Abstract
This study examines the plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA) and metabolites 4-hydroxy-3-methoxymethamphetamine (HMMA), 3,4-methylenedioxyamphetamine (MDA), and 4-hydroxy-3-methoxyamphetamine (HMA) in young adults for up to 143 hours after drug administration. Seventeen female and male participants (black, white, and Hispanic) received placebo, low (1.0 mg/kg), and high (1.6 mg/kg) oral MDMA doses (comparable to recreational doses) in a double-blind, randomized, balanced, within-subject design while residing on a closed research unit. Doses were separated by 1 week or more. A fully validated two-dimensional gas chromatography/mass spectrometry method simultaneously quantified MDMA, HMMA, MDA, and HMA. Calibration curves were MDA, 1 to 100 ng/mL; HMA, 2.5 to 100 ng/mL; and MDMA and HMMA, 2.5 to 400 ng/mL. Mean ± standard deviation maximum plasma concentrations (Cmax) of 162.9 ± 39.8 and 171.9 ± 79.5 ng/mL were observed for MDMA and HMMA, respectively, after low-dose MDMA. After the high dose, mean MDMA Cmax significantly increased to 291.8 ± 76.5 ng/mL, whereas mean HMMA Cmax was unchanged at 173.5 ± 66.3 ng/mL. High intersubject variability in Cmax was observed. Mean MDA Cmax were 8.4 ± 2.1 (low) and 13.8 ± 3.8 (high) ng/mL. HMA Cmax were 3.5 ± 0.4 and 3.9 ± 0.9 ng/mL after the low and high doses, respectively. AUC∞ displayed similar trends to Cmax, demonstrating nonlinear pharmacokinetics. Times of last plasma detection were generally HMA < MDA < MDMA < HMMA. Mean half-lives (t1/2) of MDMA, MDA, and HMMA were approximately 7 to 8 hours, 10.5 to 12.5 hours, and 11.5 to 13.5 hours, respectively. HMA t1/2 showed high variability. Mean MDMA volume of distribution was constant for low and high doses; clearance was significantly higher after the low dose. This study presents MDMA plasma pharmacokinetic data for the first time from blacks and females as well as measurement of HMMA and HMA concentrations after low and high MDMA doses and more frequent and extended plasma sampling than in prior studies.
Keywords: MDMA, plasma, pharmacokinetics, ecstasy, metabolites, nonlinear
INTRODUCTION
3,4-Methylenedioxymethamphetamine (MDMA or ecstasy) is an illicit drug popular with young adults at clubs or “raves”1 as well as in more intimate settings.2 MDMA is known as the “love drug” for its entactogenic properties of enhanced euphoria, loving feelings, self-acceptance, communication, empathy, and understanding.3-9 In addition, MDMA exerts a number of sympathomimetic effects, including marked increases in cardiovascular parameters, pupil dilation, dry mouth, and loss of appetite.5,6,10,11
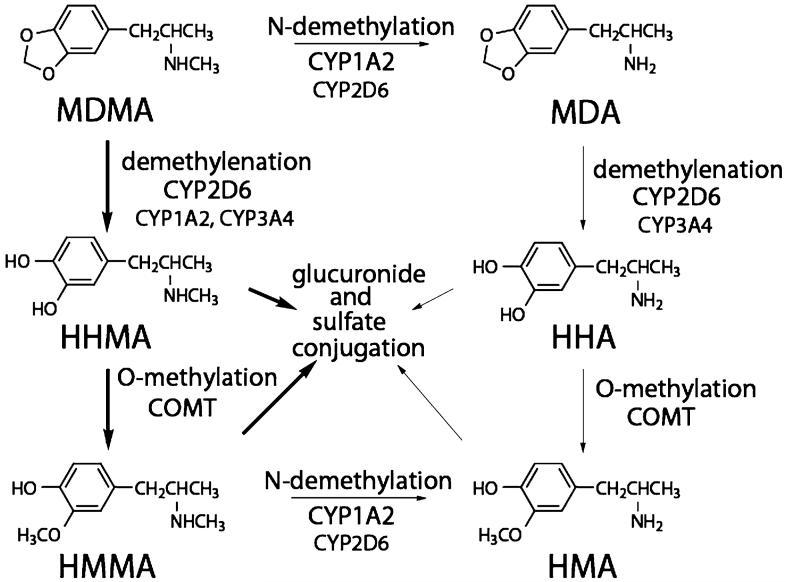
MDMA is primarily ingested orally, although there have been reports of intravenous injections of crushed tablets,12,13 insufflation,14 and accidental sublingual absorption.15 MDMA absorption is rapid after oral administration.16-22 Figure 1 shows the two main metabolic pathways for MDMA in humans and the associated hepatic microsomal enzymes. 4-Hydroxy-3-methoxymethamphetamine (HMMA) is the major plasma and urinary metabolite6 and 3,4-dihydroxymethamphetamine (HHMA) is a reported additional major metabolite.23 Two metabolites, HHMA and 3,4-dihydroxyamphetamine (HHA), are not commercially available and, therefore, were not included in the present analysis.
