Abstract
Apoptosis of tubular epithelial cells is a hallmark of acute kidney injury (AKI), but the cellular events preceding apoptosis in this setting are incompletely understood. Because matrix metalloproteinase 9 (MMP9) degrades matrix components involved in cell survival, we studied the role of MMP9 in AKI. In the mouse model of folic acid–induced AKI, we observed a marked increase of MMP9 activity in the S3 segment of the proximal tubule (S3PT), correlating with the apoptotic phase. MMP9 deficiency increased apoptosis and the severity of renal lesions and substantially delayed recovery of renal function. MMP9−/− mice exhibited significant apoptosis in the S3PT and the intercalated cells of the collecting duct (I-CD), whereas wild-type mice exhibited none in these segments. Stem cell factor (SCF), an MMP9 substrate, was identified in the S3PT, and its receptor, c-Kit, was expressed in both the S3PT and I-CD. MMP9 released the soluble form of SCF (sSCF) from kidney cells in vivo and in vitro. In addition, SCF inhibited apoptosis of tubular cells in vitro, rescued MMP9−/− S3PT and I-CD from apoptosis in vivo, and improved renal function. An ischemia-reperfusion model of AKI produced similar results. In patients with AKI, urinary sSCF increased with acute tubular necrosis but not with prerenal azotemia. In conclusion, these data show that MMP9 protects the S3 segment of the proximal tubule and the I-CD from apoptosis in AKI, most likely by releasing sSCF.
Acute kidney injury (AKI) is a common renal disease with a prevalence of 10 to 30% in patients in critical care units. Despite technological advances in renal replacement therapy, the high mortality of patients with AKI has not changed in the past decades and remains >50%. The course of the illness is highly variable, ranging from a transient disease associated with full recovery of renal function to a disease requiring dialysis and intensive care management. The promotion of cell survival in AKI is important for restitution of renal function. It is therefore mandatory to identify the mechanisms underlying the apoptosis of the tubular cells.1
In the adult kidney, matrix metalloproteinase 9 (MMP9) is mainly expressed in collecting duct cells and to a lesser extent in proximal tubule and podocytes.2–4 The principal substrate of MMP9 is type IV collagen, but MMP9 can degrade other basement membrane molecules as well as nonmatrix components.5 We previously showed that crescentic proliferative glomerulonephritis was more severe in MMP9-deficient (MMP9−/−) mice because of MMP9's ability to cleave fibrin.6
We investigated the folic acid (FA) model of AKI in mice, which is characterized by an apoptotic phase 12 to 24 h after the FA injection.7 We expected that MMP9 deficiency would have a protective effect on apoptosis because MMP9 degrades matrix components5 that play a role in cell survival.8–10 On the contrary, MMP9−/− mice developed a more severe AKI than their wild-type mates, with exacerbated renal lesions, a two-fold increase of apoptosis, and delayed renal function recovery. Because MMP9 releases soluble stem cell factor (sSCF), the c-Kit ligand,11 and the SCF/c-Kit system has an important antiapoptotic role in many tissues,12 we investigated the expression and role of that system in AKI. Here we show that SCF and c-Kit are expressed at the cell membrane in specific segments of renal epithelial tubules. We provide evidence that MMP9 protects from apoptosis the S3 segment of the mouse proximal tubule (S3PT) and the intercalated cells of the collecting duct (I-CD) after FA and ischemia reperfusion injury and releases the sSCF in kidney tissue. Our results suggest that SCF is a novel survival factor in AKI.
RESULTS
MMP9 Deficiency Delays Renal Function Recovery, Aggravates Histologic Lesions, and Markedly Increases Apoptosis in Mice Treated with FA
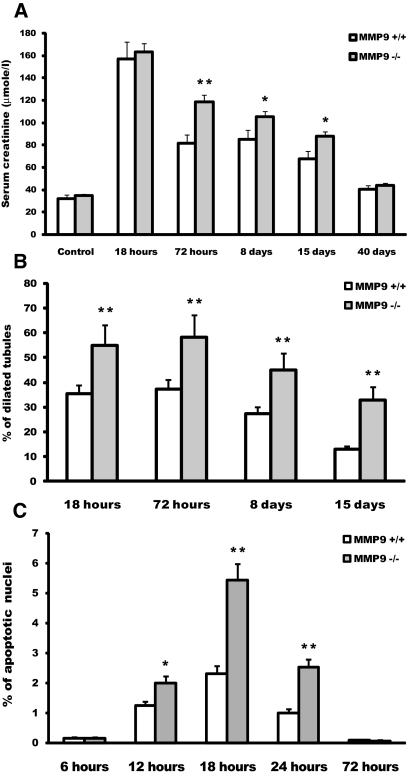
After FA injection, both groups of mice developed severe renal failure, which peaked at 18 h (Figure 1A). Renal function progressively returned to normal values at 40 d, but the recovery was much slower in MMP9−/− mice, with serum creatinine values significantly higher at 3, 8, and 15 d (Figure 1A).
Figure 1.
Effect of MMP9 deficiency on renal function, tubular dilation, and apoptosis at different time points after FA injection. (A) Serum creatinine level in MMP9+/+ (□) and MMP9−/− (  ) mice injected with bicarbonate vehicle (Control) or FA. (B) Percentage of dilated tubules in MMP9+/+ and MMP9−/− kidney sections. Five microphotographs were taken from six different MMP9−/− and MMP9+/+ kidneys. (C) Percentage of apoptotic nuclei. Six microphotographs were taken from six different MMP9+/+ and MMP9−/− kidneys. Data are means ± SEM. **P < 0.001 versus MMP9+/+ injected mice; *P < 0.01 versus MMP9+/+ injected mice. Magnification, ×400.
) mice injected with bicarbonate vehicle (Control) or FA. (B) Percentage of dilated tubules in MMP9+/+ and MMP9−/− kidney sections. Five microphotographs were taken from six different MMP9−/− and MMP9+/+ kidneys. (C) Percentage of apoptotic nuclei. Six microphotographs were taken from six different MMP9+/+ and MMP9−/− kidneys. Data are means ± SEM. **P < 0.001 versus MMP9+/+ injected mice; *P < 0.01 versus MMP9+/+ injected mice. Magnification, ×400.
Histologic lesions were more severe in MMP9−/− mice, with a marked increase in the number of dilated tubules from 18 h to 15 d after FA injection (Figure 1B).
Time-course studies from 6 to 72 h after FA injection established that the peak of apoptosis occurred at 18 h in both groups of mice, with the percentage of apoptotic cells in kidney sections being double in MMP9−/− kidneys (Figure 1C). We therefore performed further experiments at this time point. We verified that nuclei stained by the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) method were morphologically apoptotic with condensed chromatin and that they were not proliferating cell nuclear antigen (PCNA) positive (data not shown).
