Abstract
Multipotent mesenchymal stromal cells from the bone marrow ameliorate acute kidney injury through a mechanism other than transdifferentiation into renal tissue. Stromal cells exert immunoregulatory effects on dendritic and T cells, both of which are important in the pathophysiology of immune-mediated kidney injury. We hypothesized that similar cells with immunoregulatory function exist within the adult kidney. We isolated murine kidney-derived cells with morphologic features, growth properties, and an immunophenotype characteristic of mesenchymal stromal cells. These cells lacked lineage markers and could be differentiated into mesodermal cell lineages, including osteocytes and adipocytes. Furthermore, these kidney-derived cells induced the generation of bone marrow–derived dendritic cells with significantly reduced MHC II expression, increased CD80 expression, increased IL-10 production and the inability to stimulate CD4+ T cell proliferation in allogeneic and nominal antigen-specific cultures. Experiments in mixed and transwell cultures demonstrated that the production of soluble immune modulators, such as IL-6, was responsible for these effects on dendritic cell differentiation and maturation. Contact-dependent mechanisms, however, inhibited mitogenic T cell proliferation. In summary, kidney-derived cells may suppress inflammation in the kidney in vivo; a better understanding of their biology could have therapeutic implications in a wide variety of immune-mediated kidney diseases.
The kidney undergoes continuous, slow cellular turnover for tissue maintenance and rapid cell replacement after injury, as evidenced by cellular proliferation and functional recovery after ischemic tubular necrosis.1,2 Considerable debate exists, however, over the origin of newly generated renal cells.3 Bone marrow (BM) contains at least two populations of progenitor cells: (1) Hematopoietic stem cells (HSCs), which give rise to all differentiated blood cell types, and (2) multipotent mesenchymal stromal cells (MSCs),4,5 which differentiate into cells of mesodermal lineage. Early reports of injection of crude BM preparations during acute kidney injury (AKI) seemed to demonstrate transdifferentiation and ability to replace damaged tissue.6,7 A subsequent study demonstrated that injection of BM-MSCs but not HSCs was protective during AKI8; however, these cells seemed to ameliorate renal injury and accelerate repair without localization to the kidney,9,10 questioning their ability to regenerate renal tissue. Moreover, Bi et al.11 showed that passive transfer of conditioned medium from cultured BM-MSCs afforded the same renoprotection, indicating humoral factors were sufficient to protect or to promote repair of injured nephrons. Effects of BM-MSCs or their conditioned medium on inflammation during AKI were not addressed in these studies.
There is considerable evidence that human BM-MSCs exert immunoregulatory effects,12 including inhibition of dendritic cell (DC) alloantigen recognition and processing13–15; suppression of T cell responses16; induction of regulatory T cells17–19; suppression of B lymphocyte proliferation and antibody production20; and suppression of proliferation, cytokine production, and cytotoxicity of NK cells.21 These effects seem to be mediated, in part, through soluble factors.18,22 Outside the BM, MSCs have been identified in a variety of postnatal organs,23–27 including kidney23,28–31; however, their immunomodulatory properties are only beginning to be studied.24 Dekel et al.31 isolated MSCs from mouse kidney that inhibited alloreactive CD8+ T cell responses, although a comprehensive assessment of their immunoregulatory properties was not performed. Given the importance of inflammation in the pathophysiology of AKI,32,33 the immunoregulatory properties of kidney-resident MSCs or their soluble products may play a significant role in renoprotection.34 In addition to AKI, these properties could be important in other immune-mediated diseases, including glomerulonephritis, interstitial nephritis, and allograft rejection.
T cells play a direct pathogenic role in renal dysfunction during AKI.33,35 Although the antigen specificity of these T cells is not known, it is implicit from these studies that T cell antigen-presenting cell (APC) interactions occur after AKI and result in injury to the kidney. Dendritic cells (DCs) are potent APCs with ability to induce tolerance or immunity and are positioned within the renal parenchyma to respond to changes in the interstitial compartment.36,37 During inflammation, steady-state DCs upregulate surface expression of MHC class II and migrate to regional lymph nodes (LNs) to activate T cells. It has been shown that AKI augments the capacity for DC-mediated T cell activation in the LNs of the kidney.38 Furthermore, resident DCs seem to be the predominant “first responders” in secretion of TNF and other inflammatory mediators active during AKI.39 In nephrotoxic glomerulonephritis, focal DC accumulation has been demonstrated within tubulointerstitial mononuclear infiltrates,40 whereas more recent reports indicated that renal DCs exert a renoprotective effect in this model.41 Thus, efforts to ameliorate renal damage during immune-mediated kidney diseases should address these important cell types.
The aim of this study was to determine whether multipotent mesenchymal stromal-like cells with immunoregulatory function exist within adult kidney and to assess their effects on immune cells active during kidney injury. Using a clinically applicable method reported for isolation and expansion of adult human progenitor cells from endomyocardial biopsy specimens,42 we identified a population of sphere-derived cells from adult mouse kidney with characteristics of MSCs, including immunoregulatory effects on BM-derived DCs and splenic T cells. Collectively, these data support the hypothesis that kidney sphere–derived cells (KSCs) influence host immunity and may be responsible for suppressing local inflammation in the kidney during immune-mediated kidney diseases.
RESULTS
Isolation, Expansion, and Characterization of KSCs
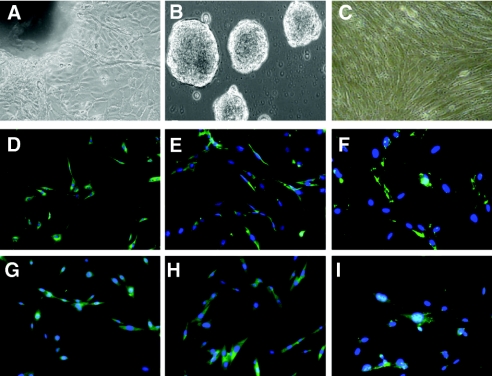
The KSC isolation and expansion technique is detailed in the Concise Methods section. Seven days after minced kidney tissue was transferred to nutrient medium and firmly attached to fibronectin plates, the mixed cells that spontaneously shed from the explants (Figure 1A) were harvested and transferred to poly-d-lysine plates to generate spherical multicellular clusters. These kidney spheres emerged after 5 more days of culture and detached from the plates (Figure 1B). Floating spheres were isolated and plated for expansion, and the resultant sphere-derived monolayer spindle-shaped cells were passaged at 4- to 8-d intervals (Figure 1C).
