Abstract
Patients with lupus membranous nephropathy (LMN) are at substantial long-term risk for morbidity and mortality associated with protracted nephrotic syndrome, including ESRD. The optimal treatment for this condition is controversial. Forty-two patients with LMN participated in a randomized, controlled trial to compare adjunctive immunosuppressive drugs with prednisone alone. Adjunctive regimens included either cyclosporine (CsA) for 11 mo or alternate-month intravenous pulse cyclophosphamide (IVCY) for six doses; the control group received alternate-day prednisone alone. Median proteinuria was 5.4 g/d (range 2.7 to 15.4 g/d). We assessed the primary outcome, time to remission of proteinuria during the 12-mo protocol, by univariate survival analysis. At 1 yr, the cumulative probability of remission was 27% with prednisone, 60% with IVCY, and 83% with CsA. Although both IVCY and CsA were more effective than prednisone in inducing remissions of proteinuria, relapse of nephrotic syndrome occurred significantly more often after completion of CsA than after IVCY. By multivariate survival analysis, treatment with prednisone and high-grade proteinuria (>5 g/d) but not race or ethnicity were independently associated with a decreased probability of remission. Adverse effects during the 12-mo protocol included insulin-requiring diabetes (one with prednisone and two with CsA), pneumonia (one with prednisone and two with CsA), and localized herpes zoster (two with IVCY). In conclusion, regimens containing CsA or IVCY are each more effective than prednisone alone in inducing remission of proteinuria among patients with LMN.
Approximately 50% of patients with systemic lupus erythematosus develop clinically evident renal disease that may be an important cause of morbidity and mortality.1,2 Therapeutic trials have focused on the treatment of proliferative lupus nephritis because of the short-term risk for end-stage renal failure observed in this population of patients. Lupus membranous nephropathy (LMN) accounts for approximately 10 to 20% of cases of lupus nephritis.3 This characteristically young female population of patients has an increased long-term risk for ESRD; on average, 20% require dialysis or renal transplantation within 10 yr of diagnosis.4–6 Furthermore, Mercadal et al.6 observed renal survival in only 50% of their patients with LMN at 20 yr.
Cardiovascular and cerebrovascular events account for approximately 25 to 50% of deaths among patients who had lupus nephritis and were followed for an extended period.7–10 Dyslipidemia, hypertension, and thrombotic diatheses place patients with lupus nephritis at increased risk for these complications.11 Ordonez et al.12 reported that patients with nephrotic syndrome had a 5.5-fold increased risk for myocardial infarction and a 2.8-fold increased risk for coronary death compared with age- and gender-matched non-nephrotic patients followed in the same health plan.
Furthermore, patients with LMN are at increased risk for thromboembolic events; the incidence was 23% in two cohorts followed long-term in Italy13 and France.6 Thrombotic events were associated with antiphospholipid antibodies in the former study and with the nephrotic syndrome during follow-up in the latter.
The potential for adverse outcomes associated with LMN has prompted a number of studies to refine the treatment of this disorder. High- and low-dosage corticosteroid regimens have been evaluated in a few retrospective studies.14,15 Although there were no significant trends in the data to support the benefits of corticosteroids, high-dosage alternate-day regimens of prednisone are widely used for 2 to 4 mo. Additional insights into potentially effective regimens for LMN have been obtained from studies of cytotoxic drugs16–19 or cyclosporine (CsA).20–22 We report the results of a prospective, randomized, controlled trial to evaluate the effects of adding alternate-month intravenous pulse cyclophosphamide (IVCY) or CsA to alternate-day prednisone in patients with LMN.
RESULTS
Patient Characteristics
Forty-two patients were enrolled in the randomized clinical study. At study entry, the median age was 40 yr (range 13 to 60 yr). There were 24 black women, three black men, eight white women, four white men, and three Hispanic women. The median duration of clinically suspected LMN before study entry was 7 mo (range 1 to 120 mo). The median GFR was 83 ml/min per 1.73 m2 body surface area (range 32 to 189 ml/min per 1.73 m2 body surface area), and the median urine protein excretion rate was 5.4 g/d (range 2.7 to 15.4 g/d). The median serum albumin was 2.8 mg/dl (range 1.3 to 3.6 mg/dl) and the median serum cholesterol was 279 mg/dl (range 173 to 631 mg/dl). Sixteen patients received stable dosages of angiotensin antagonist therapy that began at least 1 mo before baseline testing and continued during the 12-mo protocol treatment period (six in the prednisone group, six in the IVCY group, and four in the CsA group).
The distributions of demographic and clinical attributes at study entry among patients in the three treatment groups are depicted in Table 1. The proportion of white patients in the IVCY group was relatively high but did not differ significantly from the proportion of white patients in the prednisone control group (Fisher exact test, P = 0.13). The proportion of white patients was significantly higher (P = 0.02) in the IVCY group than in the CsA group. Otherwise, the distributions of these demographic and clinical characteristics were not statistically different among the treatment groups.
Table 1.
Characteristics of patients at study entry by treatment group
| Characteristics | Prednisone Group 1 | IVCY Group 2 | CsA Group 3 |
|---|---|---|---|
| Gender (female/male) | 12/3 | 12/3 | 11/1 |
| Age (yr; median [range]) | 40 (20 to 58) | 41 (17 to 60) | 34 (13 to 56) |
| Race/ethnicity (white/other)a | 3/12 | 8/7 | 1/11 |
| Duration of LMN before study entry (mo; median [range]) | 11.5 (3 to 120) | 7 (2 to 24) | 6 (1 to 16) |
| GFR (ml/min per 1.73 m2; median [range]) | 80 (32 to 112) | 80 (61 to 112) | 89 (68 to 189) |
| Proteinuria (g/d; median [range]) | 5.7 (2.8 to 10.6) | 5.0 (2.7 to 15.4) | 5.8 (2.7 to 13.8) |
| Serum albumin (g/dl; median [range]) | 2.7 (1.3 to 3.6) | 3.0 (1.6 to 3.6) | 2.5 (1.9 to 3.3) |
| Cholesterol (mg/dl; median [range]) | 297 (207 to 501) | 268 (173 to 339) | 279 (194 to 631) |
| Hematocrit (%; median [range]) | 36 (31 to 45) | 38 (33 to 45) | 36 (27 to 42) |
| Anti dsDNA antibodies (patients) | 4 | 3 | 2 |
| Low C3 complement (patients) | 1 | 1 | 1 |
| Low C4 complement (patients) | 2 | 0 | 1 |
White refers to non-Hispanic white patients, and other refers to black and Hispanic patients.
Prognostic Factors
We sought to determine whether demographic or clinical features affected the probability of remission. We examined the prognostic impacts of the variables shown in Table 2 by cumulative survival analysis using time from study entry to remission of proteinuria as the measure of outcome. By univariate analysis, baseline urinary protein excretion rate and race/ethnicity emerged as statistically significant predictors of remission (Table 2). Protein excretion rate ≤5 g/d was associated with an increased probability of remission (P = 0.003). Non-Hispanic white patients were more likely to achieve a remission (P = 0.03) than black or Hispanic individuals.
