Abstract
Glomerulonephritis (GN) is the leading cause of chronic kidney disease among recipients of renal transplants. Because modern immunosuppressive regimens have reduced the incidence of rejection-related graft loss, the probability and clinical significance of posttransplantation GN (PTGN) requires reevaluation. In this Canadian epidemiologic study, we monitored 2026 sequential renal transplant recipients whose original renal disease resulted from biopsy-proven GN (36%), from presumed GN (7.8%), or from disorders other than GN (56%) for 15 yr without loss to follow-up. Kaplan-Meier estimates of PTGN in the whole population were 5.5% at 5 yr, 10.1% at 10 yr, and 15.7% at 15 yr. PTGN was diagnosed in 24.3% of patients whose original renal disease resulted from biopsy-proven GN, compared with 11.8% of those with presumed GN and 10.5% of those with disorders other than GN. Biopsy-proven GN in the native kidney, male gender, younger age, and nonwhite ethnicity predicted PTGN. Current immunosuppressive regimens did not associate with a reduced frequency of PTGN. Patients who developed PTGN had significantly reduced graft survival (10.2 versus 69.7%; P < 0.0001). In summary, in the Canadian population, PTGN is a common and serious complication that causes accelerated graft failure, despite the use of modern immunosuppressive regimens.
The success of renal transplantation has increased dramatically during the past two decades driven by advances in understanding of biologic processes of graft injury and the selective inhibition of key molecular signals by new immunosuppressive drugs.1–4 Patient and graft survival now exceed 95 and 90%, respectively, during the first year; >80% of patients remain free from acute rejection, and graft half-life is exceeds 13 and 21 yr, respectively, for deceased- and live-donor organs.5–7 Complications have diminished in frequency and severity, life-threatening infections are now uncommon, and there has been a corresponding improvement in both quality of life and overall cost-effectiveness.8–10 As a result, transplantation is now the optimal therapy for chronic kidney disease (CKD). Up to 40% of patients with CKD in Canada and other developed countries are now maintained with a functioning transplant,5–7,11 and care is increasingly delivered by nephrologists and physicians distant from the transplanting team.
Glomerulonephritis (GN) is the leading cause of chronic renal disease in patients undergoing renal transplantation, present in up to 50% of such patients.12 Since the earliest reports by Hume and colleagues,13–19 it has been recognized that patients are at risk for the recurrence of their original disorder or development of de novo GN in the graft. Posttransplantation GN (PTGN) has been reported in up to 40% of patients, and the cumulative probability increases with time after transplantation, although with great variation in frequency and severity.14–27 In some reports, histologic recurrence may have only a minor clinical expression, whereas in others, it is associated with premature graft failure.12,18,20,21,28,29 Despite the marked reduction in graft rejection and improvement in survival observed with new immunosuppressive agents,30 the occurrence and progression of PTGN seem to be uninfluenced by these potent drugs, and PTGN is now one of the most common causes of death-censored graft failure.12,20,25,31
The risk for allograft loss as a result of PTGN is thus an important factor in the decision to proceed with transplantation, and an accurate understanding of the probability and clinical significance of PTGN is essential for the referring physician, the transplant team, the patient, and a potential live donor.31 This study was therefore undertaken to provide precise information on the probability, predictors, and prognosis of PTGN in a health system with universal access to care and comprehensive follow-up and to determine the evolution of this complication with modern immunosuppressive therapy.
RESULTS
Patient Demographics and Treatment
We evaluated, a total of 2026 patients who had end-stage renal failure and received a renal transplant between January 1, 1990, and December 31, 2005. All 2026 patients received a single transplant during the period of observation; 36.6% of these were from a living donor, 56.3% were from a cadaver donor, and donor source was not available for 7.1% of patients who underwent transplantation elsewhere. A total of 104 patients received a second and a third transplant during the observation period, but these were not included in the analysis. The mean duration of follow-up for the study was 80 mo (range 0 to 204 mo). A total of 2784 renal graft biopsies were performed on 1331 patients during the period of observation. Of these, 1580 were performed in the first 3 mo primarily for diagnosis of acute rejection, whereas 1204 occurred after this time for investigation of graft dysfunction. These occurred in 38, 35, and 27% of patients across the three time intervals outlined next.
As shown in Table 1, 734 (36.2%) patients had chronic kidney failure as a result of biopsy-proven GN, with IgA nephropathy, FSGS, lupus nephritis, and type 1 mesangioproliferative GN (MPGN) being the most common histologic forms. An additional 159 (7.8%) patients received a diagnosis of GN on clinical criteria, but renal biopsy was not performed for patient safety or other reasons (presumed GN). The true cause was thus uncertain. The remaining 1133 (56%) patients had CKD as a result of other disorders, the principal causes being diabetes, cystic kidney disease, and chronic interstitial disease.
Table 1.