Figure 1.
Isolation, expansion, and characterization of KSCs. (A) Edge of kidney explant 7 d after plating on fibronectin, showing mixed cell types. (B) Formation of kidney spherical multicellular cells 5 d after transfer of mixed cells to poly-d-lysine plates. (C) KSCs during passage 2, plated on fibronectin for expansion. Analysis of KSCs (D through F) and BM-MSCs (G through I) by fluorescence microscopy on whole mount for α-smooth muscle actin (D and G), vimentin (E and H), and fibronectin (F and I), double stained with DAPI. Magnifications: ×10 to 20 in A through C; ×20 in D through I.
Flow cytometric analysis of surface markers is shown in Table 1. KSCs expressed a combination of classic stem cell antigens including Sca-1 (90%), CD34 (41%), and CD24 (52%) but not CD117, CD31, CD90, or CD133. In addition, KSCs stained positive for MSC markers such as CD29 (95%), CD44 (75%), CD105 (67%), CD166 (56%), CD106 (50%), CD49e (32%), CD73 (28%), Stro-1 (21%), and CD13 (5%). Slight expression of MHC class I (15%) was also detected. No expression of leukocyte markers CD45 (leukocyte common antigen), CD3 (pan T cell), CD4, CD8, CD19 (B cell), CD45R/B220 (B cell), CD11c (DC), Ly-6G (granulocyte), co-stimulatory molecules, or MHC class II could be demonstrated. Expression of mesenchymal markers, vimentin, α-smooth muscle actin, and fibronectin (weak; Figure 1D, E, and F, respectively) but not epithelial cell markers cytokeratin or ZO-1 (data not shown) could be detected by immunofluorescence. Markers expressed on renal tubular cells, including CD326 (EpCAM) or MUC1 (Mucin), were not detected using freshly harvested renal cells as positive control. Immunophenotype analysis of BM-MSCs obtained for comparison was consistent with that reported in the literature43 and very similar to that of KSCs (Figure 1, G through I; Table 1).
Table 1.
Immunophenotype of KSC and BM-MSCa
| Nomenclature | Cell Type or Molecule Name | Detection
|
|
|---|---|---|---|
| KSC | BM-MSC | ||
| CD29 | Integrin ß1 | +++ | +++ |
| Sca-1 | Stem cell antigen | +++ | +++ |
| CD105 | Endoglin | ++ | +++ |
| CD44 | HCAM, Pgp-1, Ly-24 | ++ | ++ |
| CD34 | Mucosialin | ++ | ++ |
| CD49e | Integrin α5 | ++ | ++ |
| CD73 | Ecto-5′-nucleotidase | ++ | ++ |
| CD24 | Heat-stable antigen | ++ | + |
| Stro-1 | Stromal cell | ++ | + |
| CD166 | ALCAM | ++ | − |
| CD106 | VCAM-1 | ++ | + |
| H-2Kb | MHC I | + | + |
| CD13 | Aminopeptidase N | + | − |
| CD3e | Pan T cell | − | − |
| CD4 | Helper T cell | − | − |
| CD8a | Cytotoxic T cell | − | − |
| CD11c | Dendritic cell | − | − |
| CD19 | Pan B cell | − | − |
| CD25 | IL-2 receptor | − | − |
| CD31 | PECAM-1 | − | − |
| CD45 | Leukocyte common marker | − | − |
| CD45R/B220 | B cell | − | − |
| CD54 | ICAM-1 | − | − |
| CD62L | l-selectin | − | − |
| CD86 | B7-2 co-stimulator | − | − |
| CD90.2 | Thy-1 | − | − |
| CD117 | c-Kit | − | − |
| CD133 | Prominin 1 | − | − |
| CD197 | CCR7, EBI-1 | − | − |
| CD326 | EpCAM | − | − |
| IA/IE | MHC II | − | − |
| LT-α | Lymphotoxin α | − | − |
| Ly-6G | Granulocytes | − | − |
| MUC1 | Mucin | − | − |
Mouse KSCs and BM-MSCs were analyzed by FACS using a panel of antibodies with proper isotype controls. The expression of individual molecules was scored as follows: −, 0 to 2% positive; +, 2 to 20% positive; ++, 20 to 80% positive; and +++, >80% positive.
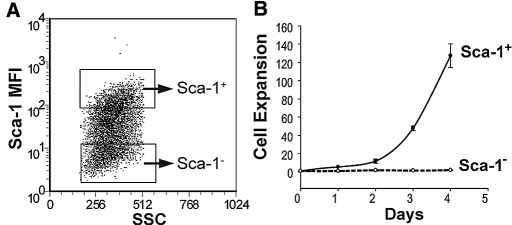
We found that KSC expression of Sca-1 increased from 40 to 60% at passage 2 to 90% by passage 3 or higher. Purified Sca-1− KSCs (>99% purity) from passage 2 showed significantly slower cell expansion when compared with Sca-1+ cells (Figure 2). Moreover, with later passages, Sca-1− cells became positive, suggesting either acquisition of this antigen over time or, alternatively, a selective advantage of contaminating Sca-1+ cells in our culture conditions.
Figure 2.
Sorting and expansion of Sca-1+ or Sca-1− KSCs. (A) Representative FACS plot showing Sca-1+ and Sca-1− cells in passage 2 before flow sorting. (B) Equal numbers of purified Sca-1+ and Sca-1− cells were cultured and counted daily. Cell expansion was calculated as the daily cell number divided by the initial cell number.
In Vitro Differentiation Capacity
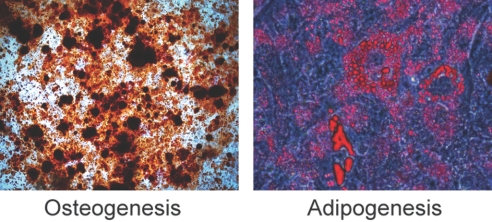
To examine their differentiation capacity, we cultured KSCs under conditions favorable for osteogenesis or adipogenesis. After 3 to 4 wk, KSCs transformed into mineralized nodules staining positive for Alizarin Red (osteogenesis). Likewise, cytoplasmic accumulation of oil droplets stained with Oil Red O, indicating adipogenesis (Figure 3). Staining was absent on control KSCs grown in standard KSC medium (data not shown).
Figure 3.
KSC differentiation capacity. KSCs were incubated with osteogenic or adipogenic culture medium for 3 to 4 wk. KSC differentiation to osteocytes or adipocytes was assessed by Alizarin Red and Oil Red O staining, respectively.