Table 2.
Prognostic significance of patient characteristics at study entry determined by univariate survival analysis
| Characteristics | No. of Patients | Probability of Remission at 1 yr (%)a | Pb |
|---|---|---|---|
| Age (yr) | |||
| <40 | 21 | 52 | 0.830 |
| ≥40 | 21 | 57 | |
| Duration of LMNc (mo) | |||
| ≤6 | 18 | 61 | 0.500 |
| >6 | 23 | 52 | |
| Race/ethnicity | |||
| non-Hispanic white | 12 | 75 | 0.030 |
| black and Hispanic | 30 | 47 | |
| Proteinuria (g/d) | |||
| ≤5 | 19 | 74 | 0.003 |
| >5 | 23 | 39 | |
| GFR (ml/min per 1.73 m2) | |||
| <70 | 10 | 40 | 0.410 |
| ≥70 | 32 | 59 | |
| Hematocrit | |||
| <34 | 10 | 50 | 0.550 |
| ≥34 | 32 | 56 | |
| Anti dsDNA antibodies | |||
| present | 9 | 44 | 0.180 |
| absent | 30 | 57 | |
| Treatmentd | |||
| prednisone alone | 15 | 27 | 0.007 |
| adjunctive IVCY or CSA | 27 | 70 | |
| Angiotensin antagoniste | |||
| yes | 16 | 50 | 0.850 |
| no | 26 | 58 |
Remission is defined as complete or partial remission of proteinuria (see the Concise Methods section).
Significance level for the Gehan test that evaluates the equality of the survival curves that were obtained for subgroups of patients identified according to the level of the prognostic variable.
Information regarding the duration of clinically suspected LMN before study entry was available for 41 of 42 patients.
Shows the probability of remission for all patients who were randomly assigned to either prednisone alone or one of the adjunctive immunosuppressive regimens of IVCY or CSA. Details of the analyses of therapeutic outcomes are shown in Figure 1.
Shows the probability of remission for 16 patients who were on stable dosages of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker that began at least 1 mo before baseline testing and continued during the 12-mo protocol treatment period compared with 26 patients who were not on concurrent angiotensin antagonist therapy.
We also examined by univariate survival analysis the prognostic impact of adding either IVCY or CsA to standard alternate-day prednisone. Patients who were randomly assigned to one of the adjunctive immunosuppressive regimens of IVCY or CsA were significantly more likely to achieve remission (P = 0.007) of proteinuria than patients who were randomly assigned to the control regimen of alternate-day prednisone alone.
The univariate survival analyses underscored the prognostic significance of baseline urinary protein excretion rate, race/ethnicity, and the immunosuppressive treatment regimen (Table 2). We used multivariate survival analysis to determine whether both protein excretion rate and race/ethnicity were independent baseline predictors of remission (Table 3, model 1). In this statistical model, protein excretion rate was a strong independent predictor of remission. In the context of proteinuria, race/ethnicity did not contribute significantly to the prediction of remission. The prognostic information provided by protein excretion rate incorporated and superseded that provided by race/ethnicity in this study. Consequently, deletion of race/ethnicity from the statistical model did not significantly degrade the ability of the model to predict remission.
Table 3.
Statistical models that predict time to remission
| Survival Model | Prognostic Factorsa
|
||
|---|---|---|---|
| Proteinuria (g/d) | Race and Ethnicity | Adjunctive Immunosuppressive Treatmentd | |
| 1. Clinical and demographic predictors identified by univariate survival analysis | |||
| coefficientb | 0.980 | −0.671 | |
| Pc | 0.010 | 0.100 | |
| 2. Treatment and proteinuria (independent clinical predictor identified in model 1) | |||
| coefficientb | 1.075 | −1.252 | |
| Pc | 0.003 | 0.002 | |
Variables in the model are categorized as shown in Table 2. Values of each variable are assigned to one of two levels (0 and 1); proteinuria >5 g/d, non-Hispanic white patients, and adjunctive immunosuppressive treatment (prednisone combined with IVCY or CsA) were each designated level 1.
Estimated regression coefficients in the Weibull survival model; a negative coefficient indicates that level 1 of the variable (e.g., adjunctive immunosuppressive treatment) is associated with a relatively short time to remission.
P value of the likelihood ratio test that evaluates the impact on outcome predictions of eliminating each variable from the model. Prognostic factors that significantly (P < 0.05) enhance the prediction of remission remain in the model.
Prognostic impact of adjunctive immunosuppressive treatment (IVCY or CsA) compared with prednisone alone. Concurrent angiotensin antagonist therapy is not an independent predictor of remission in this study and is not included in this model.
To evaluate more fully the impact of combination immunosuppressive therapy on the probability of remission, we added treatment to a statistical model (Table 3, model 2) that contained protein excretion rate (the independent baseline clinical predictor of remission identified in model 1). Urinary protein excretion rate and adjunctive immunosuppressive treatment emerged as independent predictors of remission in this model; both factors contributed significantly to predictions of remission. Proteinuria >5 g/d and treatment with prednisone alone were each associated with a decreased probability of remission.
Treatment Effectiveness
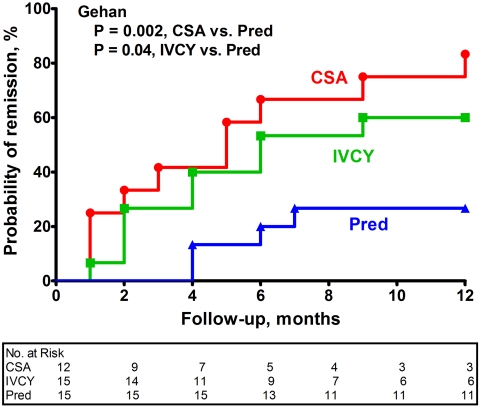
The probabilities of achieving remission in each of the treatment groups are depicted in Figure 1. At 1 yr, the cumulative probability of remission of proteinuria was 27% with prednisone (four of 15 patients), 60% with IVCY (nine of 15 patients), and 83% with CsA (10 of 12 patients). Remission was more likely among patients who were treated with CsA (Gehan test, P = 0.002) and those who were treated with IVCY (Gehan test, P = 0.04) compared with those who were treated with prednisone alone. Patients who were treated with CsA tended to achieve a remission more rapidly than those who were treated with IVCY, but the difference was not statistically significant (Gehan test, P = 0.28). During the 12-mo protocol treatment period, the incidence of remission per 100 patient-months of follow-up was 2.6 with prednisone, 8.3 with IVCY, and 14.5 with CSA. Two thirds of those who achieved remission in each adjunctive treatment group (six of nine with IVCY and six of 10 with CsA) and half of those who achieved remission in the control group (two of four) experienced a complete remission. Changes in protein excretion are illustrated in Figure 2.
Figure 1.
Cumulative probability of remission of proteinuria during the 12-mo protocol treatment period by treatment group. No patients were lost to follow-up or censored during this period. PRED, prednisone alone.
Figure 2.