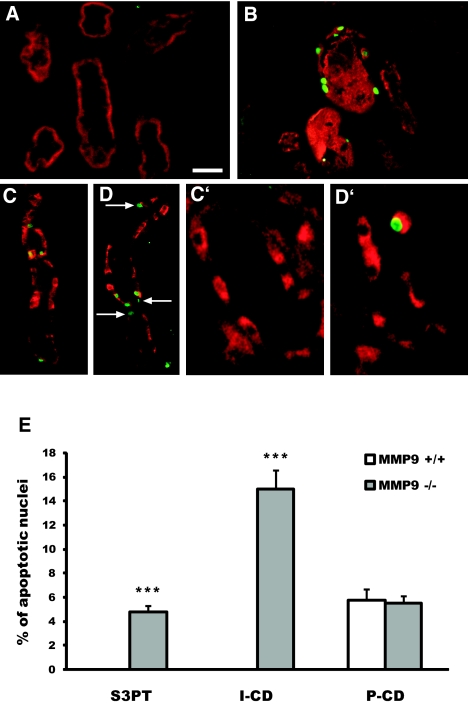
Double-staining experiments showed that apoptosis occurred in all tubular segments, with the exception of the S1 and S2 segments of the proximal tubule of MMP9+/+ and MMP9−/− mice and of the S3PT of MMP9+/+ mice. Two remarkable differences were observed between MMP9+/+ and MMP9−/− mice. First, apoptotic nuclei were detected in the S3PT in MMP9−/− kidneys, whereas no apoptosis was seen in any part of the proximal tubule of MMP9+/+ kidneys (Figure 2, B versus A, and E). Second, in collecting ducts, apoptosis was exclusively observed in principal cells (P-CD) of MMP9+/+ kidneys (Figure 2, C, C′, and E), whereas it was seen in both P-CD (Figure 2D) and intercalated cells (I-CD; Figure 2D, arrow and Figure 2D′) of MMP9−/− kidneys (Figure 2E).
Figure 2.
Impact of MMP9 deficiency on the distribution of apoptosis along tubule segments 18 h after FA injection. (A and B) Representative kidney sections from MMP9+/+ (A) and MMP9−/− (B) mice stained with TUNEL method (apoptotic nuclei, green) and CD10 (S3 segment, red). Note that apoptosis is observed in S3 segment of proximal tubules in MMP9−/− kidney only. (C through D′) Representative collecting duct sections from MMP9+/+ (C and C′) and MMP9−/− (D and D′) mice stained with TUNEL method and DBA lectin, a marker of principal cells (C and D) or H+ATPase, a marker of intercalated cells (C′ and D′). The number of apoptotic nuclei is increased in MMP9−/− compared with MMP9+/+ collecting ducts (D versus C). In MMP9+/+ mice, apoptotic nuclei are observed only in principal cells (C), whereas in MMP9−/− mice, they are also seen in intercalated cells (D, arrow, and D′). (E) Percentage of apoptotic nuclei in S3PT I-CD and P-CD. Six microphotographs of six different kidneys’ samples from MMP9+/+ and MMP9−/− mice. Bar = 100 μm in A, B, C, and D and 60 μm in C′ and D′. Data are means ± SEM. **P < 0.001 versus MMP9+/+ injected mice.
MMP9 Activity is Induced in Proximal Tubule at the Onset of Renal Failure
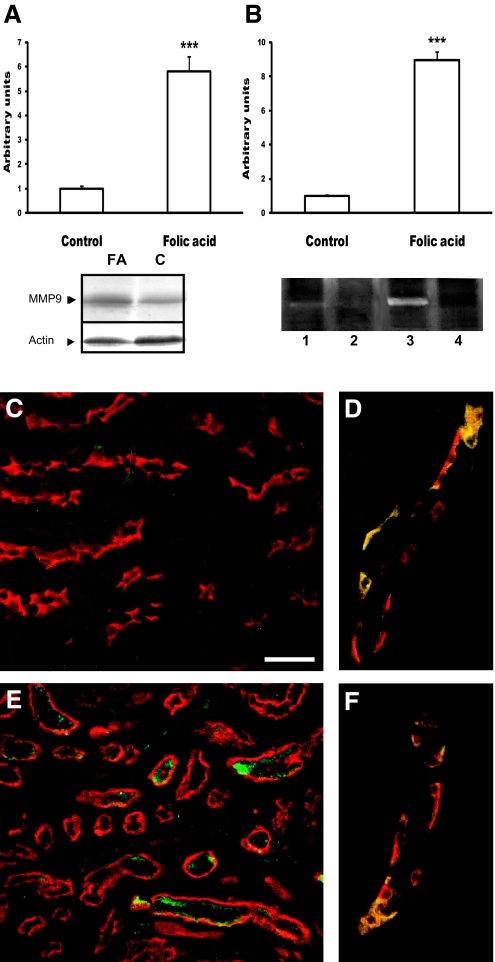
We first showed that MMP9 antigen and activity, assessed by Western blot (Figure 3A) and zymography (Figure 3B), increased in MMP9+/+ mice 18 h after FA injection. Quantitative analysis of Western blots normalized to β-actin expression showed a six-fold increase in protein expression, whereas scanning of zymograms evidenced a nine-fold increase in MMP9 activity (Figure 3B), suggesting a role for MMP9 at the onset of the disease. MMP9 expression in collecting duct cells was similar in control (Figure 3D) and FA-treated (Figure 3F) mice. In contrast, 18 h after FA injection, MMP9 expression mainly increased in S3PT (Figure 3, E versus C). MMP9 was never detected in MMP9−/− mice (data not shown).
Figure 3.
Induction of MMP9 in MMP9+/+ mice 18 h after injection of FA. (A) Western blot performed with 20 μg of protein extracts from control MMP9+/+ kidney or kidney from MMP9+/+ mice 18 h after injection of FA. Quantitative analysis of five blots, using β-actin as an internal control, showed that MMP9 protein expression was increased by six-fold in mice treated with FA compared with control mice injected with vehicle. (B) Zymograms performed with protein extracts from MMP9+/+ (lanes 1 and 3) and MMP9−/− (lanes 2 and 4) control kidneys (lanes 1 and 2) and kidneys injected with FA (lanes 3 and 4). Quantitative analysis of six zymograms showed that MMP9 enzymatic activity was increased by nine-fold in mice treated with FA compared with control mice injected with vehicle. (C through F) Expression of MMP9, revealed by FITC, in representative paraffin kidney sections of control MMP9+/+ mice (C and D) and of MMP9+/+ mice injected with FA (E and F). (C and E) Proximal tubule S3 segment was stained with CD10 and revealed by TRITC. Immunoreactive MMP9 (green) was induced 18 h after FA injection (E), whereas it was barely detected in control kidney (C). (D and F) Principal cells from collecting duct were stained with DBA lectin conjugated to TRITC. Bar = 120 μm in C and E and 100 μm in D and F. Data are means ± SEM. ***P < 0.001.
SCF and Its Receptor c-Kit Are Expressed in Adult Kidney Epithelial Cells
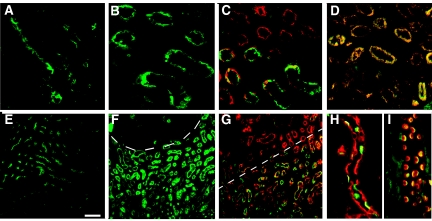
Given the apical localization of MMP9, we next asked what could be the critical substrate for MMP9 that could mediate its antiapoptotic activity. SCF was expressed at the apical side of tubular cells both in normal kidney (Figure 4A) and in FA-treated (Figure 4B) mice. In normal kidney, double labeling with megalin (a protein expressed over the cortical and medullary segments of proximal tubule)13 showed that SCF was expressed in the medulla only (i.e., in S3PT; data not shown). At the apoptotic phase of the FA model, SCF had the same location in S3PT (Figure 4C), where it co-localized with MMP9 (Figure 4D). SCF was also localized in S3PT in MMP9−/− mice injected or not with FA (data not shown).
Figure 4.
