Abstract
Although management of warfarin is challenging for patients with chronic kidney disease (CKD), no prospective studies have compared response to warfarin among patients with minimal, moderate, and severe CKD. This secondary analysis of a prospective cohort of 578 patients evaluated the influence of kidney function on warfarin dosage, anticoagulation control, and risk for hemorrhagic complications. We adjusted all multivariable regression and proportional hazard analyses for clinical and genetic factors. Patients with severe CKD (estimated GFR <30 ml/min per 1.73 kg/m2) required significantly lower warfarin dosages (P = 0.0002), spent less time with their international normalized ratio within the target range (P = 0.049), and were at a higher risk for overanticoagulation (international normalized ratio >4; P = 0.052), compared with patients with no, mild, or moderate CKD. Patients with severe CKD had a risk for major hemorrhage more than double that of patients with lesser degrees of renal dysfunction (hazard ratio 2.4, 95% confidence interval 1.1 to 5.3). In conclusion, patients with reduced kidney function require lower dosages of warfarin, have poorer control of anticoagulation, and are at a higher risk for major hemorrhage. These observations suggest that warfarin may need to be initiated at a lower dosage and monitored more closely in patients with moderate or severe CKD compared with the general population. Diminished renal function may have implications for a larger proportion of warfarin users than previously estimated.
Despite its proven benefits, warfarin is frequently underused because of the complexity of clinical management and concerns about the risk for hemorrhagic complications.1,2 The intricacies of warfarin metabolism, the complexities of the coagulation system, and the multitude of factors affecting level of anticoagulation each pose significant challenges for optimal management in the general medical population.3 These challenges are even greater among patients with chronic kidney disease (CKD) and ESRD4; however, anticoagulation is typically prescribed and managed similarly in patients with CKD as in the general medical population.
Although limited, previous reports suggest that patients with reduced renal function may require lower warfarin dosages and have a higher incidence of overanticoagulation and underanticoagulation and a two-fold higher risk for major hemorrhage5,6; however, these estimates are derived predominantly from studies that have evaluated the risk for hemorrhage among dialysis patients prescribed warfarin for prevention of vascular access thrombosis.6 The risks and benefits of long-term anticoagulation with warfarin are largely unknown in the CKD population. To our knowledge, no prospective studies have compared the risk for hemorrhage among patients with none/minimal, moderate, or severe decrease in kidney function.
There is recent evidence that genetic factors have a significant impact on warfarin response. Two genes, cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex 1 (VKORC1), have demonstrated a strong, consistent influence across various racial groups on warfarin dosage7–10 and hemorrhagic complications.11,12 This evidence provided the impetus for the recent warfarin package insert update (http://www.fda.gov/cder/drug/infopage/warfarin/default.htm) by the US Food and Drug Administration. The influence of the interaction between CKD and genetic factors on warfarin response has not been reported. The secondary analysis of this prospective study evaluates the influence of reduced kidney function on warfarin dosage, anticoagulation control, and risk for hemorrhagic complications after adjustment for clinical and genetic factors in a racially diverse cohort.
RESULTS
Of the 621 eligible patients in the Pharmacogenetic Optimization of Anticoagulation Therapy (POAT) cohort, 43 (6.9%) declined participation in the study and 13 (2.1%) were excluded because of missing creatinine values. The remaining 565 participants (mean 61 ± 16 yr, 51.1% male, 47.6% African American) were included in analysis for this study. Patients were categorized into three groups on the basis of GFR (≥60 ml/min per 1.73 m2 no/mild CKD; 30 to 59 ml/min per 1.73 m2 moderate CKD; <30 ml/min per 1.73 m2 severe CKD).
The majority (59.5%) of the participants had no/mild CKD, 31.2% had moderate CKD, and 9.4% had severe CKD (Table 1). Forty-seven of the 53 participants with severe CKD were on dialysis. Patients with severe CKD were younger (P < 0.0001) and were more likely to be African American (P < 0.0001), be female (P = 0.005), have medical insurance (P = 0.002), have higher comorbidity (P < 0.0001), have venous thromboembolism (P = 0.007), and have low annual income (P = 0.005) and shorter duration of warfarin therapy (P = 0.001). There were no significant differences in body mass index (BMI), use of concurrent medications, or distribution of CYP2C9 and VKORC1 genotypes across CKD groups.
Table 1.
