Abstract
It is unknown whether screening for albuminuria in the general population identifies individuals at increased risk for renal replacement therapy (RRT) or accelerated loss of renal function. Here, in a general population-based cohort of 40,854 individuals aged 28 to 75 yr, we collected a first morning void for measurement of urinary albumin. In a subset of 6879 individuals, we measured 24-h urinary albumin excretion and estimated GFR at baseline and during 6 yr of follow-up. Linkage with the national RRT registry identified 45 individuals who started RRT during 9 yr of follow-up. The quantity of albuminuria was associated with increased renal risk: the higher the level of albuminuria, the higher the risk of need for renal replacement therapy and the more rapid renal function decline. A urinary albumin concentration of ≥20 mg/L identified individuals who started RRT during follow-up with 58% sensitivity and 92% specificity. Of the identified individuals, 39% were previously unknown to have impaired renal function, and 50% were not being medically treated. Restricting screening to high-risk groups (e.g., known hypertension, diabetes, cardiovascular disease [CVD], older age) reduced the sensitivity of the test only marginally but failed to identify 45% of individuals with micro- and macroalbuminuria. In conclusion, individuals with elevated levels of urinary albumin are at increased risk for RRT and accelerated loss of renal function. Screening for albuminuria identifies patients at increased risk for progressive renal disease, 40 to 50% of whom were previously undiagnosed or untreated.
Worldwide, the incidence of ESRD is increasing, resulting in an increased need for long-term renal replacement therapy (RRT).1,2 Timely start of renoprotective therapy, which consists predominantly of lowering BP and reducing albuminuria or proteinuria,3 may slow progression of chronic kidney disease (CKD) to ESRD, thereby preventing the need for RRT. Because many individuals are unaware of having CKD, screening may be necessary. Moreover, identifying individuals with CKD is necessary for the purpose of treating the associated health problems associated with CKD (e.g., increased risk for CVD,4,5 anemia, and renal osteodystrophy6).
Different screening strategies for detection of CKD have been proposed.7 Some advocate targeted screening of populations that are at increased risk for CKD, such as patients known with hypertension or diabetes.8,9 Others have suggested screening in the general population.10 It is not yet clear how many patients may benefit from screening because they have unknown CKD and/or are untreated before screening.11 Elevated levels of albuminuria have been shown to predict renal failure in patients with diabetes12 and de novo renal function impairment in the general population13 and might be a stronger predictor of ESRD than low estimated GFR (eGFR).14 This study was designed first to answer the question of whether albuminuria is associated with increased risk for need for RRT and accelerated renal function decline in the general population and second to evaluate measurement of albuminuria as a screening test to identify individuals at increased renal risk in the general population and in subpopulations at increased risk for CKD.6
RESULTS
In the overall Groningen population of individuals who were aged 28 to 75 yr and were invited for the baseline screening in 1997 (n = 85,421), 100 patients started RRT during 9 yr of follow-up. Of the individuals who actually participated in screening (n = 40,854), 45 started RRT during follow-up. The characteristics of the overall population and the individuals who started RRT are listed in Table 1, with those who participated in screening and those who did not participate listed separately. It seemed that screening participants were slightly older and more often female compared with nonparticipants. Of the individuals who started RRT (n = 100), screening participants had significantly longer time between screening and start of RRT and less often diabetes compared with nonparticipants. When comparing the characteristics of screening participants who started RRT with those who did not start RRT, those who started RRT were older (P < 0.001) and were more often known to have hypertension (P < 0.001), insulin-dependent diabetes (P < 0.001), CVD (P < 0.001), and a urine albumin concentration (UAC) >20 mg/L (P < 0.001).
Table 1.
Baseline characteristics of the general population and incident RRT patientsa
| Characteristic | General Population
|
Incident RRT Patients
|
||||
|---|---|---|---|---|---|---|
| Screened (n = 40,854) | Not Screened (n = 44,565) | Pb | Screened (n = 45) | Not Screened (n = 55) | Pc | |
| Age (yr; mean ± SD) | 49 ± 13 | 44 ± 12 | <0.001 | 60 ± 10 | 57 ± 10 | 0.170 |
| Female (%) | 54 | 46 | <0.001 | 58 | 51 | 0.493 |
| Time from screening to dialysis (mo; mean ± SD) | – | – | – | 65 ± 30 | 42 ± 27 | <0.001 |
| Not known to have renal disease (%) | – | – | – | 51 | 36 | 0.138 |
| Not treated with ACEI/ARB (%) | – | – | – | 65 | 53 | 0.275 |
| Cause of ESRD (%) | – | – | – | |||
| glomerulonephritis | – | – | – | 13 | 4 | 0.075 |
| pyelonephritis/interstitial nephritis | – | – | – | 4 | 9 | 0.365 |
| cystic kidney disease and other congenital diseases | – | – | – | 18 | 9 | 0.199 |
| renal vascular disease | – | – | – | 24 | 33 | 0.364 |
| diabetes | – | – | – | 11 | 36 | 0.004 |
| other multisystem diseases | – | – | – | 18 | 6 | 0.050 |
| unknown/other | – | – | – | 11 | 4 | 0.145 |
| Known hypertension (%) | 11 | – | – | 67 | 57 | 0.346 |
| Known insulin-dependent diabetes (%) | 1 | – | – | 7 | 22 | 0.035 |
| Known CVD history (%) | 4 | – | – | 20 | 20 | 1.000 |
| UAC (mg/L; median [IQR]) | 6 (4 to 10) | – | – | 54 (5 to 726) | – | – |
| UAC (%) | ||||||
| normoalbuminuria (<10 mg/L) | 75.5 | – | – | 33.3 | – | – |
| high normoalbuminuria (10 to 20 mg/L) | 16.5 | – | – | 8.9 | – | – |
| microalbuminuria (20 to 100 mg/L) | 6.5 | – | – | 8.9 | – | – |
| microalbuminuria (100 to 200 mg/L) | 0.6 | – | – | 13.3 | – | – |
| macroalbuminuria (>200 mg/L) | 0.7 | – | – | 35.6 | – | – |
IQR, interquartile range.