Cause of CKD among 2026 patients before receiving a renal transplant
| Original Renal Disease | n (%) |
|---|---|
| Group 1: Biopsy-proven GN | 734 (36.2) |
| IgA nephropathy | 246 (12.1) |
| FSGS | 196 (9.7) |
| lupus nephritis | 79 (3.9) |
| membranoproliferative GN, type 1 | 42 (2.1) |
| pauci-immune crescentic glomerulonephritis | 26 (1.3) |
| membranous nephropathy | 20 (0.9) |
| other types of glomerulonephritis | 125 (6.2) |
| Group 2: Presumed glomerulonephritis | 159 (7.8) |
| Group 3: Other disorders | 1133 (56.0) |
| diabetic kidney disease | 280 (13.8) |
| cystic kidney disease | 237 (11.7) |
| chronic interstitial disease | 167 (8.2) |
| renal vascular disease | 130 (6.4) |
| uncertain cause | 123 (6.1) |
| congenital renal disease | 84 (4.2) |
| nephrotoxicity | 28 (1.4) |
| miscellaneous | 84 (4.2) |
The characteristics of the patients are shown in Table 2. The median age at transplantation was 46 yr; 1217 (60.1%) patients were male; 1393 (69.1%) were white, 366 (18.1%) were Asian, and the remainder were of African, Indian, or First Nations origin. Patients with biopsy-proven GN as a cause of primary kidney disease were younger, more frequently of Asian origin and received a graft from a living donor, were less likely to have received depleting antibody but more likely to have received mycophenolate mofetil (MMF) than those with other causes of renal disease (P = 0.01 to <0.001).
Table 2.
Characteristics of patients, donors, and immunosuppressive treatmenta
| Characteristic | Biopsy-Proven GN (n = 734) | Presumed GN (n = 159) | Other Disorders (n = 1133) | Total (N = 2026) |
|---|---|---|---|---|
| Patients | ||||
| male (n [%]) | 454 (61.9) | 99 (62.3) | 664 (58.6) | 1217 (60.1) |
| age at transplantation (yr; median [range])b | 42 (33 to 54) | 50 (40 to 60) | 48 (37 to 58) | 46 (35 to 57) |
| race (n [%]) | ||||
| Asianb | 188 (25.6) | 39 (24.5) | 139 (12.4) | 366 (18.1) |
| whiteb | 442 (60.2) | 98 (61.6) | 853 (75.9) | 1393 (69.1) |
| other | 104 (14.2) | 22 (13.8) | 132 (11.7) | 258 (12.8) |
| HBsAg positive (n [%])c | 13 (1.8) | 9 (6.7) | 21 (2.2) | 43 (2.5) |
| HCV positive (n [%]) | 22 (5.2) | 6 (8.2) | 32 (5.5) | 60 (5.6) |
| CMV positive (n [%])b | 440 (64.1) | 121 (82.3) | 637 (61.5) | 1198 (64.1) |
| Donors | ||||
| cadaver donor (n [%])b | 394 (53.7) | 110 (69.2) | 636 (56.1) | 1140 (56.3) |
| first graft (n [%]) | 671 (91.4) | 136 (85.5) | 1043 (92.1) | 1850 (91.3) |
| HLA mismatch (median [range]) | 3 (2 to 4) | 3 (2 to 4) | 3 (2 to 4) | 3 (2 to 4) |
| Immunosuppression (n [%]) | ||||
| ATG or OKT3d | 86 (11.7) | 20 (12.6) | 191 (16.9) | 297 (14.7) |
| anti-CD25d | 173 (23.6) | 20 (12.6) | 242 (21.4) | 435 (21.5) |
| CsA | 474 (64.6) | 110 (69.2) | 723 (63.8) | 1307 (64.5) |
| tacrolimus | 193 (26.3) | 33 (20.8) | 269 (23.7) | 495 (24.4) |
| MMFb | 357 (48.6) | 55 (34.6) | 472 (41.7) | 884 (43.6) |
CMV, cytomegalovirus; CsA, cyclosporine; HCV, hepatitis C virus.
P < 0.001.
P = 0.01.
P = 0.006.
Probability and Predictors of PTGN
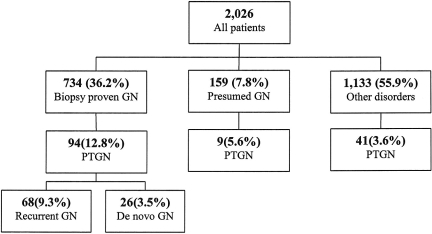
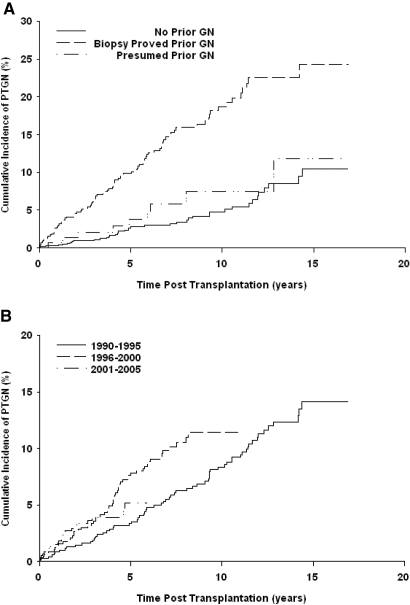
A total of 144 of 2026 patients received a diagnosis of PTGN between 6 and 5246 d after transplantation (mean 57.8 mo; Figure 1). Kaplan-Meier estimates of cumulative probability were 5.5, 10.1, and 15.7% by 5, 10, and 15 yr after transplantation, respectively. PTGN was diagnosed in 94 of 734 patients who had biopsy-proven GN in their native kidney, compared with nine of 159 whose disease was not confirmed by biopsy (P = 0.004) and 41 of 1133 who had CKD as a result of other causes (P < 0.001). As shown in Figure 2A, the cumulative probability of PTGN in patients with previous GN was 9.9, 18.7, and 24.3% by 5, 10, and 15 yr, respectively, compared with 3.8, 7.4, and 11.8% in patients with presumed GN and 2.8, 4.8, and 10.5% in those with other primary disorders. Among patients with previous GN, the probability of recurrent disease was 7.4, 13.1, and 17.8% by 5, 10, and 15 yr, respectively, while de novo disease occurred in 2.8, 5.4, and 9.6% of patients at these time points.