Baseline cohort characteristics among African American (n = 269) and European American (n = 296) participants with different levels of renal functiona
| Characteristic | GFR (ml/min per 1.73 m2)
|
||
|---|---|---|---|
| ≥60 (n = 336) | 30 to 59 (n = 176) | <30 (n = 53) | |
| CKD categoryb | No/mild | Moderate | Severe |
| Age (yr; mean ± SD) | 58.6 ± 15.5 | 67.7 ± 13.2 | 55.9 ± 16.0 |
| Follow-up (mo; mean ± SD) | 16.8 ± 11.1 | 16.4 ± 10.8 | 11.5 ± 9.6 |
| BMI (kg/m2; mean ± SD) | 29.8 ± 7.4 | 29.3 ± 7.2 | 30.0 ± 6.5 |
| BUN (mg/dl; mean ± SD) | 13.7 ± 5.1 | 21.5 ± 9.8 | 37.8 ± 20.7 |
| Serum creatinine (mg/dl; mean ± SD) | 0.94 ± 0.20 | 1.35 ± 0.30 | 6.54 ± 4.30 |
| Race (n [%]) | |||
| African American | 176 (52.4) | 59 (33.5) | 34 (64.1) |
| European American | 160 (47.6) | 117 (66.5) | 19 (35.8) |
| Gender (n [%]) | |||
| female | 145 (43.2) | 101 (57.4) | 30 (56.6) |
| male | 191 (56.8) | 75 (42.6) | 23 (43.4) |
| No alcohol intake (n [%]) | 245 (72.9) | 134 (76.1) | 46 (86.8) |
| Current smokers (n [%]) | 48 (14.3) | 16 (9.1) | 10 (18.9) |
| Education (n [%]) | |||
| high school or less | 194 (57.7) | 108 (61.4) | 33 (62.3) |
| more than high school | 142 (42.3) | 68 (36.9) | 20 (37.7) |
| Annual household income (n [%]) | |||
| <$50,000 | 257 (76.5) | 121 (68.7) | 48 (90.5) |
| ≥$50,000 | 78 (23.2) | 54 (30.7) | 5 (9.5) |
| Medical insurance (n [%]) | 269 (79.8) | 159 (90.3) | 49 (92.4) |
| Indication for warfarin (n [%])c | |||
| atrial fibrillation | 134 (40.0) | 99 (56.2) | 23 (43.4) |
| stroke | 56 (16.7) | 33 (18.7) | 5 (9.4) |
| transient ischemic attack | 22 (6.6) | 13 (7.4) | 1 (1.9) |
| deep vein thrombosis | 94 (28.1) | 37 (21.0) | 23 (43.4) |
| pulmonary embolism | 52 (15.5) | 18 (10.2) | 6 (11.3) |
| myocardial infarction/ | |||
| cardiomyopathy | 96 (28.7) | 50 (28.4) | 14 (26.4) |
| No. of comorbid conditions (n [%])c | |||
| low (0 or 1) | 18 (35.2) | 39 (22.2) | 1 (1.9) |
| medium (2 to 4) | 151 (44.9) | 91 (51.7) | 23 (43.4) |
| high (≥5) | 67 (19.9) | 46 (26.1) | 29 (54.7) |
| Concurrent medications (n [%]) | |||
| antiplatelet agents | 125 (37.2) | 71 (40.3) | 26 (49.1) |
| CYP2C9 substrate | 54 (16.1) | 39 (22.2) | 16 (30.2) |
| CYP2C9 inhibitors | 38 (11.3) | 28 (15.9) | 12 (22.6) |
| CYP2C9 (n [%])d | |||
| NN | 254 (77.7) | 132 (76.7) | 37 (72.5) |
| NV | 65 (19.9) | 35 (20.3) | 11 (21.6) |
| VV | 8 (2.4) | 5 (2.9) | 3 (5.9) |
| VKORC1-1173C/T (n [%])e | |||
| CC | 195 (61.9) | 87 (51.8) | 30 (63.8) |
| CT | 97 (30.8) | 71 (42.3) | 13 (27.7) |
| TT | 23 (7.3) | 10 (5.9) | 4 (8.5) |
All patients had a prescribed target INR range of 2 to 3. Patients with orthopedic surgery were excluded because of short (3 to 6 mo) treatment duration, and patients with mechanical heart valve and hypercoagulable state were excluded because of higher intensity of anticoagulation required. Three Hispanic patients were excluded. Income missing for three patients, and education missing for four. BUN, blood urea nitrogen.
Patients were categorized into three categories: GFR ≥60 (no CKD or stages 1 and 2 CKD), GFR 30 to 59 (stage 3 CKD), and GFR <30 (stages 4 and 5 CKD).
Patients can have more than one indication for therapy and comorbid conditions. Comorbid conditions include cardiomyopathy, congestive heart failure, diabetes, hyperlipidemia, hypertension, malignancy, coronary artery disease, renal insufficiency and renal failure.
CYP2C9 variant genotype includes one or two copies of *2 and *3 alleles among European Americans and *2, *3, *5, *6, and *11 alleles among African Americans. CYP2C9 genotype information missing for 25 and VKORC1-1173 missing for 45 patients.
Variant VKORC1-1173C/T (rs9934438) includes TT or CT'.
As reported previously, European Americans had a higher frequency of variant CYP2C9 (33.1%), VKORC1 1173 (60.4%) genotype as compared with African Americans (11.6 and 20.1%, respectively; P < 0.0001).9 Genotype distributions for CYP2C9 and VKORC1 were in Hardy-Weinberg equilibrium among European Americans (all P > 0.5) and African Americans (all P > 0.25).
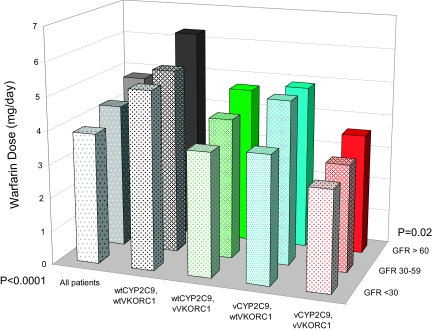
After adjustment for clinical and genetic factors, the patients with severe CKD had significantly lower warfarin dosage requirements compared with those with no/mild and moderate CKD (P = 0.0002). This finding remained significant after stratification by genotype and adjustment for clinical factors. Thus, a lower GFR was associated with lower warfarin dosage within each CYP2C9 and VKORC1 genotype (Table 2, Figure 1).
Table 2.
