Abstract
Damaged mitochondria generate an excess of superoxide, which may mediate tissue injury in diabetes. We hypothesized that in diabetic nephropathy, advanced glycation end-products (AGEs) lead to increases in cytosolic reactive oxygen species (ROS), which facilitate the production of mitochondrial superoxide. In normoglycemic conditions, exposure of primary renal cells to AGEs, transient overexpression of the receptor for AGEs (RAGE) with an adenoviral vector, and infusion of AGEs to healthy rodents each induced renal cytosolic oxidative stress, which led to mitochondrial permeability transition and deficiency of mitochondrial complex I. Because of a lack of glucose-derived NADH, which is the substrate for complex I, these changes did not lead to excess production of mitochondrial superoxide; however, when we performed these experiments in hyperglycemic conditions in vitro or in diabetic rats, we observed significant generation of mitochondrial superoxide at the level of complex I, fueled by a sustained supply of NADH. Pharmacologic inhibition of AGE-RAGE–induced mitochondrial permeability transition in vitro abrogated production of mitochondrial superoxide; we observed a similar effect in vivo after inhibiting cytosolic ROS production with apocynin or lowering AGEs with alagebrium. Furthermore, RAGE deficiency prevented diabetes-induced increases in renal mitochondrial superoxide and renal cortical apoptosis in mice. Taken together, these studies suggest that AGE-RAGE–induced cytosolic ROS production facilitates mitochondrial superoxide production in hyperglycemic environments, providing further evidence of a role for the advanced glycation pathway in the development and progression of diabetic nephropathy.
During oxidative phosphorylation (OXPHOS) under physiologic conditions, there is minimal superoxide leakage, which is immediately scavenged by the antioxidant enzyme manganese superoxide dismutase (MnSOD, SOD2); however, damaged or dysfunctional mitochondria generate excessive superoxide, creating a state of redox imbalance.1 In diabetic complications, a unifying hypothesis has been postulated: That the excessive generation of mitochondrial superoxide by hyperglycemia is the primary initiating event that activates all other pathways of tissue damage.2 There remains debate, however, as to whether oxidative stress is an important early link between hyperglycemia and complications or is a byproduct of other primary pathogenic mechanisms.3
It is thought that excess concentrations of mitochondrial reactive oxygen species (ROS) are mediated by disruption of the activity of OXPHOS enzymes via electron leakage at complex I (NADH:ubiquinone oxidoreductase) or complex III (ubiquinol:cytochrome c oxidoreductase).1,4,5 For example, in Friedreich ataxia, a genetically inherited mitochondrial disease, deficiencies of OXPHOS complexes I, II, and III are associated with excessive steady-state generation of mitochondrial superoxide.6 Of interest is that progressive renal disease has been reported in patients with mitochondrial respiratory chain abnormalities, with some patients presenting with renal abnormalities as their primary pathology.7,8 In addition, a decline in the activity of complex III9 and enhanced susceptibility to mitochondrial permeability transition (mPT)10 have been shown in diabetic kidneys. In other contexts, mPT decreases the activity of complex I, leading to a specific leakage of superoxide.11 Furthermore, the hyperglycemia characteristic of diabetes increases the complex I substrate NADH,12 which is likely to potentiate ROS production by the respiratory chain.13
Advanced glycation end products (AGEs), formed by the nonenzymatic irreversible modification of proteins, are known contributors to the pathogenesis of diabetic renal disease,14 in particular as effector molecules for the receptor for AGEs (RAGE).15 Ligand engagement of RAGE by AGEs also results in the production of cellular ROS.16,17
In this study, we investigated the in vitro and in vivo effects of the AGE-RAGE interaction on mitochondrial function, under both normal- and high-glucose environments. We show that engagement of RAGE by AGEs induces mPT, which subsequently leads to NADH-dependent excessive generation of mitochondrial superoxide radical via complex I deficiency in a high-glucose milieu.
RESULTS
Exogenous AGEs Induce RAGE Expression and Generate Cytosolic Hydrogen Peroxide in Normal-Glucose Environments
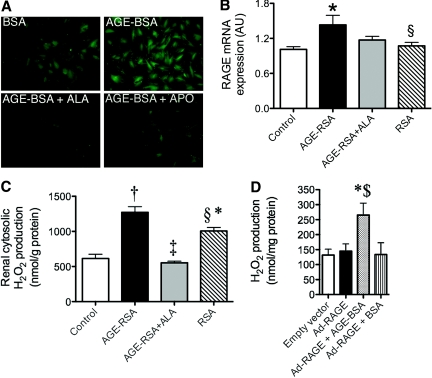
Primary rat mesangial cells exposed to 100 μg/ml AGE-modified BSA (AGE-BSA) had increased cellular hydrogen peroxide (H2O2) production, as compared with BSA vehicle–treated cells alone (Figure 1A). H2O2 production was attenuated by the inhibitor of AGE accumulation alagebrium (ALA) and by the cytosolic NADPH-oxidase inhibitor apocynin (APO; Figure 1A).
Figure 1.
AGEs induce RAGE expression and generate cytosolic H2O2 in normal glucose. Primary rat mesangial cells cultured in the presence of 5.5 mM d-glucose were treated with 100 μg/ml glycated BSA (AGE-BSA) or native BSA for 3 d (n = 3 separate experiments). (A) Fluorescence micrographs of intracellular H2O2 production (5 μM CM-H2DCFDA for 10 min at 37°C; ALA 40 μM, APO 1 μM). (B and C) In vivo, rats were injected with saline (control), AGE-RSA or native RSA (20 mg/kg per d) for 16 wk (n = 6 to 10 rats per group). One group of rats was co-administered ALA (AGE-RSA+ALA, 10 mg/kg per d). (B) RAGE gene expression in renal cortex. (C) Cytosolic H2O2 production as assessed using the Amplex Red reagent. (D) Cytosolic H2O2 in primary rat mesangial cells infected with the human full-length (FL) Ad-RAGE or the control empty vector for 72 h. At 24 h after infection, AGE-BSA (100 μg/ml) was added to one group (Ad-RAGE+AGEs). Samples shown are representative of three independent experiments. Bars represent means ± SEM, n = 3. *P < 0.05 versus control or empty vector; †P < 0.001 versus control; ‡P < 0.001 versus AGE-RSA; §P < 0.05 versus AGE-RSA; $P < 0.05 versus Ad-RAGE.