Differences between individuals from the general population comparing those who participated in screening with those who had not.
Difference between RRT patients who participated in screening and those who had not.
Albuminuria as a Risk Factor for RRT
We evaluated the risk for developing ESRD and the consequent need for RRT by Cox proportional hazard analysis per UAC category. We showed that a UAC ≥20 mg/L was a significant risk factor for start of RRT during follow-up, also after adjustment for age and gender; however, the risk for the “lower microalbuminuria category” (UAC 20 to 100 mg/L) was relatively small (hazard ratio [HR] 3.0; 95% confidence interval [CI] 1.0 to 9.0) compared with the increased risk for RRT in the “high microalbuminuria” (UAC 100 to 200 mg/L) and the macroalbuminuria category (UAC >200 mg/L) with HRs of 47 (95% CI 18 to 122) and 120 (95% CI 58 to 246), respectively. After exclusion of those with known diabetes, HRs (age- and gender-adjusted) for start of RRT (n = 38) were 2.4 (95% CI 0.7 to 8.3) for UAC 20 to 100 mg/L, 44 (95% CI 15 to 123) for UAC 100 to 200 mg/L, and 112 (95% CI 51 to 247) for UAC >200 mg/L. For comparison, age- and gender-adjusted HRs of other risk factors for start of RRT were 10.7 (95% CI 5.3 to 21.5) for known hypertension, 5.1 (95% CI 1.6 to 16.6) for known insulin-dependent diabetes, and 3.4 (95% CI 1.5 to 7.6) for known CVD.
We established curves for RRT-free survival using Kaplan-Meier graphs. Figure 1 shows the RRT-free survival for the five UAC categories. It demonstrates graphically that beyond UAC ≥100 mg/L, the risk for need for RRT increased considerably. These survival curves were censored for death. During the follow-up period of this study, the cumulative incidence of total mortality per UAC category was 5.0, 6.3, 12.5, 22.9, and 28.7% for the categories UAC <10, 10 to 20, 20 to 100, 100 to 200, and >200 mg/L, respectively, with those who died after starting RRT not accounted for in these numbers. The cumulative incidence of cardiovascular mortality was 1.3, 1.6, 4.1, 6.9, and 11.3%, respectively, for the UAC categories, whereas the cumulative incidence of fatal and nonfatal cardiovascular events during follow-up was 5.4, 6.4, 12.3, 19.2, and 19.5%, respectively. The percentage of patients who started RRT, per UAC category, was 0.05, 0.06, 0.15, 2.45, and 5.67, respectively.
Figure 1.
RRT-free survival per UAC category.
We evaluated possible differences between men and women in albuminuria as a risk factor for start of RRT. Gender seemed not to be a significant risk factor for RRT in this population; neither did the relation between the level of albuminuria and the need for RRT differ between men and women.
Albuminuria as a Screening Test for RRT
Of the 45 RRT patients who had a UAC measurement available, 26 (58%) had a UAC of ≥20 mg/L and could therefore be identified by screening for microalbuminuria. Characteristics of patients with normoalbuminuria versus those with UAC ≥20 mg/L are shown in Table 2. Patients with elevated albuminuria were more often known to have renal disease, more often treated with angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEIs/ARBs), and had more comorbidities. Still, 39% of patients with UAC ≥20 mg/L were not known to have renal disease at the time of screening, and 50% of these patients were not treated with a renin-angiotensin system blocker. The lower the albuminuria level, the more individuals were not known to have renal disease at time of screening: 75% of individuals with UAC 20 to 100 were not known to have renal disease, compared with 50% of individuals with UAC 100 to 200 and 25% with UAC >200 mg/L.
Table 2.
Incident RRT patients subdivided according to albuminuria levela
| Parameter | Normoalbuminuria (UAC <20 mg/L; n = 19) | Elevated Albuminuria (UAC ≥20 mg/L; n = 26) |
|---|---|---|
| Age (yr; mean ± SD) | 60 ± 9 | 60 ± 10 |
| Female (%) | 69 | 50 |
| Time from screening to dialysis (mo; mean ± SD) | 71 ± 30 | 61 ± 30 |
| No history of renal disease at time of screening (%) | 68 | 39 |
| Not treated with ACEI/ARB at time of screening (%) | 88 | 50 |
| Known hypertension (%) | 53 | 77 |
| Known insulin-dependent diabetes (%) | 0 | 12 |
| Known CVD history (%) | 11 | 27 |
| Cause of ESRD (%) | ||
| glomerulonephritis | 11 | 15 |
| pyelonephritis/TIN | 11 | 0 |
| cystic kidney disease and other congenital disease | 21 | 15 |
| renal vascular disease | 16 | 31 |
| diabetes | 0 | 19 |
| other multisystem diseases | 26 | 12 |
| unknown and other | 16 | 8 |
| UAC (mg/L; median [IQR]) | 5 (4 to 7) | 506 (140 to 1040) |
TIN, tubulointerstitial nephritis.
The prevalence of albuminuria and the incidence of RRT per UAC category are listed in Table 3 for individuals with a CVD history, known hypertension or diabetes, and/or age >55 yr. The prevalence of elevated albuminuria (UAC ≥20 mg/L) was highest among individuals with a history of CVD, namely 17% ([175 + 33 + 42]/1475; Table 3); however, these individuals represented only 8% of the total number of individuals with elevated albuminuria ([175 + 33 + 42]/3200; Table 3) and only 16% of the total number of individuals who started RRT ([2 + 2 + 3]/45). Screening all individuals with at least one of the risk factors (n = 15257) would identify 55% of those with UAC ≥20 mg/L and 24 of the 26 RRT events that could be identified by screening for UAC ≥20 mg/L.
Table 3.