Warfarin dosage requirements (mg/d) among patients stratified by CYP2C9 and VKORC-1173 genotype and GFRa
| Parameter | eGFR
|
P | |||||
|---|---|---|---|---|---|---|---|
| ≥60 (No/Mild)
|
30 to 59 (Moderate)
|
<30 (Severe)
|
|||||
| n | Dosage (95% CI) | n | Dosage (95% CI) | n | Dosage (95% CI) | ||
| All patients (adjusted) | 314 | 4.8 (4.6 to 5.0) | 168 | 4.3 (4.0 to 4.6) | 47 | 3.9 (3.5 to 4.4) | 0.0002 |
| By genotype | |||||||
| wtCYP2C9, wtVKORC1 | 157 | 6.3 (5.9 to 6.7) | 73 | 5.5 (5.0 to 6.0) | 21 | 5.3 (4.5 to 6.2) | 0.0180 |
| wtCYP2C9, vVKORC1 | 89 | 4.7 (4.3 to 5.1) | 57 | 4.2 (3.8 to 4.6) | 13 | 3.7 (3.0 to 4.6) | 0.0450 |
| vCYP2C9, wtVKORC1 | 37 | 4.9 (4.4 to 5.5) | 14 | 4.9 (4.0 to 5.9) | 9 | 3.8 (3.0 to 4.8) | 0.1400 |
| vCYP2C9, vVKORC1 | 31 | 3.6 (3.1 to 4.1) | 24 | 3.2 (2.8 to 3.7) | 4 | 3.0 (2.1 to 4.3) | 0.3600 |
Least square means of back-transformed log-dosage adjusted for VKORC1-1173, CYP2C9, age, race, gender, BMI, vitamin K intake, alcohol, education, insurance, income, smoking, number of comorbid conditions, and concomitant therapy with CYP2C9 inhibitors and HMG-CoA inhibitors. Values given for a 60-yr-old white man with BMI 30, consuming two servings of vitamin K–rich foods per week, nondrinker, nonsmoker, with no comorbid conditions, and not on concomitant therapy with amiodarone or HMG-CoA inhibitors. CYP2C9 Variant genotype includes *2 and *3 alleles among European Americans and *2, *3, *5, *6, and *11 alleles among African Americans. Variant VKORC1-1173C/T includes 'TT or CT'.
Figure 1.
Average warfarin dosage (mg/d) by CKD stages 1 and 2 (no/mild) versus stage 3 (moderate) versus stages 4 and 5 (severe) stratified by CYP2C9 and VKORC1 genotype. Least square means of back-transformed log-dosage adjusted for VKORC1-1173, CYP2C9, GFR, age, race, gender, BMI, vitamin K intake, alcohol, education, insurance, income, smoking, number of comorbid conditions, and concomitant therapy with CYP2C9 inhibitors and HMG-CoA inhibitors. Values given for a 60-yr-old white man with BMI 30, consuming two servings of vitamin K–rich foods per week, nondrinker, nonsmoker, with no other comorbid conditions, no CYP2C9 inhibitors or HMG-CoA reductase inhibitors. Wt, wild-type; v, variant. CYP2C9 variant genotype includes *2 and *3 alleles among European Americans and *2, *3, *5, *6, and *11 alleles among African Americans. Variant VKORC1-1173C/T includes TT or CT.
Lower GFR was associated with poorer anticoagulation control as assessed by the proportion of out-of-range international normalized ratios (INRs) in both univariate and multivariable analyses. Specifically, patients with severe CKD spent less time in target INR range and had more INRs >3 as compared with patients with no/mild or moderate CKD (Table 3).
Table 3.
Association of CYP2C9 and VKORC1 genotypes with anticoagulation control among POAT participants after attainment of first target INR (range 2 to 3)a
| Parameter | GFR (ml/min per 1.73 m2)
|
P | ||
|---|---|---|---|---|
| ≥60 (No/Mild) | 30 to 59 (Moderate) | <30 (Severe) | ||
| No. of patients | 314 | 168 | 47 | |
| No. of visits | 8104 | 4500 | 1257 | |
| Follow-up (mo) | 16.8 ± 11.1 | 16.4 ± 10.8 | 11.5 ± 9.6 | |
| Unadjusted analysesb | ||||
| % INRs below range (INR <2) | 32.2 | 31.2 | 35.7 | 0.290 |
| % INRs in range (INR 2 to 3) | 49.7 | 48.2 | 40.1 | 0.002 |
| % INRs above range (INR >3) | 18.0 | 20.6 | 24.2 | 0.010 |
| Adjusted analysesb,c | ||||
| % INRs below range (INR <2) | 31.4 | 33.2 | 31.6 | 0.610 |
| % INRs in range (INR 2 to 3) | 49.7 | 45.7 | 45.6 | 0.049 |
| % INRs above range (INR >3) | 18.9 | 21.1 | 22.8 | 0.110 |
All patients had a prescribed target INR range of 2 to 3. Patients with orthopedic surgery were excluded because of short (3 to 6 mo) treatment duration, and patients with mechanical heart valve and hypercoagulable state were excluded because of higher intensity of anticoagulation required. Hispanic patients were excluded. CYP2C9 Variant genotype includes *2 and *3 alleles among European Americans and *2, *3, *5, *6, and *11 alleles among African Americans. Variant VKORC1-1173C/T (rs9934438) includes ‘TT or CT’ for both race groups.
Percentage INRs in target range, below range, and above range were assessed after attainment of first INR in target range.
Adjusted for VKORC1-1173, CYP2C9, age, race, gender, BMI, vitamin K intake, alcohol, education, insurance, income, smoking, number of comorbid conditions, concomitant therapy with CYP2C9 inhibitors and HMG-CoA inhibitors, frequency of visits, and unobserved heterogeneity.
Overanticoagulation (INR >4) was more frequently encountered among patients with severe CKD, as compared with patients with moderate CKD (incidence rate ratio [IRR] 1.8, 95% confidence interval [CI] 1.4 to 2.3; P < 0.0001). Likewise, patients with moderate CKD had a higher incidence of overanticoagulation as compared with those with no/mild CKD (IRR 1.20; 95% CI 1.10 to 1.46; P = 0.007; Table 4). The risk for overanticoagulation was higher among patients with severe CKD after adjustment for clinical and genetic factors (P = 0.052; Table 5).
Table 4.