Exogenously prepared AGE-BSA and AGE-modified rat serum albumin (AGE-RSA) had approximately 100-fold increases in CML modification (AGE-BSA 67.0 ± 1.2 mmol/mol lysine; AGE-RSA 38.2 ± 3.6 mmol/mol lysine) as compared with the original preparations (BSA 0.5 ± 0.0 mmol/mol lysine and RSA 0.3 ± 0.0 mmol/mol lysine).18,19 Importantly, no endotoxin was detected in any of the albumin preparations (as measured by the Limulus Amoebocyte Lysate assay). In addition, no anti-CML antibodies in excess of those seen in RSA-injected rats could be detected in the plasma from AGE-RSA–injected rats by ELISA (data not shown).
AGE-RSA injection did not change plasma glucose, glycated hemoglobin, renal function, or systolic BP (Table 1) when compared with RSA-injected rats. Renal cortical RAGE gene (Figure 1B, gene accession no. L33413) and protein expression were increased in AGE-RSA–injected rats as compared with RSA (densitometric analysis of RAGE protein expression as determined by Western immunoblotting and corrected to β-actin RSA 202.3 ± 5.7 arbitrary units versus AGE-RSA 220.1 ± 1.3 arbitrary units; n = 3 rats per group; P < 0.05). The cytosol from the renal cortex of AGE-RSA–injected rats also had markedly increased H2O2 generation, which was normalized with concomitant ALA therapy (Figure 1C). The RSA vehicle also modestly increased cytosolic H2O2 production, although this was significantly lower when compared with AGE-RSA–injected rodents (Figure 1C).
Table 1.
Rat biochemical and metabolic parameters: AGE injectiona
| Parameter | Control | AGE-RSA | AGE-RSA+ALA | RSA |
|---|---|---|---|---|
| Plasma glucose (mmol/L) | 7.4 ± 0.2 | 7.4 ± 0.2 | 7.6 ± 0.2 | 7.9 ± 0.3 |
| GHb (%) | 4.1 ± 0.2 | 3.9 ± 0.2 | 3.6 ± 0.2 | 4.1 ± 0.5 |
| Kidney wt:body wt (×10−3) | 5.1 ± 0.1 | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.4 ± 0.1 |
| GFR (ml/min) | 4.3 ± 0.1 | 4.4 ± 0.2 | 4.1 ± 0.2 | 4.3 ± 0.3 |
| Systolic BP (mmHg) | 114 ± 2 | 118 ± 2 | 123 ± 3b | 121 ± 2 |
| AER (mg/24 h) | 1.2 ± 0.8 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.1 |
Data are means ± SEM; n = 6 to 10 per group. AER, albumin excretion rate; GHb, glycated hemoglobin.
P < 0.01 versus control.
To verify the combined effects of AGE-RAGE interactions on renal H2O2 production, we treated RAGE adenoviral vector (Ad-RAGE)-infected cells with AGE-BSA (Ad-RAGE+AGEs) or BSA (Ad-RAGE+BSA). Cytosolic H2O2 production was unchanged with Ad-RAGE transfection alone (Figure 1D, Ad-RAGE) or BSA but was significantly increased by more than two-fold with addition of ligand (Ad-RAGE+AGEs), clearly indicating that the AGE-RAGE interaction was required for increased cytosolic H2O2 generation in mesangial cells.
AGE-Induced Cytosolic H2O2 Liberation Induces mPT and Disrupts Mitochondrial Membrane Potential
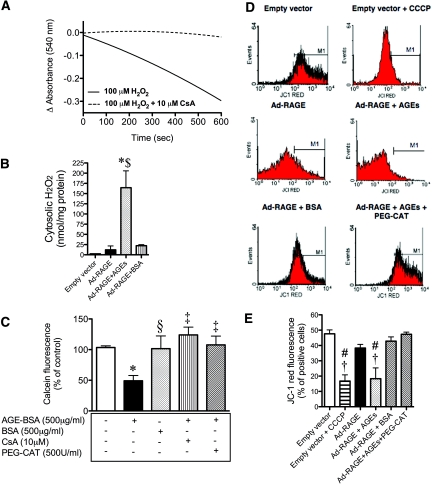
Exposure of renal mitochondria to H2O2 led to a sequential decrease in absorbance at 540 nm, reflecting induction of mPT (Figure 2A). This decrease in absorbance was inhibited by cyclosporin A (CsA), a classically described agent known to inhibit mPT.20–22 In addition, an acute AGE-BSA treatment of mesangial cells infected with the RAGE adenovirus led to the liberation of cytosolic H2O2 (Figure 2B).
Figure 2.
AGE-induced cytosolic H2O2 liberation induces mPT and disrupts mitochondrial membrane potential. (A) H2O2-induced swelling of renal cortical mitochondria. Mitochondria were incubated with 100 μM H2O2 with or without 10 μM CsA for 10 min, and mPT was followed by a decrease in absorbance at 540 nm. Trace is representative of three independent experiments. (B) Cytosolic H2O2 in primary rat mesangial cells infected with the human FL Ad-RAGE or the control empty vector for 72 h. At 24 h after infection, either AGE-BSA (500 μg/ml) or BSA (500 μg/ml) was added for 15 min. Samples shown are representative of three independent experiments. Bars represent means ± SEM, n = 3. (C) Primary rat mesangial cells were treated with 500 μg/ml AGE-BSA or 500 μg/ml BSA for 30 min, and cells were loaded with 2 μM calcein-AM with or without 1 mM cobalt chloride for 30 min at 37°C to assess mPT. A decrease in fluorescence indicates opening of the mitochondrial transition pore. Appropriate cells were pretreated with either 10 μM CsA or 500 U/ml PEG-CAT for 30 min at 37°C. Data are representative of three independent experiments. Bars represent means ± SEM, n = 3. (D) FACS analysis of mitochondrial membrane potential (Δψm) in primary rat mesangial cells infected with the human FL Ad-RAGE or the control empty vector for 72 h. At 24 h after infection, either AGE-BSA (100 μg/ml) or BSA (100 μg/ml) was added to one group. Cells were stained with 2 μM JC-1 and analyzed on a flow cytometer. CCCP, a mitochondrial membrane potential disrupter, was used as a positive control, and PEG-CAT (500U/ml) was used to detoxify intracellular H2O2. (E) JC-1 red fluorescence quantification. Data are representative of four independent experiments. *P < 0.05 versus control or empty vector; †P < 0.01 versus Ad-RAGE; ‡P < 0.01 versus AGE-BSA; §P < 0.05 versus AGE-BSA; #P < 0.001 versus empty vector; $P < 0.001 versus Ad-RAGE.