Prevalence of various albuminuria classes and corresponding incidence of RRT in the general as well as in high-risk populationsa
| UAC (mg/L) | n | % | RRT (n) | IR |
|---|---|---|---|---|
| General population | 40,854 | 45 | ||
| <10 | 30,888 | 75.6 | 15 | 0.1 |
| 10 to 20 | 6766 | 16.6 | 4 | 0.1 |
| 20 to 100 | 2673 | 6.5 | 4 | 0.2 |
| 100 to 200 | 245 | 0.6 | 6 | 3.2 |
| >200 | 282 | 0.7 | 16 | 8.0 |
| CVD history | 1475 | 8 | ||
| <10 | 996 | 67.5 | 1 | 0.1 |
| 10 to 20 | 229 | 15.5 | 0 | 0.0 |
| 20 to 100 | 175 | 11.9 | 2 | 1.6 |
| 100 to 200 | 33 | 2.2 | 2 | 9.8 |
| >200 | 42 | 2.8 | 3 | 12.2 |
| No CVD history | 39,379 | 37 | ||
| <10 | 29,892 | 75.9 | 14 | 0.1 |
| 10 to 20 | 6537 | 16.6 | 4 | 0.1 |
| 20 to 100 | 2498 | 6.3 | 2 | 0.1 |
| 100 to 200 | 212 | 0.5 | 4 | 2.4 |
| >200 | 240 | 0.6 | 13 | 7.4 |
| With known hypertension/diabetes | 5083 | 31 | ||
| <10 | 3484 | 68.5 | 9 | 0.3 |
| 10 to 20 | 831 | 16.3 | 2 | 0.3 |
| 20 to 100 | 565 | 11.1 | 3 | 0.7 |
| 100 to 200 | 82 | 1.6 | 3 | 5.1 |
| >200 | 121 | 2.4 | 14 | 18.1 |
| Without known hypertension/diabetes | 35,771 | 14 | ||
| <10 | 27,404 | 76.6 | 6 | 0.0 |
| 10 to 20 | 5935 | 16.6 | 2 | 0.0 |
| 20 to 100 | 2108 | 5.9 | 1 | 0.1 |
| 100 to 200 | 163 | 0.5 | 3 | 2.3 |
| >200 | 161 | 0.5 | 2 | 1.6 |
| Age >55 | 13,616 | 33 | ||
| <10 | 10,133 | 74.4 | 12 | 0.1 |
| 10 to 20 | 1969 | 14.5 | 1 | 0.1 |
| 20 to 100 | 1206 | 8.9 | 4 | 0.4 |
| 100 to 200 | 138 | 1.0 | 4 | 4.1 |
| >200 | 170 | 1.2 | 12 | 10.9 |
| Age ≤55 | 27,238 | 12 | ||
| <10 | 20,755 | 76.2 | 3 | 0.0 |
| 10 to 20 | 4797 | 17.6 | 3 | 0.1 |
| 20 to 100 | 1467 | 5.4 | 0 | 0.0 |
| 100 to 200 | 107 | 0.4 | 2 | 2.2 |
| >200 | 112 | 0.4 | 4 | 4.4 |
| At least one of above risk factors | 15,257 | 39 | ||
| <10 | 11,284 | 74.0 | 13 | 0.1 |
| 10 to 20 | 2251 | 14.8 | 2 | 0.1 |
| 20 to 100 | 1369 | 9.0 | 4 | 0.4 |
| 100 to 200 | 156 | 1.0 | 5 | 4.5 |
| >200 | 197 | 1.3 | 15 | 11.7 |
| No risk factors | 25,597 | 6 | ||
| <10 | 19,604 | 76.6 | 2 | 0.0 |
| 10 to 20 | 4515 | 17.6 | 2 | 0.1 |
| 20 to 100 | 1304 | 5.1 | 0 | 0.0 |
| 100 to 200 | 89 | 0.3 | 1 | 1.3 |
| >200 | 85 | 0.3 | 1 | 1.4 |
IR, incidence rate (events per 1000 person-years).
We calculated screening test characteristics for the general population, as well as for high-risk subgroups (Table 4). Because of the low prevalence of RRT, specificity is high at almost every cutoff point and is even higher when the screening test takes place in individuals with CKD risk factors. A negative test result adds little information to what was known before the test, as is reflected by the likelihood ratio of a negative test. In contrast, a positive test result beyond UAC ≥20 has a high positive likelihood ratio and adds significant information, especially in the high-risk groups. For example, an individual with a history of hypertension or diabetes and a UAC ≥20 mg/L has 23 times the previous odds to start RRT during follow-up; for a UAC >200 this figure is 115 (Table 4).
Table 4.
Screening test characteristics for various cutoff points of UAC in a single first morning void urine to identify individuals who started RRT during follow-upa
| UAC (mg/L) | Test Characteristics UAC
|
|||||
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR− | |
| General population | ||||||
| >10 | 67 | 76 | 0.3 | 99.9 | 2.74 | 0.44 |
| >20 | 58 | 92 | 0.8 | 99.9 | 7.43 | 0.46 |
| >100 | 49 | 99 | 4.2 | 99.9 | 39.51 | 0.52 |
| >200 | 36 | 99 | 5.7 | 99.9 | 54.55 | 0.65 |
| CVD history | ||||||
| >10 | 16 | 99 | 1.5 | 99.9 | 13.5 | 0.85 |
| >20 | 16 | 99 | 2.8 | 99.9 | 26.1 | 0.85 |
| >100 | 11 | 99 | 6.7 | 99.9 | 64.8 | 0.89 |
| >200 | 7 | 99 | 7.1 | 99.8 | 69.8 | 0.93 |
| With known hypertension/diabetes | ||||||
| >10 | 49 | 96 | 1.4 | 99.9 | 12.7 | 0.53 |
| >20 | 44 | 98 | 2.6 | 99.9 | 24.3 | 0.57 |
| >100 | 38 | 99 | 8.4 | 99.9 | 82.9 | 0.63 |
| >200 | 31 | 99 | 11.6 | 99.9 | 118.7 | 0.69 |
| Age >55 yr | ||||||
| >10 | 47 | 92 | 0.6 | 99.9 | 5.5 | 0.58 |
| >20 | 44 | 96 | 1.3 | 99.9 | 12.1 | 0.58 |
| >100 | 36 | 99 | 5.2 | 99.9 | 46.7 | 0.65 |
| >200 | 27 | 99 | 7.1 | 99.9 | 68.9 | 0.74 |
| At least one of above risk factors | ||||||
| >10 | 58 | 90 | 0.7 | 99.9 | 6.0 | 0.47 |
| >20 | 53 | 96 | 1.4 | 99.9 | 12.8 | 0.49 |
| >100 | 44 | 99 | 5.7 | 99.9 | 54.5 | 0.56 |
| >200 | 33 | 99 | 7.6 | 99.9 | 74.7 | 0.67 |
LR+, likelihood ratio of a positive test; LR−, likelihood ratio of a negative test; NPV, negative predictive value; PPV, positive predictive value.