Incidence rate of minor and major hemorrhagic complications stratified by GFR (per 100 patient-years)a
| Parameter | GFR (ml/min per 1.73 m2)
|
||
|---|---|---|---|
| ≥60 (No/Mild; n = 336) | 30 to 59 (Moderate; n = 176) | <30 (Severe; n = 53) | |
| Patient-years | 467.8 | 240.7 | 49.2 |
| INR >4 | |||
| no. of events | 392 | 251 | 93 |
| incidence rate | 83.8 (75.7 to 92.5) | 104.3 (91.7 to 118.0) | 189.0 (152.6 to 231.6) |
| Minor hemorrhage | |||
| no. of events | 147 | 78 | 52 |
| incidence rate | 31.4 (26.5 to 36.9) | 32.4 (25.6 to 40.4) | 105.7 (78.9 to 138.6) |
| Major hemorrhage | |||
| no. of events | 29 | 20 | 15 |
| incidence rate | 6.2 (4.1 to 8.9) | 8.3 (5.1 to 12.8) | 30.5 (17.0 to 50.3) |
Minor hemorrhagic complications included mild nosebleeds (lasting <30 min), microscopic hematuria, mild bruising, and mild hemorrhoidal bleeding and mild access-related bleeding. Major hemorrhagic complications included serious, life-threatening and fatal bleeding episodes as defined by Fihn et al.36 Among patients with variant CYP2C9 genotype, nine major hemorrhages occurred in patients with *1/*3, eight in *1/*2, one in *2/*3, and one in *5/*6. Five patients with GFR ≥60, two patients with GFR 30 to 59, and three patients with GFR <30 had a second major hemorrhagic event.
Table 5.
Risk for overanticoagulation and hemorrhagic complications among POAT participants by renal functiona
| Parameter | Unadjusted
|
Adjusting for Clinical Factorsb
|
Adjusting for Clinical and Genetic Factorsc
|
||
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) Robust Variance Estimation | HR (95% CI) | HR (95% CI) Robust Variance Estimation | |
| Overanticoagulation | |||||
| eGFR ≥60 | Referent | Referent | Referent | Referent | Referent |
| eGFR 30 to 59 | 1.26 (1.07 to 1.47) | 1.22 (1.03 to 1.45) | 1.22 (0.98 to 1.53) | 1.19 (1.00 to 1.42) | 1.19 (0.94 to 1.50) |
| P | 0.0040 | 0.0200 | 0.0760 | 0.0490 | 0.1400 |
| eGFR <30 | 1.89 (1.52 to 2.36) | 1.48 (1.16 to 1.90) | 1.48 (1.01 to 2.19) | 1.49 (1.16 to 1.93) | 1.49 (0.99 to 2.24) |
| P | <0.0001 | 0.0020 | 0.0460 | 0.0020 | 0.0520 |
| Major hemorrhaged (restricting analysis to first event) | |||||
| eGFR ≥60 | Referent | Referent | – | Referent | – |
| eGFR 30 to 59 | 1.52 (0.82 to 2.81) | 1.28 (0.65 to 2.51) | – | 1.33 (0.66 to 2.68) | – |
| P | P = 0.19 | P = 0.48 | P = 0.42 | ||
| eGFR <30 | 4.31 (2.14 to 8.69) | 2.65 (1.19 to 5.92) | – | 2.27 (0.97 to 5.30) | – |
| P | <0.0001 | 0.0170 | 0.0570 | ||
| Major hemorrhaged (allowing repeat events in an individual patient) | |||||
| eGFR ≥60 | Referent | Referent | Referent | Referent | Referent |
| eGFR 30 to 59 | 1.32 (0.75 to 2.34) | 1.05 (0.57 to 1.93) | 1.05 (0.53 to 2.11) | 1.18 (0.63 to 2.22) | 1.18 (0.58 to 2.40) |
| P | 0.3400 | 0.8800 | 0.8500 | 0.6000 | 0.6500 |
| eGFR <30 | 4.20 (2.25 to 7.85) | 2.39 (1.19 to 4.98) | 2.39 (1.20 to 4.78) | 2.42 (1.17 to 4.99) | 2.42 (1.11 to 5.29) |
| P | <0.0001 | 0.0160 | 0.0130 | 0.0170 | 0.0270 |
| Minor hemorrhagee (restricting analysis to first event) | |||||
| eGFR ≥60 | Referent | Referent | – | Referent | – |
| eGFR 30 to 59 | 1.07 (0.76 to 1.51) | 1.0 (0.70 to 1.44) | – | 1.01 (0.69 to 1.46) | – |
| P | P = 0.68 | P = 0.98 | P = 0.97 | ||
| eGFR <30 | 2.75 (1.80 to 4.19) | 2.33 (1.44 to 3.75) | – | 2.45 (1.48 to 4.05) | – |
| P | <0.0001 | 0.0005 | 0.0005 | ||
| Minor hemorrhagee (allowing repeat events in an individual patient) | |||||
| eGFR ≥60 | Referent | Referent | Referent | Referent | Referent |
| eGFR 30 to 59 | 1.01 (0.77 to 1.33) | 0.96 (0.72 to 1.29) | 0.96 (0.66 to 1.40) | 0.97 (0.72 to 1.30) | 0.97 (0.66 to 1.45) |
| P | 0.9300 | 0.8100 | 0.8500 | 0.8300 | 0.8700 |
| eGFR <30 | 2.87 (2.09 to 3.94) | 2.16 (1.52 to 3.09) | 2.16 (1.27 to 3.68) | 2.24 (1.56 to 3.22) | 2.24 (1.29 to 3.09) |
| P | <0.0001 | <0.0001 | 0.0040 | <0.0001 | 0.0040 |
Target INR range for all patients in the study was 2.0 to 3.0. CYP2C9 variant genotype includes *2 and *3 alleles among European Americans and *2, *3, *5, *6, and *11 alleles among African Americans. Variant VKORC1-1173C/T (rs9934438) includes 'TT or CT'.
Adjusted for age, gender, race, BMI, vitamin K and alcohol intake, warfarin dosage, smoking, interacting drugs, number of comorbid conditions, and INR at the time of the event.
Adjusted for age, gender, race, BMI, CYP2C9, VKORC1-1173C/T, vitamin K and alcohol intake, warfarin dosage, smoking, interacting drugs, number of comorbid conditions, and INR at the time of the event.
Major hemorrhagic complications included serious, life-threatening and fatal bleeding episodes as defined by Fihn et al.36 All major hemorrhagic complications were adjudicated by the director of the Anticoagulation Clinic, who was blinded to genotype. Five patients with GFR ≥60, two patients with GFR 30 to 59, and three patients with GFR <30 had a second major hemorrhagic event.