To assess AGE-induced mPT more directly, we treated primary rat mesangial cells with AGE-BSA or BSA for 30 min and cells loaded with calcein-AM with or without cobalt chloride. Mesangial cells that were exposed to AGE-BSA had a significant decrease in calcein fluorescence, indicative of mPT, when compared with cells treated with BSA (Figure 2C). The AGE-mediated decrease in calcein fluorescence was prevented by co-incubation with CsA, confirming the process of mPT. In addition, pretreatment with PEG-catalase (PEG-CAT), a cell-permeable form of catalase, prevented the AGE-BSA–induced mPT (Figure 2C), implicating intracellular H2O2 in the process.
To explore further AGE-RAGE–induced mitochondrial dysfunction, we used flow cytometric analysis of cells loaded with the potentiometric dye JC-1 to assess changes in mitochondrial membrane potential (Δψm). Primary rat mesangial cells were exposed to the uncoupler CCCP as a positive control, which demonstrated a significant loss in red fluorescence in the third (103) and fourth (104) log, resulting in a leftward shift (Figure 2D, top right), compared with the control empty vector (Figure 2D, top left). This finding is consistent with a decrease in Δψm (i.e., depolarization of the inner mitochondrial membrane).23 Mesangial cells overexpressing RAGE via infection with the human full-length Ad-RAGE had a modest but NS loss in red fluorescence compared with the empty vector (Figure 2D, middle left). After addition of the AGE ligand, this leftward shift became more pronounced (Figure 2D, middle right). This was not seen with BSA treatment (Figure 2D, bottom left). In addition, pretreatment with PEG-CAT prevented the AGE-BSA–induced shift (Figure 2D, bottom right). Data from four independent experiments are collated in Figure 2E. Taken together, these data clearly demonstrate AGE-RAGE–induced disruption of Δψm and enhanced susceptibility to mPT.
AGE-RAGE Interaction Disrupts the Mitochondrial Respiratory Chain in Normoglycemia, but Hyperglycemia Is Essential for Superoxide Overproduction
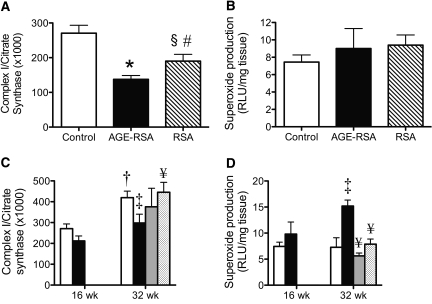
To evaluate further whether AGE-induced cytosolic oxidative stress and subsequent mPT could be associated with or could influence changes in the activity of the respiratory chain, we investigated the enzyme complexes I through IV. Initially we identified in an advanced model of diabetes (streptozotocin [STZ]-induced diabetes in Sprague-Dawley rats with no insulin treatment) that complex I activity is selectively decreased by 50% in renal glomeruli compared with control rats (50 ± 10% of control; n = 6 rats per group; P < 0.001), whereas the remaining OXPHOS complexes were unchanged (complex II 110 ± 8, complexes II+III 105 ± 6, complex III 145 ± 30, and complex IV 125 ± 30% of control; n = 6 rats per group). In addition, another previous study9 would have shown this pattern of electron transport complexes in long-term diabetes if it had corrected for the marker of mitochondrial activity, citrate synthase, which is elevated in renal mitochondria from diabetic rats (control 76.9 ± 9.1 versus diabetic 114.5 ± 4.0 nmol/min per mg; n = 5 rats per group; P < 0.01). On the basis of these results, we focused on complex I alone as a target of dysfunction in our rodent models.
In vivo, long-term injection of AGE-RSA (16 wk) induced a 50% suppression of complex I activity in renal glomeruli as compared with both sham-injected rats (control) and rats injected with RSA (Figure 3A). Of note, modest inhibition of complex I activity was also seen in rodents injected long-term with RSA alone. Generation of mitochondrial superoxide was unchanged by long-term AGE-RSA injection in the absence of hyperglycemia (Figure 3B).
Figure 3.
The AGE-RAGE interaction disrupts the mitochondrial respiratory chain in normoglycemia, but hyperglycemia is essential for superoxide overproduction. (A and B) Representative of renal glomeruli or renal cortex of AGE-RSA or RSA-injected (20 mg/kg per d) rats for 16 wk (n = 6 to 10 rats per group). (A) Glomerular complex I (NADH:ubiquinone oxidoreductase) activity expressed relative to citrate synthase activity. (B) NADH-driven (mitochondrial) superoxide production was measured by lucigenin-enhanced chemiluminescence. (C and D) Representative of renal glomeruli or renal cortex of diabetic rats (▪) or control rats (□) followed for 16 or 32 wk. A subset of diabetic rats received the NADPH oxidase inhibitor APO (  15 mg/kg per d) or the AGE inhibitor ALA chloride (□ 10 mg/kg per d) from week 16 to 32. (C) Glomerular complex I activity expressed relative to citrate synthase activity. (D) NADH-driven (mitochondrial) superoxide production. Bars represent means ± SEM, n = 6 to 10 rats per group. *P < 0.001 versus control; †P < 0.001 versus 16-wk control; ‡P < 0.05 versus 32-wk control; §P < 0.05 versus AGE-RSA; #P < 0.05 versus control; ¥P < 0.05 versus 32-wk diabetes.
15 mg/kg per d) or the AGE inhibitor ALA chloride (□ 10 mg/kg per d) from week 16 to 32. (C) Glomerular complex I activity expressed relative to citrate synthase activity. (D) NADH-driven (mitochondrial) superoxide production. Bars represent means ± SEM, n = 6 to 10 rats per group. *P < 0.001 versus control; †P < 0.001 versus 16-wk control; ‡P < 0.05 versus 32-wk control; §P < 0.05 versus AGE-RSA; #P < 0.05 versus control; ¥P < 0.05 versus 32-wk diabetes.