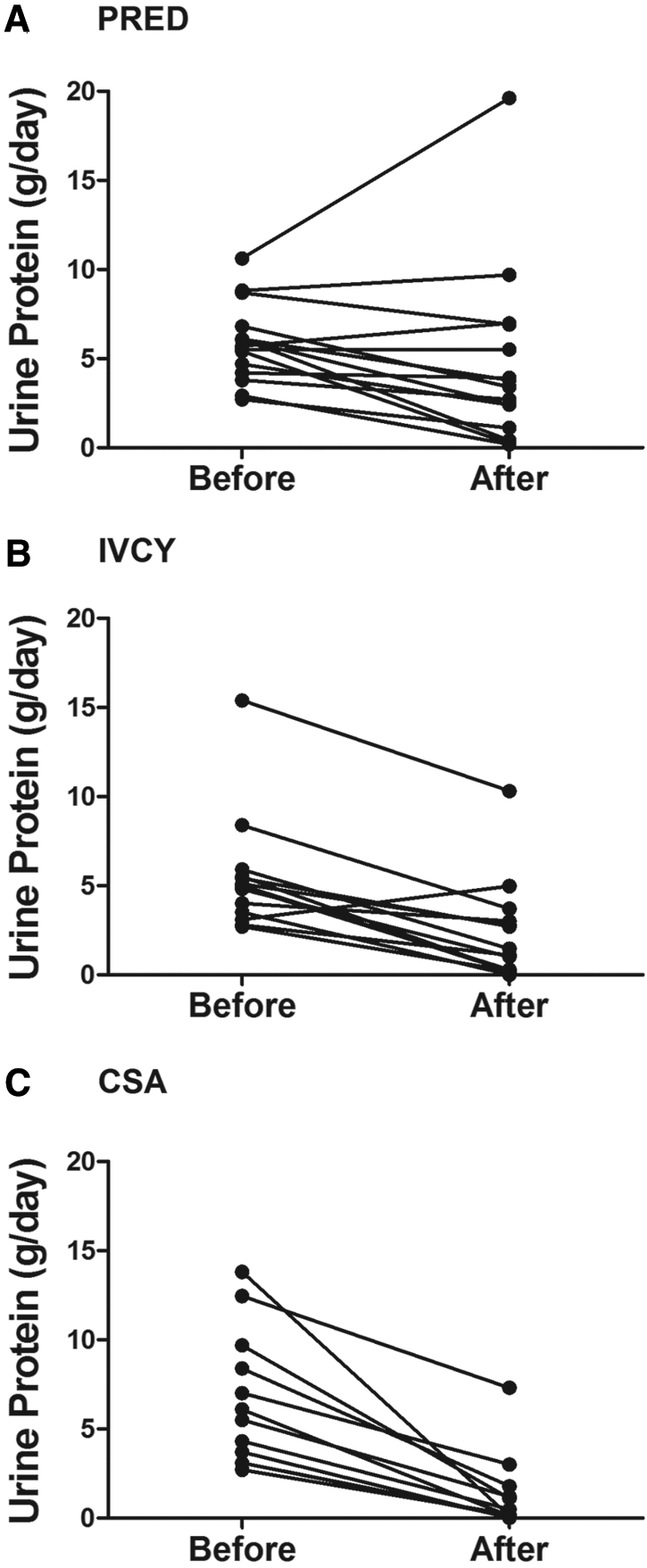
(A through C) Protein excretion rates for individual patients who were randomly assigned to PRED (A), IVCY (B), and CsA (C) before and after protocol treatment.
On average, the GFRs at month 12 were not significantly different from the baseline value in each treatment group (paired t test, P > 0.35 for each comparison of baseline versus month 12). Five patients experienced a ≥20% reduction in GFR (two in the prednisone group, one in the IVCY group, and two in the CsA group).
After extended follow-up, the median serum creatinine had increased statistically significantly for patients in each treatment group from 0.8 to 1.0 mg/dl in the prednisone group, from 0.8 to 0.9 mg/dl in the IVCY group, and from 0.7 to 0.85 mg/dl in the CsA group (paired t test, P < 0.05 for each comparison). Four patients experienced a doubling of the serum creatinine (two in the prednisone group, one in the IVCY group, and one in the CsA group). For 14 patients who achieved during the 12-mo protocol treatment period a remission of proteinuria that was sustained during extended follow-up, the average increase in serum creatinine was 0.09 compared with 0.29 mg/dl for patients who did not have a sustained remission after the 12-mo protocol treatment (t test, P = 0.04). The average change in serum creatinine was not different for 27 patients who received an angiotensin antagonist during the 12-mo protocol treatment period and/or during extended follow-up compared with those who did not receive an angiotensin antagonist.
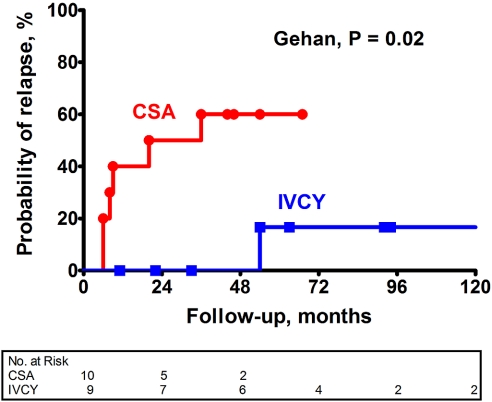
Risk for Relapse
Patients who had a remission of proteinuria were subsequently followed on low-dosage alternate-day prednisone (10 mg/m2 body-surface area). Figure 3 shows that patients who were randomly assigned to CsA were significantly more likely (Gehan test, P = 0.02) to experience a relapse of nephrotic syndrome than those who were randomly assigned to IVCY. During extended follow-up, the incidence of relapse per 100 patient-months was 2.0 with CsA and 0.2 with IVCY. Figure 4 shows changes in protein excretion after completion of the 12-mo protocol treatment for individual patients who achieved a remission on IVCY and CsA.
Figure 3.
Cumulative probability of relapse of nephrotic syndrome after completion of protocol treatment with IVCY versus CSA. Follow-up begins at the end of the 12-mo protocol treatment period. Patients were censored when they were lost to follow-up.
Figure 4.