Minor hemorrhagic complications included mild nosebleeds (lasting <30 min), microscopic hematuria, mild bruising, and mild access-related bleeding.
The overall incidence of major hemorrhage was 8.4 (95% CI 6.5 to 10.7) per 100 patient-years. Patients with severe CKD had higher incidence of major hemorrhage (Table 4) as compared with those with moderate CKD (IRR 3.7; 95% CI 1.8 to 7.2; P = 0.0003) and those with no/mild CKD (IRR 4.9; 95% CI 2.6 to 9.1; P < 0.0001). The incidence of major hemorrhage was not significantly different among patients with moderate CKD compared with those with no/mild CKD (IRR 1.30; 95% CI 0.74 to 2.40; P = 0.31). Severe CKD was associated with a two-fold higher risk for major hemorrhage (P = 0.027) after adjustment for clinical and genotypic variables and correction for dependence (Table 5, Figure 2A).
Figure 2.
(A and B) Time to major (A) and minor (B) hemorrhage stratified by CKD stages 1 and (no/mild) versus stage 3 (moderate) versus stages 4 and 5 (severe). Estimated survival curve from Cox PH model adjusted for age, race, warfarin dosage, gender, BMI, INR, alcohol intake, smoking, vitamin K intake, number of comorbid conditions, drug interactions, and CYP2C9 and VKORC1 genotype.
Patients with severe CKD also had higher incidence of minor hemorrhage (Table 4), as compared with those with moderate CKD (IRR 3.2; 95% CI 2.3 to 4.6; P < 0.0001) and those with no/mild CKD (IRR 3.3; 95% CI 2.4 to 4.6; P < 0.0001). The incidence of minor hemorrhage did not differ between patients with no/mild CKD and those with moderate CKD (IRR 1.03; 95% CI 0.78 to 1.35; P = 0.82). Severe CKD was associated with a two-fold higher risk for minor hemorrhage (P = 0.004) after adjustment for clinical and genotypic variables and correction for dependence (Table 5; Figure 2B). Recognizing that access-related minor bleeding after dialysis may contribute to this increased risk, we reevaluated the association of CKD and minor hemorrhage after excluding access-related minor bleeding events. Although patients with severe CKD still demonstrated a 50% increase in risk for minor hemorrhage after exclusion of access-related events, this finding was no longer statistically significant (hazard ratio [HR] 1.54; 95% CI 0.78 to 3.03; P = 0.21).
DISCUSSION
To our knowledge, this is the first prospective study to demonstrate the influence of reduced kidney function on warfarin dosage, anticoagulation control, and risk for hemorrhage after accounting for clinical and genetic factors. Unlike previous reports that focused solely on patients with ESRD and hemodialysis patients, the inclusion of warfarin users with no/mild, moderate, and severe decrease in kidney function highlights the generalizability of these results.
There is abundant evidence that CKD and ESRD alter drug disposition by reducing systemic clearance of drugs with significant renal excretion. Less appreciated is that CKD can significantly reduce nonrenal clearance and alter the bioavailability of drugs predominantly metabolized by the liver.13 Animal studies in CKD have shown a significant downregulation (40 to 85%) of hepatic cytochrome P-450 metabolism.14,15 This is corroborated by clinical data demonstrating significant alterations in nonrenal clearance in CKD for a number of drugs. Dreisbach et al.5 demonstrated a 50% increase in the plasma warfarin S/R ratio among patients with ESRD relative to control subjects, perhaps reflecting a selective decrease in hepatic CYP2C9 activity in renal failure. Although this suggests that patients with reduced kidney function may require lower warfarin dosages, this association has not been documented. Herein, we demonstrate that patients with reduced kidney function maintained therapeutic anticoagulation (INR 2 to 3) with lower warfarin dosages independent of CYP2C9 and VKORC1 genotype after accounting for clinical factors. This suggests that warfarin may need to be initiated at a lower dosage in patients with moderate or severe decrease in kidney function, as compared with those with relatively normal kidney function.
It has been suggested that patients with reduced renal function may have a higher incidence of overanticoagulation and underanticoagulation. Moreover, the consequences of poor anticoagulation control may be more drastic in these vulnerable patients.3,16 In our study, despite the lower warfarin dosage and follow-up in an anticoagulation clinic, patients with reduced kidney function had worse anticoagulation control (lower proportion of INRs in target range) after accounting for clinical and genetic factors and after adjustment for the frequency of clinic visits. Reduced renal function was associated with a higher incidence of overanticoagulation (INR >4). Thus, this subgroup of patients may require more frequent monitoring to maintain therapeutic anticoagulation.
Patients with severe CKD and ESRD also have an increased risk for hemorrhagic complications.6,16 Three retrospective studies and one observational cohort study of hemodialysis patients receiving high-intensity anticoagulation documented a frequency of bleeding events ranging between 0.10 and 0.54 per patient-year.17–20 Two additional prospective studies of dialysis patients receiving low-intensity anticoagulation observed a bleeding frequency of 0.0 to 0.2 events per year21,22; however, these estimates were derived from studies comparing hemorrhagic risk among warfarin users and warfarin nonusers who had ESRD and were on dialysis. The use of warfarin for prevention of vascular access thrombosis and the varying intensity of anticoagulation prescribed make generalizability of these findings to the general medical population tenuous.