Consistent with previous studies, diabetic rats had significantly elevated plasma glucose and glycated hemoglobin and an increase in kidney-to-body weight ratio compared with control rats (Table 2). By 16 wk of diabetes, significant elevations in systolic BP and albumin excretion rate were evident, which were further increased by 32 wk of diabetes (Table 2). Induction of RAGE gene expression was seen at both 16 and 32 wk of diabetes (see Supplemental Appendix; Figure 2). As observed in the AGE injection model, there was increased cytosolic H2O2 at weeks 16 and 32 of diabetes (see Supplemental Appendix; Figure 3).
Table 2.
Rat biochemical and metabolic parameters: STZ-induced diabetesa
| Parameter | 16 Wk
|
32 Wk
|
||
|---|---|---|---|---|
| Control | Diabetes | Control | Diabetes | |
| Plasma glucose (mmol/L) | 7.4 ± 0.2 | 34.5 ± 0.5b | 6.9 ± 0.2 | 33.2 ± 0.7b |
| GHb (%) | 4.1 ± 0.2 | 17.3 ± 1.0b | 5.5 ± 0.2 | 18.3 ± 0.7b |
| Kidney wt:body wt (×10−3) | 5.1 ± 0.1 | 10.9 ± 0.6b | 5.3 ± 0.2 | 11.3 ± 0.4b |
| GFR (ml/min) | 4.3 ± 0.1 | 4.5 ± 0.3 | 4.8 ± 0.2 | 4.6 ± 0.1 |
| Systolic BP (mmHg) | 106 ± 2 | 140 ± 4c | 114 ± 2 | 134 ± 3c |
| AER (mg/24 h) | 1.2 ± 0.8 | 29.9 ± 7.5b | 5.6 ± 1.9 | 48.9 ± 16.0b |
Data are means ± SEM; n = 6 to 10 per group.
P < 0.001 versus respective control.
P < 0.05 versus respective control.
Renal glomerular complex I activity was unchanged after 16 wk of diabetes; however, by 32 wk, there was a significant decline in glomerular complex I activity of 30% compared with control (Figure 3C). In contrast to results for AGE-injected rodents, by 32 wk of disease, complex I deficiency in diabetic glomeruli was accompanied by a significant overproduction of mitochondrial superoxide (Figure 3D). Strikingly, renal mitochondria from diabetic rats treated with the cytosolic NADPH oxidase inhibitor, APO, or ALA, the inhibitor of AGE accumulation had less superoxide production with levels similar to those seen in healthy rats (Figure 3D). Furthermore, ALA restored glomerular complex I activity to control levels (Figure 3C). The superoxide overproduction was observed in the context of decreased activity of MnSOD in renal mitochondria from diabetic rats (see Supplemental Appendix; Figure 4).
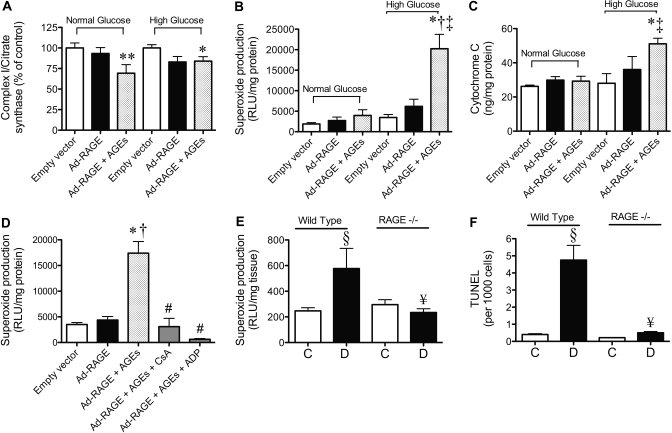
Figure 4.
The AGE-RAGE interaction and high glucose amplify renal mitochondrial superoxide generation, facilitated by induction of mPT. (A through C) Representative of primary rat mesangial cells cultured in normal glucose (5.5 mM) or high glucose (25 mM) and infected with the human FL Ad-RAGE or the control empty vector for 72 h. At 24 h after infection, AGE-BSA (100 μg/ml) was added to one group (Ad-RAGE+AGEs). (A) Mitochondrial complex I activity expressed relative to citrate synthase activity. (B) Mitochondrial NADH-driven superoxide generation was measured by lucigenin-enhanced chemiluminescence. (C) Cytochrome C release from mitochondria determined by ELISA. Inhibitors of mPT, CsA (10 μM) and ADP (100 μM), were also given to some groups 24 h after infection in the presence of 100 μg/ml AGE-BSA. (D) Mitochondrial NADH-driven superoxide generation from cells treated with high glucose (25 mM) after inhibition of mPT. Bars represent means ± SEM, n = 3 independent cell culture experiments. (E and F) Representative of renal cortical samples from control (□) and diabetic (▪) WT (C57BL/6J) and RAGE−/− mice at week 24 after the induction of STZ diabetes (n = 10 mice per group). (E) NADH-driven superoxide production. (F) Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling in renal cortices expressed per 1000 cells counted. *P < 0.001 versus control empty vector high glucose; **P < 0.05 versus control empty vector normal glucose; †P < 0.01 versus Ad-RAGE in high glucose; ‡P < 0.05 versus Ad-RAGE+AGEs in normal glucose; §P < 0.05 versus wild type control 24W; #P < 0.001 versus Ad-RAGE+AGEs; ¥P < 0.05 versus WT diabetic 24 wk.
AGE-RAGE Interaction and High Glucose Are Critical for Superoxide Overproduction, Facilitated by Induction of mPT
Mesangial cells overexpressing RAGE and exposed to AGEs (Ad-RAGE+AGE) had decreased complex I activity in both normal- and high-glucose environments (Figure 4A). There was, however, no increase in mitochondrial superoxide production in Ad-RAGE–infected cells exposed to normal glucose and AGEs (Figure 4B). Although no excess superoxide generation was evident in Ad-RAGE–infected cells incubated in high glucose in the absence of AGE-BSA (Figure 4B), there was a three-fold increase upon exposure to both high glucose and AGEs (Figure 4B). Similarly, increased cytochrome C release from the mitochondria was evident only in Ad-RAGE–infected cells incubated with both high glucose and AGEs (Figure 4C). In addition, caspase 3 was activated in Ad-RAGE–infected mesangial cells exposed to AGEs and high glucose, and this increase in apoptosis was inhibited by the mPT inhibitor adenosine 5′diphosphate (ADP; see Supplemental Appendix; Figure 5).