Whereas specificity increased in the high-risk population approach, sensitivity decreased, because all individuals without the risk factor that defines that high-risk subgroup were left undetected. For each of the high-risk groups, sensitivity was <50% at all UAC cutoff points, except for the group in which all risk factors were combined. Because specificity was high in high-risk groups, the cutoff point defining abnormal albuminuria could be lower in these high-risk groups in comparison with the situation in the general population, in which this cutoff point should probably be higher because of the lower specificity.
Albuminuria and the Rate of Renal Function Decline
We evaluated the rate of decline in eGFR during follow-up (i.e., the slope of a linear regression line between the serial measurements of eGFR) per urinary albumin excretion (UAE) category. The average rate of decline in eGFR was −0.45 ± 1.6 ml/min per 1.73 m2/yr. Figure 2 shows the mean slopes for every UAE category with CIs, unadjusted and adjusted for age and gender. Figure 2 shows there was a gradual relation between UAE and rate of decline in eGFR; those with higher levels of UAE had a more rapid decline in eGFR, especially beyond a UAE ≥150 mg/24 h. The average rate of renal function decline in the category UAE >300 mg/24 h was four times as rapid as the average rate of eGFR decline for UAE <15 mg/24 h. Table 5 shows slope and baseline age and eGFR per UAE category for those with and without risk factors for CKD. It seems that those with CKD risk factors and elevated levels of UAE had a more rapid decline of eGFR than those without risk factors. Of note, approximately 40% of the individuals with micro- and macroalbuminuria had no CKD risk factors (Tables 3 and 5) and would therefore not have been identified if only high-risk populations were to be subjected to screening. These individuals had a rate of renal function loss up to three times as fast as those with UAE <15 mg/24 h and were relatively young and had well-preserved renal function at baseline. Also, the cumulative incidence of cardiovascular morbidity and mortality was increased beyond UAC ≥20, as was described already.
Figure 2.
UAE versus mean rate of eGFR decline. *P < 0.05, **P < 0.01, and ***P < 0.001 versus UAE <15 mg/24 h.
Table 5.
Distribution of UAE categories and slope (age and gender adjusted) in PREVEND cohort
| UAE (mg/L) | n (%) | Age (SD) | eGFR (SD) | Slope (SD) |
|---|---|---|---|---|
| PREVEND cohort | 6879 | |||
| <15 | 4990 (72.5) | 47 (12) | 81 (13) | −0.39 (1.60) |
| 15 to 30 | 977 (14.2) | 51 (12) | 81 (15) | −0.53 (1.60) |
| 30 to 150 | 748 (10.9) | 55 (12) | 78 (16) | −0.59 (1.70) |
| 150 to 300 | 77 (1.1) | 57 (10) | 75 (15) | −0.99 (1.60) |
| >300 | 87 (1.3) | 57 (12) | 68 (19) | −1.68 (1.60) |
| CVD history | 215 | |||
| <15 | 98 (45.6) | 59 (10) | 77 (14) | −0.45 (2.93) |
| 15 to 30 | 44 (20.5) | 61 (8) | 75 (13) | −0.78 (2.79) |
| 30 to 150 | 54 (25.1) | 63 (8) | 69 (19) | −0.76 (2.98) |
| 150 to 300 | 9 (4.2) | 61 (9) | 72 (14) | −1.97 (2.38) |
| >300 | 10 (4.7) | 63 (7) | 67 (18) | −3.37 (2.47) |
| No CVD history | 6664 | |||
| <15 | 4892 (73.4) | 47 (13) | 81 (13) | −0.39 (1.61) |
| 15 to 30 | 933 (14.0) | 51 (12) | 82 (15) | −0.52 (1.62) |
| 30 to 150 | 694 (10.4) | 54 (12) | 79 (15) | −0.59 (1.63) |
| 150 to 300 | 68 (1.0) | 57 (10) | 76 (15) | −0.86 (1.62) |
| >300 | 77 (1.2) | 56 (13) | 68 (19) | −1.44 (1.61) |
| With known hypertension/diabetes | 774 | |||
| <15 | 395 (51.0) | 59 (9) | 74 (14) | −0.34 (2.48) |
| 15 to 30 | 165 (21.3) | 59 (10) | 76 (16) | −0.59 (2.17) |
| 30 to 150 | 166 (21.4) | 61 (9) | 72 (17) | −0.64 (2.27) |
| 150 to 300 | 18 (2.3) | 61 (9) | 71 (12) | −1.90 (1.99) |
| >300 | 30 (3.9) | 64 (6) | 60 (16) | −1.86 (2.04) |
| Without known hypertension/diabetes | 6105 | |||
| <15 | 4595 (75.3) | 46 (11) | 82 (13) | −0.38 (1.63) |
| 15 to 30 | 812 (13.3) | 50 (12) | 82 (14) | −0.50 (1.60) |
| 30 to 150 | 582 (9.5) | 53 (12) | 80 (15) | −0.55 (1.62) |
| 150 to 300 | 59 (1.0) | 56 (10) | 77 (15) | −0.68 (1.60) |
| >300 | 57 (0.9) | 53 (13) | 72 (19) | −1.47 (1.60) |
| Age >55 yr | 2053 | |||
| <15 | 1223 (59.7) | 64 (5) | 74 (13) | −0.26 (4.44) |
| 15 to 30 | 357 (17.4) | 64 (5) | 75 (14) | −0.59 (2.89) |
| 30 to 150 | 373 (18.2) | 65 (5) | 72 (15) | −0.62 (2.97) |
| 150 to 300 | 43 (2.1) | 65 (6) | 72 (15) | −1.18 (1.95) |
| >300 | 53 (2.6) | 65 (6) | 61 (17) | −1.85 (2.01) |
| Age <55 yr | 4842 | |||
| <15 | 3767 (78.0) | 42 (6) | 84 (13) | −0.33 (2.09) |
| 15 to 30 | 620 (12.8) | 44 (8) | 85 (14) | −0.39 (1.62) |
| 30 to 150 | 375 (8.0) | 45 (7) | 84 (15) | −0.41 (1.59) |
| 150 to 300 | 34 (0.7) | 48 (6) | 79 (14) | −0.58 (1.57) |
| >300 | 34 (0.7) | 44 (8) | 79 (16) | −1.11 (1.57) |
| At least one of above risk factors | 2334 | |||
| <15 | 1389 (59.5) | 62 (7) | 75 (13) | −0.31 (3.06) |
| 15 to 30 | 418 (17.9) | 62 (8) | 76 (15) | −0.61 (2.22) |
| 30 to 150 | 422 (18.1) | 63 (7) | 73 (15) | −0.65 (2.32) |
| 150 to 300 | 48 (2.1) | 63 (7) | 72 (14) | −1.18 (1.86) |
| >300 | 57 (2.4) | 64 (6) | 61 (18) | −1.86 (1.90) |
| No risk factors | 4545 | |||