(A through I) Immunoreactive SCF (A through D) and c-Kit (E through I) expression (FITC, green) in MMP9+/+ paraffin kidney sections. (A and E) SCF (A) and c-Kit (E) expression in normal kidney. (B through D and F through I) Expression in kidney 18 h after FA injection. (B and F) The localization of SCF (B) and c-Kit (F) at the apex of dilated tubules is similar to A and E, respectively. (C and G) Proximal tubules were stained with megalin and revealed by TRITC. The corticomedullary junction is delineated by a dotted line with the medulla in the lower part of the figure. Note that both SCF (C) and c-Kit (G) co-localized with megalin in medulla only. (D) Co-localization of SCF and MMP9 revealed by TRITC in proximal tubules. (H and I) Expression of c-Kit in collecting duct cells. Collecting duct cells were stained in red with DBA lectin, a marker of principal cells (H) or with H+ATPase, a marker of intercalated cells (I). Note that c-Kit was observed in intercalated cells only, where it co-localized with H+ATPase. Bar = 100 μm in A through D, H, and I; and 140 μm in E through G.
c-Kit was detected at the apical side of tubules of normal (Figure 4E) and FA-treated (Figure 4, F through I) mice. Double-labeling experiments with megalin showed that, like SCF, c-Kit was present in S3PT (Figure 4G). c-Kit was also detected in the collecting ducts, where it was coexpressed with H+ATPase, a marker of I-CD (Figure 4I), but not with the Dolichos Biflorus Agglutinin (DBA) lectin, a marker of principal cells (Figure 4H). Localization of c-Kit was identical in MMP9−/− mice, regardless of whether they were injected with FA (data not shown). Control experiments performed with an excess of blocking peptide (for SCF) or with mouse IgG (for c-Kit) did not show staining (data not shown).
MMP9 Releases sSCF in Kidney Cells Both In Vivo and In Vitro
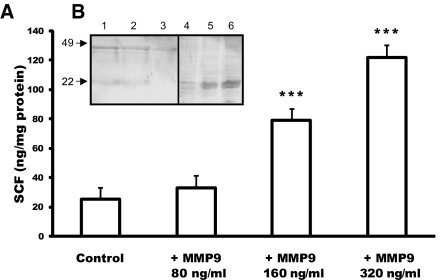
We then asked whether MMP9 could release sSCF in kidney. We first determined the sensitivity of SCF to MMP9 proteolysis by an in vitro approach using the proximal tubular cell line RC.SV1.14 ELISA of sSCF showed that recombinant MMP9 incubated with confluent RC.SV1 cells increased the release of SCF in culture medium in a concentration-dependent manner (Figure 5A). Western blot analysis of RC.SV1 cell extracts and medium confirmed that the amount of sSCF released in medium (molecular weight 22 kD) increased with MMP9 concentration, whereas the 49-kD membrane form decreased (Figure 5B).
Figure 5.
Sensitivity of membrane SCF to MMP9 proteolysis in vitro. (A) ELISA of sSCF in the culture medium of confluent proximal tubular cell line incubated with 4-aminophenylmercuric acetate in gelatinase substrate buffer (Control) or with activated MMP9 at increasing concentrations (+MMP9 80 ng/ml, +MMP9 160 ng/ml, and +MMP9 320 ng/ml). Data are means ± SEM. ***P < 0.001 control. (B) Western blot analysis of SCF expression in cell extracts (lanes 1 through 3) and conditioned media (lanes 4 through 6) of RC-SV1 control cells (lanes 1 and 4) and cells incubated with 160 ng/ml (lanes 2 and 5) and 320 ng/ml (lanes 3 and 6) recombinant activated MMP9. Recombinant MMP9 decreased expression of the 49-kD intact form of SCF in cell extracts (compare lanes 2 and 3 with lane 1), whereas it increased the release of the 22-kD sSCF in culture medium (compare lanes 5 and 6 with lane 4).
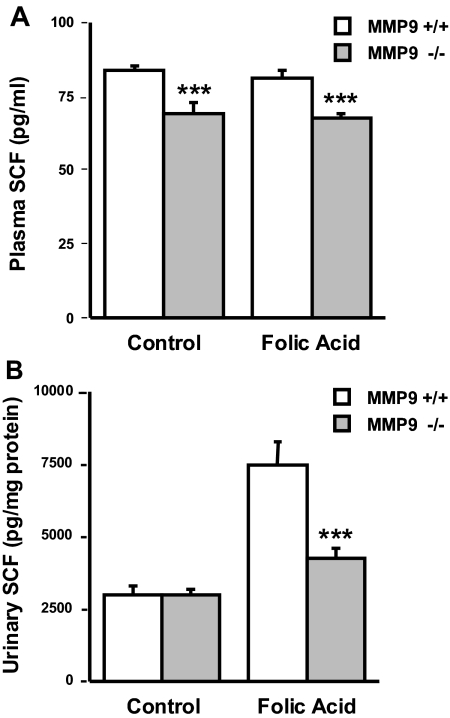
We then verified in vivo the proteolytic effect of MMP9 on membrane SCF by measuring the concentration of sSCF in plasma and urine by ELISA. SCF levels were significantly lower in the plasma of MMP9−/− mice compared with MMP9+/+ mice (Figure 6A). SCF plasma levels were unchanged after FA injection. In contrast, in the urine, the ratio of sSCF to urinary protein was markedly increased at the apoptotic peak of the FA model in both groups of mice. There was significantly less sSCF in the urine of MMP9−/− mice compared with MMP9+/+ mice (Figure 6B).
Figure 6.
Effect of MMP9 deficiency on plasma and urine SCF levels. (A) sSCF concentration in plasma of MMP9+/+ (□) and MMP9−/− (  ) mice injected with bicarbonate vehicle (Control) or with FA at 18 h. SCF concentration was significantly lower (P < 0.005; n = 5) in the plasma of MMP9−/− compared with MMP9+/+ mice. (B) Urinary sSCF normalized to urinary protein in MMP9+/+ (□) and MMP9−/− (
) mice injected with bicarbonate vehicle (Control) or with FA at 18 h. SCF concentration was significantly lower (P < 0.005; n = 5) in the plasma of MMP9−/− compared with MMP9+/+ mice. (B) Urinary sSCF normalized to urinary protein in MMP9+/+ (□) and MMP9−/− ( ) mice injected with vehicle (Control) or FA. Urinary SCF was not significantly different between control MMP9+/+ and MMP9−/− mice. It significantly increased in both groups of FA-injected mice compared with their respective controls (MMP9+/+ mice P < 0.001; MMP9−/− mice P < 0.002; n = 6), but the increase was significantly less in MMP9−/− mice compared with MMP9+/+ mice. ***P < 0.001 (n = 6).
) mice injected with vehicle (Control) or FA. Urinary SCF was not significantly different between control MMP9+/+ and MMP9−/− mice. It significantly increased in both groups of FA-injected mice compared with their respective controls (MMP9+/+ mice P < 0.001; MMP9−/− mice P < 0.002; n = 6), but the increase was significantly less in MMP9−/− mice compared with MMP9+/+ mice. ***P < 0.001 (n = 6).
SCF Prevents Collecting Duct Cell Apoptosis In Vitro
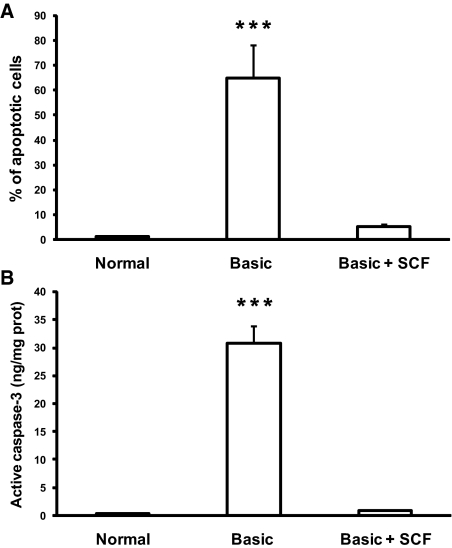
Collecting duct cells were grown in three different conditions, including a medium supplemented with serum and hormones, a medium deprived of serum and hormones (basic medium), and the basic medium supplemented with human recombinant SCF (rSCF) at the same concentration (15 pg/ml) as the one measured by ELISA in the urine of mice supplemented with sSCF. Serum and hormone deprivation induced dramatic cell apoptosis, assessed by TUNEL method and caspase-3 activity, which was completely inhibited by addition of rSCF to the culture medium (Figure 7).