KSCs Induce the Differentiation of BM-Derived DCs with Reduced MHC Class II and Increased CD80 Expression
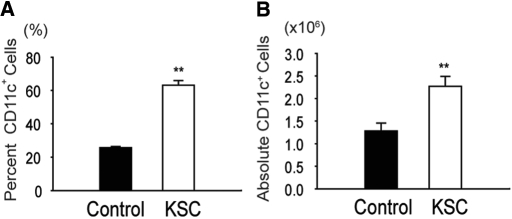
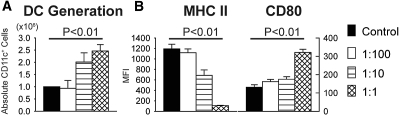
To address whether KSCs altered DC differentiation from BM cells, we cultured KSCs pretreated with mitomycin to inhibit their proliferation with C57BL/6 BM cells in the presence of GM-CSF/IL-4, as described in the Concise Methods section. When added to incipient DC transwell cultures (day 0), KSCs significantly increased (1) DC frequency (CD11c+ percentage of total live cells) and (2) DC number (absolute CD11c+ cells per well; Figure 4). No difference in DC necrosis (7-AAD+) was observed between cultures (12.5 ± 0.6 versus 12.6 ± 2.5%; NS), indicating KSCs induced DC differentiation from BM precursors rather than preventing DC death. Negligible DC differentiation (<1% CD11c+ cells) was observed in any cultures without GM-CSF/IL-4. Likewise, control experiments with KSCs cultured alone with GM-CSF/IL-4 showed no differentiation into CD11c+ cells (data not shown).
Figure 4.
Generation of BM-derived DCs in the presence of KSCs. (A and B) Addition of KSCs to transwells of incipient DC culture (day 0) markedly induced the differentiation of DC (CD11c+) from BM cells, as determined by percentage of total live cells (7-AAD negative; A) and absolute DCs per well(B). **P < 0.01 versus control wells.
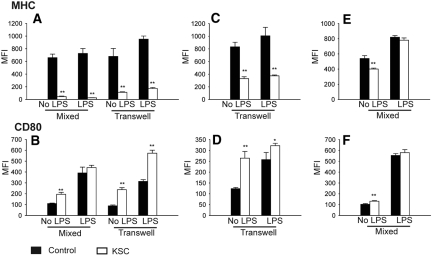
To determine whether KSCs modulated DC activation/maturation, we assessed expression of MHC class II, CD80, and CD86. KSCs treated DCs were characterized by significantly reduced MHC class II (Figure 5A) and increased CD80 (Figure 5B) expression before and after LPS stimulation. In separate experiments, DCs were isolated from KSC-DC co-culture and recultured alone for 2 more days. Removal of DCs from the KSC co-culture milieu failed to reverse the KSC effect on DC MHC class II (Figure 5C) and CD80 (Figure 5D) expression, even after LPS stimulation. Furthermore, analysis of culture supernatants from these LPS-stimulated DCs revealed significantly higher IL-10 levels compared with control (135.7 ± 12.4 versus 46.1 ± 6.9 pg/ml; P < 0.01). When added to later DC culture (day 5), KSCs induced significant but less dramatic effects (Figure 5, E and F) at baseline, although significance was lost after LPS stimulation. Effects of KSCs on DC CD86 expression were equivocal (data not shown). Separate experiments with mitomycin added to control wells showed no significant differences, ruling out an effect of this compound (data not shown). Statistically significant but relatively small differences were demonstrated between mixed and transwell culture systems (Figure 5, A and B), indicating that although cell–cell contact mechanisms may be contributory, soluble factors clearly predominate. Furthermore, addition of conditioned medium from KSC culture to incipient DC culture mirrored the findings seen with KSC-DC co-culture (Figure 6, A and B). Thus, KSCs exert immunomodulatory effects early during DC differentiation that seem predominately due to soluble factors, yielding an IL-10–producing DC with very low expression of MHC class II.
Figure 5.
DC expression of MHC class II and CD80 after KSC-DC co-culture. (A through D) Significantly decreased MHC class II and increased CD80 expression were noted on DCs with and without LPS stimulation compared with control after KSC addition to incipient DC culture (day 0) and analysis on day 5 (A and B) or KSC addition to incipient DC culture but DC removal at day 6 and re-culture for 2 more days before analysis (C and D). (E and F) After KSC addition to late DC culture (day 5), these same effects were demonstrated on DCs at baseline but were less pronounced after LPS stimulation. **P < 0.01 versus control wells.
Figure 6.
DC generation and expression of MHC class II and CD80 after addition of KSC-conditioned medium (CM). (A and B) When added to incipient DC culture, KSC-CM induced the differentiation of DC (absolute CD11c+ cells per well; A) with decreased MHC class II and increased CD80 expression (B) in a dosage-dependent manner compared with wells after addition of control medium alone. Ratio of KSC-CM:control medium at 1:1, 1:10, and 1:100.
Effect of KSCs on DC Phenotype Is Partially Due to an IL-6–Dependent Mechanism
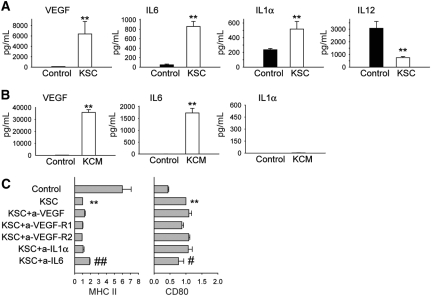
In an effort to identify the soluble factor(s) responsible for the effects on DC differentiation, we performed analysis of KSC-DC co-culture supernatants, with selection of candidate proteins based on previous BM-MSC studies showing them to be active or upregulated.44–47 Results revealed a large increase in vascular endothelial growth factor (VEGF), IL-6, and IL-1α, with a reduction in IL-12 concentrations compared with control DC culture (Figure 7A). KSCs cultured alone revealed heavy VEGF and IL-6 but negligible IL-1α production (Figure 7B), suggesting that KSCs did not produce but rather induced IL-1α production from cells in DC culture. Addition of neutralizing antibodies to VEGF, VEGF receptors 1 and 2, and IL-1α individually or in combination failed to reverse the KSC effect, although a small but significant diminution was demonstrated after neutralization of IL-6 (Figure 7C). Thus, KSC effects on DC MHC class II and CD80 surface marker expression seem to be partially due to an IL-6–dependent mechanism, although additional soluble factors contribute.