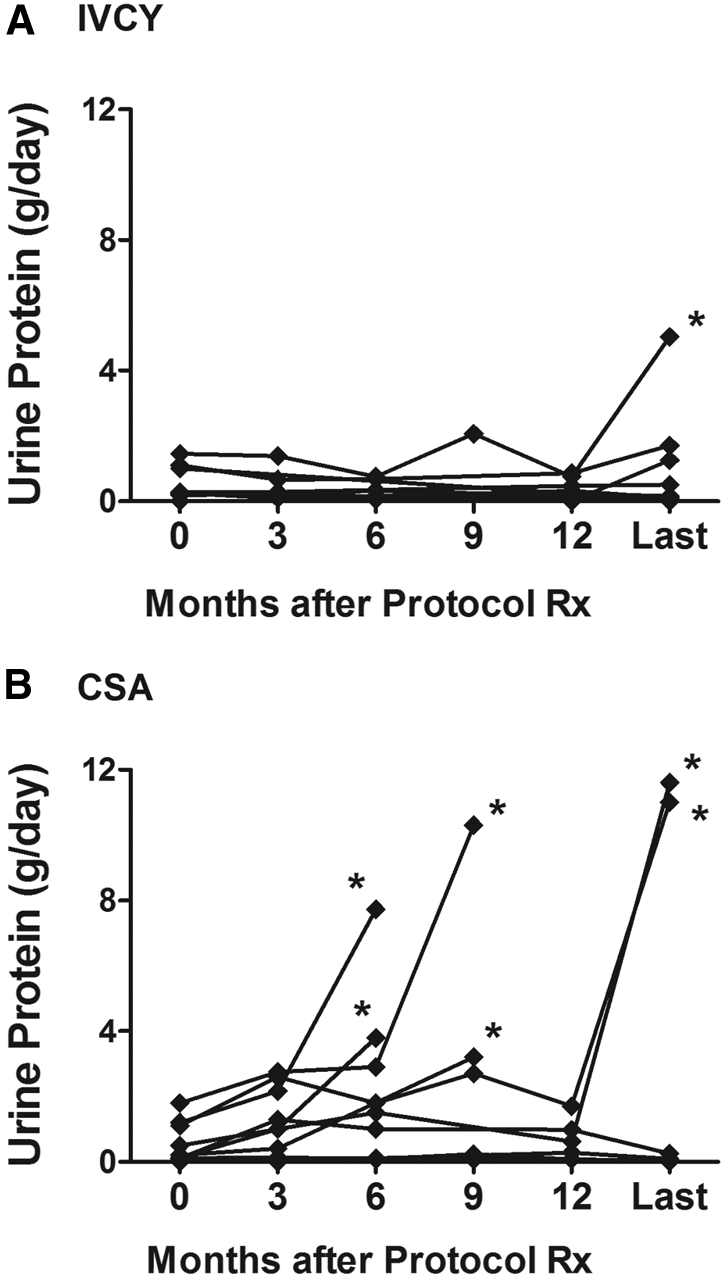
(A and B) Protein excretion rates after completion of the 12-mo protocol treatment for individual patients who achieved a remission on IVCY (A; n = 9) and CsA (B; n = 10). “Last” refers to the last observation during extended follow-up for the effects of IVCY or CsA (i.e., before the patient was lost to follow-up or at the time of relapse, before the patient started an alternative immunosuppressive regimen). *Relapse (urine protein excretion >3 g/d).
Treatment of Persistent or Recurrent Nephrotic Syndrome
Ten patients who failed to respond to prednisone alone (n = 4) or CsA (n = 1) or who relapsed into the nephrotic syndrome after responding to CsA (n = 5) were treated with IVCY for a median duration of 24 mo (range 10 to 48 mo; every 2 mo for the first year and then quarterly). There were no indications to alter the immunosuppressive regimen because of extrarenal lupus activity. Eight of these patients were black, one was Hispanic, and one was white. The median protein excretion rate was 6.8 g/d (range 3.2 to 22 g/d), the median serum albumin was 2.3 mg/dl (range 1.4 to 3.7 mg/dl), and the median serum creatinine was 0.9 mg/dl (range 0.5 to 2 mg/dl). Eight of 10 patients achieved a remission; the probability of remission was 70% at 18 mo and 80% at 36 mo. Only two of these patients had a complete remission. Among the responders, the protein excretion rate decreased from a medium of 6.8 to 0.8 g/d at the end of follow-up (median follow-up 55 mo after starting IVCY). The serum creatinine did not change statistically during IVCY treatment and follow-up of these 10 patients (paired t test, P = 0.11). None of these 10 patients experienced a doubling of the serum creatinine during long-term follow-up from the initiation of IVCY to the last observation. Two patients received anticoagulant therapy for deep vein thromboses and pulmonary emboli.
One patient who had persistent nephrotic syndrome after the initial 12-mo protocol treatment with IVCY and one patient who relapsed into the nephrotic syndrome after responding to IVCY were subsequently treated with CsA. One patient achieved a partial remission after 4 mo of treatment; the other failed to respond to 1 yr of CsA therapy. The other five patients who did not achieve a remission after IVCY were not offered CsA because of declining renal function (n = 1), leukocytoclastic vasculitis treated with daily oral cyclophosphamide (n = 1), and decreased proteinuria that had declined into the subnephrotic range (n = 3).
Two of the four patients who achieved a remission during the 12-mo protocol treatment with prednisone alone subsequently relapsed into the nephrotic syndrome and were treated with additional immunosuppressive therapy (one in a National Institutes of Health study of cladribine and one with azathioprine prescribed by her referring physician). One patient in the prednisone group experienced a transient ischemic attack during long-term follow-up; she was treated with antiplatelet agents as well as antihypertensive and lipid-lowering medications.
Adverse Effects
Three patients developed insulin-requiring diabetes: One in the prednisone group and two in the CsA group (Table 4). Patients received the same prednisone regimen in each treatment group. Localized herpes zoster occurred in two patients in the IVCY group. Four patients developed pneumonia: One in the prednisone group, two in the CsA group, and one during extended IVCY treatment. Two patients in the IVCY group experienced leukopenia below the target range for this protocol. Twenty-five percent of women (two of eight) who received IVCY and who were younger than 40 yr and had not previously undergone hysterectomy experienced amenorrhea. One patient in the IVCY group subsequently developed a cutaneous basal cell carcinoma that was resected. Nine of 12 patients in the CsA group required adjustments in CsA dosages because of increased serum creatinine and/or hypertension; two patients required CsA dosage reduction because of gastrointestinal intolerance.
Table 4.
Adverse events by treatment group
| Adverse Events | Prednisone Group 1 (n = 15) | IVCY Group 2 (n = 15) | CsA Group 3 (n = 12) | Extended Cyclophosphamide (n = 10) |
|---|---|---|---|---|
| Diabetes | 1 | 2 | ||
| Infections | ||||
| pneumonia | 1 | 2 | 1 | |
| herpes zoster | 2 | |||
| othera | 3 | 8 | 5 | 8 |
| Leukopeniab | 2 | |||
| Amenorrhea | 1 of 4c | 1 of 4c | ||
| Increased BP with or without increased creatinine (required decrease in CSA dosage) | 9 | |||
| Nausea/anorexia | 3 | 2 | ||
| Paresthesias/tremor | 4 | |||
| Gingival hyperplasia/increased facial hair | 8 | |||
| Osteoporosis/avascular necrosis of the hip | 4 | 3 | 2 | |
| Basal cell skin cancer | 1 |
Other infections were sinusitis, bronchitis, otitis media, dental abscess, upper respiratory tract infection, sty, pelvic inflammatory disease, cholecystitis, and herpes simplex virus skin infection.
Leukopenia below the target range for this protocol.
One of four women in these treatment groups who were younger than 40 yr and had not previously undergone hysterectomy experienced amenorrhea.