| <15 | 3601 (79.2) | 41 (7) | 84 (13) | −0.34 (2.10) |
| 15 to 30 | 559 (12.3) | 43 (8) | 85 (13) | −0.39 (1.61) |
| 30 to 150 | 326 (7.2) | 44 (7) | 84 (14) | −0.39 (1.57) |
| 150 to 300 | 29 (0.6) | 47 (6) | 80 (15) | −0.54 (1.55) |
| >300 | 30 (0.7) | 43 (8) | 80 (14) | −1.06 (1.56) |
We evaluated possible gender differences in the association between albuminuria and the rate of renal function decline. In men, the average rate of decline in eGFR was −0.55 ml/min per 1.73 m2, whereas, in women, this was −0.36 ml/min per 1.73 m2 (P < 0.001). We found no significant interaction in multivariate regression analysis between gender and UAE for the rate of decline in eGFR.
DISCUSSION
This study demonstrates that in the general population, albuminuria is associated with renal risk: The higher the level of albuminuria, the higher the risk for need for RRT and the more rapid the rate of renal function decline. Screening for increased albuminuria levels in the general population detects more than half of the patients who started RRT during follow-up, approximately 40% of whom were previously not known to have renal disease. Restricting screening to those with known hypertension, known diabetes, CVD history, or age >55 yr would identify nearly all incident cases of RRT during follow-up; however, nearly half of the individuals with UAC ≥20 mg/L would be left undetected, but these individuals are at considerable cardiovascular and renal risk.
The first objective of this study was to investigate whether elevated albuminuria is associated with increased renal risk in the general population. Albuminuria has long been established as a risk factor for overt diabetic nephropathy15–18 and for ESRD in patients with diabetes.12 In the nondiabetic population, elevated UAE has been related to renal function abnormalities in cross-sectional studies and de novo development of renal function impairment, defined as an eGFR <60 ml/min, in longitudinal studies.13,19 Iseki et al.14 showed that dipstick-positive proteinuria, which in general corresponds with UAC >200 mg/L, predicted need for RRT during follow-up in the general population; however, to our knowledge, no previous study investigated the association between quantitatively measured albuminuria and the risk for need for RRT in the general population. In this study, albuminuria was measured by nephelometry, allowing adequate study of the predictive value of albuminuria, also in its lower range. It was shown that microalbuminuria is a risk factor for need for RRT, especially beyond UAC ≥100 mg/L. This holds true even after exclusion of individuals with known diabetes.
Our results also show that the rate of renal function decline increases continuously with increasing levels of albuminuria (Figure 2), in the lower range of albuminuria, but especially beyond UAE ≥150 mg/24 h. Accelerated renal function loss is associated with many adverse effects, of which the increased risk for cardiovascular death is probably the most detrimental.4,5,20 Data from this study are consistent with these premises, because the rate of renal function decline, the incidence of death, and the incidence of CVD were associated with baseline albuminuria.
Previous findings from the Prevention of Renal and Vascular Endstage Disease (PREVEND) study group21 and others22 have indicated that there might be a difference in the association between baseline albuminuria and rate of renal function decline during follow-up in men versus women. This study, however, shows that the risk associated with elevated albuminuria is comparable for men and women. These seemingly contradictory findings are explained by the fact that this study investigated albuminuria as the risk factor of interest, crude or with adjustment for age and gender only, whereas the other studies investigated albuminuria as one of many variables entered in a multivariable model.
The second objective of this study was to investigate whether screening for albuminuria can identify individuals at risk for need for RRT. A total of 58% of individuals who started RRT during follow-up had a UAC ≥20 mg/L at baseline (specificity 92%). Of note, to our knowledge no other study has yet published characteristics of screening tests that can identify individuals who start RRT in the general population. Our numbers can therefore not be compared with other studies. The percentage of individuals who start RRT during follow-up and are identified by screening may seem insufficient, but several considerations should be kept in mind. First, some of the individuals who started RRT during follow-up and who could not be identified by screening had causes of ESRD that are not open for preventive measures (e.g., nephrectomy in case of malignancy). Second, the numbers are based on a one-time screening for UAC. Screening at regular intervals might increase the number of patients who can be identified before they develop ESRD. Third, inherent in identifying individuals at risk for a specific disease is that not all individuals at risk will develop the disease; therefore, screening to identify patients at higher risk will never have perfect test results.