Figure 7.
Analysis of cytokine data. (A) Supernatants from KSC-DC co-culture after 5 d demonstrated significantly increased VEGF, IL-6, IL-1α and decreased IL-12 concentrations compared with control DC cultures. (B) Analysis of supernatants from culture of KSCs alone (KCM) revealed increases in VEGF and IL-6 but not IL-1α levels. (C) Neutralizing antibodies to VEGF, VEGF receptors 1 and 2, and IL-1α individually or in combination failed to reverse the effects on DC surface MHC class II and CD80 expression after KSC-DC co-culture, although a small but statistically significant effect was demonstrated after neutralization of IL-6. **P < 0.01 versus control wells; ##P < 0.01, #P < 0.05 versus KSC wells.
KSCs Inhibit the T Cell Stimulatory Capacity of BM-Derived DCs
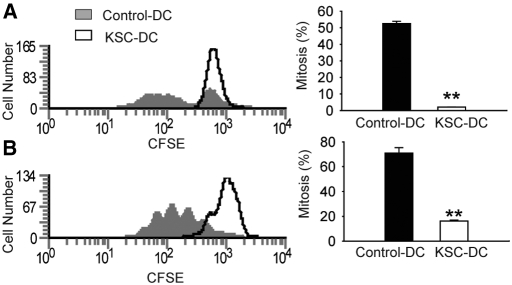
To investigate the effects of KSCs on DC stimulatory capacity, we purified LPS-treated C57BL/6 DC from transwell KSC-DC co-cultures and mixed them with purified carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+ splenic T cells from (1) BALB/c mice (allogeneic MLR) or (2) transgenic OT-II mice on syngeneic C57BL/6 background in the presence of OVA323-339 (nominal antigen-specific T cell response). Results showed that DCs from KSC-DC transwell co-cultures stimulated allogeneic (Figure 8A) and nominal antigen-specific T cell proliferation (Figure 8B) poorly compared with control DC. The small amount of OT-II transgenic T cell proliferation observed in the KSC-DC group is consistent with that observed with OVA323-339 peptide alone (KSC-DC versus OVA peptide alone 16 ± 0.8 versus 15 ± 1.7%; P = 0.41) and thus reflects proliferation stimulated by contaminating OT-II APCs or autopresentation of peptide in a T cell–to–T cell manner, rather than KSC-DC stimulation. Addition of blocking anti-CD80 antibody abrogated KSC-DC–mediated T cell suppression in allogeneic cultures (control versus anti-CD80 9.7 ± 3.6 versus 44.1 ± 1%; P < 0.01), arguing for involvement of the increased KSC-DC CD80 expression mechanistically in the observed suppression.
Figure 8.
KSC-DC stimulated T cell proliferation. (A and B) LPS-stimulated C57BL/6 KSC-DCs or control DCs were isolated from transwell co-culture on day 5 and mixed with CFSE-labeled CD4+ BALB/c T cells (allogeneic response; A) or OT-II transgenic T cells plus OVA323-339 peptide (nominal antigen antigen-specific response; B). Proliferation is expressed as percentage mitosis, as determined by loss of CFSE fluorescence by flow cytometric analysis. Proliferation was significantly less with KSC-DC versus control DC stimulators in either culture system. **P < 0.01 versus control wells.
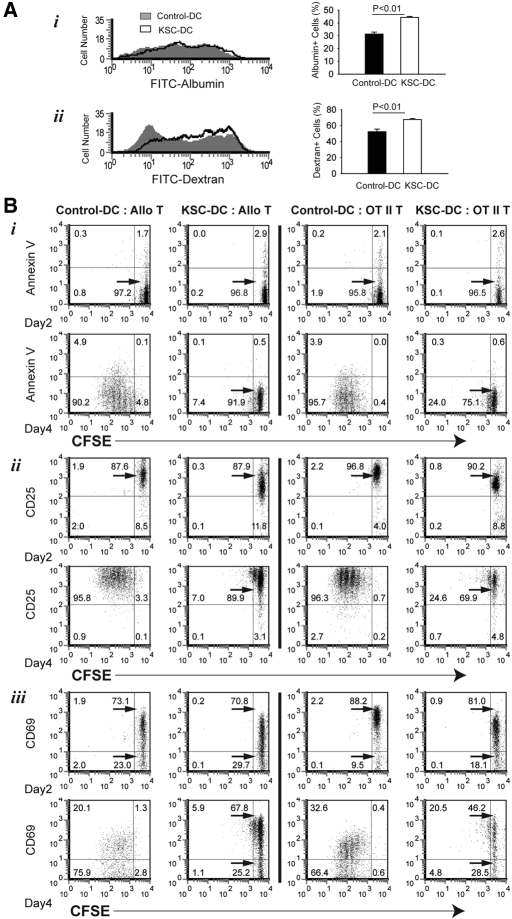
Because antigen uptake is a critical DC biologic function, we evaluated the ability of KSC-DCs to perform this function in vitro. Isolated KSC-DCs were analyzed by flow cytometry after culture with FITC-albumin (macropinocytosis) or FITC-dextran (mannose receptor–mediated endocytosis). Results showed KSC-DCs to have increased antigen uptake ability compared with control DCs, consistent with their more immature state (Figure 9A). We next analyzed whether KSC-DCs inhibited T cell proliferation via proapoptotic effects. Proliferation and apoptosis were measured using double staining with CFSE and Annexin V after 2 and 4 d of culture. Consistent with the data presented in Figure 8, CFSE-labeled T cells proliferated significantly less in allogeneic and nominal antigen-specific cultures at 4 d after stimulation by KSC-DCs compared with control DCs (Figure 9Bi). Low level, presumably activation-induced, apoptosis (4 to 5%) was demonstrated in control DC-stimulated cultures at this same time point; however, negligible apoptosis was noted in KSC-DC–stimulated cultures, arguing against this process as a mechanism for inhibition of T cell proliferation. Finally, KSC-DCs did not prevent T cell activation, because the vast majority of T cells in either culture system expressed CD25 and CD69 2 d after stimulation (Figure 9B, ii and iii, respectively). Loss of CD69 expression occurred by day 4 in all cultures but was more pronounced after control DC stimulation, consistent with more proliferation in that group. Thus, KSCs inhibit the ability of DCs to stimulate T cell proliferation but not by impairing their ability to process or present antigen to T cells.
Figure 9.