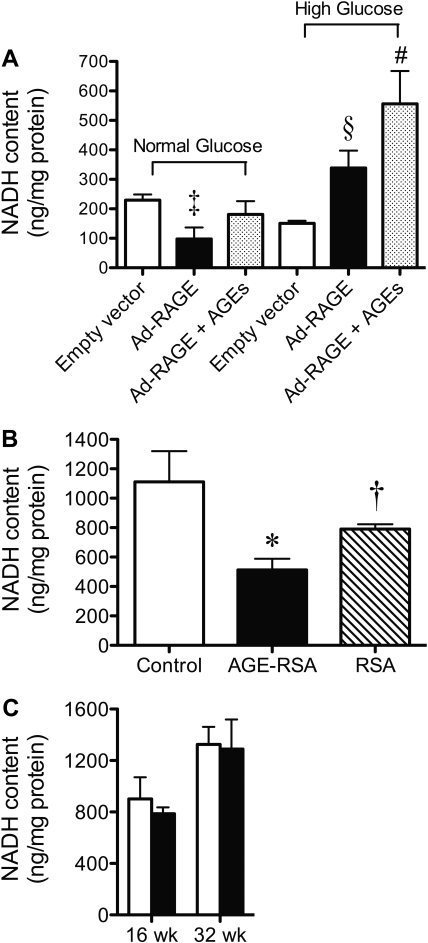
Figure 5.
OXPHOS substrate availability is crucial for superoxide-induced renal dysfunction in diabetes. (A) Primary mesangial cells cultured in high glucose (25 mM) were infected with the human FL Ad-RAGE or the control empty vector for 72 h. At 24 h after infection, AGE-BSA (100 μg/ml) was added to one group (Ad-RAGE+AGEs). NADH content of isolated mitochondria. Mitochondria were isolated from the renal cortex of STZ-induced diabetic rats followed for 16 or 32 wk and from rats injected with AGE-RSA for 16 wk. (B and C) NADH content was determined in mitochondria from AGE-injected rats (B) and mitochondria from STZ-induced diabetic rats (C). □, control; ▪, diabetes group. Bars represent means ± SEM, n = 6 to 10 rats per group. *P < 0.05 versus control; †P < 0.05 versus AGE-RSA; ‡P < 0.05 versus empty vector cultured in normal glucose; §P < 0.05 versus Ad-RAGE cultured in normal glucose; #P < 0.05 versus Ad-RAGE+AGEs cultured in normal glucose.
To confirm that AGE-RAGE interactions caused excess mitochondrial superoxide generation via mPT in high-glucose environments, we incubated Ad-RAGE–infected primary mesangial cells with various mPT inhibitors in the presence and absence of AGE-BSA. Indeed, significant inhibition of AGE-RAGE–induced mitochondrial superoxide generation was observed with the addition of two disparate inhibitors of mPT, CsA and ADP (Figure 4D).
Mitochondrial superoxide generation was also elevated in renal cortices from wild-type (WT) mice after 24 wk of diabetes when compared with control WT mice (Figure 4E). Conversely, no increase in the production of mitochondrial superoxide was seen in renal cortices from diabetic RAGE−/− mice. This lack of mitochondrial ROS production in diabetic RAGE−/− mice occurred in the context of reduced albuminuria in these mice when compared with WT diabetic mice (WT control 155 ± 41, WT diabetic 410 ± 75 μg/24 h [P < 0.05 WT control versus WT diabetic]; RAGE−/− control 142 ± 51; RAGE−/− diabetic 158 ± 50 μg/24 h [P < 0.05 WT diabetic versus RAGE−/− diabetic]). Furthermore, analysis of apoptotic cells in renal cortices demonstrated a diabetes-specific increase in WT mice when compared with control WT mice (Figure 4F). Diabetes, however, was not associated with an increase in apoptosis in renal cortices from diabetic RAGE−/− mice. In addition to this, cytosolic ROS production was blocked in diabetic RAGE−/− mice compared with WT diabetic mice (Supplemental Figure 6).
OXPHOS Substrate Availability Is Crucial for Superoxide-Induced Renal Dysfunction in Diabetes
Finally, we examined the substrate availability of the pyridine nucleotide NADH, which is essential for complex I function in vivo. Mesangial cells infected with Ad-RAGE in high-glucose environments had significant increases in mitochondrial NADH content that were not seen in Ad-RAGE cells incubated in normal-glucose conditions (Figure 5A). Indeed, in normal glucose, Ad-RAGE–infected cells had a depletion of NADH content within the mitochondria when compared with the empty vector alone. In addition, in rat renal cortical mitochondria, NADH content was depleted 50% after long-term AGE injection in healthy normoglycemic rodents when compared with control and RSA-treated rats (Figure 5B). This depletion was not via an inhibition of glycolysis, because cytosolic lactate concentration was similar between groups (data not shown). In contrast, there was no depletion of NADH content in renal mitochondria from diabetic rodents at week 16 or 32 compared with nondiabetic rats (Figure 5C), consistent with the presence of sufficient substrate levels to drive complex I and thereby generate superoxide radicals.
DISCUSSION
This series of in vitro and in vivo studies demonstrated that both impairment of function and adequate substrate availability for the respiratory chain are critical for the subsequent amplification of mitochondrial superoxide in the kidney in diabetes. We demonstrated that the interaction between AGEs and RAGE liberates cytosolic ROS, which leads to the induction of mPT, a phenomenon that proceeded even in the absence of hyperglycemia. Opening of the mitochondrial transition pore and resultant swelling seemed to induce a decrease in the activity of complex I of the respiratory chain. Perhaps the most striking finding in our study was that once electron leakage from the respiratory chain occurred, which was not glucose dependent, generation of excess mitochondrial superoxide was seen only in the setting of high glucose. Our studies support and extend the hypothesis that overproduction of mitochondrial ROS is a key event in activating pathways implicated in the development of the complications of diabetes.2 Furthermore, we specifically highlighted the importance of the cytosolic compartment in enhancing and indeed facilitating mitochondrial generation of ROS. This indicates that cytosolic events, as a result of the AGE-RAGE interaction, play a key role in amplifying mitochondrial ROS generation in the kidney.