Screening for CKD is usually performed using one of two measures: Impaired eGFR and/or increased albuminuria/proteinuria. Interestingly, it was recently shown that individuals with impaired eGFR and without elevated albuminuria had only mildly increased risk for ESRD.14,23 In contrast, individuals with increased albuminuria are at risk for ESRD, irrespective of their eGFR level. These data suggest that screening for albuminuria might be more effective than screening for impaired eGFR to identify individuals at risk for developing ESRD. It is furthermore to be expected that screening for impaired eGFR will be associated with more costs than screening for increased albuminuria, because blood samples need to be drawn of large numbers of patients at medical facilities, whereas a urine sample for albuminuria measurement can be sent by post, as was done in the PREVEND study. A simpler test to measure the presence of albuminuria is a urine dipstick test. Unfortunately, in the PREVEND study, no data on dipstick analysis are available; however, Konta et al.24 were able to show that the sensitivity of urine dipstick for trace positivity or more for micro- and macroalbuminuria was only 37.1%. This suggests that by using dipsticks, 63% of individuals with micro- and macroalbuminuria will not be identified. These individuals could be at considerable risk for progressive renal disease and CVD.
Whether the benefits of screening for albuminuria to prevent ESRD are sufficient to outweigh the costs and efforts is debatable. In this study, 40,856 people had to be screened to find 3199 (7.8%) people with UAC >20 mg/L, only 26 of whom reached RRT during 9 yr of follow-up. The costs of inviting 85,421 individuals to participate in a one-time screening and to analyze 40,854 urine samples added up to a total of €282,700, as we recently published.25 RRT costs approximately €75,000 per person per year in the Netherlands. At first sight, delaying the onset of ESRD by 4 yr for one individual seems to outweigh the costs associated with screening; however, the costs of a screening program depend on more factors: Whether all patients with albuminuria will be medically treated or only those who have progressive renal disease during follow-up, the costs of medication, costs of follow-up tests, etc. It is clear that additional research is needed to answer the question of whether screening for albuminuria will be cost-effective to prevent or delay the need for RRT; however, it should be kept in mind that individuals with higher levels of albuminuria are also at increased risk for CVD.26,27 Screening for albuminuria may therefore benefit not only those at risk for renal disease but also those at risk for CVD.28 When combining these goals, screening for albuminuria and consequent treatment of those at increased risk for renal and CVD has been calculated to be cost-effective.25
Limiting screening to specific risk groups could be a different strategy to reduce the number needed to screen and thereby reduce the costs of screening. Hallan et al.9 found that 93.2% of patients with an eGFR <60 ml/min can be identified when the population to be screened is restricted to patients with known diabetes, known hypertension, or age >55 yr, reducing the number needed to screen from 21 to nine. We studied the prevalence of elevated albuminuria and the incidence of RRT in various risk groups. When only those with risk factors were screened, 1404 (45%) individuals with micro- and macroalbuminuria were left unidentified. Our data have shown that these undetected individuals are at increased risk for progressive renal (Table 5) and CVD,27 and it is questionable whether this should be ignored, especially when taking into account that such undetected individuals are relatively young and have well-preserved renal function at baseline. Considering these characteristics, timely start of reno- and cardioprotective intervention in such individuals seems especially worth the effort.
This study has a number of limitations. First, patients with progressive CKD often die before they need RRT, mostly as a result of CVD. This may lead to an underestimation of the association between albuminuria and the risk for RRT. Second, the number of patients who started RRT is relatively small because of the low incidence of ESRD. This limits the power of our study. To include more events, longer follow-up is needed. Third, in our cohort of 40,854 individuals, albuminuria was measured only once as UAC in a first morning void urine sample. The validity of this strategy for population screening was evaluated previously.29 It was shown that sensitivity and specificity in comparison with the gold standard (measurement of UAE in a 24-h urine sample) was high (85 and 85%, respectively) and comparable to measurement of the albumin:creatinine ratio. Fourth, this study was not able to answer the question of whether early identification and start of preventive treatment will result in a decrease in need for RRT or in a decrease in rate of renal function decline or associated morbidity. Future studies will have to address these questions and combine the results with formal cost-effectiveness analyses. This study should be regarded as a strong rationale to start such studies.
In conclusion, in the general population, an increased level of albuminuria is associated with increased risk for need for RRT and an increased rate of renal function loss, especially when albuminuria is ≥100 mg/L. Screening for UAC ≥20 mg/L identified the majority of individuals who started RRT during follow-up, approximately half of whom were not treated and were unaware of kidney disease at the time of screening. Restricting screening to high-risk groups would identify nearly all incident cases of RRT during follow-up but would leave nearly half of the individuals with UAC ≥20 mg/L undetected, but these individuals have been shown to be at considerable renal and cardiovascular risk. To answer the question of whether screening for albuminuria to detect (and treat) individuals at increased renal and cardiovascular risk may be (cost-)effective requires additional studies that should also take into account whether such screening can best take place in the general population or in specific subgroups. These data provide a base to start such studies.