Effects of KSCs on DC antigen uptake and DC-mediated T cell apoptosis/activation. (A) Isolated KSC-DCs or control DCs were analyzed by flow cytometry after culture with FITC-labeled albumin (constitutive macropinocytosis; i) or FITC-labeled dextran (mannose receptor-mediated endocytosis; ii). Significantly increased uptake for both molecules could be demonstrated in KSC-DCs versus control DCs. (B) After co-culture of C57BL/6 KSC-DCs or control DCs with CFSE-labeled BALB/c T cells (Allo T) or syngeneic OT-II transgenic T cells plus OVA323-339 peptide (OTII T), cells were stained on day 2 or 4 with Annexin V (gate = 7AAD−CD4+ cells; i) or APC-conjugated CD25 (ii) and PE-conjugated CD69 (gate = CD4+ cells; iii) and analyzed by flow cytometry. No significant increase in apoptosis could be demonstrated in T cells stimulated by KSC-DCs versus control DCs in either culture system. T cell activation markers CD25 and CD69 were upregulated on the majority of T cells at day 2 and remained upregulated by day 4 in the case of CD25. Loss of CD69 occurred by day 4 to a larger degree in control DC- versus KSC-DC–stimulated T cells.
KSCs Directly Inhibit T Cell Proliferation by a Contact-Dependent Mechanism
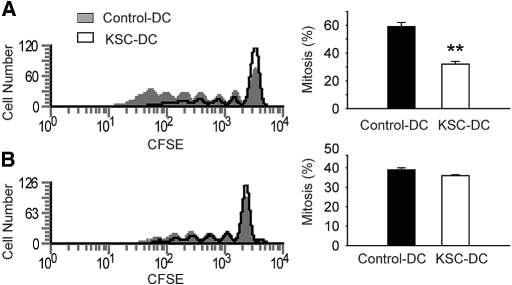
To determine the direct effects of KSC on T cell proliferation, we added KSCs to mitogen-stimulated CFSE-labeled splenic CD4+ T cell cultures. In these experiments, KSCs significantly reduced T cell proliferation (Figure 10A). Interestingly, addition of conditioned medium from KSC culture failed to inhibit T cell proliferation (Figure 10B). Thus, in contrast to the inhibitory effects of soluble factors on DC-induced T cell proliferation, these results suggest a requirement for cell–cell contact between KSC and T cells for direct T cell suppression.
Figure 10.
Direct effects of KSCs on anti-CD3/CD28–stimulated T cell proliferation. CFSE-labeled isolated CD4+ splenic T cells were stimulated with anti-CD3/CD28, and proliferation was assessed by loss of fluorescence on day 5. (A) Addition of KSCs directly to culture significantly inhibited T cell proliferation. (B) Addition of KSC-CM failed to inhibit T cell proliferation. **P < 0.01 versus control wells.
DISCUSSION
Several groups have isolated multipotent mesenchymal stromal cells from adult mammalian organs,23–27 including kidney.23,28–31 In our study, we used a technique42 that requires no antigen selection step and uses the emerging kidney spherical multicellular clusters from initial culture as the basis for cell expansion. This approach provides consistent starting material for cell expansion and minimizes fibroblast contamination, because only nonadherent kidney spheres are plated to generate KSCs. Multilineage differentiation was demonstrated by KSCs into cells of mesodermal lineage, including osteocytes and adipocytes, a well-described characteristic of MSCs. Analysis of KSC surface markers in parallel with BM-MSCs from the identical mouse strain revealed a similar profile. Likewise, KSC surface marker expression is consistent with that of kidney-derived MSC reported by others,23,28,30 although Dekel et al.31 noted no expression of CD44. Interestingly, KSC Sca-1 positivity increased with successive passages to >90% by passage 3, as described previously for BM-MSCs.43 As with all reports for mouse kidney-derived MSCs, KSCs were negative for hematopoietic progenitor cell markers, including c-kit, CD133, and CD31. Thus, the KSC immunophenotype is consistent with that reported for MSCs. Dekel et al.31 identified Sca-1+ cells in the kidney interstitium in close proximity to but separate from tubules, most notably in the renal papilla. This region has been suggested previously as a niche for stem/progenitor cells in the adult kidney.48 Although it was not the focus of our report to detect KSCs in situ in the kidney, the surface marker analysis suggests a nontubular origin, because no renal tubular markers could be detected. Our report does not answer the question of whether KSCs are derived from progenitors within the kidney or, alternatively, are continuously replenished from BM stores; however, we do not believe KSCs represent MSC contamination from blood, because kidneys were perfused extensively before culture, and cells clearly shed from tissue explants in culture. Furthermore, using our technique, KSC could not be expanded from peripheral blood, consistent with previous studies that were similarly unable to culture MSCs using standard techniques.23,49 Finally, donor-derived MSCs have been isolated from allografts >10 yr after transplantation, arguing for a resident progenitor.27
Numerous studies have confirmed that human and mouse BM-derived MSCs hold immunomodulatory properties.12,22 Although the report by Dekel et al. demonstrated inhibition of alloreactive CD8+ T cell proliferation, no study has addressed in a comprehensive manner the immunomodulatory properties of kidney-derived MSCs, including effects on DCs. Given the importance of both DC and T cells in the pathophysiology of acute kidney injury,33,38,39 we evaluated the immunologic effects of KSCs on these populations. In our experiments, KSCs promoted the differentiation of DCs from BM precursor characterized by a phenotype with significant reduction of MHC class II and, interestingly, increased CD80 expression. KSC-DCs were refractory to maturation with LPS, even after removal from co-culture, and produced increased IL-10. Although KSC addition to later DC culture also produced an altered DC phenotype with low MHC class II expression, the effect was less pronounced, and maturation occurred normally after stimulation with LPS. Thus, KSCs seem to act early during the differentiation of DCs from BM precursors to maintain DCs in an immature or “semimature” state, a phenotype associated with immunoregulation and self-tolerance.50
Several recent studies examined the effects of human BM-MSCs on DC differentiation and maturation from DC precursors, including adult peripheral blood monocytes15,46,51 and CD34+ cells isolated from umbilical cord blood46,52 or adult peripheral blood.17 In these reports, human BM-MSCs inhibited rather than promoted the differentiation from precursors and yielded a DC phenotype with reduced expression of MHC class II but also reduced CD80. English et al.14 demonstrated that mouse BM-MSCs inhibited the upregulation of MHC class II during TNF-α–driven BM-derived DC maturation. In their report, effects on DC differentiation from BM precursors were not studied, and DC surface expression of CD80 was not reported. In direct contrast to our findings, Djouad et al.44 found that mouse BM-MSC–conditioned medium inhibited BM-derived DC differentiation and reduced CD80 expression; however, the conditioned medium was obtained by co-culture of BM-MSCs with splenocytes, whereas, in our experiments, KSCs were cultured alone. Thus, our findings that mouse KSC induced differentiation of BM-derived DCs with increased CD80 expression may reflect species-specific differences, the type of culture system or DC progenitor used, but also differential properties of kidney versus BM-MSC. Admittedly, our findings are limited to in vitro cultures using BM-derived DC and, therefore, may not reflect in vivo effects on renal DCs; however, considerable debate exists over the origin of peripheral organ DCs,53,54 and a recent report identified both resident and infiltrating (presumably BM-derived) DC populations during AKI.39 Thus, our results may indicate an organ-specific role for KSCs in the differentiation and recruitment of tolerogenic DCs from the BM to the kidney during injury. Our findings that soluble factors were active in DC immunomodulation support such an endocrine mechanism.