To our knowledge, the incidence of and risk for hemorrhagic complications among warfarin users with varying degrees of kidney function is not documented. We present results of our analyses of the kidney function hemorrhage association before adjustment for clinical factors, after adjustment for clinical factors, and after adjustment for clinical and genetic factors. Although the analyses restricted to the first event have been widely published, they ignore repeated events within the same patient (if warfarin was not discontinued). This could potentially bias an exposure–outcome association (in either direction) because all of the observed data/outcomes are not used; therefore, we present results of analyses restricted to the first hemorrhagic event and of analyses that allow multiple events per patient correcting for dependence using robust variance estimation. This level of detail is provided to facilitate comparisons with previous studies that may not have adjusted the analyses for clinical or genetic (CYP2C9, VKORC1) factors, may have restricted to analyses of the first event, or may have ignored the dependence between repeat events within the same patient. As reported previously,12 the risk for hemorrhage was higher among patients with variant CYP2C9 genotype (HR 3.1; 95% CI 1.5 to 6.3; P = 0.002) but not among those with variant VKORC1 1173 genotype (HR 0.95; 95% CI 0.48 to 1.85; P = 0.87).
Our findings indicate that severe CKD is associated with a higher risk for major hemorrhage. Although severe CKD was also associated with a higher risk for minor hemorrhage, the excess risk was likely driven by access-related minor bleeding after dialysis. The risk for hemorrhagic complications from this and previous studies of dialysis patients may have important clinical implications.23–25 First, these results suggest that simply extrapolating the results of anticoagulation trials performed with patients with normal kidney function to dialysis patients may not be appropriate, because it assumes that the benefits of and risks for anticoagulation are comparable in both patient subgroups. Second, if the risk of hemorrhage is higher in dialysis patients, then the net benefit of chronic anticoagulation therapy may not be as obvious in this patient population.
Although severe CKD has been associated with a higher risk for major hemorrhage, it is important to recognize there are limited data on its effectiveness in preventing recurrent systemic thromboembolism in this subgroup; therefore, we hesitate to recommend the use of renal function in making treatment decisions; “treat or withhold warfarin.” We need to understand the thromboembolic risk associated with withholding of warfarin therapy in patients with severe CKD. Perhaps ongoing and future research efforts evaluating both thromboembolic and hemorrhagic events will facilitate a more robust assessment of risk estimates. These risk estimates in turn will enable clinical decision making in this unique and medically challenging patient population.
These findings add fuel to the debate regarding the use of warfarin among patients with ESRD.23,24 Although the use of warfarin in prevention of vascular access thrombosis has fallen out of favor, recently, the debate has shifted to focus on warfarin use for other accepted indications, such as atrial fibrillation.4,20,23,25,26 At the crux of this debate is the realization that the risk–benefit of warfarin therapy for a patient with normal renal function cannot be extrapolated to patients with severe CKD. Certainly, the observations in this study highlight the need for larger studies to evaluate the risk–benefit of long-term warfarin therapy in patients with reduced kidney function, particularly those who are on dialysis.
This racially diverse cohort is representative of the aging population of warfarin users. Forty percent of participants in this study had reduced renal function (estimated GFR <60 ml/min per 1.73 m2). These findings highlight that diminished renal function may have implications for a larger proportion of warfarin users than previously estimated.
CONCISE METHODS
The POAT is an ongoing prospective cohort study aimed at defining the influence of polymorphisms in CYP2C9 and other genes on warfarin response during a 2-yr follow-up period.9
Inclusion and Exclusion
Patients ≥20 yr of age were considered eligible when the intended duration of anticoagulation therapy was ≥2 yr, therapy was managed at the anticoagulation clinic, and the target INR range was 2 to 3. Patients with higher target INR (range 2.5 to 3.5) were excluded. The study was conducted under the approval of the institutional review boards of the University of Alabama at Birmingham and Jefferson County Health System.
Data Collection
A detailed history documented information including self-reported race, patient demographics, education, income, medical insurance, height and weight, blood urea nitrogen, serum creatinine, hemoglobin and hematocrit, indication for therapy, comorbid conditions, medications, smoking, alcohol use, vitamin K intake, and physical activity as detailed in recent publications.9,12,27,28
All patients were followed monthly for up to 2 yr from initiation of therapy. Factors influencing warfarin response were recorded at each visit, including warfarin dosage, INR, concurrent medications, dietary vitamin K intake (number of servings of foods rich in vitamin K consumed per week),29 alcohol intake, and compliance. Changes in concomitant medications such as nonsteroidal anti-inflammatory drugs or antiplatelet agents or drugs that alter warfarin pharmacokinetics, including CYP2C9 inhibitors (e.g., amiodarone), CYP2C9 inducers (e.g., rifampin), or CYP2C9 substrates (e.g., losartan)30,31 were documented at each visit.
GFR was estimated by using the four-variable Modification of Diet in Renal Disease (MDRD) Study equation: (eGFR = 175 × standardized serum creatinine−1.154 × age−0.203 × 1.212 [if black] × 0.742 [if female]).32 Patients were categorized into three groups on the basis of GFR. Patients with GFR ≥60 (no CKD or stages 1 and 2 CKD) were categorized as having no/mild CKD, those with GFR 30 to 59 ml/min per 1.73 m (stage 3 CKD) were categorized as having moderate CKD, and those with GFR <30 ml/min per 1.73 m2 (stages 4 and 5 CKD) were categorized as having severe CKD. Patients receiving maintenance dialysis were categorized as having severe CKD (stage 5 CKD; http://www.kidney.org/kidneydisease/ckd/knowGFR.cfm). CYP2C9 and VKORC1 genotypes were determined using PCR–restriction fragment length polymorphism, pyrosequencing, and Sequenom iPLEX technology (Broad Institute, Cambridge, MA) from DNA extracted from whole blood as detailed recently.9,12,28
Statistical Analysis
ANOVA was used to assess group differences for continuous variables and χ2 test of independence for categorical variables. The assumption of Hardy-Weinberg equilibrium was tested using the χ2 test of independence, and exact statistics were obtained using a Markov Chain Monte Carlo algorithm.33
Warfarin dosage was defined as the average maintenance dosage required to maintain therapeutic anticoagulation for the duration of therapy. Because the distribution of dosage was marginally skewed, log transformation was done to attain normality. Multivariable regression analysis was used to assess the influence of renal function, CYP2C9, VKORC1 genotype on log-transformed dosage after adjustment for age, gender, BMI, smoking status, level of physical activity, alcohol intake, vitamin K intake, comorbid conditions, and drug interactions.