Figure 1.
Schematic of primary renal disease and PTGN.
Figure 2.
Cumulative probability of PTGN by original disease and by treatment era.
Principal covariates of the Cox model are shown in Table 3. Principal predictors of PTGN were the presence of biopsy-proven GN in the native kidney (P < 0.001), male gender (P < 0.001), lower age (P = 0.009), and nonwhite ethnicity (P < 0.02). Of the 2026 patients evaluated, 297 (14.7%) received induction therapy with depleting antibody and 435 (21.5%) with nondepleting anti-CD25 antibody; 1307 (65%) received cyclosporine and 495 (24%) received tacrolimus; and 884 (44%) received MMF. The individual immunosuppressive modalities used did not influence the probability of PTGN within these models.
Table 3.
Principal outcome predictors from Cox models
| Parameter | Adjusted HR | 95% CI | P |
|---|---|---|---|
| Development of PTGN | |||
| age at transplantation | 0.98 | 0.97 to 0.99 | 0.009 |
| male gender | 1.98 | 1.36 to 2.89 | <0.001 |
| Asian | 1.71 | 1.11 to 2.62 | 0.010 |
| black | 4.26 | 1.33 to 13.63 | 0.010 |
| Filipino | 5.32 | 2.72 to 10.41 | <0.001 |
| Indian subcontinent | 1.93 | 1.10 to 3.36 | 0.020 |
| North American First Nations | 2.59 | 1.32 to 5.07 | 0.005 |
| other nonwhite | 4.03 | 1.26 to 12.82 | 0.020 |
| previous GN | 3.22 | 2.21 to 4.69 | <0.001 |
| Graft failure | |||
| PTGN | 7.45 | 5.47 to 10.16 | <0.001 |
| living kidney donor | 0.57 | <0.44 to 0.75 | <0.001 |
| Asian race | 0.68 | 0.49 to 0.93 | 0.010 |
| North American First Nations | 1.63 | 1.09 to 2.42 | 0.010 |
| CsA | 0.39 | 0.27 to 0.59 | <0.001 |
| tacrolimus | 0.29 | 0.16 to 0.53 | <0.001 |
| MMF | 0.61 | 0.45 to 0.84 | 0.002 |
| Patient death | |||
| biopsy-proven GN as primary disease | 0.55 | 0.43 to 0.72 | <0.001 |
| age | 1.05 | 1.04 to 1.06 | <0.001 |
| Asian | 0.63 | 0.44 to 0.90 | 0.010 |
| living kidney donor | 0.65 | 0.48 to 0.87 | 0.004 |
| tacrolimus | 0.56 | 0.33 to 0.94 | 0.030 |
We performed an analysis to evaluate the influence of immunosuppression and other treatment modalities comparing three time periods of 1990 through 1995 (reflecting principally the use of cyclosporine, imuran, and steroids), 1996 through 2000 (use of cyclosporine, MMF, and steroids) and 2001 through 2005 (use of basiliximab, tacrolimus, and MMF with steroid minimization). The incidence of PTGN among patients who underwent a transplant during 1990 through 1995 was significantly lower than in those who underwent a transplant after this period (P = 0.02), suggesting that current potent immunosuppression has not reduced the risk for this complication (Figure 2B). This was not explained simply by a differential frequency of graft biopsy in stable patients, which was performed in 38, 35 and 27%, respectively, in the three time periods.
Histologic Forms of PTGN
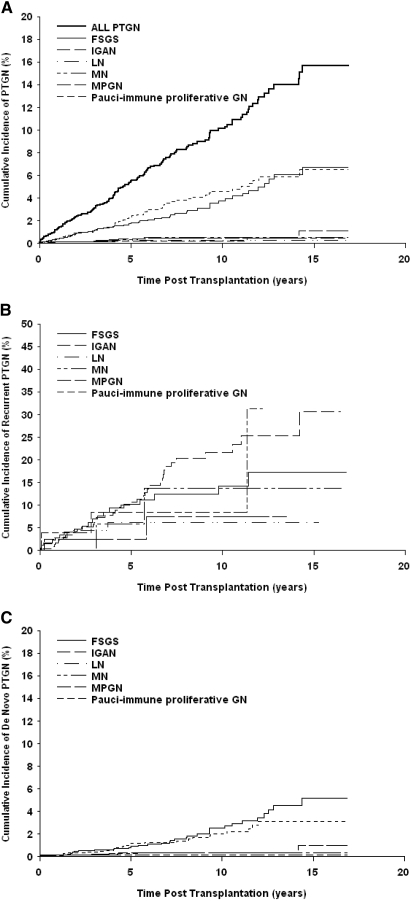
There was a marked disparity in risk for PTGN according to histologic type as shown in Figure 3. The most common forms were IgA nephropathy and FSGS, whereas all other forms occurred in <1% of patients (Table 4). The cumulative probabilities of IgA nephropathy and FSGS at 15 yr after transplantation were 6.5 and 6.7%, respectively, whereas other principal histologic categories, including MPGN 1(1.1%), membranous nephropathy (0.5%), and lupus nephritis and pauci-immune GN (0.7%), were substantially lower (Figure 3A).