The mechanisms by which MSC modulate DC differentiation and maturation have not been elucidated fully. Studies of human BM-MSCs suggest immune hyporesponsiveness is mediated in part through soluble factors.13,16,18,22 Our transwell experiments clearly demonstrated that soluble factors predominate in altering DC phenotype, which was further confirmed by the KSC-conditioned medium transfer experiments. Reports in both humans and mouse have demonstrated a role for IL-6 in MSC modulation of DC function.44,46 We noted that KSCs cultured alone produce IL-6, although neutralization of this cytokine during KSC-DC co-culture only partially inhibited the KSC effect on DC phenotype. Thus, KSC modulation of DCs likely involves the contribution of additional soluble factors, as has been suggested for BM-MSCs.
In addition to modulation of DC differentiation and maturation, KSCs inhibited DC-stimulated T cell proliferation in both allogeneic and nominal antigen-specific culture systems, consistent with the findings of English et al.14 using mouse BM-MSCs. Inhibition of DC-induced T cell proliferation was not due to impaired DC antigen uptake, and antigen presentation remained intact, as the majority of T cells expressed markers of activation after culture with KSC-DCs in both culture systems. Neither was the inhibition of T cell proliferation explained by greater apoptosis of the responder population in KSC-DC cultures, suggesting either T cell anergy or regulatory phenomena. Blocking antibody to CD80 abrogated KSC-DC–mediated T cell suppression. Recent studies demonstrated that indoleamine 2,3 dioxygenase–dependent DC suppression of T cells is mediated by CTLA4-B7 engagement.55,56 Thus, KSC-DC upregulation of CD80 may indicate an indoleamine 2,3 dioxygenase–dependent mechanism is active. Finally, when added directly to T cell culture, KSCs inhibited proliferation after mitogen stimulation. Interestingly, no inhibition could be demonstrated after addition of KSC-conditioned medium alone. Thus, KSCs indirectly inhibited T cell proliferation via contact-independent effects on DC function while directly inhibiting T cells via contact-dependent mechanisms.
In conclusion, we show that KSCs exert immunoregulatory effects on DCs and T cells in vitro. A possible role of KSCs in vivo may be to suppress local inflammation in the kidney after injury, allowing subsequent repair of renal tissue. In a recent clinical study, infusion of BM-MSCs successfully treated severe, steroid-resistant graft-versus-host disease after hematopoietic stem cell transplantation.57 Our findings raise the possibility of KSC manipulation in vivo or expansion ex vivo for use therapeutically in a wide variety of immune-mediated kidney diseases, including glomerulonephritis, interstitial nephritis, and allograft rejection.
CONCISE METHODS
Animals
Six-week-old male C57BL/6, BALB/c, and TCR transgenic OT II mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions, according to institutional guidelines. Protocols were approved by the Johns Hopkins University Animal Care and Use Committee.
Isolation and Expansion of Adult Mouse Kidney Sphere–Derived Cells
We used a technique modified from that previously described for human cardiac sphere–derived cells42 to generate mouse KSCs. Briefly, under sterile conditions, adult C57BL/6 mouse kidneys were first perfused in situ with PBS to remove contaminating blood cells, then harvested and removed of vessels and connective tissue. Kidneys were then minced, partially digested with collagenase IV (Sigma, St. Louis, MO) for 45 min at 37°C, and placed as explants on fibronectin-coated plates. After several days, mixed cell types arose from adherent tissue explants. Once confluent, these cells were harvested by gentle enzymatic digestion and seeded on poly-d-lysine–coated plates (Sigma) at 4 × 104 cells/ml in medium containing Iscove's Modified Dulbecco's Medium (IMDM), 20% FBS, 2-mercaptoethanol, l-glutamine, and penicillin-streptomycin (PSN). Several days later, cells remaining adherent to the plates were discarded to minimize contamination with other cells (e.g., fibroblasts, epithelial cells), whereas detached floating kidney spheres were plated on fibronectin-coated flasks and expanded as monolayers. This postkidney sphere expansion step allowed for the generation of large numbers of KSCs for experimentation. KSC-conditioned medium was harvested after 5 d of culture for cytokine analysis and transfer experiments. Flow cytometry and immunofluorescence microscopy were used to characterize KSC phenotype, as described in the next section. KSCs within 14 passages were used for experiments, with strict interbatch quality control measures established. Sca-1+ and Sca-1− KSCs were isolated from KSCs within passage 2 using flow sorting (BD VantageSE). Equal numbers of Sca-1+ and Sca-1− cells were then plated at 2 × 103 cells/cm2 in individual wells and harvested every day for 4 d, stained with trypan blue, and counted manually.
Differentiation Cultures
KSCs were plated in 25-cm2 flasks until confluence in standard KSC medium. For osteogenesis, culture medium containing IMDM, 10% FBS, 10% horse serum (Invitrogen, Carlsbad, CA), PSN, 2 mM l-glutamine, 20 mM β-glycerol phosphate (Sigma), 50 ng/ml thyroxine (Sigma), 1 nM dexamethasone (Sigma), and 0.5 μM ascorbate 2-phosphate (Sigma) was used. The medium was changed twice weekly for 4 wk. Cells were fixed with 10% formalin for 20 min at room temperature and stained with Alizarin Red (Sigma) for 20 min. For adipogenesis, KSCs were incubated in medium containing IMDM, 10% FBS, 10% horse serum, PSN, 2 mM l-glutamine, 5 μg/ml insulin (Sigma), 50 μM indomethacin (Sigma), 1 μM dexamethasone, and 0.5 μM 3-isobutyl-1-methylxanthine (Sigma). The medium was changed twice weekly for 3 wk. The cells were fixed with 10% formalin for 20 min at room temperature and stained with 0.5% Oil Red O (Sigma) in isopropyl alcohol/PBS for 20 min.