Figure 7.
Effect of sSCF on apoptosis in human collecting duct cell line in vitro, (A) Percentage of apoptotic nuclei in cells grown in media supplemented (Normal) or not (Basic) with FCS and hormones and in cells grown in basic medium supplemented with soluble recombinant SCF (Basic + SCF). (B) Concentration of active caspase-3 in cells grown in Normal, Basic and Basic media supplemented with SCF. Note that apoptosis (assessed by both methods) was induced in cells grown in Basic medium and inhibited by addition of SCF. Data are means ± SEM. ***P < 0.001.
Injection of sSCF to MMP9−/− Mice Improves Renal Function Recovery, Severity of Histologic Lesions, and Apoptosis
We hypothesized that induction of apoptosis in S3PT of MMP9−/− mice was the result of a decreased availability of sSCF. We first verified by ELISA that injection of 15 ng of mouse rSCF restored plasma concentration of SCF in MMP9−/− mice (MMP9+/+ 81.2 ± 2.6; MMP9−/− 67.8 ± 1.6; MMP9−/− injected with soluble rSCF 79.3 ± 5.5 pg/ml; n = 9; P < 0.01 versus MMP9−/−) as well as SCF urine concentration (MMP9+/+ 7453 ± 828; MMP9−/− 4223 ± 343; MMP9−/− injected with soluble rSCF 6816 ± 964 pg/mg protein; n = 6; P < 0.01 versus MMP9−/−).
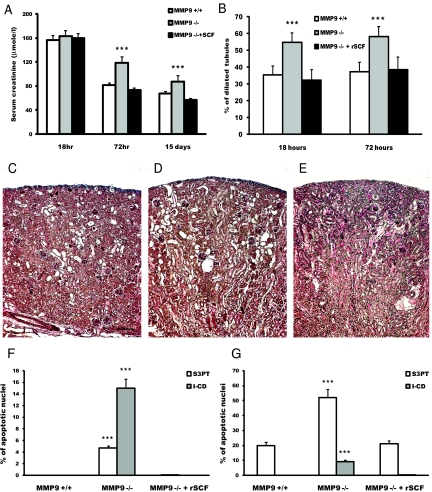
Supplementation of MMP9−/− mice with SCF at the time of FA injection dramatically reduced the percentage of apoptotic cells in S3PT and in I-CD down to levels of control MMP9+/+ mice (Figure 8F). The defect of sSCF availability was most likely responsible for more severe lesions and delayed recovery of renal function in MMP9−/− mice because serum creatinine was similar in MMP9+/+ mice and SCF-rescued MMP9−/− mice 3 and 15 d after FA injection (Figure 8A). Accordingly, kidney morphology was improved by SCF rescue (Figure 8, E versus C and D) with the number of dilated tubules being similar to that of MMP9+/+ mice 18 and 72 h after FA injection (Figure 8B).
Figure 8.
Effect of sSCF on renal function, renal histology, and apoptosis. (A) Serum creatinine level in MMP9+/+ (□), MMP9−/− (  ) and MMP9−/− mice rescued with SCF (▪) 18 h, 72 h, and 15 d after FA injection. Serum creatinine concentrations were not statistically different in MMP9+/+ mice and in MMP9−/− mice injected with recombinant SCF. Data are means ± SEM, ***P < 0.001. (B) Percentage of dilated tubules in MMP9+/+, MMP9−/−, and MMP9−/− mice rescued with SCF 18 and 72 h after FA injection. Five microphotographs taken from six different kidneys were analyzed. Percentage of dilated tubules was not statistically different in MMP9+/+ mice and in MMP9−/− mice injected with rSCF. Data are means ± SEM. ***P < 0.001. (C through E) Photomicrographs of representative paraffin kidney sections stained with periodic-acid Schiff from MMP9+/+ (C), MMP9−/− (D), and MMP9−/− mice rescued with SCF (E) 18 h after FA injection. Bar = 170 μm. (F and G) Percentage of apoptotic cells in S3PT and I-CD. Six microphotographs of six different kidneys sampled from MMP9+/+, MMP9−/−, and MMP9−/− mice rescued with SCF were analyzed 18 h after FA injection (F) and 24 h after reperfusion in the clamped kidney (G). Apoptosis observed in S3PT and I-CD was not statistically different in MMP9+/+ mice and in MMP9−/− mice injected with recombinant SCF+. Data are means ± SEM, ***P < 0.001; **P < 0.01 versus MMP9+/+ or MMP9−/− injected with SCF.
) and MMP9−/− mice rescued with SCF (▪) 18 h, 72 h, and 15 d after FA injection. Serum creatinine concentrations were not statistically different in MMP9+/+ mice and in MMP9−/− mice injected with recombinant SCF. Data are means ± SEM, ***P < 0.001. (B) Percentage of dilated tubules in MMP9+/+, MMP9−/−, and MMP9−/− mice rescued with SCF 18 and 72 h after FA injection. Five microphotographs taken from six different kidneys were analyzed. Percentage of dilated tubules was not statistically different in MMP9+/+ mice and in MMP9−/− mice injected with rSCF. Data are means ± SEM. ***P < 0.001. (C through E) Photomicrographs of representative paraffin kidney sections stained with periodic-acid Schiff from MMP9+/+ (C), MMP9−/− (D), and MMP9−/− mice rescued with SCF (E) 18 h after FA injection. Bar = 170 μm. (F and G) Percentage of apoptotic cells in S3PT and I-CD. Six microphotographs of six different kidneys sampled from MMP9+/+, MMP9−/−, and MMP9−/− mice rescued with SCF were analyzed 18 h after FA injection (F) and 24 h after reperfusion in the clamped kidney (G). Apoptosis observed in S3PT and I-CD was not statistically different in MMP9+/+ mice and in MMP9−/− mice injected with recombinant SCF+. Data are means ± SEM, ***P < 0.001; **P < 0.01 versus MMP9+/+ or MMP9−/− injected with SCF.