Figure 3.
Cumulative probability of PTGN by histologic type. (A) all cases of PTGN. (B) Cases of recurrent PTGN (n = 68 of 734). (C) All cases of de novo PTGN (n = 76 of 2026). IGAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy.
Table 4.
Principal histologic forms of PTGN according to classification of original renal diseasea
| Parameter | Biopsy-Proven GN (n = 734)
|
Presumed GN (n = 159) | Other Disorders (n = 1133) | Total (N = 2026) | |
|---|---|---|---|---|---|
| Recurrent | De Novo | ||||
| IgA nephropathy | 38 | 7 | 4 | 12 | 61 |
| FSGS | 19 | 11 | 4 | 19 | 53 |
| Lupus nephritis | 4 | – | – | 0 | 4 |
| Membranoproliferative GN, type 1 | 2 | 1 | – | 5 | 8 |
| Membranous nephropathy | 2 | 4 | – | 1 | 7 |
| Pauci-immune proliferative GN | 3 | 1 | – | 1 | 5 |
| MPGN | – | 2 | – | 2 | 4 |
| IgM nephropathy | – | – | 1 | 1 | 2 |
| Total | 68 | 26 | 9 | 41 | 144 |
Number of patients with biopsy-proven GN according to diagnostic type: IgA nephropathy = 246; FSGS = 196; lupus nephritis = 79; membranoproliferative GN type 1 = 42; membranous nephritis = 20; pauci-immune proliferative GN = 26; mesangial proliferative nephritis = 12; IgM nephropathy = 0.
Relative frequencies varied slightly between groups according to original disease (Table 4). IgA nephropathy was most frequent among patients with biopsy-proven GN, followed by FSGS, membranous nephropathy, lupus nephritis and pauci-immune nephritis, MPGN type 1, and mesangial proliferative GN (Figure 3B). IgA nephropathy and FSGS developed with equal frequency among patients with undefined disease, whereas FSGS was most common among patients without previous GN, followed by IgA nephropathy, type 1 MPGN, mesangial proliferative GN and IgM nephropathy, membranous nephropathy, and pauci-immune crescentic GN (Figure 3C).
As shown in Figure 1, 68 of 734 patients with previous GN had recurrence of their original histologic disease, diagnosed at a median of 38 mo (range 0.5 to 172) after transplantation. IgA nephropathy recurred in 25.3% of patients after 15 yr of follow-up, FSGS in 12.6%, pauci-immune crescentic GN in 8%, membranous nephropathy in 11.3%, lupus nephritis in 5.3%, and type 1 MPGN in 5.8% (Kaplan-Meier estimate). An additional 26 patients developed PTGN distinct from that in their native kidney, diagnosed after a median of 50 mo (range 0.3 to 134). Of these, 1% developed IgA nephropathy, 1.4% FSGS, 0.1% type 1 MPGN, 0.5% membranous nephropathy, 0.3% mesangial proliferative GN, and 0.1% pauci-immune crescentic GN.
Graft and Patient Outcomes
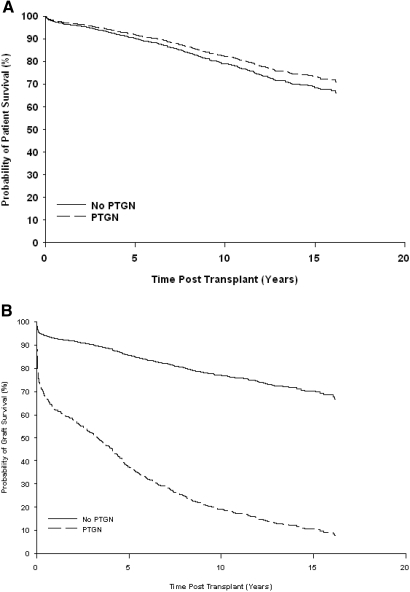
A total of 370 Kaplan-Meiers died during the period of follow-up as a result of cardiovascular disease (n = 107), malignancy (n = 52), infection (n = 40), and other causes (n = 171). Estimated patient survival at 15 yr after transplantation was comparable between patients with or without PTGN (73.2 versus 68.5%; P = 0.47; Figure 4A).
Figure 4.
Cox model estimates of probability of patient and graft survival censored by death according to the occurrence of PTGN.
An additional 443 patients lost their grafts; 63 (43.8%) of 144 graft losses occurred in patients with PTGN, and 380 (20.2%) of 1882 occurred in patients without PTGN. As shown in Figure 4B, estimated graft survival at 15 yr after transplantation was significantly reduced in patients who developed PTGN (10.2 versus 69.7%; P < 0.001).