Activation of RAGE is known to induce NADPH oxidase and cellular ROS production in endothelial cells in vitro.24 In the diabetic context, this study extends these findings by demonstrating that interactions between AGEs and RAGE stimulate the cytosolic production of ROS within the renal cortex, and this is inhibited by the NADPH oxidase inhibitor APO. It was also of interest that cellular RAGE overexpression alone in the absence of elevated AGEs could not increase cytosolic H2O2 production that was seen with concomitant AGE exposure. This suggests that ligand binding is an important event in AGE-RAGE–mediated production of cytosolic ROS. Furthermore, our own recent study demonstrated the utility of APO as a therapy for diabetic renal disease, confirming in vivo that AGE-RAGE ligation can activate NADPH oxidase.25
ROS in particular, the more stable H2O2, are widely known inducers of mPT in tissues other than the kidney.26,27 Hence, we confirmed these findings in renal mitochondria by demonstrating that addition of exogenous H2O2 induces mPT. Furthermore, AGE-BSA exposure caused mPT in excess of that seen in untreated mesangial cells, in addition to disrupting the mitochondrial membrane potential, both of which were reversed by detoxifying intracellular H2O2 with catalase. Importantly, these changes were independent of ambient glucose concentrations. Thus, it was proposed that AGE-induced intracellular H2O2 formation played a significant role in enhancing susceptibility to the mPT; however, cytochrome C release from mitochondria, indicative of induction of apoptosis,28 was observed only in groups cultured in high-glucose environments. Furthermore, diabetic RAGE−/− mice were protected from renal cellular apoptosis when compared with WT diabetic mice. Indeed, there is increased apoptosis in mesangial cells isolated from STZ-induced diabetic rats,29 and AGE-RAGE interactions were postulated to contribute to podocyte apoptosis.30 Furthermore, other groups demonstrated protection against the induction of diabetic nephropathy by inhibition of cellular apoptosis,31,32 which is consistent with the improved renal function in the context of less renal apoptosis seen in RAGE−/− mice from this study.
Studies have suggested that patients with genetic mutations leading to a dysfunctional mitochondrial respiratory chain can present with renal disease as their primary pathology.7,8 One such disorder, Friedreich ataxia, has specific electron leakage at complexes I and III leading to excessive generation of superoxide.6 In our study, we showed that within diabetic glomeruli, there seems to be a specific defect in complex I rather than other complexes of the respiratory chain. In other contexts, opening of the mPT pore induces electron leakage, leading to increased mitochondrial ROS production at the level of complex I.11 In that study, mitochondrial ROS production was observed only in the presence of a continued supply of the complex I substrate NADH to the respiratory chain, because mPT led to depletion of NADH through diffusion of pyridine nucleotides out of the mitochondria. Hence, these investigators postulated that mPT induces a decline in the activity of complex I, which dramatically increases ROS production as long as electrons are provided to complex I.11 In addition, a recent study elegantly demonstrated that transient opening of the mPT pore stimulated spontaneous bursts of superoxide generation by the mitochondrial respiratory chain.33 Indeed, our own studies in RAGE overexpressing cells exposed to AGE-BSA demonstrated an abrogation of excess mitochondrial superoxide production in the presence of inhibitors of mPT. Furthermore, although a decline in complex I activity could be seen in various settings of normoglycemia in this series of studies, there was no increase in superoxide generation as a result of the depletion of mitochondrial NADH. Indeed, our AGE-injected nondiabetic rats had little evidence of progressive nephropathy at the time point studied. In our diabetic rats, however, the mitochondrial NADH pool seemed sufficient to supply reducing equivalents to complex I, thereby sustaining mitochondrial superoxide production. Furthermore, this mitochondrial superoxide overproduction was exacerbated by decreased activity of mitochondrial MnSOD.
A previous study found that mitochondria from the renal cortex of rats with diabetes exhibited a diminution of OXPHOS via decreased complex III activity and increased superoxide formation.9 In that particular study, however, the measurement of OXPHOS enzymes was not normalized to citrate synthase activity, a procedure considered critical to correct for changes in total mitochondrial number between kidneys from control and diabetic rats; therefore, in that study, one cannot exclude that the decreased OXPHOS reported is in fact due to changes in other subunits of the respiratory chain. Nevertheless, the observation in that study of increased renal mitochondrial superoxide production in STZ-induced diabetes is consistent with the work described in this study. This is further supported by our studies, which included evaluation of the role of RAGE per se. Specifically, the diabetes-induced increases in renal mitochondrial superoxide generation were observed in WT mice but not in kidneys from diabetic RAGE−/− mice.
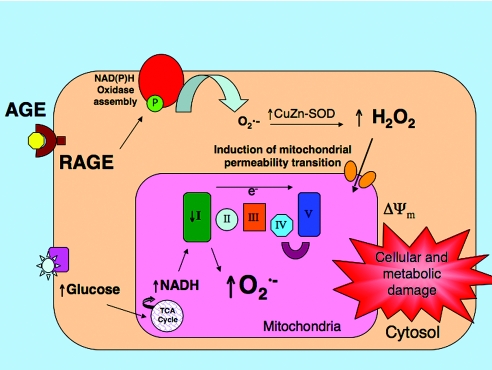
In conclusion, our data indicate that chronic hyperglycemia, leading to enhanced NADH-derived electron delivery to complex I, is required in conjunction with AGE-RAGE–induced cytosolic H2O2 activation of mPT to generate excessive mitochondrial superoxide (Figure 6). This indicates not only does the cytosol act in concert with the mitochondria to generate cellular ROS but also that cytosolic ROS generation may cause or amplify some of the specific mitochondrial defects observed in diabetes. These defects include complex I deficiency, enhanced susceptibility to mPT, and ultimately overproduction of superoxide, which is considered to play a central role in inducing diabetic end-organ injury. The observations presented here suggest that pharmacologic inhibition of cytosolic ROS production may be an appropriate strategy to reduce excessive mitochondrial superoxide production in diabetic complications. In addition, it is postulated that therapies that target complex I may restore mitochondrial integrity and potentially confer a degree of renoprotection in diabetes.