To confirm that SCF can prevent induction of apoptosis in S3PT and in I-CD, we tested the effect of SCF rescue in another model of AKI, ischemia reperfusion injury.15 Twenty-four hours after reperfusion, MMP9 deficiency increased apoptosis in S3PT and induced apoptosis in I-CD cells, where no apoptotic nuclei were detected in control MMP9+/+ mice. Apoptosis in S3PT and in I-CD cells could be reversed in MMP9−/− mice by SCF injection before clamping (Figure 8G).
sSCF Does not Affect the Number of CD34+ Stem Cells
To exclude a role of MMP9 and SCF in stem cell mobilization in our model, we verified by FACS analysis that the percentage of CD34+ cells after FA injection was not modified by MMP9 deficiency and by recombinant SCF injection (MMP9+/+ 1.9 ± 0.06; MMP9−/− 2.2 ± 0.04; MMP9−/− injected with sSCF 2.1 ± 0.07% CD34+/total peripheral blood cells; n = 9).
sSCF Is a Potential Biomarker of AKI in Patients
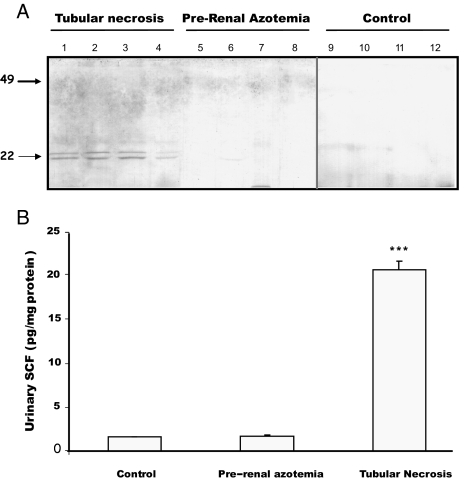
sSCF was detected by Western blotting (Figure 9A) and by ELISA (Figure 9B) in urine of patients with tubular necrosis (Figure 9, A, lanes 1 through 4, and B), whereas it was not or was barely detectable in healthy controls (Figure 9, A, lanes 9 through 12, and B) and in patients with prerenal azotemia (Figure 9, A, lanes 5 through 8, and B).
Figure 9.
(A and B) Detection of SCF in urine of patients with AKI by Western blotting (A) and ELISA (B). (A) Western blot performed with 50 μg of total protein from urine of patients with tubular necrosis (lanes 1 through 4), patients with prerenal azotemia (lanes 5 through 8), and control healthy individuals (lanes 9 through 12). Note that SCF was observed only in urine of patients with tubular necrosis. (B) Urinary sSCF concentration normalized to protein in healthy individuals (control), patients with prerenal azotemia, and patients with tubular necrosis. Data are means ± SEM. ***P < 0.001 versus urine of control subjects or patients with prerenal azotemia (n = 4).
DISCUSSION
Our data show that MMP9 and SCF are important regulators of renal function in AKI. After FA injection, MMP9−/− mice11 showed delayed recovery of renal function caused by more severe renal lesions, particularly a marked increase of apoptosis. This provides the first evidence that MMP9 protects mice from apoptosis in AKI. Results suggest that MMP9 acts by its ability to release sSCF in kidney tissue. Conversely, both increased apoptosis and delay in renal function recovery could be suppressed by injecting MMP9−/− mice with recombinant SCF, whereas the number of circulating stem cells was not affected. The antiapoptotic effect of MMP9 and the rescue by SCF were confirmed in the ischemia-reperfusion model, which suggests that MMP9-induced mobilization of SCF may be a survival factor in AKI.
Increased MMP9 expression was documented during ischemia in mice.16 In the rat models of AKI, MMP9 expression and/or activity and the effects of MMP pharmacologic inhibitors are the subject of controversy. MMP9 was reported to be either unchanged17 or increased in various nephron segments including glomeruli18 and renal tubules and in interstitial cells, tubulointerstitial space, and perivascular cells.19,20 Increased MMP9 activity was observed in the endothelial cells, suggesting a role for MMP9 in vascular permeability.21 This hypothesis is further supported by the finding that minocycline, which reduced the perivascular increase in MMP2 and MMP9 protein expression and the increase in MMP2 activity, and ABT-518, a specific inhibitor of MMP2 and MMP9, reduced the increased vascular permeability after ischemia reperfusion.20 Moreover, minocycline inhibited tubular cell apoptosis and improved renal function,22 whereas the MMP inhibitor batimastat did not alter outcome of renal function.17 We found that, at the onset of FA AKI in mice, MMP9 expression and activity were markedly induced in S3PT (in the segment where apoptosis appeared in MMP9−/− but not in wild-type mice). We also observed a substantial induction of apoptosis in S3PT after ischemia reperfusion in MMP9−/− mice. The discrepancy between previously published data on the outcome of renal function and our results may be related to the fact that batimastat and minocycline inhibit both MMP2 and MMP9,17,20,22 and minocycline is likely to have additional effects on the apoptosis pathway.22,23
The role of MMP9 is unpredictable because of the variety of its substrates.5 In many tissues, including the kidney, extracellular matrix behaves as a survival factor.8–10 The effect of MMP9 on extracellular matrix degradation should rather favor apoptosis, and, reciprocally, MMP9−/− mice should be protected. Decreased apoptosis was indeed observed in MMP9−/− mice after partial hepatectomy24 and cerebral hypoxia-ischemia,25 although the underlying mechanisms were poorly understood; however, we found that MMP9 was protective in AKI. We did not observe an accumulation of extracellular matrix in MMP9−/− kidney, although we cannot rule out an antiapoptotic effect of MMP9 through the release of still unidentified matrix fragment. Because MMP9 was expressed apically on principal cells and was induced at the cell apex in S3PT, we hypothesized that the antiapoptotic effect of MMP9 could be unrelated to its matrix-degrading activity. Thus, we looked for the critical substrate of MMP9 in AKI.
SCF and its receptor c-Kit emerged as a candidate system for three reasons. First, MMP9 is an important player in regulating SCF/c-Kit function. In irradiated mice with bone marrow ablation, MMP9 released sSCF, which was instrumental in promoting stem cell mobilization and differentiation and thus hematopoietic reconstitution.11 Second, c-Kit/SCF has an important antiapoptotic role in many tissues, although such a role has so far not been demonstrated in the kidney.12 Third, both c-Kit and SCF were detected in the metanephros.26–28 Recent studies reported SCF staining in rat tubules, although SCF subcellular location and the identity of tubules expressing SCF were not specified.29 c-Kit was also identified in human kidney in the cytoplasm of proximal and distal tubules30,31 and in murine collecting ducts of late developing kidney32; however, to be functional, c-Kit needs to be expressed in the cell membrane. We demonstrated the expression of SCF and c-Kit at the cell membrane of mature tubular epithelial cells, in S3PT. In addition, c-Kit was observed at the apex of I-CD. This localization is reminiscent of its overexpression in chromophobe renal cell carcinoma and oncocytomas, which originate from these cells.31
Our in vitro and in vivo data strongly suggest that the release of sSCF by MMP9 described in the bone marrow11 also occurs in the kidney. Because cells of the S3PT and I-CD were protected from apoptosis in MMP9+/+ mice whereas they were severely affected in MMP9−/− mice after FA injection, we hypothesized that sSCF released in the urine by MMP9 at the apex of S3PT cells had an autocrine/paracrine effect by activating c-Kit in S3 segment and a paracrine effect downstream the nephron on I-CD. First, we showed that MMP9 could release SCF from kidney proximal tubule cells. Second, recombinant sSCF inhibited CD cell apoptosis in vitro and rescued MMP9−/− S3PT and I-CD from apoptosis both in the FA and in the ischemia-reperfusion models of AKI. Restoring the level of circulating SCF in MMP9−/− mice reduced the severity of lesions assessed by the percentage of dilated tubules in MMP9−/− kidney 18 and 72 h after FA injection and suppressed the delay in renal function recovery. In contrast, in cisplatin-induced AKI in mice, recombinant SCF did not improve apoptosis and renal function 96 h after cisplatin administration,33 suggesting that other mediators may play a role in this model. In both models we used, the effect of SCF injection on kidney cell apoptosis were shown to be restricted to S3PT and I-CD in the absence of MMP9 expression, which indicates that regulation of apoptosis in AKI may vary in the different segments of the nephron. There is indeed evidence that a combination of survival and lethal mediators including growth factors, cytokines, or guanidine nucleotides can control apoptosis in various animal models of AKI.34,35 Preliminary data indicate that MMP9-induced SCF release may also occur in human AKI because sSCF was dramatically increased in patients with tubular necrosis, whereas it was barely detectable in those with prerenal azotemia.