Cox analysis showed that the risk for graft failure was significantly increased among patients with PTGN (hazard ratio [HR] 7.45; P < 0.001) and North American First Nations (HR 1.63; P = 0.01), whereas Asian patients (HR 0.68; P = 0.01); recipients of a kidney from a live donor (HR 0.57; P < 0.001); and those treated with cyclosporine (HR 0.39; P < 0.001), tacrolimus (HR 0.29; P < 0.001), or MMF (HR 0.61; P = 0.002) had a decreased risk for graft failure. The risk for death was significantly higher among older patients (HR 1.05; P < 0.001) and was decreased among patients of Asian origin (HR 0.63; P = 0.01), those with biopsy-proven GN as their original disease (HR 0.55; P < 0.001), recipients of a kidney from a live donor (HR 0.65; P = 0.004), and those treated with tacrolimus (HR 0.56; P = 0.03).
DISCUSSION
Improvements in immunosuppression have markedly reduced the frequency and severity of graft rejection, gradually attenuating the impact of alloimmune injury on long-term survival after renal transplantation.32 This has highlighted the clinical importance of PTGN, which is rapidly emerging as a leading cause of graft failure.12,20,31 Estimates of the frequency and severity of this problem vary between reports, often confounded by issues of sample size, duration of observation, heterogeneity of treatment or diagnosis, and completeness of follow-up.20,21,25,33 This analysis attempted to minimize these limitations by ensuring universal health care, standardized clinical management, uniform histologic sampling and interpretation, and continuous and uniform patient supervision throughout the whole transplant course, although the regional focus, relative frequency of Asian rather than black minorities, and other characteristics of the Canadian system must temper extrapolation.
The epidemiology of primary renal failure in this study was comparable to that reported for patients receiving a renal transplant in other regions.5,6 More than one third of patients had chronic kidney failure as a result of biopsy proven GN, with IgA nephropathy, FSGS, lupus nephritis, and type 1 MPGN the most common histologic forms. More than half had CKD as a result of other disorders, principally diabetes, cystic kidney disease, and chronic interstitial disease. In a small remaining group who received a diagnosis from their physicians of probable GN, renal biopsy was not performed for patient safety or other reasons. The true cause in these patients therefore remains uncertain, and these patients were analyzed separately to avoid confounding inherent in certain previous analyses.27 Minor demographic differences were noted between these groups, and patients with GN as a cause of primary renal failure were in general younger, were more frequently of Asian origin, and received a graft from a living donor. Donor origin was consistent with Canadian and international norms,5–7 and to minimize bias and co-linearity, we considered only the first transplant performed within the observation period for each patient.
All biopsy specimens, including those with chronic allograft nephropathy or transplant glomerulopathy, were routinely evaluated by immunofluorescence and electron microscopy unless precluded by inadequate tissue, and the biopsy criteria were the same throughout all time periods. Although PTGN was diagnosed at a mean of 5 yr, individual presentations ranged from 6 d to 14 yr, and the cumulative probability rose from 5% at 5 yr to 16% by 15 yr after transplantation. The most common histologic forms of PTGN were IgA nephropathy and FSGS, both of which occurred with an overall cumulative probability of approximately 7%, whereas other forms were substantially less frequent. These frequencies parallel the relative incidence rates of these disorders in patients presenting for renal transplantation, lending credence to the suggestion that the pathologic process leading to primary renal failure remains operative in the recipient after transplantation.28 To what extent these changes represent primary disease or reflect ischemic or alloimmune injury resulting in secondary FSGS or MPGN, respectively, is difficult to determine from this study; however, as shown in Figure 3, the cumulative probability of FSGS is substantially higher in patients with previous biopsy-proven GN than in those with other disorders (17.2 versus 5.2% at 15 yr), whereas the incidence of MPGN is similar between the two groups (0 and 0.5% at 15 yr), providing a “lowest-case” scenario for comparison.
Cox analysis showed that the principal predictors of PTGN were the presence of biopsy-proven GN in the native kidney,12 male gender,12,20 lower age,34 and nonwhite ethnicity. In contrast to other reports, donor source (living versus deceased) did not alter the risk for PTGN.35,36 The individual immunosuppressive modalities used did not influence the probability of PTGN within these models, and sequential cohort analysis indicated that advances in immunosuppression have not reduced the risk for this complication.31 Neither was this due to detection bias related to frequency of biopsy, which was comparable in all three periods. Interestingly, preliminary data suggest the possibility that acute rejection and PTGN may occur with reciprocal frequency and that the frequency of PTGN is not increased by early steroid withdrawal.37,38
PTGN was most common among patients with biopsy-proven GN in their native kidney, reaching a cumulative probability of 25% after 15 yr. Approximately 70% of cases in this setting were consistent with recurrence of a primary disease, with IgA nephropathy the most common histologic form; the remaining 30% represented a different histology, among which FSGS predominated. Other forms of PTGN were uncommon in both settings.26,29,35 In contrast, PTGN occurred in approximately 10% of patients whose primary disease with not GN and 12% of patients with “uncertain disease” that was not biopsy proven.