To assess anticoagulation control, we calculated the proportion of INRs in target range by dividing the number of INRs within range by the total number of INRs for each patient. Computation of this measure encompassed the period of time from attainment of first target INR until the end of the follow-up period. Multivariable regression analysis was conducted to assess the influence of renal function on proportion of INRs in target range after adjustment for clinical and genetic factors and frequency of measurements/unobserved heterogeneity.
Accounting for Frequency of Measurements and Unobserved Heterogeneity
In any given patient, INR fluctuates over time in response to medication or dietary changes. Moreover, as the interval between INR measurements decreases, the variation in INR decreases34; therefore, by default, patients who are evaluated more often may tend to have lower variation (heterogeneity) in the INR and a higher proportion of INRs in target range.35 To capture the potential influence of such factors, we computed a patient-specific variance growth rate (Vscore), a cumulative measure of time-weighted variance of the INR for each time interval (between visits) as proposed by Fihn et al.,36 with minor modification.9 This measure adjusts for the influence of the frequency of visits and the interval between visits on anticoagulation control for each patient.
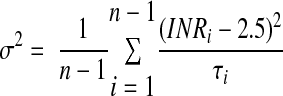 |
σ2 = variance growth rate (Vscore)
n = number of visits (n − 1 will compute the Vscore up to the preceding visit)
τi = duration in weeks since preceding clinic visit (INR measurement)
INRi = INR at the ith visit
Modified from Fihn et al.36
To assess the risk for overanticoagulation (INR >4) and hemorrhagic events, we obtained the HRs and 95% CIs using the counting process format in the proportional hazard (PH) model.37,38 This format allows individuals to contribute more than one event. Valid CIs were obtained by correction of dependence using robust variance estimation. Departures from the PH assumption were assessed by evaluating interactions of the predictors and a function of survival time. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC) at a nondirectional α level of 0.05.
Classification of Events
The review of complications included objective documentation of complication site (e.g., endoscopy of gastrointestinal tract), gravity of the event (e.g., requiring transfusion, surgical intervention), and laboratory findings (e.g., INR, hemoglobin/hematocrit) at the time of the event. Isolated subtherapeutic or supratherapeutic INRs in the absence of evidence of bleeding were not classified as events.
Hemorrhagic complications were classified using the scheme detailed by Fihn et al.36 Minor hemorrhages included mild nosebleeds, microscopic hematuria, mild bruising, and mild hemorrhoidal bleeding. Among patients with ESRD, oozing/bleeding from the vascular access site after dialysis were considered minor bleeding events. Major hemorrhages included serious, life-threatening and fatal bleeding episodes. If the severity of the bleed warranted discontinuation of warfarin, then the patient data were censored at that time point.
Patient Follow-up, Event Documentation, Verification, and Adjudication
During the 2-yr follow-up, all major hemorrhagic complications were captured and verified through review of admissions and emergency department visits, and minor hemorrhages were obtained by patient self-report. Only medically documented events were included in the analyses. The Alabama Center for Health Statistics was queried to verify cause of death for all deceased to ensure inclusion of deaths as a result of hemorrhagic complications. All complications were documented by the study nurse, confirmed by the principal investigator, and finally reviewed independently by the medical director of the Anticoagulation Clinic. Genotype information was maintained separately until data analysis.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by a grant from the National Heart Lung and Blood Institute (RO1HL092173); National Institute of Neurologic Disorders and Stroke (NINDS; K23NS45598); and the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. The Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278-01 from the National Center for Research Resources.
This study has contributed samples to the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds), NINDS Repository sample numbers corresponding to the samples used are ND04466, ND04556, ND04604, ND04605, ND04626, ND04869, ND04907, ND04934, ND04951, ND05036, ND05108, ND05175, ND05176, ND05239, ND05605, ND05606, ND05701, ND05702, ND05735, ND06147, ND06207, ND06385, ND06424, ND06480, ND06706, ND06814, ND06871, ND06983, ND07057, ND07234, ND07304, ND07494, ND07602, ND07711, ND07712, ND08065, ND08596, ND08864, ND08932, ND09079, ND09172, ND09760, ND09761, and ND09809.
