness, Inc., NY, NY.
LITERATURE CITED
- Abbott A. Biology’s new dimension. Nature. 2003;424:870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- Abraham VC, Krishnamurthi V, Taylor DL, Lanni F. The actin-based nanomachine at the leading edge of migrating cells. Biophys J. 1999;77:1721–1732. doi: 10.1016/S0006-3495(99)77018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ledet T, Ehlers N. Keratocyte migration and peptide growth factors: the effect of PDGF, bFGF, EGF, IGF-1, aFGF and TGF-beta on human keratocyte migration in a collagen gel. Curr Eye Res. 1997;16:605–613. doi: 10.1076/ceyr.16.6.605.5081. [DOI] [PubMed] [Google Scholar]
- Bard JBL, Hay ED. The behavior of fibroblasts from the developing avian cornea: Morphology and movement in situ and in vitro. J Cell Biol. 1975;67:400–418. doi: 10.1083/jcb.67.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153(4):881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Hamao K, Dembo M, Wang Y-L, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Archives of Biochemistry and Biophysics. 2006;456:224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnet S, Ananthakrishnan R, Mogilner A, Meister J-J, Verkhovsky AB. Weak force stalls protrusion at the leading edge of the lamellipodium. Biophys J. 2006;90:1810–1820. doi: 10.1529/biophysj.105.064600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzeska H, Szczepanowska J, Matsumua F, Korn ED. Rac-induced increase of phoaphorylation of myosin regulatory light chain in Hela cells. Cell Motil Cytoskeleton. 2004;58:186–199. doi: 10.1002/cm.20009. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka C, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994;107:3643–3564. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- Craig SW, Johnson RP. Assembly of focal adhesions: Progress, paradigms, and portents. Curr Opin Cell Biol. 1996;8:74–85. doi: 10.1016/s0955-0674(96)80051-2. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- Demali KA, Burridge K. Coupling membrane protrusion and cell adhesion. J Cell Sci. 2003;116:2389–2397. doi: 10.1242/jcs.00605. [DOI] [PubMed] [Google Scholar]
- Doane KJ, Birk DE. Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Exp Cell Res. 1991;195:432–442. doi: 10.1016/0014-4827(91)90394-a. [DOI] [PubMed] [Google Scholar]
- Friedl P, Brocker E-B. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Ho C-H, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Cell Biol. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: A new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behavior. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, Shay JW. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest Ophthal Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Grinnell F. Cell-matrix entanglement and mechanical anchorage of fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2005;16:5070–5076. doi: 10.1091/mbc.E05-01-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilkine A, Maree AFM, Edelstein-Keshet L. Mathematical model for spatial segregation of the Rho-family GTPases based on inhibitory crosstalk. Bull Math Biol. 2007;69:1943–1978. doi: 10.1007/s11538-007-9200-6. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by Rho familiy GTPase in Mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002 doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of Myosin-binding Subunit (MBS) of Myosin Phosphatase by Rho-Kinase In Vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Lakshman N, Petroll WM. Quantitative assessment of local collagen matrix reorganization in 3-D culture: The role of Rho kinase. Exp Cell Res. 2006;312:3683–3692. doi: 10.1016/j.yexcr.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993;268:23850–23855. [PubMed] [Google Scholar]
- Kraemer A, Goodwin M, Verma S, Yap AS, Ali RG. Rac is a dominant regulator of cadherin directed actin assembly that is activated by adhesive ligation independently of Tiam1. Am J Physiol Cell Physiol. 2007;292:C1061–C1069. doi: 10.1152/ajpcell.00073.2006. [DOI] [PubMed] [Google Scholar]
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:462–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Marcy Y, Prost J, Carlier M-F, Sykes C. Forces generated during actin-based propulsion: A direct measurement by micromanipulation. Proc Natl Acad Sci. 2004;101:5992–5997. doi: 10.1073/pnas.0307704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic transport mechanisms of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7:157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998a;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGFβ modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998b;17:736–747. [PubMed] [Google Scholar]
- Moller-Pedersen T, Vogel MD, Li H, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze following photorefractive keratectomy using in vivo confocal microscopy. Ophthalmol. 1997;104:360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Hutcheon AEK, Zieske JD, Wilson SE. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of rho, myosin light chain kinase, and myosin light chain phosphotase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- Pestenjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and urupod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Ma L. Direct, dynamic assessment of cell-matrix interactions inside fibrillar collagen lattices. Cell Motil Cytoskeleton. 2003;55:254–264. doi: 10.1002/cm.10126. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Ma L, Jester JV. Direct Correlation of Collagen Matrix Deformation with Focal Adhesion Dynamics in Living Corneal Fibroblasts. J Cell Sci. 2003;116:1481–1491. doi: 10.1242/jcs.00357. [DOI] [PubMed] [Google Scholar]
- Petroll WM, New K, Sachdev M, Cavanagh HD, Jester JV. Radial Keratotomy III. Relationship between wound gape and corneal curvature in primate eyes. Invest Ophthal Vis Sci. 1992;33:3283–3291. [PubMed] [Google Scholar]