The results suggest that sSCF controls apoptosis in kidney S3PT and in I-CD. This might be explained by different c-Kit signal transduction pathways being stimulated by soluble and membrane forms of SCF. For instance, it was shown that sSCF activates phosphatidylinositol-3 kinase and subsequently Akt pathways, which are known to control the expression of molecules involved in apoptosis such as Bcl-2, Bad, or Bim.12,36
Our data also suggest that proteases other than MMP9 might release the sSCF because in the absence of MMP9, sSCF was still increased in the urine after FA injection. Whatever the nature of the surrogate protease(s), the amount of released SCF did not seem to be sufficient to prevent apoptosis in S3PT and I-CD.
In conclusion, our results show that MMP9 is able to release sSCF in the kidney and thus participates in the control of apoptosis in two models of AKI. They also suggest that the S3PT and I-CD may play a key role in renal function recovery because suppression of apoptosis in these structures was associated with faster recovery of renal function.
CONCISE METHODS
Mice
MMP9−/− 129SV (MMP9−/−) mice were generated as described previously37 and were used after 12 backcrosses to C57BL/6J background. Ten-week-old female MMP9−/− and MMP9+/+ female mice were used for the study. The mice were maintained with a 12-h light/12-h dark cycle and had free access to food and water. Mouse studies followed the Institutional Animal Care and Use Guidelines, and all protocols were approved by the Institut National de la Santé et de la Recherche Médicale.
Human Urine Samples and Clinical Data
Urine samples were collected prospectively in a group of four control subjects without renal dysfunction, four patients with prerenal azotemia, and four patients with tubular necrosis. The diagnosis of prerenal azotemia caused by renal hypoperfusion was based on decreased fractional excretion of sodium <1% and a rapid recovery of GFR after restoration of appropriate renal perfusion. Patients with tubular necrosis were a 78-yr-old man (serum creatinine 516 μmol/L), a 68-yr-old man (serum creatinine 752 μmol/L), a 76-yr-old man (serum creatinine 523 μmol/L), and a 78-yr-old man (serum creatinine 588 μmol/L). They presented an acute renal dysfunction (without rapid improvement after correction of a possible prerenal cause and a fractional excretion of sodium >2%) and displayed on their renal biopsy signs of acute tubular injury (necrosis, loss of brush border, de-differentiation). Urine samples were frozen quickly after collection and maintained at −80°C until analysis. All samples and clinical data were obtained from patients after their informed consent.
Antibodies and Reagents
Goat polyclonal and rabbit polyclonal anti-SCF and blocking peptide were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti–c-Kit was obtained from Eurodiagnostica (clone T595; AbCys, Paris, France) and rabbit polyclonal anti-MMP9 from Chemicon Int. (Temecula, CA). Mouse mAb directed against PCNA (clone PC10) conjugated to horseradish peroxidase was obtained from DakoCytomation SAS (Glostrup, Denmark), and mouse mAb directed against CD10 was purchased from Novocastra (Newcastle upon Tyne, UK). Rabbit polyclonal antibody directed against β-actin was purchased from IMGENEX (San Diego, CA). Thick ascending limbs of Henle's loops and proximal tubules were identified with rabbit anti–Tamm-Horsfall protein antibody38 and rabbit anti-megalin antibody,39 respectively. A rabbit anti-β subunit of H+ ATPase was used to identify intercalated cells in the collecting duct.40 DBA conjugated to TRITC; biotinylated anti-goat, anti-rabbit, and anti-mouse IgGs; and goat anti-rabbit IgG conjugated to alkaline phosphatase were purchased from Vector Laboratories (Burlingame, CA). Extravidin FITC and extravidin TRITC were obtained from Sigma Aldrich (St. Louis, MO). Mouse rSCF and recombinant human MMP9 were obtained from Biosource Int. (Clinisciences, Montrouge, France). Mouse and human ELISA SCF kits were purchased from R&D Systems (Minneapolis, MN).
Induction of AKI
Two models of AKI were used, the FA model and ischemia reperfusion. Four sets of 10 MMP9+/+ and 10 MMP9−/− 3-mo-old female mice received an intraperitoneal injection of FA (500 mg/kg body wt diluted in 0.3 M NaHCO3). Control groups of MMP9+/+ and MMP9−/− mice received an intraperitoneal injection of vehicle only (0.2 ml of 0.3 M NaHCO3). Rescue experiments were performed by injecting into the tail vein 15 ng of soluble recombinant mouse SCF diluted in isotonic saline in a final volume of 150 μl, before FA injection.
In another set of experiments, six MMP9+/+ and six MMP9−/− female mice were anesthetized by intraperitoneal administration of xylazine 2% (0.5 ml/kg body wt) and ketamine 1000 (1.5 ml/kg body wt), a flank incision was performed, and the left renal pedicle was clamped for 45 min. The clamp was then removed and the organ was allowed to reperfuse. Twenty-four hours after reperfusion, the mice were anesthetized again and the renal tissues were removed. The right kidney was used as a control in each mouse. Details of the technique have been previously reported.15 Recombinant mouse SCF was injected into four MMP9−/− female mice as described already before clamping of the renal pedicle.
Assessment of Renal Function and Proteinuria
Mice were placed in metabolic cages after FA injection and urine was collected. During this time, they were supplied with food and tap water ad libitum. Serum and urine creatinine (alkaline picric acid method), and urine total proteins (Biuret method) were measured on an autoanalyzer (Instrumentation Laboratory, Paris, France).
Histology and Morphometric Analysis
Renal tissue was fixed in Dubosq-Brazil's fixative, embedded in paraffin, cut in 3-μm sections and stained with periodic acid-Schiff stain and Masson's trichrome for histologic analysis. Tubular lesions were assessed by a morphometric method using intercepts. For each mouse, microphotographs of five randomly selected nonoverlapping fields of periodic acid-Schiff stained kidneys were digitized and displayed using Photoshop Software 6.0 (Adobe System). A tracing paper was used to count normal or injured tubules among 35 intercepts. Six MMP9−/− and six MMP9+/+ female mice killed 18 h after FA injection were used for these studies. Counts were performed in double-blind manner.
Immunohistochemistry
Immunofluorescent studies were performed on kidneys transversally sectioned, fixed with 4% paraformaldehyde overnight, and embedded in paraffin. Antigen was unmasked either by proteinase K (20 μg/ml) treatment for 15 min at room temperature or by boiling the slides in citrate buffer (0.01 M, pH 6.0) at 500 W in a microwave oven three times for 5 min. Proteinase treatment was performed before SCF (10 μg/ml) and H+ATPase (dilution 1:4) staining, and slides were incubated in a microwave-heated citrate buffer before stainings for c-Kit (5 μg/ml), MMP9 (20 μg/ml), and CD10 (1:50). Double stainings with DBA lectin (80 μg/ml), megalin (1:1000), and Tamm-Horsfall (20 μg/ml) were performed after either proteinase K treatment or citrate buffer incubation. Primary antibody was applied for 1 h at room temperature followed by incubation with biotinylated antibody (7.5 μg/ml) for 45 min at 37°C and a final incubation with extravidin conjugated to FITC or to TRITC (5 μg/ml) for 30 min at 37°C.