The incidence of PTGN among patients with CKD as a result of causes other than GN is striking, far exceeding that in the normal population and suggesting active nephritogenic mechanisms operative within the graft recipient. Diverse mechanisms may contribute to specific patterns of injury.39 Predisposition to the nephrotic syndrome is associated with mutations in several genes, including NPHS1, NPHS2, ACTN4, CD2AP, WT1, TRPC6, and LAMB2, coding for proteins (nephrin, podocin, α-actinin-4, an adapter protein anchoring CD2 and others) that influence the function of the podocytes,39–41 and other genetic loci, ACTN4, TRPC6, and CD2AP connected with autosomal dominant forms of FSGS.42 Such mutations may be transferred with the graft but should not exceed population frequencies. Infection is increased in the graft recipient, and viral, bacterial, or fungal infection may play an important contributory role.43–47 Hepatitis C virus is perhaps the most frequently defined cause of membranoproliferative GN, membranous GN, or thrombotic microangiopathy disease in the graft,46,48,49 although hepatitis G virus and other viruses may contribute.45,50 Finally, immunosuppressive therapy itself may play a role: Cyclosporine may cause a spectrum of renal injury including FSGS, whereas sirolimus toxicity may lead to the appearance of FSGS independently or as a co-factor in patients with a genetic susceptibility to this disorder.51,52
In concert with recent publications, the data reported here emphasize that PTGN is an important cause of graft loss in renal transplant recipients and is associated with a dramatic reduction in graft survival.12,20,21 The prognosis of each of the forms observed may depend on various factors, including the severity or type of histologic lesion53,54 or whether the disease is recurrent or de novo.38 Nonetheless, for most forms of PTGN, there is as yet no effective treatment, although intensive plasma exchange or rituximab may be of benefit in some cases of FSGS.55,56 A cogent case may therefore be made to support a unified approach to define the specific causative factors in the diverse forms of this devastating disorder and to explore options for therapy in a controlled manner.34
CONCISE METHODS
Setting
The study was undertaken within the Canadian health care system. The British Columbia transplant program, established in 1986, provides free, universal access to treatment for a population of more than 4 million with continuous follow-up of all transplant recipients in a network of clinics across the province. Patients are followed according to a defined schedule, and clinical management is conducted according to standardized protocols reviewed annually by the transplant team. The clinical course, treatment, and key outcomes for all patients are recorded prospectively in an electronic information system providing detailed and continuous information on every patient who receives a transplant within the program.
Patients
This prospective cohort study included 2026 renal transplants recipients from 1990 through 2005 in the British Columbia transplant program. All recipients were followed by the specialist staff in the regional nephrology units and were referred for evaluation to an integrated medical/surgical team at the transplant program. All donors and recipients were ABO compatible, and all recipients had a negative donor T cell cross-match. All patients were followed by the transplant program up to the point of graft loss or death, and there was no loss to follow-up during the period of observation. Clinical, therapeutic, and histologic data were recorded prospectively in the provincial electronic database, and detailed chart review was conducted for any patients for whom discrepancies existed to ensure concordance with the archived data.
Treatment
For purposes of this analysis, patients were divided into three treatment eras. From 1990 to 1995, immunosuppression normally consisted of cyclosporine, azathioprine, and prednisone. During 1996 to 2000, immunosuppression was changed to cyclosporine or tacrolimus, MMF for the first transplant year then switched to azathioprine, and prednisone. From January 2001, routine immunosuppression consisted of a nondepleting anti-CD25 antibody (basiliximab, 20 mg on days 0 and 4), with tacrolimus or cyclosporine, MMF, and prednisone minimization. Acute rejection episodes were diagnosed using standard clinical and laboratory criteria and were confirmed by graft biopsy. Acute rejection episodes were treated with intravenous pulse methylprednisolone, and steroid-resistant episodes then received either muromonab-CD3 or antithymocyte globulin. Delayed graft function was defined as a delay in the fall of serum creatinine with the need for hemodialysis within the first week after transplantation. Graft loss was defined as the commencement of permanent dialysis, retransplantation, or death.
Histopathology
Renal histopathology was provided by a single expert team in the Department of Pathology and Laboratory Medicine at the University of British Columbia. Biopsy of the native kidney was performed routinely for all patients with progressive renal disease of uncertain cause, unless impossible for reasons of medical risk or patient compliance. Biopsy of the renal allograft was performed routinely after renal transplantation in the presence of an increase in the serum creatinine level of 20% over baseline, particularly accompanied by persistent microscopic hematuria or proteinuria. All biopsy specimens were examined by light microscopy, with immunofluorescence and electron microscopy, unless precluded by inadequate tissue, and reviewed routinely with the clinical team in conference. Recurrent disease was differentiated from de novo disease by evaluating the native kidney biopsy specimen when available.
Statistical Analysis
Categorical variables were summarized using frequency and percentages; continuous variables were summarized using the median and first and third quartiles (Q1, Q3), unless otherwise indicated. Categorical demographic, clinical, biologic, and histologic variables were compared by Fisher exact test; continuous variables were compared by nonparametric tests (Wilcoxon or Kruskal-Wallis). Kaplan-Meier estimates were used for actuarial survival analysis, log-rank tests were used to compare the probability of PTGN according to original renal disease and treatment era, and the probability of graft failure was censored for death according to original renal disease. Multiple risk factors for PTGN were explored using Cox proportional hazard models. Covariates included previous GN; number of previous transplants; immunosuppression; living or deceased donor; number of the HLA mismatches; and donor and recipient age, gender, and race. Time-dependent Cox models examined the influence of PTGN on graft failure censored for death or patient survival. P values were two-sided, and P ≤ 0.05 was considered to indicate statistical significance. Analyses were performed using SAS 9.13 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Acknowledgments
W.C. is a member of the Division of Nephrology, Department of Medicine, Bhumibol Adulyadej Hospital, Bangkok, Thailand, and was supported by a fellowship from the Kidney Foundation of Thailand.