Figure 6.
Schematic representation of the interplay between AGE and RAGE and high glucose in promoting mitochondrial superoxide production in the diabetic kidney. AGEs binding to RAGE induce cytosolic H2O2 production. Cytosolic H2O2 facilitates induction of mPT, promoting a deficiency in complex I of the mitochondrial respiratory chain. Hyperglycemia provides increased mitochondrial NADH availability for OXPHOS, which, when coupled with a deficient complex I activity, amplifies mitochondrial superoxide generation. Both the AGE-RAGE interaction and hyperglycemia synergistically coordinate overproduction of mitochondrial superoxide and promote diabetic kidney disease.
CONCISE METHODS
An expanded version of the Concise Methods section is available in the Supplemental Appendix.
In Vivo Rodent Studies
All animal experiments were performed in accordance with the Alfred Medical Research and Education Precinct Animal Ethics committee.
STZ-Induced Diabetes.
Experimental diabetes was induced in male Sprague-Dawley rats34 or WT (C57BL/6J; Jackson Laboratories, Bar Harbor, ME) and RAGE-deficient mice (RAGE−/−)35,36 with STZ as described previously37 with control rodents followed concurrently. Diabetic and control animals were randomized (n = 10 per group) and followed for 16 or 32 wk for rats or 24 wk for mice. A subset of diabetic rats (n = 10) received either the NADPH oxidase inhibitor APO (15 mg/kg per d, oral gavage) or the AGE inhibitor ALA chloride [4,5-dimethyl-3-(2-oxo2-phenylethyl)-thiazolium chloride, a gift from Synvista, Ramsey, NJ; 10 mg/kg per d, oral gavage] from weeks 16 to 32. Two units of Ultralente insulin (Novo Nordisk, Bagsvjrd, Denmark) were administered daily to diabetic rats to prevent ketoacidosis and improve survival.
Exogenous AGE Administration.
Endotoxin-free AGE-RSA was prepared as described previously.19 Exogenous AGE-RSA was administered daily to healthy 8-wk-old Sprague-Dawley rats (n = 10 per group); AGE-RSA (20 mg/kg per d, intraperitoneally), RSA (20 mg/kg per d, intraperitoneally), or saline (controls) for 16 wk.38 An additional AGE-RSA subgroup (n = 10) was randomized to receive treatment with the AGE inhibitor ALA chloride (10 mg/kg per d, oral gavage) for the duration of the study. Sixteen weeks was chosen on the basis of previous studies that documented the time course of renal disease in this rat model.38
Construction and Generation of Recombinant Adenovirus Vectors.
A recombinant adenovirus encoding human RAGE was constructed and generated. Full-length human RAGE cDNA (gift of Prof. H. Yamamoto39) was inserted into the shuttle plasmid pAdTrack-CMV containing a GFP reporter and was then subcloned into the adenoviral backbone plasmid AdEasy-1 (Ad-RAGE). Human embryonic kidney 293 cells were then infected to package the virus using lipofectamine 2000 reagent (Invitrogen, Melbourne, Australia). The pAdTrack-CMV shuttle vector without the gene of interest was used as a control (empty vector). RAGE expression was verified using Western immunoblotting (see Supplemental Appendix, Figure 1).
Adenovirus-Mediated RAGE Gene Transfer In Vitro.
Primary mesangial cells were isolated from rat kidney as described previously.40 Mesangial cells seeded into 75-cm2 tissue culture flasks were grown for 3 d to 60% confluence in 5.5 mM d-glucose in DMEM containing 10% FCS; then the medium was replaced with DMEM containing either 5.5 or 25.0 mM d-glucose and the purified Ad-RAGE vector or empty vector (2.85 × 108 optical particle units). After 24 h, 100 μg/ml AGE-BSA or 100 μg/ml BSA was added, and cells were cultured for an additional 2 d. In some studies, inhibitors of mitochondrial pore transition, ADP (100 μM) and CsA (10 μM), or the antioxidant PEG-catalase (500 U/ml), were concomitantly administered with AGE-BSA. Transfection efficiency was 80 to 90% on the basis of GFP fluorescence.
Mitochondrial Superoxide Production.
Superoxide production in renal cortex was determined by lucigenin-enhanced chemiluminescence as described previously.34 Superoxide production in freshly isolated mitochondria from primary mesangial cells was measured as described previously.1
H2O2 Production.
Please refer to the expanded version of the Methods section of the Supplemental Appendix.
Superoxide Dismutase Activity.
Please refer to the expanded version of the Methods section of the Supplemental Appendix.
Mitochondrial Membrane Potential.
Mitochondrial membrane potential was assessed using the MitoProbe JC-1 assay kit (Molecular Probes, Eugene, Oregon) by flow cytometry, according to the manufacturer's instructions.
OXPHOS Complex Activity.
Isolation of renal glomeruli was performed as described previously.41 Respiratory chain complexes and citrate synthase were assayed in glomerular supernatants and in mesangial cell mitochondrial preparations as described previously for other tissues.42 For accounting for variations in mitochondrial number between samples, complex activity was expressed relative to citrate synthase, which is much more resistant to oxidative inactivation than the respiratory chain complexes.43
Apoptosis.
Please refer to the expanded version of the Methods section of the Supplemental Appendix.
Mitochondrial Permeability Transition.
Please refer to the expanded version of the Methods section of the Supplemental Appendix.
Mitochondrial NADH Content.
Mitochondria (50-μl suspension) from renal cortex or from primary rat mesangial cells were alkaline treated (50 μl of 1 M NaOH) at 90°C for 5 min to destroy the oxidized NAD+ isoform. Samples were quenched on ice and neutralized, and NADH content was measured using a modification of the method originally described by Nisselbaum and Green.44
Real-Time Reverse Transcription–PCR.
Please refer to the expanded version of the Methods section of the Supplemental Appendix.