CONCISE METHODS
Population
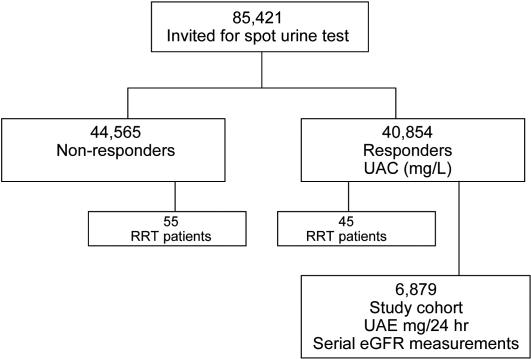
This study is part of the PREVEND study, a prospective cohort study that was designed to investigate the predictive value of albuminuria for ESRD and CVD in the general population. The study was initiated in 1997, when all 85,421 inhabitants of Groningen, Netherlands, aged 28 to 75 yr, were invited to fill out a brief questionnaire and to collect a first morning void urine sample. These were sent by post to a central laboratory for UAC measurement. A total of 40,854 individuals responded and participated in baseline screening. A study cohort was selected from the 40,854 responders on the basis of level of UAC in the spot morning urine sample to enrich the cohort for the presence of higher albuminuria. These PREVEND study cohort participants were invited to visit the study outpatient clinic every 3 yr for measurements of renal and cardiovascular risk factors and outcome (Figure 3). Further details of this study have been published elsewhere.19 The PREVEND study is approved by the medical ethics committee of our institution and conducted in accordance with the guidelines of the declaration of Helsinki.
Figure 3.
Study design.
Albuminuria as a Risk Factor for Need for RRT
Data on the 85,421 invited inhabitants of Groningen were used to evaluate the incidence of RRT during 9 yr of follow-up, until January to November 2006. RRT status and cause of ESRD were obtained from the national registry of RRT (RENINE). For this purpose, the PREVEND database was linked to the RENINE database using date of birth, gender, last name, and postal code. Incomplete matches were checked for authenticity. For survival analysis, data on mortality were received from the national Central Bureau of Statistics and the municipal registry. Death as a result of CVD was defined as International Classification of Diseases, 10th Revision code 100 through 199 as primary cause of death. RRT-free survival was established according to UAC level, with UAC divided into five clinical categories: <10, 10 to 20, 20 to 100, 100 to 200, and >200 mg/L. Information on history of hypertension, diabetes, and CVD was obtained from the baseline screening questionnaire. For the individuals who started RRT during follow-up, information on the history of these diseases, kidney disease, and the use of renoprotective medication was obtained from the patient's medical record.
Albuminuria as a Screening Test for RRT
UAC as a screening test to detect individuals at increased risk for RRT was evaluated using clinical cutoff points of UAC, both in the general population and in individuals at increased risk for CKD. For the risk groups, the screening test was considered to be positive when the risk factor was present and the UAC level was above the determined cutoff point. When the risk factor was not present, the test result was regarded as negative.
Screening test characteristics were calculated for the entire study population as well as for specific subgroups for various cutoff points of albuminuria. Sensitivity was defined as the number of individuals who had a positive test result and started RRT during follow-up (true-positive test) divided by the total number of individuals who started RRT during follow-up. Specificity was defined as the number of true-negative test results divided by the total number of individuals who did not start RRT during follow-up. The positive predictive value was defined as the number of true-positive test results divided by the total number of positive test results; the negative predictive value was defined as the number of true-negative test results divided by the total number of negative test results. The positive likelihood ratio was defined as sensitivity divided by 1 − specificity; the negative likelihood ratio was defined as 1 − sensitivity divided by specificity.
Albuminuria and the Rate of Renal Function Decline
To evaluate the relation between albuminuria and the rate of decline in eGFR per year, we obtained data from the PREVEND cohort participants. For these individuals, albuminuria was defined by the average UAE in two 24-h urine samples, collected on 2 consecutive days at baseline. Individuals who had UAE measured at their first outpatient clinic visit and had serum creatinine measured at two (n = 1110) or three (n = 5769) consecutive visits were included in this study (total n = 6879). Mean time between first and second study visit was 4.2 ± 0.4 yr and between second and third visit was 2.2 ± 0.3 yr. The rate of decline of renal function was depicted by the slope of a linear regression line, fitted between the two or three serial estimates of GFR, using the least squares principle. Rate of loss of eGFR was calculated according to UAE level, with UAE divided into five clinical categories: <15, 15 to 30, 30 to 150, 150 to 300, and >300 mg/24 h. Given an average 24-h urinary volume of approximately 1.5 L, these UAE figures correspond with the cutoff values for UAC in first morning void urine samples, as were used in the analysis of albuminuria versus risk for need for RRT.
Measurements and Definitions
UAC was determined by nephelometry (BNII; Dade Behring Diagnostic, Marburg, Germany). Plasma creatinine was determined by Kodak Ektachem dry chemistry (Eastman Kodak, Rochester, NY), an automated enzymatic method. eGFR was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) formula.30
CVD as a baseline characteristic was defined as having a history of myocardial infarction and/or a history of a cerebrovascular accident for which the individual had been admitted to a hospital, as indicated at the baseline questionnaire. Insulin-dependent diabetes and hypertension were noted when individuals received medical treatment for these conditions, whereas “known with diabetes” was assigned when the patient indicated being known to have diabetes. A patient was assigned to “known with renal disease” when an internist or nephrologist had established the diagnosis of renal disease or renal insufficiency before the patient was invited for the PREVEND screening. When a patient was treated with ACEIs or ARBs during 1997, the patient was assigned as “being treated with ACEI/ARB.”
Cardiovascular events during follow-up were obtained from the Dutch national registries of hospital discharge diagnoses and death certificates. Cardiovascular events were defined as the incidence of an acute myocardial infarction (International Classification of Diseases, 9th Revision code 410), acute and subacute ischemic heart disease (411), coronary artery bypass grafting or percutaneous transluminal coronary angioplasty, subarachnoid hemorrhage (430), intracerebral hemorrhage (431), other intracranial hemorrhage (432), occlusion or stenosis of the precerebral (433) or cerebral arteries (434), or vascular interventions (percutaneous transluminal angioplasty or bypass grafting of aorta or peripheral vessels).