- Petroll WM, Vishwanath M, Ma L. Corneal fibroblasts respond rapidly to changes in local mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:3466–3474. doi: 10.1167/iovs.04-0361. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Prass M, Jacobson K, Mogilner A, Radmacher M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol. 2006;174:767–772. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF and LPA-stimulated collagen matrix contractin by human fibroblats. J Cell Biol. 2006;172:423–432. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S, Jiang H, Ho C-H, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci. 2007;104:5425–5430. doi: 10.1073/pnas.0608030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano F, Chiarenza C, Fioretta P, Filippini A, Padula F, Ziparo E, De Cesaris P. Platelet-derived growht factor-BB-induced hypertrophy of peritubular smooth muscle cells is mediated by activation of p38 MAP-kinase and Rho-Kinase. J Cell Physiol. 2006;207:123–131. doi: 10.1002/jcp.20554. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Rottner K, Krause M, Gimona M, Small JV, Wehland J. Zyxin is not colocalized with vasodilator-stimulated phosphoprotein (VASP) at lamellipodial tips and exhibits different dynamics to vinculin, paxillin, and VASP in focal adhesions. Mol Biol Cell. 2001;12:3103–3113. doi: 10.1091/mbc.12.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell biol. 1999;147:1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiber DI, Enever PAJ, Tranquillo RT. Effects of PDGF-BB on rat dermal fibroblast behavior in mechnically stressed and unstressed collagen and fibrin gels. Exp Cell Res. 2001;266:155–166. doi: 10.1006/excr.2001.5208. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokenesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Kurisu S, Oikawa T, Yamazaki D, Oda A, Takenawa T. Optimization of WAVE2 complex-induced actin polymerization by membrane bound IRSp53, PIP(3), and Rac. J Cell Biol. 2006;173:571–585. doi: 10.1083/jcb.200509067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamariz E, Grinnel F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell. 2002;13:3915–3929. doi: 10.1091/mbc.E02-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Klooster JP, Evers EE, Janssen L, Machesky LM, Michiels F, Hordijk P, Collard JG. Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem J. 2006;397:39–45. doi: 10.1042/BJ20051957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: Distribution of actin, α-actinin and myosin. Dev Biol. 1982;92:107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, McConathy WJ, Checovich WJ, Mosher DF. Lipoproteins promote fibroblast-mediated collagen lattice contraction. Mol Biol Cell. 1992;3:234A. [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath M, Ma L, Jester JV, Otey CA, Petroll WM. Modulation of corneal fibroblast contractility within fibrillar collagen matrices. Invest Ophthalmol Vis Sci. 2003;44:4724–4735. doi: 10.1167/iovs.03-0513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDGF-induced cell spreading of an HTK cell plated inside a 3-D collagen matrix, cultured for 24 hours in serum-free media, and transferred to the microscope stage. In general, relaxation (decompression) of the ECM was observed along the cell body (yellow arrows), whereas tractional forces were generated by extending cell processes, as indicated by centripetal displacement of collagen fibrils (red arrows).
Cell-matrix mechanical interactions in response to PDGF. Tracking of the ECM displacements showed minimal collagen displacement prior to the addition of PDGF (red tracks, crosses mark position at time 0:00). However, following addition of PDGF, the matrix in front of the cell was pulled inward by the extending pseudopodial processes, resulting in compression of the ECM.
Cell-matrix mechanical interactions during PDGF-induced spreading. Tractional forces generated by an extending cell processes were often transient, and sometimes dissipated as processes continued to branch and spread (note lower left process).
High magnification assessment of cellular interactions with individual collagen fibrils in response to PDGF. In this example, a collagen fibril (arrowhead) in front of an extending process is aligned somewhat parallel to the direction of spreading. The extending process engages the fibril, pulls it into alignment, then continues to spread along it. This results in a final alignment of the collagen fibril parallel with the pseudopodia.
Cellular interactions with two collagen fibrils (arrowheads) aligned somewhat perpendicular to the direction of spreading. Following PDGF treatment, the extending process (arrows) engages the first fibril, pushes past it to engage the second fibril, then pulls the fibrils together. This results in compaction of the collagen fibrils in a direction perpendicular to the extending process.
Time-lapse color overlays of GFP-zyxin (green) and DIC (red). Formation of focal adhesions at the leading edge of an extending pseudopodia was associated with centripetal displacement and/or bending of the collagen fibrils with which it interacted. Cytochalasain D induced disassembly of focal adhesions and matrix relaxation.
The role of ROCK on the subcellular pattern of force generation and cell-matrix interactions in response to PDGF. Addition of the ROCK inhibitor Y-27632 (10 μM) to a PDGF treated cell induced additional cell spreading and elongation. The cell also assumed a more convoluted shape with thinner cell processes, suggesting a reduction of cellular tension.
Following 30 minutes of ROCK inhibition, Y-27632 (10 μM) was washed out by switching the perfusion back to PDGF alone. Cell processes became thicker, and increased tractional forces were observed, particularly at the base of pseudopodial processes. Subsequent treatment with cytochalasin D resulted in elongation and thinning of cellular processes, and ECM decompression.
Cell matrix interactions at the end of a pseudopodia following ROCK inhibition. Movie begins after cell was treated with PDGF (50ng/ml) for 40 minutes, then with PDGF plus Y-27632 (100 μM) for 22 minutes. As the cell spreads, a pseudopodia branches off from another process and displaces a collagen fibril inward.
Cell matrix interactions at the end of a spreading pseudopodia following both ROCK and myosin II inhibition. Movie begins after cell was treated with Y-27632 and blebbistatin for 90 minutes, then Y-27632, blebbistatin and PDGF for 30 minutes.