We thank Joyce Blaisdell for work with CYP2C9 genotyping. N.A.L. acknowledges Dr. Edward Faught for support and mentorship. We are grateful to all of the patients who participated in the study. We thank Janice Ware for untiring efforts with patient recruitment and the staff of the Anticoagulation Clinic at the Kirklin Clinic, the Cooper Green Hospital, and Jefferson Clinic PC for help with identification of potential participants. We also thank the physicians, especially Drs. Mark Wilson and Melissa Baird, at the University of Alabama at Birmingham and the Health Service Foundation for support of this research. Thanks to Steve Duncan and Darlene Green and the Office of Data Resources for work with the POAT database and quality assurance.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Budnitz DS, Shehab N, Kegler SR, Richards CL: Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 147: 755–765, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Wittkowsky AK: Effective anticoagulation therapy: Defining the gap between clinical studies and clinical practice. Am J Manag Care 10: S297–S306, discussion S312–S317, 2004 [PubMed] [Google Scholar]
- 3.Wittkowsky AK, Devine EB: Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy 24: 1311–1316, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, Valsecchi MG: Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis 51: 255–262, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Dreisbach AW, Japa S, Gebrekal AB, Mowry SE, Lertora JJ, Kamath BL, Rettie AE: Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther 73: 475–477, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Elliott MJ, Zimmerman D, Holden RM: Warfarin anticoagulation in hemodialysis patients: A systematic review of bleeding rates. Am J Kidney Dis 50: 433–440, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, Bentley D, McGinnis R, Deloukas P: Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121: 23–34, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClain MR, Palomaki GE, Piper M, Haddow JE: A rapid-ACCE review of CYP2C9 and VKORC1 alleles testing to inform warfarin dosing in adults at elevated risk for thrombotic events to avoid serious bleeding. Genet Med 10: 89–98, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Limdi NA, Arnett DK, Goldstein JA, Beasley TM, McGwin G, Adler BK, Acton RT: Influence of CYP2C9 and VKORC1 polymorphisms on warfarin dose, anticoagulation attainment and maintenance among European American and African Americans. Pharmacogenomics 9: 511–526, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schelleman H, Chen Z, Kealey C, Whitehead AS, Christie J, Price M, Brensinger CM, Newcomb CW, Thorn CF, Samaha FF, Kimmel SE: Warfarin response and vitamin K epoxide reductase complex 1 in African Americans and Caucasians. Clin Pharmacol Ther 81: 742–747, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, Rettie AE: Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287: 1690–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Limdi N, McGwin G, Goldstein J, Beasley T, Arnett D, Adler B, Baird M, Acton R: Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Mol Ther 83: 312–321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichette V, Leblond FA: Drug metabolism in chronic renal failure. Curr Drug Metab 4: 91–103, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Dreisbach AW, Lertora JJ: The effect of chronic renal failure on hepatic drug metabolism and drug disposition. Semin Dial 16: 45–50, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V: Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol 12: 326–332, 2001 [DOI] [PubMed] [Google Scholar]
- 16.McMahan DA, Smith DM, Carey MA, Zhou XH: Risk of major hemorrhage for outpatients treated with warfarin. J Gen Intern Med 13: 311–316, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biggers JA, Remmers AR Jr, Glassford DM, Sarles HE, Lindley JD, Fish JC: The risk of anticoagulation in hemodialysis patients. Nephron 18: 109–113, 1977 [DOI] [PubMed] [Google Scholar]
- 18.LeSar CJ, Merrick HW, Smith MR: Thrombotic complications resulting from hypercoagulable states in chronic hemodialysis vascular access. J Am Coll Surg 189: 73–79, discussion 79–81, 1999 [DOI] [PubMed] [Google Scholar]
- 19.O'Shea SI, Lawson JH, Reddan D, Murphy M, Ortel TL: Hypercoagulable states and antithrombotic strategies in recurrent vascular access site thrombosis. J Vasc Surg 38: 541–548, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Vazquez E, Sanchez-Perales C, Garcia-Cortes MJ, Borrego F, Lozano C, Guzman M, Gil JM, Liebana A, Perez P, Borrego MJ, Perez V: Ought dialysis patients with atrial fibrillation be treated with oral anticoagulants? Int J Cardiol 87: 135–139, discussion 139–141, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Crowther MA, Clase CM, Margetts PJ, Julian J, Lambert K, Sneath D, Nagai R, Wilson S, Ingram AJ: Low-intensity warfarin is ineffective for the prevention of PTFE graft failure in patients on hemodialysis: A randomized controlled trial. J Am Soc Nephrol 13: 2331–2337, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Zellweger M, Bouchard J, Raymond-Carrier S, Laforest-Renald A, Querin S, Madore F: Systemic anticoagulation and prevention of hemodialysis catheter malfunction. ASAIO J 51: 360–365, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Abbott KC, Neff RT, Bohen EM, Narayan R: Anticoagulation for chronic atrial fibrillation in hemodialysis patients: Which fruit from the decision tree? Am J Kidney Dis 50: 345–348, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Bennett WM: Should dialysis patients ever receive warfarin and for what reasons? Clin J Am Soc Nephrol 1: 1357–1359, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Quinn RR, Naimark DM, Oliver MJ, Bayoumi AM: Should hemodialysis patients with atrial fibrillation undergo systemic anticoagulation? A cost-utility analysis. Am J Kidney Dis 50: 421–432, 2007 [DOI] [PubMed] [Google Scholar]
- 26.To AC, Yehia M, Collins JF: Atrial fibrillation in haemodialysis patients: Do the guidelines for anticoagulation apply? Nephrology (Carlton) 12: 441–447, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Limdi NA, Beasley TM, Allison DB, Rivers CA, Acton RT: Racial differences in the prevalence of factor V Leiden mutation among patients on chronic warfarin therapy. Blood Cells Mol Dis 37: 100–106, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limdi NA, Goldstein JA, Blaisdell JA, Beasley TM, Rivers CA, Acton RT: Influence of CYP2C9 Genotype on warfarin dose among African American and European Americans. Pers Med 4: 157–169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth SL, Saowski SJ, Weihrauch JL, Ferland G: Vitamin K (phylloquinone) content of foods. J Food Consump Anal 6: 109–120, 1993 [Google Scholar]
- 30.Miners JO, Birkett DJ: Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45: 525–538, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rettie AE, Jones JP: Clinical and toxicological relevance of CYP2C9: Drug-drug interactions and pharmacogenetics. Annu Rev Pharmacol Toxicol 45: 477–494, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Guo SW, Thompson EA: Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 48: 361–372, 1992 [PubMed] [Google Scholar]
- 34.Ansell J, Hollowell J, Pengo V, Martinez-Brotons F, Caro J, Drouet L: Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: The international study of anticoagulation management (ISAM). J Thromb Thrombolysis 23: 83–91, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Shalev V, Rogowski O, Shimron O, Sheinberg B, Shapira I, Seligsohn U, Berliner S, Misgav M: The interval between prothrombin time tests and the quality of oral anticoagulants treatment in patients with chronic atrial fibrillation. Thromb Res 120: 201–206, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Fihn SD, McDonell M, Martin D, Henikoff J, Vermes D, Kent D, White RH: Risk factors for complications of chronic anticoagulation: A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med 118: 511–520, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Ake CF, Carpenter AL: Extending the Use of PROC PHREG in Survival Analysis: Proceedings of the 11th Annual Western Users of SAS, SAS Institute Inc., Cary, NC, 2003
- 38.Therneau TM, Grambsch PM: Multiple events per subject. In: Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health), edited by Therneau TM, Grambsch PM, New York, Springer-Verlag, 2001, pp 169–229