Culture of BM Mesenchymal Cells
BM mesenchymal stem cells from C57BL/6 mice were obtained from the Tulane Center for Gene Therapy, Tulane University (New Orleans, LA). Cells were expanded in medium containing IMDM, 10% FBS, 10% horse serum, PSN, and l-glutamine as described previously.58
BM-Derived DC Culture
BM-derived DCs were generated as described previously.59 Briefly, BM cells from C57BL/6 mice were cultured for 5 d in RPMI 1640 with 10% FBS, l-glutamine, 2-mercaptoethanol, PSN, mouse GM-CSF (500 U/ml), and mouse IL-4 (500 U/ml). Half of the supernatant was replaced with fresh cytokine-containing medium every other day. LPS (1 μg/ml) was added for an additional 24 h to induce DC activation/maturation. To study the effect of KSCs on DC differentiation and maturation, we treated KSCs with mitomycin (50 μg/ml) at 37°C for 30 min to inhibit their proliferation, washed, and added into DC culture at different time points (KSC:DC at 1:10 ratio) using Corning Transwell (pore size 0.4 μm; no cell contact; Corning T, Lowel) or mixed chamber systems (cell–cell contact). KSC-conditioned medium was added into DC culture at various concentrations to study the influence of KSC-soluble product, with culture media alone serving as control. DCs were identified as CD11c+ cells, with apoptosis/necrosis (7-AAD, Annexin V), differentiation (percentage of total cells and absolute number), and activation/maturation (MHC class II, CD80, CD86) analyzed by flow cytometry.
DC-Stimulated T Cell Responses
Magnetic bead separation (Miltenyi Biotec, Auburn, CA) was used to isolate CD11c+ DCs from KSC:DC co-cultures and CD4+ T cells from mouse spleen and LNs. Purity of isolated fractions was consistently approximately 90% for DCs and >95% for CD4+ T cells. Isolated DCs were co-cultured with CFSE-labeled CD4+ T cells from BALB/c (allogeneic response) in the presence of low dosage anti-CD3 (1 μg/ml) or from TCR-transgenic OT II mice (C57BL/6 background) in the presence of OVA323-339 (400 ng/ml; nominal antigen-specific response) at a ratio of 1:5 (DC:T) for 2 to 5 d. T cell phenotype and function were characterized using flow cytometric analysis of viability (7-AAD+ = necrosis; 7-AAD− and Annexin V+ = apoptosis), proliferation (percentage of cells exhibiting CFSE fluorescence dilution), and surface markers of activation (CD25, CD69).
Antigen Uptake Assay
Quantitative analysis of DC endocytosis was performed as described previously,60 with minor modifications. KSC-conditioned medium was added to incipient BM-derived DC culture (ratio 1:1), with DCs harvested on day 5. To study constitutive macropinocytosis or mannose receptor mediated endocytosis, we incubated 5 × 105 DCs with 5 μg/ml FITC-albumin (Sigma) or 0.1 mg/ml FITC-dextran (MW 42000; Sigma), respectively, at either 37 or 4°C (as negative control) for 1 h. Endocytosis was stopped by cold wash in 0.1% sodium azide/1% FBS/PBS. Cells were stained with anti-CD11c and 7-AAD, followed by flow cytometric analysis.
Anti-CD3/CD28–Stimulated T Cell Responses
To study the direct effect of KSCs on T cells, we stimulated CFSE-labeled CD4+ T cells from C57BL/6 mice with anti-CD3 (25 μg/ml) and anti-CD28 (1 μg/ml) and co-cultured them with KSCs (KSC:T as 1:10) or KSC-conditioned medium (KSC-CM:T cell culture medium at 1:1 or 1:10) for 2 to 5 d. T cell proliferation was assessed as described already.
Flow Cytometric Analysis
Cells were subjected to flow cytometric analysis using a FACScalibur flow cytometer (Becton Dickinson, Mansfield, MA). Data were analyzed using the FCS Express analysis software (De Novo, Los Angeles, CA). Fluorochrome-conjugated antibodies against the following mouse cell surface antigens (as well as relevant isotype controls) were purchased from BD Pharmingen (San Diego, CA) and eBioscience, Los Angeles, CA, unless specifically mentioned: CD3ɛ chain (145–2C11), CD4 (L3T4; RM4-5), CD8 (Ly-2; 53-607), CD11b (Mac-1 α chain; M1/70), CD11c (Integrin ax chain; HL3), CD13 (R3-242), CD19 (1D3), CD25 (IL-2 receptor α chain; HL3), CD29 (Integrin β1; eBioHMb1-1), CD31 (PECAM-1; MEC 13.3), CD34 (RAM34), CD44 (H-CAM; IM7), CD45 (Ly-5; 30-F11), CD45R (B220; RA3-6B2), CD54 (ICAM-1; 3 E2), CD55 (DAF; RIKO-5), CD69 (VEAA; H1.2F3), CD80 (B7–1; 16-10-A1), CD86 (B7-2; GL-1), CD90 (Thy1.2; OX-7), CD105 (Endoglin; MJ7/18), CD117 (C-kit; 2B8), CD133 (Prominin 1; 13A4), CD166 (ALCAM; eBioALC-48), MHC class II (I-A/I-E; M5/114.15.2), Sca-1 (Ly6-6A/E; D7), TCRβ (H57-597), CD49e (5H10-27), CD73 (TY/23), CCR7 (4B12), Ly-6G (1A8), CD62L (MEL-14), CD106 (429), CD24 (C57BL/10), MHC I H2-Kb (CTKb; Abcam), MUC1 (EP1024Y; Abcam, Cambridge, MA), Stro-1 (Stro-1; Invitrogen), Ep-CAM (G8.8), mouse anti-Armenian and Syrian hamster IgG, and mouse anti-rat IgG2a. Gates were established on live cells by 7-AAD negative fluorescence.