DISCUSSION
Patients with LMN were randomly assigned to receive prednisone alone (control group), IVCY every other month for six doses, or CsA for 11 mo. Although the sample size may have been too small to detect minor differences in efficacy between the treatment groups, given the inherent risk of immunosuppressive therapy, our goal was to detect substantial clinically significant differences in response rate. Both adjunctive IVCY and CsA were clinically and statistically significantly more effective than alternate-day prednisone alone in inducing remissions of proteinuria. Patients who were randomly assigned to adjunctive immunosuppressive therapy were more than twice as likely as those who were randomly assigned to prednisone alone to experience a remission of proteinuria. Relapse of high-grade proteinuria occurred significantly more often after completion of CsA than after IVCY. Ten patients who had failed to respond to prednisone or CsA or who had relapsed after CsA was stopped were subsequently treated with a regimen of IVCY that was comparable to the original protocol, followed by quarterly pulse cyclophosphamide. Eight of these high-risk patients achieved a remission of proteinuria.
The clinical course of patients with lupus nephritis is influenced by a number of clinical, demographic, and histologic features. The impact of these prognostic factors has been studied extensively among patients with proliferative lupus nephritis and in several studies of LMN as well. Sloan et al.23 showed that the initial serum creatinine was an independent predictor of death and/or end-stage renal failure when patients with “pure” LMN and those with mixed membranous and proliferative lupus nephritis were considered together; however, when the analysis was restricted to patients with pure LMN (comparable to those included in this study), the initial serum creatinine was no longer an independent risk factor. Moreover, several investigators reported that the degree of proteinuria at baseline was not a statistically significant predictor of renal function deterioration among patients with LMN.6,23,24 Conversely, Mercadal et al.6 showed (by univariate analysis) that patients with nephrotic syndrome at any time during follow-up were at increased risk for renal failure. By multivariate analysis, they found that nephrotic proteinuria was not an independent predictor of renal failure when other factors indicative of lupus activity were included in the model. We used a somewhat different approach in this study and identified high-risk patients according to relatively severe proteinuria (>5 g/d), comparable to the approach in several studies of idiopathic membranous nephropathy.25,26 This level of high-grade proteinuria was an independent predictor of time to remission in this study.
Compared with white patients, black and Hispanic lupus patients have been reported to have higher overall lupus activity,27 a greater risk for developing lupus nephritis,28,29 histologic features of aggressive lupus nephritis,30,31 and an increased risk for chronic renal failure.31–37 Barr et al.36 observed that after statistical adjustment for socioeconomic factors, Hispanic ethnicity but not black race was an independent predictor of renal function deterioration. Although race, ethnicity, and poverty have emerged as significant predictors of renal outcomes in studies that included predominantly cases of proliferative lupus nephritis, the impact of these demographic factors on the prognosis of LMN is unclear. The predictive value of socioeconomic factors in patients with LMN has not been reported, and we did not obtain this information when patients enrolled in this study. Bakir et al.38 reported that the prognosis of black patients with LMN in their study was comparable to that observed in white patients. We observed (by univariate analysis) that non-Hispanic white patients had a relatively favorable prognosis, but the predictive value of race and ethnicity seemed to diminish (by multivariate analysis) in the context of high-grade proteinuria (the strongest clinical predictor of time to remission in this study). Although race and ethnicity were not independent predictors of remission in this study, larger studies will be needed to clarify the predictive value of these factors in populations of patients with LMN.
This is a prospective, randomized study of immunosuppressive therapy for LMN. The entry criteria for this study, including the range of baseline proteinuria, were comparable to those reported for most studies of the treatment LMN.14,15,20–22,39–43 We compared two approaches (corticosteroids plus IVCY or corticosteroids plus CsA) with corticosteroids alone. Our results were comparable to those of Moroni et al.,16 who described a retrospective study in which most patients with <5 g/d proteinuria were treated with corticosteroids alone and most patients with more severe proteinuria received an alternate-month regimen of methylprednisolone and chlorambucil that has been effective in idiopathic membranous nephropathy. Patients who were treated with alternate-month cycles of methylprednisolone and chlorambucil were more likely to achieve a complete or partial remission of proteinuria than those who were treated with corticosteroids alone. Wang et al.17 reported that six Chinese patients who were treated with this regimen for LMN developed severe pancytopenia. Chan et al.18 observed a high rate of complete or partial remission in 20 patients who had LMN and were treated initially with prednisolone and daily oral cyclophosphamide for 6 mo followed by low-dosage methylprednisolone and azathioprine maintenance therapy. Of concern, 40% of their patients experienced herpes zoster and 20% developed pulmonary tuberculosis.
Previous reports of CsA treatment for LMN provided encouraging evidence of potential benefit.20–22 For example, Radhakrishnan et al.20 reported the results of a pilot study of CsA for patients with LMN (seven patients) and mixed membranous and proliferative lupus nephritis (three patients); eight received prednisone as well. Proteinuria decreased to <1 g/d in six patients and to 1 to 2 g/d in two other patients. The three patients with mixed membranous and proliferative glomerular changes had renal and extrarenal flares of lupus while on CsA and required additional immunosuppressive therapy.
Several investigators reported their experience using mycophenolate mofetil (MMF) as treatment for LMN.39–44 Kasitanon et al.43 at Johns Hopkins observed that only 11% of their patients with nephrotic proteinuria experienced a complete remission, in contrast to 50% of their patients with milder proteinuria. They noted a similar response rate among patients with pure membranous lesions compared with those with mixed membranous and proliferative glomerular changes. Kapitsinou et al.40 reported that two of six patients with LMN achieved a complete or partial remission, but other investigators observed more favorable responses.39,41,42 For example, Spetie et al.39 reported that 10 of 13 patients (with a mean baseline urine protein to creatinine ratio of 5.1) experienced a complete or partial remission at 6 mo of therapy. Radhakrishnan et al.44 described a subgroup analysis of 27 patients who had LMN and participated in a multicenter study that compared MMF with IVCY as induction therapy in 140 patients, most of whom had proliferative lupus nephritis. The response to MMF and IVCY was similar in the patients with LMN, but the analysis is somewhat complicated by the large number of early withdrawals (five from MMF and three from IVCY).
The choice of immunosuppressive treatment for patients with LMN must be based on a careful consideration of potential risks and benefits. A short course of high-dosage alternate-day corticosteroids may be tried, but comparable to the experience treating proliferative lupus nephritis45,46 and idiopathic membranous nephropathy,47,48 this treatment is unlikely to induce a sustained remission and may be associated with insidious, serious adverse effects. We observed that alternate-month IVCY was more effective than corticosteroids alone during the first 12 mo of treatment. Furthermore, most patients, who had failed to respond to prednisone alone or CsA or who had relapsed after completing CsA therapy subsequently responded to an extended course of IVCY. Conversely, typically, patients did not respond rapidly to this regimen of IVCY. The slow response may reflect the alternate-month schedule, which led to a relatively modest cumulative dosage of cyclophosphamide every 2 mo compared with more intensive regimens of monthly IVCY or daily oral cyclophosphamide.
A high percentage of patients responded relatively quickly to CsA in this study even though the demographic composition of the CsA group suggested that it was at increased risk for adverse outcomes compared with the IVCY group. Because GFR was not a statistically significant predictor of remission in this study, it is unlikely that the favorable outcomes observed in the CsA treatment group are a consequence of the decision not to randomly assign patients with impaired renal function to CsA.