Assessment of Apoptosis
Apoptotic cells were detected by morphologic criteria, including cell shrinking, formation of apoptotic bodies, and condensation of chromatin, and by in situ TUNEL method (Apoptag; QBiogene, Irvine, CA). We first tested different fixatives to avoid unspecific staining. Kidneys stained overnight at 4°C with 4% PFA did not exhibit nonspecific positive staining because kidney sections from MMP9+/+ and MMP9−/− untreated mice or mice injected with bicarbonate vehicle exhibited no more than two positive nuclei (data not shown). Mouse kidneys were therefore fixed with 4% PFA overnight and embedded in paraffin.
Three-micrometer sections were dewaxed and rehydrated through graded series of alcohol. Immunofluorescence (FITC) TUNEL technique was performed according to the manufacturer's instructions. Kidney sections were counterstained with an antibody directed against PCNA conjugated to horseradish peroxidase (for 90 min at room temperature) to detect proliferative cells. Peroxidase was revealed by incubation with its substrate 3-amino-9-ethyl-carbazole for 30 min at room temperature. Kidney sections were also counterstained as described already with DBA lectin (80 μg/ml) and with antibodies directed against H+ATPase (1:4), CD10 (1:50), megalin (1:1000), and Tamm-Horsfall (20 μg/ml) protein to identify the nephron segments that exhibited apoptosis. Three washes of the slides with PBS for 10 min each were performed between antibody incubations. Sections were counterstained with DAPI (2 μg/ml for 30 s at room temperature) to stain nuclei. Slides were then mounted in Tris glycerol buffer and viewed under a Zeiss Axiophot microscope (Carl Zeiss S.A., Le Pecq, France). The percentage of apoptotic nuclei related to kidney surface area was determined on six microphotographs taken from each of six MMP9−/− and six MMP9+/+ kidneys sampled 18 h after FA injection.
In another set of experiments, the percentage of apoptotic cells in S3PT and collecting duct was estimated on kidney sections from six different MMP9+/+, MMP9−/−, and MMP9−/− mice rescued with an injection of 15 ng of recombinant sSCF. Microphotographs of six randomly selected nonoverlapping fields were digitized and displayed using Photoshop Software 6.0 (Adobe System). Apoptotic and normal nuclei were counted on printed pictures by two independent examiners who were unaware of the genotypes of the mice.
Assessment of MMP9 Activity by Zymography
The gelatinolytic activity of MMP9 was demonstrated by zymography as described previously3 on 20 μg of proteins. The presence of metalloproteinases was indicated by an unstained proteolytic zone of the substrate. Zymograms were analyzed with ImageJ (National Institutes of Health, Bethesda, MD) and converted to a graphic format.
Assessment of Membrane SCF Sensitivity to MMP9 Proteolysis In Vitro
In vitro studies were conducted on RC-SV1, a proximal tubule cell line established in the laboratory.14 For these studies, recombinant human MMP9 was activated with 1 mM 4-aminophenylmercuric acetate (Sigma) in 5 μl of gelatinase substrate buffer at 37°C for 3 h. Confluent RC-SV1 cells were then incubated for 2 h with various concentrations of activated recombinant human MMP9 (80, 160, and 320 ng/ml). Control experiments included omission of activated MMP9. Cells and medium were collected and subjected to Western blotting or immunohistochemistry. The concentration of sSCF was measured by ELISA (see Measurement of sSCF).
In another set of experiments, 30-μm cryostat kidney sections were cut. Three sections from six different MMP9+/+ and MMP9−/− kidneys were incubated for 24 h at 37°C in gelatin substrate buffer. sSCF released from tissue in medium was measured by ELISA (see Measurement of sSCF).
Inhibition of Tubular Cell Apoptosis by SCF In Vitro
Studies were conducted on HCD, a human cortical collecting duct cell line established in the laboratory and presenting features of both intercalated and principal cells.41 Cells were plated and grown for 48 h in a hormonally defined medium (DMEM/Ham's F12 1:1 [vol/vol], 30 nM sodium selenate, 5 μg/ml transferrin, 2 mM glutamine, 5 10−8 M dexamethasone, 5 μg/ml insulin, and 20 mM HEPES) supplemented with 2% FCS. Then cells were divided into three experimental groups. One group was maintained in this medium for 96 h until confluence, the second group was grown in a hormone-free/serum-free medium (basic medium), and the third group was grown in the basic medium supplemented with 15 pg/ml human rSCF. Media were changed after 48 h. Apoptosis was estimated by TUNEL method (see Assessment of Apoptosis) and measurement of caspase-3 activity using an ELISA assay developed by R&D Systems (Quantikine Human Active Caspase-3 F). The detection limit of the active caspase-3 immunoassay was 0.1 ng/ml.
Western Blotting
Western blots were performed with 20 μg of kidney lysate proteins to determine expression of MMP9 in control MMP9+/+ mice after FA injection. SCF was determined on 50 μg of proteins from concentrated human urine or 20 μg of RC-SV1 cell lysate proteins and 50 μg of proteins from concentrated conditioned culture medium. Proteins were submitted to SDS-PAGE under reducing conditions in a 12% (SCF) or 8% (MMP9) polyacrylamide gel and electrotransferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA) for 90 min at a constant current of 190 mA. Afterward, nitrocellulose sheet was saturated with 5% dry milk in TBS-Tween (Tris 50 mM, NaCl 150 mM, Tween 20 0.1% [pH 8.0]) for 1 h at 37°C, extensively washed in TBS-Tween, and incubated overnight at 4°C with polyclonal rabbit IgG anti-SCF (0.2 μg/ml) or polyclonal rabbit IgG anti-MMP9 (0.1 μg/ml) and polyclonal rabbit IgG anti-actin (0.5 μg/ml). Polyvinylidene difluoride membrane was then incubated for 2 h at room temperature with goat anti-rabbit IgG conjugated to alkaline phosphatase (0.02 μg/ml). Alkaline phosphatase activity was revealed by adding the NBT/BCI substrate (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate complex in 100 mM Tris-HCl, 100 mM NaCl, and 5 mM MgCl2 [pH 9.5]). The reaction was stopped in 20 mM Tris-HCl and 5 mM EDTA (pH 8.0). Blots were analyzed with ImageJ and converted to a graphic format.
Measurement of sSCF
sSCF was measured in mouse plasma and urine, human urine, and culture media from the proximal tubule cell line RC-SV1. Urine and culture media were ultracentrifuged (100,000 × g for 10 min) to remove cell debris.
Urine and plasma were collected from 5 MMP9+/+ and 5 MMP9−/− mice before and 18 h after FA injection. SCF was measured by an ELISA assay developed by R&D Systems (Quantikine M mouse SCF). The detection limit of the mouse SCF immunoassay was 5 pg/ml.
Similarly, urine was collected from four normal healthy control subjects, four patients with prerenal azotemia, and four patients with a tubular necrosis. SCF was detected by ELISA (Quantikine human SCF; R&D Systems). sSCF values in urine were normalized to urinary proteins. The same assay was used to measure sSCF in conditioned culture medium of the proximal tubule cell line untreated or incubated with 80, 160, and 320 ng/ml of recombinant human activated MMP9. The detection limit of the human SCF immunoassay was 9 pg/ml.
FACS Analysis
To determine the number of circulating CD34+ cells, we killed anesthetized control MMP9+/+ mice, MMP9−/− mice, and MMP9−/− mice injected with soluble recombinant mouse SCF 18 h after FA injection. Peripheral blood was incubated in hemolysis buffer (150 mM NH4Cl, 12 mM NaHCO3, and 0.1 mM EDTA [pH 7.2]) for 5 min at room temperature, then incubated with conjugated isotype control or phycoerythrin-conjugated anti-mouse CD34 for 30 min at 37°C. As positive control, staining was performed on bone marrow cells isolated from tibia and femur of the same mice. The samples were analyzed by a flow cytometer EPICS Elite (Beckman Coulter, Fullerton, CA).