Immunocytochemistry
Cell slides were fixed in 4% paraformaldehyde, permeabilized with Triton X-100, and blocked with 5% BSA. Slides were then incubated with primary antibodies overnight at 4°C, washed, and incubated with secondary fluorochrome-conjugated antibodies for 1 h at room temperature. Nuclei were counterstained with DAPI and viewed under a Nikon Eclipse E600 microscope (Nikon, Melville, NY). The following antibodies were used: Rabbit anti–α smooth muscle actin (1:100 dilution; Abcam), mouse anti-vimentin (1:50 dilution; BD), mouse anti-fibronectin (1:100 dilution; BD), rabbit anti-ZO1 (1:50 dilution; Abcam), FITC mouse anti-cytokeratin (1:100 dilution; Sigma), FITC goat anti-mouse IgG (1:100 dilution; Sigma), and FITC goat anti-rabbit IgG (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). For directly conjugated primary antibodies, fluorescently labeled isotype antibodies were used as a negative control. For all other immunocytochemistry staining, a negative control was run using the same technique but omitting the primary antibody while adding the labeled secondary antibody.
Cytokine Measurement
Supernatants from cell cultures were subjected to cytokine analysis, by either ELISA or Bio-Plex multiplex cytokine detection system, according to the manufacturer's protocol (Bio-Rad, Hercules, CA).
Statistical Analysis
Data are presented as mean ± SD. Results shown are representative of at least three independent experiments. Statistical comparisons between groups were performed by t and ANOVA tests, where appropriate. P < 0.05 was deemed significant. Analysis was accomplished using SigmaStat 3.5 (Systat Software Inc., San Jose, CA).
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants K08DK066319 (K.L.W.) and R01DK054770 (H.R.), American Heart Association Mid-Atlantic Affiliate (K.L.W.), and the Maryland Technology Development Corporation (H.R.). Some of the materials used in this work were provided by the Tulane Center for Gene Therapy through a grant P40RR017447 from National Center for Research Resources of the National Institutes of Health.
Portions of this work were presented in abstract form at the annual scientific meetings of the American Society of Nephrology (October 31 through November 5, 2007 and November 4 through 9, 2008, San Francisco, CA), the Transplantation Society (August 10 through 14, 2008, Sydney, Australia), and the American Society of Transplantation (May 31 through June 4, 2008, Toronto, Ontario, Canada).
We thank Patricia Agreda for expert technical assistance during preliminary studies.
Published online ahead of print. Publication date available at www.jasn.org.
E.M.'s current affiliation is Heart Institute, Cedars Sinai Medical Center, Los Angeles, California.
See related editorial, “Interfacing Kidney Stroma with Dendritic Cells,” on pages 685–686.
REFERENCES
- 1.Finn WF, Chevalier RL: Recovery from postischemic acute renal failure in the rat. Kidney Int 16: 113–123, 1979 [DOI] [PubMed] [Google Scholar]
- 2.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney: Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A: Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7: 393–395, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Robey PG, Simmons PJ: Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2: 313–319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG: Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA: Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G: Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15: 1794–1804, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG: Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Fibbe WE: Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499–3506, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J: Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105: 2214–2219, 2005 [DOI] [PubMed] [Google Scholar]
- 14.English K, Barry FP, Mahon BP: Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 115: 50–58, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC: Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 13: 263–271, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F: Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722–3729, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang JP, Stoltz JF, Miossec P, Eljaafari A: Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol 180: 1598–1608, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal S, Pittenger MF: Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F: Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90: 516–525, 2005 [PubMed] [Google Scholar]
- 20.Rasmusson I, Le Blanc K, Sundberg B, Ringden O: Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol 65: 336–343, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Rasmusson I, Ringden O, Sundberg B, Le Blanc K: Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76: 1208–1213, 2003 [DOI] [PubMed] [Google Scholar]
- 22.McTaggart SJ, Atkinson K: Mesenchymal stem cells: Immunobiology and therapeutic potential in kidney disease. Nephrology (Carlton) 12: 44–52, 2007 [DOI] [PubMed] [Google Scholar]
- 23.da Silva Meirelles L, Chagastelles PC, Nardi NB: Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN: Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181: 4389–4396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B: A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH: Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 7: 211–228, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ: Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117: 989–996, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challen GA, Bertoncello I, Deane JA, Ricardo SD, Little MH: Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol 17: 1896–1912, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Park HC, Addabbo F, Ni J, Pelger E, Li H, Plotkin M, Goligorsky MS: Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int 74: 879–889, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plotkin MD, Goligorsky MS: Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol 291: F902–F912, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y: Isolation and characterization of nontubular sca-1+lin− multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 17: 3300–3314, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Bonventre JV, Zuk A: Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Rabb H, Womer KL: Ischemia-reperfusion and immediate T cell responses. Cell Immunol 248: 4–11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabb H: Paracrine and differentiation mechanisms underlying stem cell therapy for the damaged kidney. Am J Physiol Renal Physiol 289: F29–F30, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Yokota N, Daniels F, Crosson J, Rabb H: Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation 74: 759–763, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Coates PT, Duncan FJ, Colvin BL, Wang Z, Zahorchak AF, Shufesky WJ, Morelli AE, Thomson AW: In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival. Transplantation 77: 1080–1089, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Morelli AE, Thomson AW: Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 7: 610–621, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kruger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Muller-Newen G, Floege J, Kurts C: Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Scholz J, Lukacs-Kornek V, Engel DR, Specht S, Kiss E, Eitner F, Floege J, Groene HJ, Kurts C: Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol 19: 527–537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E: Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115: 896–908, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Anjos-Afonso F, Siapati EK, Bonnet D: In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci 117: 5655–5664, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D: Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25: 2025–2032, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Xu G, Zhang Y, Zhang L, Ren G, Shi Y: The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun 361: 745–750, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE: Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 177: 2080–2087, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG: Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q: The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM: Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol 121: 368–374, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Lutz MB, Schuler G: Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol 23: 445–449, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N: Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105: 4120–4126, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Zhang W, Yue H, Han Q, Chen B, Shi M, Li J, Li B, You S, Shi Y, Zhao RC: Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells Dev 16: 719–731, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Geissmann F: The origin of dendritic cells. Nat Immunol 8: 558–560, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M: Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol 8: 578–583, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH: Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol 16: 1391–1401, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Munn DH, Sharma MD, Mellor, AL: Ligation of B7–1/B7–2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 172: 4100–4110, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O: Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 371: 1579–1586, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ: Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM: Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693–1702, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hackstein H, Taner T, Logar AJ, Thomson AW: Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood 100: 1084–1087, 2002 [DOI] [PubMed] [Google Scholar]