CsA has been shown to be effective in prospective, randomized clinical trials of treatment for patients who had idiopathic membranous nephropathy and were steroid resistant49 or had deteriorating renal function.50 The concurrent use of an angiotensin antagonist and a calcineurin inhibitor may be complicated by marked increases in serum creatinine and potassium, underscoring the need for close monitoring. The risk for nephrotoxicity and the propensity to relapse after completion of CsA treatment are serious concerns that were addressed in recently published recommendations of an international workshop on CsA for idiopathic glomerular disease associated with the nephrotic syndrome.51 They recommended CsA (initial dosage 3 to 4 mg/kg per d) and low-dosage corticosteroids or methylprednisolone and a cytotoxic drug (alternate months for 6 mo) as immunosuppressive therapy for medium- and high-risk patients with idiopathic membranous nephropathy. They would stop CsA and switch to an alternative treatment if proteinuria did not decrease by 50% at 6 mo or if CsA caused significant toxicity that could not be ameliorated by dosage reduction. They believed that CsA could be tapered off over 3 to 4 mo in patients who achieve a complete remission. Conversely, they suggested much more gradual tapering of CsA (over several years) for patients who attain a partial remission. Although this approach has not been formally tested in LMN, it offers the prospect of maintaining a remission on very low dosages of CsA. Additional prospective trials are needed to compare the efficacy and toxicity of this or similar regimens with related drugs, such as tacrolimus, with other treatments, including, for example, MMF and rituximab. It may be appropriate to reserve cytotoxic drug regimens for patients who fail to respond to or who relapse after treatment with one of these regimens. Continued investigations of basic pathogenetic mechanisms and of innovative treatments are needed to refine our approach to patients with immune-mediated kidney diseases.
CONCISE METHODS
Patients
We enrolled 42 patients with LMN into this prospective, randomized clinical study at the Clinical Center of the National Institutes of Health (Bethesda, MD) between 1988 and 1999; observation was closed after a median follow-up of 60 mo. Admission criteria included a diagnosis of systemic lupus erythematosus as defined by the American College of Rheumatology,52,53 a renal biopsy that showed typical LMN by light and electron microscopy,54 ≥2 g/d proteinuria, age ≥12 yr, and informed consent.
Exclusion criteria were endocapillary proliferation or subendothelial electron-dense deposits characteristic of proliferative lupus nephritis, clinical or histologic evidence of nonlupus renal disease, cytotoxic drug or CsA use during the 30 d period before study entry, cytotoxic drug or CsA use for >2 wk during the 10 wk period before study entry, cytotoxic drug or CsA use for >10 wk at anytime in the past, requirement for corticosteroids in dosages >20 mg/m2 body surface area per day of prednisone (or equivalent) for control of extrarenal disease at the time of study entry, active or chronic infection (including HIV infection), preexistent malignancy, pregnancy in female patients, nursing mothers, female patients who were not practicing birth control, a single functioning kidney, insulin-treated diabetes, GFR <25 ml/min per 1.73 m2 body surface area at study entry, and history of allergy or toxicity to cyclophosphamide or CsA.
The local institutional review board approved the protocol. Written informed consent was obtained from each patient before participating in this study.
Study Design
After establishing study eligibility, patients were randomly assigned to one of three treatment groups (by drawing from a masked-card sequence arranged from a table of random numbers). All patients received conventional high-dosage alternate-day oral prednisone (initiated at 40 mg/m2 body surface area [approximately 1 mg/kg body wt] every other day for 8 wk, followed by gradual tapering [5 mg/wk] to 10 mg/m2 body surface area every other day for the remainder of the 1-yr protocol treatment period). Each treatment group received one of the following regimens: (1) Corticosteroids alone (n = 15, control group); (2) IVCY every other month (six doses, ranging from 0.5 to 1.0 g/m2 body surface area to induce a leukocyte nadir no lower than 1500 cells/mm3; n = 15); (3) CsA (initiated at 200 mg/m2 body surface area [approximately 5 mg/kg body wt] per day given in two equal doses at 12-h intervals for 11 mo; n = 12). Patients in the CsA treatment group were monitored closely for signs of toxicity. The CsA dosage was reduced by 25% when the serum creatinine increased 33 to 49% or ≥0.3 mg/dl over baseline on two or more determinations. A 50% reduction in the CsA dosage was required when the serum creatinine increased 50 to 99% or ≥0.5 mg/dl over baseline or when the serum transaminase, bilirubin, or alkaline phosphatase values persistently doubled without an alternative explanation. The CsA dosage was reduced 25 to 50% for other adverse effects. When manifestations of CsA toxicity failed to improve within 2 wk, the CsA dosage was reduced further. CsA had to be discontinued when the serum creatinine increased 100% over baseline or when adverse effects persisted despite repeated CsA dosage reductions. Because of concerns for CsA-induced toxicities, seven patients with a GFR <67 ml/min per 1.73 m2 body surface area were not randomly assigned to CsA; they were randomly assigned to treatment group 1 or 2 (by drawing from a separate masked-card sequence arranged from a table of random numbers). Otherwise, randomization was not stratified according to clinical or demographic prognostic factors because inadequate information was available about the predictive value of these features in LMN.
To avoid confounding the interpretation of renal outcomes, we did not introduce angiotensin antagonist therapy (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker) during the month before study entry or during the 12-mo protocol treatment period. Otherwise, the protocol did not specify which treatments to use for hypertension or hyperlipidemia. The use of nonsteroidal anti-inflammatory drugs, including aspirin, was strongly discouraged.
Assessment
Renal biopsies were obtained before study entry and were evaluated by light and electron microscopy as described previously.55 Patients underwent a detailed clinical assessment (history, physical examination, and laboratory studies) before and 12 mo after initiating protocol treatment. The assessments included at least two 24-h urine collections for protein excretion and creatinine clearance as well as hydrated urinary clearance studies to determine GFR using 99mtechnetium-diethylene triamine pentaacetic acid before April 1996 and then inulin as the indicator of GFR.56,57 In all but two cases, the baseline and the 12-mo kidney function studies used the same indicator of GFR. During the 12-mo protocol treatment period, follow-up visits and laboratory studies (including two 24-h urine collections for protein excretion and creatinine clearance) were scheduled every 2 mo. Patients were seen more frequently as indicated. Complete remission was defined as <0.3 g/d proteinuria. Partial remission was defined as <2.0 g/d proteinuria with a >50% reduction from baseline proteinuria.
Treatment of Persistent or Recurrent Nephrotic Proteinuria
After the 12-mo evaluation, patients were followed in a natural history protocol to determine whether renal outcomes were sustained and to observe for long-term toxicities of the protocol treatments. Persistence or relapse of nephrotic proteinuria (>3 g/d) prompted a detailed clinical assessment and recommendations for additional therapy including angiotensin antagonists and lipid-lowering agents. When nephrotic proteinuria continued unabated, group 2 (IVCY) patients were offered treatment with CsA, and group 1 (prednisone) and group 3 (CsA) patients were offered treatment with IVCY. The initial dosage schedules for these treatments were the same as those described already. After 1 yr, these interventions were discontinued when the patient failed to improve, and they were gradually tapered (by reduction of the CsA dosage or prolongation of the interval between IVCY doses) when the patient had manifested a reduction in protein excretion as well as stable renal function.