We are indebted to the members of the Genome Canada Biomarkers in Transplantation Group, including Dr. Bruce McManus and Dr. Rob McMaster for contribution to and critical review of the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Kirk A: Induction immunosuppression. Transplantation 82: 593–602, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Yang H: Maintenance immunosuppression regimens: Conversion, minimization, withdrawal, and avoidance. Am J Kidney Dis 74: S37–S51, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Gaston R: Current and evolving immunosuppressive regimens in kidney transplantation. Am J Kidney Dis 47: S3–S21, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Zand M: Immunosuppression and immune monitoring after renal transplantation. Semin Dial 18: 511–519, 2005 [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System: Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
- 6.Australia and New Zealand Dialysis and Transplant Registry: ANZDATA Registry Report 2006, Adelaide, South Australia, Australia and New Zealand Dialysis and Transplant Registry, 2006
- 7.Canadian Organ Replacement Register: Treatment of End-Sage Organ Failure in Canada 1995 to 2004, Ottawa, Ontario, Canada, Canadian Institute for Health Information, 2006
- 8.Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, Raftery J, Taylor R: A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children. Health Technol Assess 10: 1–157, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Winkelmayer W, Weinstein M, Mittleman M, Glynn R, Pliskin J: Health economic evaluations: The special case of end-stage renal disease treatment. Med Decis Making 22: 417–430, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Parasuraman R, Yee J, Karthikeyan V, del Busto R: Infectious complications in renal transplant recipients. Adv Chronic Kidney Dis 13: 280–294, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk P: Renal replacement therapy in Europe. The results of a collaborative effort by the ERA-EDTA and six national or regional registries. Nephrol Dial Transplant 16: 1120–1129, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Briganti E, Russ G, McNeil J, Atkins R, Chadban S: Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 347: 103–109, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Hume D, Merrill J, Miller B, Thorn G: Experiences with renal homotransplantation in the human: Report of nine cases. J Clin Invest 34: 327–382, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron J, Turner D: Recurrent glomerulonephritis in allografted kidneys. Clin Nephrol 7: 47–54, 1977 [PubMed] [Google Scholar]
- 15.Hamburger J, Crosnier J, Noel L: Recurrent glomerulonephritis after renal transplantation. Annu Rev Med 29: 67–72, 1978 [DOI] [PubMed] [Google Scholar]
- 16.Morzycka M, Croker B, Siegler H, Tisher C: Evaluation of recurrent glomerulonephritis in kidney allografts. Am J Med 72: 588–598, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Schwarz A, Krause P, Offermann G, Keller F: Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine. Am J Kidney Dis 17: 524–531, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Mathew T: Recurrence of disease following renal transplantation. Am J Kidney Dis 12: 85–96, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Neumayer H, Kienbaum M, Graf S, Schreiber M, Mann J, Luft F: Prevalence and long-term outcome of glomerulonephritis in renal allografts. Am J Kidney Dis 22: 320–325, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Hariharan S, Adams M, Brennan D, Davis C, First M, Johnson C, Ouseph R, Peddi V, Pelz C, Roza A, Vincenti F, George V: Recurrent and de novo glomerular disease after renal transplantation: A report from Renal Allograft Disease Registry (RADR). Transplantation 68: 635–641, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Hariharan S, Peddi V, Savin V, Johnson C, First M, Roza A, Adams M: Recurrent and de novo renal diseases after renal transplantation: A report from the renal allograft disease registry. Am J Kidney Dis 31: 928–931, 1998 [DOI] [PubMed] [Google Scholar]
- 22.O'Meara Y, Green A, Carmody M: Recurrent glomerulonephritis in renal transplants: Fourteen years’ experience. Nephrol Dial Transplant 4: 730–734, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Andresdottir M, Hoitsma A, Assmann K, Koene R, Wetzels J: The impact of recurrent glomerulonephritis on graft survival in recipients of human histocompatibility leucocyte antigen-identical living related donor grafts. Transplantation 68: 623–627, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Kotanko P, Pusey C, Levy J: Recurrent glomerulonephritis following renal transplantation. Transplantation 63: 1045–1052, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Briggs J, Jones E: Recurrence of glomerulonephritis following renal transplantation. Scientific Advisory Board of the ERA-EDTA Registry. European Renal Association-European Dialysis and Transplant Association. Nephrol Dial Transplant 14: 564–565, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Odum J, Peh C, Clarkson A: Recurrent mesangial IgA nephritis following renal transplantation. Nephrol Dial Transplant 9: 309–312, 1994 [PubMed] [Google Scholar]
- 27.Chadban S: Glomerulonephritis recurrence in the renal graft. J Am Soc Nephrol 12: 394–402, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Couser W: Recurrent glomerulonephritis in the renal allograft: An update of selected areas. Exp Clin Transplant 3: 283–288, 2005 [PubMed] [Google Scholar]