Statistical Analysis
Results are expressed as means ± SEM. Significance of differences between different groups of mice were determined by ANOVA test.
DISCLOSURES
None.
Acknowledgments
This study was funded by grants from the Association pour la Recherche contre le Cancer to S.B.; the Association pour l’Utilisation du Rein Artificiel (AURA) and the Fondation pour la Recherche Médicale to M.M.; the Institut National de la Santé et de la Recherche Médicale, the Université Paris 6, and the Ministère de la Recherche (ACI 1A061G) to P.R. and B.L.; and the National Institutes of Health (P01 AI053194) to Z.W.
We thank P. Verroust and S. Nielsen for generous gifts of anti-megalin and anti-H+ATPase antibodies, respectively. We are grateful to F. Cauchard for c-Kit immunohistochemistry and C. Martin and C. Kitou for animal care. We also gratefully acknowledge U. Blank for helpful discussion and comments. We are grateful to J. Fleury and C. Prengel for FACS analysis.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Kribben A, Edelstein C, Schrier RW: Pathophysiology of acute renal failure. J Nephrol 12: S142–S151, 1999 [PubMed] [Google Scholar]
- 2.Lovett DH, Sterzel RB, Kashgarian M, Ryan JL: Neutral proteinase activity produced in vitro by cells of the glomerular mesangium. Kidney Int 23: 342–349, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Piedagnel R, Murphy G, Ronco P, Lelongt B: Matrix metalloproteinase 2 (MMP2) and MMP9 are produced by kidney collecting duct principal cells but are differentially regulated by SV40 large T, arginine vasopressin, and epidermal growth factor. J Biol Chem 274: 1614–1620, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Legallicier B, Trugnan G, Murphy G, Lelongt B, Ronco P: Expression of the type-IV collagenase during mouse kidney development and tubule segmentation. J Am Soc Nephrol 12: 2358–2369, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G: Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37: 375–536, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco P: MMP9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med 193: 793–802, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz A, Lorz C, Catalan MP, Danoff TM, Yamasaki Y, Egido J, Neilson EG: Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int 57: 969–981, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Boudreau N, Sympson CJ, Werb Z, Bissel MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267: 891–893, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stupack DG, Cheresh DA: Apoptotic cues from the extracellular matrix: Regulators of angiogenesis. Oncogene 22: 9022–9029, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Makino H, Sugiyama H, Kashihara N: Apoptosis and extracellular matrix-cell interactions in kidney disease. Kidney Int 77: S67–S75, 2000 [PubMed] [Google Scholar]
- 11.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S: Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109: 625–637, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MA, Court EL, Smith JG: Stem cell factor: Laboratory and clinical aspects. Blood Rev 15: 191–197, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kerjaschki D, Farquhar MG: The pathogenic antigen for Heyman nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A 79: 5557–5561, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandewalle A, Lelongt B, Geniteau-Legendre M, Baudouin B, Antoine M, Estrade S, Chatelet F, Verroust P, Cassingena R, Ronco P: Maintenance of proximal and distal cell functions in SV40-transformed tubular cell lines derived from rabbit kidney cortex. J Cell Physiol 141: 203–221, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Letavernier E, Perez J, Joye E, Bellocq A, Fouqueray B, Haymann JP, Heudes D, Wahli W, Desvergne B, Baud L: Peroxisome proliferator-activated receptor beta/delta exerts a strong protection from ischemic acute renal failure. J Am Soc Nephrol 16: 2395–2402, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Bellini MH, Coutinho EL, Filgueiras TC, Maciel TT, Schor N: Endostatin expression in the murine model of ischemia/reperfusion induced acute renal failure. Nephrology 12: 459–465, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ziswiler R, Daniel C, Franz E, Marti H: Renal matrix metalloproteinase activity is unaffected by experimental ischemia-reperfusion injury and matrix metalloproteinase inhibition does not alter outcome of renal function. Exp Nephrol 9: 118–124, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Caron A, Desrosiers R, Langlois S, Beliveau R: Ischemia-reperfusion injury stimulates gelatinase expression and activity in kidney glomeruli. Can J Physiol Pharmacol 83: 287–300, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Basile D, Fredrich K, Weihrauch D, Hattan N, Chilian W: Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol 286: F893–F902, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Sutton T, Kelly K, Mang H, Plotkin Z, Sandoval R, Dagher P: Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288: F91–F97, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Caron A, Desrosiers R, Langlois S, Béliveau R: Ischemia injury alters endothelial cell properties of kidney cortex: Stimulation of MMP-9. Exp Cell Res 310: 105–116, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kelly K, Sutton T, Weathered N, Ray N, Caldwell E, Plotkin Z, Dagher P: Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 287: F760–F766, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Wei Q, Wang C, Hill W, Hess D, Dong Z: Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Olle EW, Ren X, McClintock SD, Warner RL, Deogracias MP, Johnson KJ, Colletti LM: Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology 44: 540–549, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Svedin P, Haqberq H, Savman K, Zhu C, Mallard C: Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci 27: 1511–1518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ: Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development 122: 3023–3333, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Matsui Y, Zsebo KM, Hogan BL: Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature 347: 667–669, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J: c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol 299: 238–249, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Jones SE, Kelly DJ, Cox AJ, Zhang Y, Gow RM, Gilbert RE: Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int 64: 906–913, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lin ZH, Han EM, Lee ES, Kim CW, Kim HK, Kim I, Kim YS: A distinct expression pattern and point mutation of c-kit in papillary renal cell carcinomas. Mod Pathol 17: 611–616, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Miliaras D, Karasavvidou F, Papanikolaou A, Sioutopoulou D: KIT expression in fetal, normal adult, and neoplastic renal tissues. J Clin Pathol 57: 463–466, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David SG, Cebrian C, Vaughan ED, Herzlinger D: c-kit and ureteral peristalsis. J Urol 173: 292–295, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Nishida M, Fujimoto S, Toiyama K, Sato H, Hamaoka K: Effect of hematopoietic cytokines on renal function in cisplatin-induced ARF in mice. Biochem Biophys Res Commun 324: 341–347, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Ortiz A, Justo P, Sanz A, Lorz C, Egido J: Targeting apoptosis in acute tubular injury. Biochem Pharmacol 66: 1589–1594, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kaushal G, Basnakian A, Shah S: Apoptotic pathways in ischemic acute renal failure. Kidney Int 66: 500–506, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Okayama Y, Kawakami T: Development, migration, and survival of mast cells. Immunol Res 34: 97–115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z: MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunisholz M, Geniteau-Legendre M, Ronco PM, Moullier P, Pontillon F, Richet G, Verroust PJ: Characterization of monoclonal antibodies specific for human Tamm-Horsfall protein. Kidney Int 29: 971–976, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Sahali D, Mulliez N, Chatelet F, Laurent-Winter C, Citadelle D, Sabourin JC, Roux C, Ronco P, Verroust PJ: Comparative immunochemistry and ontogeny of two closely related coated pit proteins. Am J Pathol 142: 1654–1667, 1993 [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frokiaer J, Nielsen S: Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol 285: F1244–F1257, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Prié D, Friedlander G, Coureau C, Vandewalle A, Cassingéna R, Ronco P: Role of adenosine on glucagon-induced cAMP in a human cortical collecting duct cell line. Kidney Int 47: 1310–1318, 1995 [DOI] [PubMed] [Google Scholar]