Statistical Analysis
The distribution of clinical and demographic attributes among the treatment groups was examined by the Wilcoxon rank-sum test or Fisher exact test. Clinical, demographic, and treatment variables were individually tested for prognostic significance by cumulative survival analysis. Because both complete and partial remission of proteinuria have been associated with favorable outcomes in patients with idiopathic membranous nephropathy,58 we examined the length of time from study entry to complete or partial remission as the measure of outcome (defined as “remission”). For the analysis of continuous variables, groups of patients were formed by dividing the range of the variable at a cut point that we thought was clinically relevant. Because non-Hispanic white patients with lupus nephritis have been reported to have more favorable outcomes than black and Hispanic patients,31–37 we combined the last two groups and compared their outcomes with those observed among the non-Hispanic white patients in this study. The equality of survival curves obtained for different subgroups of patients was assessed by two statistical methods that are appropriate for survival analysis: The Gehan-Breslow-Wilcoxon test and the Mantel-Cox log-rank test.59 The Gehan test is relatively sensitive to early events, and the Mantel-Cox test is more sensitive to late events. Very similar results were obtained using these two tests, so we reported one of them. We chose the Gehan test because it is relatively sensitive to early remissions, which are an important goal of treatment.
To identify independent predictors of remission at the end of the 12-mo protocol treatment period, we entered prognostic factors (identified by univariate survival analysis) into a multivariate survival analysis.60 The prognostic factors were entered into equations that relate the factors, as in multiple regression, to the outcome, expressed as the natural logarithm of the time to remission. The equations, referred to as statistical models, allow inferences about one prognostic factor adjusted for variation in the others. Variables were removed from the equation in a stepwise manner until each variable that remained in the model contributed significantly (P < 0.05) to predictions of remission. The various models were compared by the likelihood ratio test to determine the impact of removing a factor from the equation.
To compare the efficacy of IVCY or CsA with prednisone alone, we used cumulative survival analysis, with the time interval from study entry to remission as the measure of outcome. We evaluated the probability of sustained remission after IVCY compared with CsA by cumulative survival analysis, with the time interval from month 12 to relapse of the nephrotic syndrome as the measure of outcome. The equality of survival curves obtained for different treatment groups was assessed by the Gehan test. Two-tailed tests were used to derive the P values reported throughout.
DISCLOSURES
None.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Portions of this study were presented at annual meetings of the American Society of Nephrology in Toronto, Ontario, Canada on October 13 through 16, 2001 and in St. Louis, Missouri on October 29 through November 1, 2004.
We are grateful to John Decker, Jack Klippel, Dimitrios Boumpas, David Webb, Paul Plotz, Alfred Steinberg, Ronald Wilder, Mark Gourley, Cheryl Yarboro, Ellen Vaughan, and Denise Knisely-Carrigan for active support of these investigations.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Treatment of Membranous Lupus Nephritis: Where Are We Now?” on pages 690–691.
REFERENCES
- 1.Estes D, Christian CL: The natural history of systemic lupus erythematosus by prospective analysis. Medicine (Baltimore) 50: 85–95, 1971 [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Urowitz MB: Clinical features. In: Rheumatology, 3rd Ed., edited by Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, Edinburgh, Mosby, 2003, pp 1359–1379
- 3.Korbet SM: Membranous lupus glomerulonephritis. In: Lupus Nephritis, edited by Lewis EJ, Schwartz MM, Korbet SM, Oxford, Oxford University Press, 1999, pp 219–240
- 4.Appel GB, Cohen DJ, Pirani CL, Meltzer JI, Estes D: Long-term follow-up of patients with lupus nephritis: A study based on the classification of the World Health Organization. Am J Med 83: 877–885, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Lupus nephritis: Prognostic factors and probability of maintaining life-supporting renal function 10 years after the diagnosis. Gruppo Italiano per lo Studio della Nefrite Lupica (GISNEL). Am J Kidney Dis 19: 473–479, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Mercadal L, Montcel ST, Nochy D, Queffeulou G, Piette JC, Isnard-Bagnis C, Martinez F: Factors affecting outcome and prognosis in membranous lupus nephropathy. Nephrol Dial Transplant 17: 1771–1778, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA: The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 60: 221–225, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Karsh J, Klippel JH, Balow JE, Decker JL: Mortality in lupus nephritis. Arthritis Rheum 22: 764–769, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Donadio JV Jr, Hart GM, Bergstralh EJ, Holley KE: Prognostic determinants in lupus nephritis: A long-term clinicopathologic study. Lupus 4: 109–115, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Bono L, Cameron JS, Hicks JA: The very long-term prognosis and complications of lupus nephritis and its treatment. QJM 92: 211–218, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Font J, Ramos-Casals M, Cervera R, Garcia-Carrasco M, Torras A, Siso A, Darnell A, Ingelmo M: Cardiovascular risk factors and the long-term outcome of lupus nephritis. QJM 94: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Ordonez JD, Hiatt RA, Killebrew EJ, Fireman BH: The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int 44: 638–642, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Pasquali S, Banfi G, Zucchelli A, Moroni G, Ponticelli C, Zucchelli P: Lupus membranous nephropathy: Long-term outcome. Clin Nephrol 39: 175–182, 1993 [PubMed] [Google Scholar]
- 14.Donadio JV Jr, Burgess JH, Holley KE: Membranous lupus nephropathy: A clinicopathologic study. Medicine (Baltimore) 56: 527–536, 1977 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Dettoni H, Tron F: Membranous glomerulopathy in systemic lupus erythematosus. Adv Nephrol 14: 347–364, 1984 [PubMed] [Google Scholar]
- 16.Moroni G, Maccario M, Banfi G, Quaglini S, Ponticelli C: Treatment of membranous lupus nephritis. Am J Kidney Dis 31: 681–686, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Wang AY, Li PK, Lai FM, Chow KM, Szeto CC, Leung CB, Lui SF: Severe bone marrow failure associated with the use of alternating steroid with chlorambucil in lupus membranous nephropathy in Chinese. Lupus 10: 295–298, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Chan TM, Li FK, Hao WK, Chan KW, Lui SL, Tang S, Lai KN: Treatment of membranous lupus nephritis with nephrotic syndrome by sequential immunosuppression. Lupus 8: 545–551, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Sqalli HT, Benabdallah L, Arrayhani M, Amar Y, Rhou H, Ouzeddoun N, Bayahia R, Benamar L: Initial presentation and course of lupus membranous glomerulonephritis. Presse Med 37: 559–563, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan J, Kunis CL, D'Agati V, Appel GB: Cyclosporine treatment of lupus membranous nephropathy. Clin Nephrol 42: 147–154, 1994 [PubMed] [Google Scholar]