Statistical Analysis
Analyses were performed using the statistical package SPSS 14.0 (SPSS, Chicago, IL). The level of significance was determined as P ≤ 0.05. Normally distributed continuous data are reported as means ± SD, whereas data with skewed distribution are given as median with interquartile range. Differences between groups were tested by t test for continuous data with a normal distribution and by the Mann-Whitney U test for skewed distributed data. Differences in prevalence or incidence were tested with a χ2 test.
Cox proportional hazards analyses were used to define the HR and 95% CI for risk for RRT. Cox proportional hazard assumptions were checked for and met. Kaplan-Meier survival analysis was used to establish survival curves for RRT-free survival. Linear regression analysis was used to compare the unadjusted and the age- and gender-adjusted rate of eGFR decline for various UAE categories. To evaluate possible gender differences regarding albuminuria as a renal risk factor, the statistical significance of the interaction term gender × albuminuria measure, with albuminuria as a continuous variable, was tested in the Cox proportional hazard model and multivariable linear regression model. The continuous variable albuminuria was log-transformed because of its skewed distribution.
DISCLOSURES
None.
Acknowledgments
We thank Dade Behring (Marburg, Germany) for supplying equipment (Behring Nephelometer II) and reagents for nephelometric measurement of urinary albumin concentration. The PREVEND study has been made possible by grants of the Dutch Kidney Foundation, for which we are grateful. The funding for this study had no role in its design, conduct, or analysis or in the decision to submit the study for publication.
An abstract of this study was presented in poster form at the annual meeting of the American Society of Nephrology; San Francisco, CA; November 2, 2007, and has been published in abstract form (J Am Soc Nephrol 18: 332A, 2007).
M.V. and R.T.G. had primary responsibility for design, gathering of data, analysis, interpretation and the report of data from the study; P.E.J. and N.H. contributed to the design and analysis of the study; F.T.C. contributed to gathering of data; and all authors assisted in the interpretation of study results, critical revision, and writing of the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Are We Ready to Screen the General Population for Microalbuminuria?” on pages 686–688.
REFERENCES
- 1.Moeller S, Gioberge S, Brown G: ESRD patients in 2001: Global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant 17: 2071–2076, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Lysaght MJ: Maintenance dialysis population dynamics: Current trends and long-term implications. J Am Soc Nephrol 13[Suppl 1]: S37–S40, 2002 [PubMed] [Google Scholar]
- 3.Adamczak M, Zeier M, Dikow R, Ritz E: Kidney and hypertension. Kidney Int Suppl 62–67, 2002 [DOI] [PubMed]
- 4.Van Biesen W, De Bacquer D, Verbeke F, Delanghe J, Lameire N, Vanholder R: The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J 28: 478–483, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD: Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int 62: 1402–1407, 2002 [DOI] [PubMed] [Google Scholar]
- 6.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 7.Bello AK, Nwankwo E, El Nahas AM: Prevention of chronic kidney disease: A global challenge. Kidney Int Suppl S11–S17, 2005 [DOI] [PubMed]
- 8.Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, King K, Klag MJ, Molony DA, Flack JM: Early detection of kidney disease in community settings: The Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 42: 22–35, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, Dekker FW: Screening strategies for chronic kidney disease in the general population: Follow-up of cross sectional health survey. BMJ 333: 1047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai E, Yamagata K, Iseki K, Iso H, Horio M, Mkino H, Hishida A, Matsuo S: Kidney disease screening program in Japan: History, outcome, and perspectives. Clin J Am Soc Nephrol 2: 1360–1366, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Clase CM: Glomerular filtration rate: Screening cannot be recommended on the basis of current knowledge. BMJ 333: 1030–1031, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld JP, Lash JP, McGill JB, Mitch WE, Remuzzi G, Shahinfar S, Snapinn SM, Toto R, Brenner BM: Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: The RENAAL study. Clin J Am Soc Nephrol 1: 761–767, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, de Zeeuw D, de Jong PE: An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl S18–S21, 2004 [DOI] [PubMed]
- 14.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Mogensen CE: Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310: 356–360, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Mogensen CE, Christensen CK: Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311: 89–93, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Mogensen CE, Keane WF, Bennett PH, Jerums G, Parving HH, Passa P, Steffes MW, Striker GE, Viberti GC: Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet 346: 1080–1084, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Basi S, Lewis JB: Microalbuminuria as a target to improve cardiovascular and renal outcomes. Am J Kidney Dis 47: 927–946, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Pinto-Sietsma SJ, Janssen WM, Hillege HL, Navis G, de Zeeuw D, de Jong PE: Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol 11: 1882–1888, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Halbesma N, Brantsma AH, Bakker S, Stolk RP, de Zeeuw D, de Jong P, Gansevoort R: Predictors of renal function decline during follow-up in the general population [Abstract]. J Am Soc Nephrol 17: 203A, 2006 [Google Scholar]
- 22.Kronborg J, Solbu M, Njolstad I, Toft I, Eriksen BO, Jenssen T: Predictors of change in estimated GFR: A population based 7-years follow-up from the Tromso study [Abstract]. J Am Soc Nephrol 18: 822A, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17: 1444–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Konta T, Hao Z, Takasaki S, Abiko H, Ishikawa M, Takahashi T, Ikeda A, Ichikawa K, Kato T, Kawata S, Kubota I: Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol 11: 51–55, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Atthobari J, Asselbergs FW, Boersma C, de Vries R, Hillege HL, van Gilst WH, Gansevoort RT, de Jong PE, de Jong-van den Berg LT, Postma MJ, PREVEND IT Study Group: Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the Prevention of Renal and Vascular Endstage Disease (PREVEND) study and the Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT). Clin Ther 28: 432–444, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 28.de Jong PE, Gansevoort RT: Prevention of chronic kidney disease: The next step forward! Nephrology (Carlton) 11: 240–244, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Gansevoort RT, Verhave JC, Hillege HL, Burgerhof JG, Bakker SJ, de Zeeuw D, de Jong PE: The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl S28–S35, 2005 [DOI] [PubMed]
- 30.Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]