- 29.Floege J: Recurrent glomerulonephritis following renal transplantation: An update. Nephrol Dial Transplant 18: 1260–1265, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Hariharan S, Johnson C, Bresnahan B, Taranto S, McIntosh M, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Choy B, Chan T, Lai K: Recurrent glomerulonephritis after kidney transplantation. Am J Transplant 6: 2535–2542, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hariharan S: Long-term kidney transplant survival. Am J Kidney Dis 38: S44–S50, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Odorico J, Knechtle S, Rayhill SC: The influence of native nephrectomy on the incidence of recurrent disease following renal transplantation for primary glomerulonephritis. Transplantation 61: 228–234, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Matas A: Recurrent disease after kidney transplantation: It is time to unite to address this problem! Am J Transplant 6: 2527–2528, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Andresdottir M, Ajubi N, Croockewit S, Assmann K, Hibrands L, Wetzels J: Recurrent focal glomerulosclerosis: Natural course and treatment with plasma exchange. Nephrol Dial Transplant 14: 2650–2656, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Glassock R, Feldman D, Reynolds E, Dammin GJ, Merrill JP: Human renal isografts: A clinical and pathologic analysis. Medicine (Baltimore) 47: 411–454, 1968 [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim H, Rogers T, Casingal V: Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation 81: 214–219, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Pardon A, Audard V, Caillard S, Moulin B, Desvaux D, Bentaarit B, Remy P, Sahali D, Roudot-Thoraval F, Lang P, Grimbert P: Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplant 21: 1053–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Daskalakis N, Winn M: Focal and segmental glomerulosclerosis: Varying biologic mechanisms underlie a final histopathologic end point. Semin Nephrol 26: 89–94, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Obeidová H, Merta M, Reiterová J, Maixnerová D, Stekrová J, Rysavá R, Tesar V: Genetic basis of nephrotic syndrome: Review. Prague Med Rep 107: 5–16, 2006 [PubMed] [Google Scholar]
- 41.Ghiggeri G, Aucella F, Caridi G, Bisceglia L, Ghio L, Gigante M, Perfumo F, Carraro M, Gesualdo L: Posttransplant recurrence of proteinuria in a case of focal segmental glomerulosclerosis associated with WT1 mutation. Am J Transplant 6: 2208–2211, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Löwik M, Groenen P, Pronk I, Lilien M, Goldschmeding R, Dijkman H, Levtchenko E, Monnens L, van den Heuvel L: Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int 72: 1198–1203, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Plumb T, Greenberg A, Smith S, Butterly D, Pham T, Fields T, Howell D: Postinfectious glomerulonephritis in renal allograft recipients. Transplantation 82: 1224–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Pillet A, Guitard J, Mehrenberger M, Kamar N, Orfila C, Ribes D, Modesto A, Rostaing L: An unusual cause of acute renal failure in a kidney transplant recipient: Salmonella enteritidis post-infectious glomerulonephritis. Clin Nephrol 67: 321–324, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Wenderfer S, Swinford R, Mauiyyedi S, Witte D, Braun M: Cytomegalovirus and recurrent idiopathic membranoproliferative glomerulonephritis type 1: Cause or consequence? Transplantation 83: 523–524, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Morales J: Hepatitis C virus infection and renal disease after renal transplantation. Transplant Proc 36: 760–762, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Egbuna O, Pavlakis M, Stillman I: Acute crescentic glomerulonephritis in a renal allograft: An unusual complication of fungal infection. Am J Kidney Dis 50: 468–470, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ozdemir B, Ozdemir F, Sezer S, Colak T, Haberal M: De novo glomerulonephritis in renal allografts with hepatitis C virus infection. Transplant Proc 38: 492–495, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Cruzado J, Carrera M, Torras J, Grinyó J: Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant 1: 171–178, 2001 [PubMed] [Google Scholar]
- 50.Berthoux P, Laurent B, Cecillon S, Berthoux F: Membranoproliferative glomerulonephritis with subendothelial deposits (type 1) associated with hepatitis G virus infection in a renal transplant recipient. Am J Nephrol 19: 513–518, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Höcker B, Knüppel T, Waldherr R, Schaefer F, Weber S, Tönshoff B: Recurrence of proteinuria 10 years post-transplant in NPHS2-associated focal segmental glomerulosclerosis after conversion from cyclosporin A to sirolimus. Pediatr Nephrol 21: 1476–1479, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Letavernier E, Bruneval P, Mandet C, Van Huyen J, Péraldi M, Helal I, Noël L, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Little M, Dupont P, Campbell E, Dorman A, Walshe J: Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int 69: 504–511, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Swaminathan S, Lager D, Qian X, Stegall M, Larson T, Griffin M: Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant 21: 2607–2614, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Gossmann J, Scheuermann E, Porubsky S, Kachel H, Geiger H, Hauser I: Abrogation of nephrotic proteinuria by rituximab treatment in a renal transplant patient with relapsed focal segmental glomerulosclerosis. Transpl Int 20: 558–562, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Gallon L, Chhabra D: Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant 6: 3017–3021, 2006 [DOI] [PubMed] [Google Scholar]