- 21.Hallegua D, Wallace DJ, Metzger AL, Rinaldi RZ, Klinenberg JR: Cyclosporine for lupus membranous nephritis: Experience with ten patients and review of the literature. Lupus 9: 241–251, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hu W, Liu Z, Shen S, Li S, Yao X, Chen H, Li L: Cyclosporine A in treatment of membranous lupus nephropathy. Chin Med J (Engl) 116: 1827–1830, 2003 [PubMed] [Google Scholar]
- 23.Sloan RP, Schwartz MM, Korbet SM, Borok RZ: Long-term outcome in systemic lupus erythematosus membranous glomerulonephritis. Lupus Nephritis Collaborative Study Group. J Am Soc Nephrol 7: 299–305, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Mok CC, Ying KY, Lau CS, Yim CW, Ng WL, Wong WS, Au TC: Treatment of pure membranous lupus nephropathy with prednisone and azathioprine: An open-label trial. Am J Kidney Dis 43: 269–276, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Pei Y, Cattran D, Greenwood C: Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int 42: 960–966, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Alarcon GS, Roseman J, Bartolucci AA, Friedman AW, Moulds JM, Goel N, Straaton KV, Reveille JD: Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 41: 1173–1180, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Bastian HM, Roseman JM, McGwin G Jr, Alarcon GS, Friedman AW, Fessler BJ, Baethge BA, Reveille JD: Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 11: 152–160, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Seligman VA, Lum RF, Olson JL, Li H, Criswell LA: Demographic differences in the development of lupus nephritis: A retrospective analysis. Am J Med 112: 726–729, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Austin HA III, Boumpas DT, Vaughan EM, Balow JE: High-risk features of lupus nephritis: Importance of race and clinical and histological factors in 166 patients. Nephrol Dial Transplant 10: 1620–1628, 1995 [PubMed] [Google Scholar]
- 31.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, Nahar N, de La Cuesta C, Hurtado A, Fornoni A, Beltran-Garcia L, Asif A, Young L, Diego J, Zachariah M, Smith-Norwood B: Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 69: 1846–1851, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Alarcon GS, McGwin G Jr, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, Bastian HM, Friedman AW, Reveille JD: Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 44: 2797–2806, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Alarcon GS, McGwin G Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP: Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus 11: 95–101, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Dooley MA, Hogan S, Jennette C, Falk R: Cyclophosphamide therapy for lupus nephritis: Poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int 51: 1188–1195, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Austin HA III, Boumpas DT, Vaughan EM, Balow JE: Predicting renal outcomes in severe lupus nephritis: Contributions of clinical and histologic data. Kidney Int 45: 544–550, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Barr RG, Seliger S, Appel GB, Zuniga R, D'Agati V, Salmon J, Radhakrishnan J: Prognosis in proliferative lupus nephritis: The role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 18: 2039–2046, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Korbet SM, Schwartz MM, Evans J, Lewis EJ: Severe lupus nephritis: Racial differences in presentation and outcome. J Am Soc Nephrol 18: 244–254, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Bakir AA, Levy PS, Dunea G: The prognosis of lupus nephritis in African-Americans: A retrospective analysis. Am J Kidney Dis 24: 159–171, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Spetie DN, Tang Y, Rovin BH, Nadasdy T, Nadasdy G, Pesavento TE, Hebert LA: Mycophenolate therapy of SLE membranous nephropathy. Kidney Int 66: 2411–2415, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Kapitsinou PP, Boletis JN, Skopouli FN, Boki KA, Moutsopoulos HM: Lupus nephritis: Treatment with mycophenolate mofetil. Rheumatology (Oxford) 43: 377–380, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Karim MY, Pisoni CN, Ferro L, Tungekar MF, Abbs IC, D'Cruz DP, Khamashta MA, Hughes GR: Reduction of proteinuria with mycophenolate mofetil in predominantly membranous lupus nephropathy. Rheumatology (Oxford) 44: 1317–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Borba EF, Guedes LK, Christmann RB, Figueiredo CP, Goncalves CR, Bonfa E: Mycophenolate mofetil is effective in reducing lupus glomerulonephritis proteinuria. Rheumatol Int 26: 1078–1083, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Kasitanon N, Petri M, Haas M, Magder LS, Fine DM: Mycophenolate mofetil as the primary treatment of membranous lupus nephritis with and without concurrent proliferative disease: A retrospective study of 29 cases. Lupus 17: 40–45, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Radhakrishnan J, Ginzler E, Appel G: Mycophenolate mofetil (MMF) vs. intravenous cyclophosphamide (IVCY) for severe lupus nephritis (LN): Subgroup analysis of patients (pts) with membranous nephropathy (SLE-V) [Abstract]. J Am Soc Nephrol 16: 8, 2005 [Google Scholar]
- 45.Donadio JV Jr, Holley KE, Ferguson RH, Ilstrup DM: Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N Engl J Med 299: 1151–1155, 1978 [DOI] [PubMed] [Google Scholar]
- 46.Dinant HJ, Decker JL, Klippel JH, Balow JE, Plotz PH, Steinberg AD: Alternative modes of cyclophosphamide and azathioprine therapy in lupus nephritis. Ann Intern Med 96: 728–736, 1982 [DOI] [PubMed] [Google Scholar]
- 47.Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, Ritchie S: A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med 320: 210–215, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Cameron JS, Healy MJ, Adu D: The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. The MRC Glomerulonephritis Working Party. Q J Med 74: 133–156, 1990 [PubMed] [Google Scholar]
- 49.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL: Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, Morrin PA, Lavoie S: A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int 47: 1130–1135, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N: Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int 72: 1429–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271–1277, 1982 [DOI] [PubMed] [Google Scholar]
- 53.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Austin HA, III, Muenz LR, Joyce KM, Antonovych TT, Balow JE: Diffuse proliferative lupus nephritis: Identification of specific pathologic features affecting renal outcome. Kidney Int 25: 689–695, 1984 [DOI] [PubMed] [Google Scholar]
- 56.Palestine AG, Austin HA III, Balow JE, Antonovych TT, Sabnis SG, Preuss HG, Nussenblatt RB: Renal histopathologic alterations in patients treated with cyclosporine for uveitis. N Engl J Med 314: 1293–1298, 1986 [DOI] [PubMed] [Google Scholar]
- 57.Nussenblatt RB, de Smet MD, Rubin B, Freidlin V, Whitcup SM, Davis J, Herman D, Bloom JN, Sran PK, Whitcher S, Palestine A, Austin H: A masked, randomized, dose-response study between cyclosporine A and G in the treatment of sight-threatening uveitis of noninfectious origin. Am J Ophthalmol 115: 583–591, 1993 [DOI] [PubMed] [Google Scholar]
- 58.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC: Idiopathic membranous nephropathy: Definition and relevance of a partial remission. Kidney Int 66: 1199–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Benedetti J, Yuen K, Young L: Life tables and survivor functions. In: BMDP Statistical Software Manual, edited by Dixon WJ, Berkeley, University of California Press, 1990, pp 739–768
- 60.Hopkins A: Survival analysis with covariates. In: BMDP Statistical Software Manual, edited by Dixon WJ, Berkeley, University of California Press, 1990, pp 769–806