Statistical Analysis
All statistical computations were performed using GraphPad Prism 4.0a for Mac OS X (GraphPad Software, San Diego, CA). Values of experimental groups are given as mean, with bars showing the SEM, unless otherwise stated. One-way ANOVA with Tukey posttest analysis or two-way ANOVA with Bonferroni posttest analysis was used to determine statistical significance. When appropriate, two-tailed t test were performed. P < 0.05 was considered to be statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This research was funded by a Juvenile Diabetes Research Foundation Center Grant (J.M.F., M.E.C., A.B., P.P.N., and M.B.), a National Health and Medical Research Council of Australia (NHMRC) Project Grant (J.F., D.T., and M.E.C.), and a Diabetes Australia Research Trust Millennium Grant (J.M.F.). J.M.F. is a recipient of a Juvenile Diabetes Research Foundation Career Development Award. D.R.T. holds an NHMRC Principal Research Fellowship, and M.E.C. holds an NHMRC Senior Principal Research Fellowship. In addition, this work was in part supported by a grant from the Deutsche Forschungsgemeinschaft (SFB405 to P.P.N.).
We thank Susan Thorpe for the analysis of the AGE-BSA and AGE-RSA for AGE content and acknowledge the technical assistance of Maryann Arnstein, Gavin Langmaid, Sandra Miljavec, Felicia Y.T. Yap, Anna Gasser, David C.K. Tong, and Josefa Pete.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Pitkanen S, Robinson BH: Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Invest 98: 345–351, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Baynes JW, Thorpe SR: Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 48: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Koopman WJ, Verkaart S, Visch HJ, van der Westhuizen FH, Murphy MP, van den Heuvel LW, Smeitink JA, Willems PH: Inhibition of complex I of the electron transport chain causes O2−.-mediated mitochondrial outgrowth. Am J Physiol Cell Physiol 288: C1440–C1450, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Beyer RE: An analysis of the role of coenzyme Q in free radical generation and as an antioxidant. Biochem Cell Biol 70: 390–403, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P: Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17: 215–217, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Martin-Hernandez E, Garcia-Silva MT, Vara J, Campos Y, Cabello A, Muley R, Del Hoyo P, Martín MA, Arenas J: Renal pathology in children with mitochondrial diseases. Pediatr Nephrol 20: 1299–1305, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Singh PJ, Santella RN, Zawada ET: Mitochondrial genome mutations and kidney disease. Am J Kidney Dis 28: 140–146, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Rosca MG, Mustata TG, Kinter MT, Ozdemir AM, Kern TS, Szweda LI, Brownlee M, Monnier VM, Weiss MF: Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol Renal Physiol 289: F420–F430, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Oliveira PJ, Esteves TC, Seica R, Moreno AJ, Santos MS: Calcium-dependent mitochondrial permeability transition is augmented in the kidney of Goto-Kakizaki diabetic rat. Diabetes Metab Res Rev 20: 131–136, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Batandier C, Leverve X, Fontaine E: Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem 279: 17197–17204, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kabat A, Ponicke K, Salameh A, Mohr FW, Dhein S: Effect of a beta 2-adrenoceptor stimulation on hyperglycemia-induced endothelial dysfunction. J Pharmacol Exp Ther 308: 564–573, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME: The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J 17: 1762–1764, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM: RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162: 1123–1137, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R: At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol 25: 1401–1407, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Cai W, He JC, Zhu L, Lu C, Vlassara H: Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci U S A 103: 13801–13806, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW: Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int 61: 939–950, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME: Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest 108: 1853–1863, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broekemeier KM, Dempsey ME, Pfeiffer DR: Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264: 7826–7830, 1989 [PubMed] [Google Scholar]
- 21.Crompton M, Ellinger H, Costi A: Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255: 357–360, 1988 [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier N, Ducet G, Crevat A: Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 19: 297–303, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Brunt KR, Fenrich KK, Kiani G, Tse MY, Pang SC, Ward CA, Melo LG: Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler Thromb Vasc Biol 26: 2027–2034, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL: Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280: E685–E694, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM: Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 57: 460–469, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Colell A, Garcia-Ruiz C, Mari M, Fernandez-Checa JC: Mitochondrial permeability transition induced by reactive oxygen species is independent of cholesterol-regulated membrane fluidity. FEBS Lett 560: 63–68, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Takeyama N, Miki S, Hirakawa A, Tanaka T: Role of the mitochondrial permeability transition and cytochrome C release in hydrogen peroxide-induced apoptosis. Exp Cell Res 274: 16–24, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Kim CN, Yang J, Jemmerson R, Wang X: Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86: 147–157, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Weissgarten J, Berman S, Efrati S, Rapoport M, Averbukh Z, Feldman L: Apoptosis and proliferation of cultured mesangial cells isolated from kidneys of rosiglitazone-treated pregnant diabetic rats. Nephrol Dial Transplant 21: 1198–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Chuang PY, Yu Q, Fang W, Uribarri J, He JC: Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72: 965–976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP: Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Susztak K, Raff AC, Schiffer M, Bottinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 33.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H: Superoxide flashes in single mitochondria. Cell 134: 279–290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlan MT, Thallas-Bonke V, Pete J, Long DM, Gasser A, Tong DC, Arnstein M, Thorpe SR, Cooper ME, Forbes JM: Combination therapy with the advanced glycation end product cross-link breaker, alagebrium, and angiotensin converting enzyme inhibitors in diabetes: Synergy or redundancy? Endocrinology 148: 886–895, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundörfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP: Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest 114: 1741–1751, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constien R, Forde A, Liliensiek B, Gröne HJ, Nawroth P, Hämmerling G, Arnold B: Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 30: 36–44, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Forbes JM, Yee LTL, Thallas V, Lassila M, Candido R, Jandeleit-Dahm K, Thomas MC, Burns WC, Deemer E, Thorpe SR, Cooper ME, Allen TJ: Advanced glycation end product interventions reduce diabetes accelerated atherosclerosis. Diabetes 53: 1813–1823, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M: Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A 91: 11704–11708, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H: Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 370: 1097–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brüne B, Sterzel RB: Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol 275: F962–F971, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Schlondorff D: Preparation and study of isolated glomeruli. Methods Enzymol 191: 130–140, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR: Leigh syndrome: Clinical features and biochemical and DNA abnormalities. Ann Neurol 39: 343–351, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Thorburn DR, Sugiana C, Salemi R, Kirby DM, Worgan L, Ohtake A, Ryan MT: Biochemical and molecular diagnosis of mitochondrial respiratory chain disorders. Biochim Biophys Acta 1659: 121–128, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Nisselbaum JS, Green S: A simple ultramicro method for determination of pyridine nucleotides in tissues. Anal Biochem 27: 212–217, 1969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.