FIGURE 1.
Metabolism of 3,4-methylenedioxymethamphetamine (MDMA). MDA, 3,4-methylenedioxyamphetamine; HHMA, 3,4-dihydroxymethamphetamine; HHA, 3,4-dihydroxyamphetamine; HMMA, 4-hydroxy-3-methoxymethamphetamine; HMA, 4-hydroxy-3-methoxyamphetamine.
CYP2D6 is a polymorphic gene containing as many as 50 alleles.24 In a recent study, 1.4% and 4.5% of blacks were genotyped as poor and ultrarapid metabolizers, respectively.25 It is estimated that 5% to 10% of the white population are poor metabolizers,26,27 with less than 5% classified as ultrarapid metabolizers.24 COMT also shows polymorphic activity with variability between ethnic groups.28 High, intermediate, and low COMT function was reported in 55%, 38%, and 7% of blacks and in 23%, 50%, and 27% of white, respectively. No gender differences were observed.
MDMA primarily affects the serotonergic system, acting as an indirect monoaminergic agonist29; however, the mechanism(s) by which MDMA causes toxicity are not fully understood. MDMA and its metabolites 3,4-methylenedioxyamphetamine (MDA), HHMA, and HMMA were nontoxic to the serotonergic system when injected directly into rat brain at concentrations achieved with systemic administration.30,31
A single controlled MDMA administration study has documented MDMA and MDA plasma pharmacokinetics after administration of two different MDMA doses to the same eight male white participants.18,21 Also in this study, one subject’s peak plasma HMMA concentration was measured after the 75-mg dose, whereas a separate subject’s peak concentration was shown after the 125-mg dose.18 In an earlier pilot study by the same group, HMMA plasma concentrations for 24 hours were displayed for one subject at each of three MDMA doses (50, 100, and 150 mg); however, the HMMA data were from a different individual at each dose.18 These data, although not collected from the same participant, suggested nonlinearity in MDMA and HMMA pharmacokinetics. Also, MDMA pharmacokinetics have not been characterized in females and blacks. Earlier characterizations of MDMA and metabolites in plasma evaluated concentrations only up to 24 hours postdose, except for two studies that collected plasma for 48 hours.32,33 Because some participants are still positive at this time, additional sampling is required to accurately determine detection windows and terminal elimination half-lives (t1/2). Specialized pharmacokinetic analyses have examined repeated MDMA dosing32 and compared poor and extensive CYP2D6 metabolizers.34
In this double-blind, randomized, balanced, within-subject study, placebo, 1.0, and 1.6 mg/kg MDMA were administered orally to 17 young adult male and female MDMA users. Plasma was collected for up to 143 hours postdose and simultaneously analyzed for MDMA, HMMA, MDA, and 4-hydroxy-3-methoxyamphetamine (HMA). Pharmacokinetic and statistical analyses were performed to compare parameters in the same subjects after low and high doses, determine MDMA pharmacokinetics in women, and characterize MDMA pharmacokinetics in a larger population and for a longer timeframe than previously assessed. We hypothesize that the MDMA dose-concentration relationship will be nonlinear in humans within the range of doses self-administered by young adult MDMA users, that there will be significant gender differences in MDMA pharmacokinetics, and that MDMA and HMMA will be detectable more than 48 hours after dosing.
MATERIALS AND METHODS
Human Participants
Participants provided written informed consent and were paid to participate in this National Institute on Drug Abuse Intramural Research Program (NIDA/IRP) Institutional Review Board-approved research study. Male and female volunteers ages 18 to 40 years with no current clinically significant medical problems were recruited by word of mouth, flyers, and television, radio, and newspaper advertisements. Subjects must have consumed at least five tablets of MDMA in their lifetime and at least one in the past 90 days. History of MDMA consumption was supported by a positive urine amphetamines or hair MDMA test in the past 90 days. Hair testing was included as an alternative matrix for verifying drug use as a result of the short detection time (30 to 48 hours) of MDMA in urine.35 A recent study found that only 20% of individuals with MDMA- or MDA-positive hair specimens had corresponding urine specimens positive for either analyte.36 Females had to use a reliable method of birth control or abstain from sexual intercourse throughout the study. Serum and urine pregnancy tests were administered at the screening visit and on the morning of each dosing session, respectively.
All potential subjects received a comprehensive medical and psychologic evaluation, including medical and drug use history and physical examination, clinical laboratory tests, 12-lead electrocardiogram with 3-minute rhythm strip, Symptom Checklist-90-Revised (SCL-90R), and computer-administered version of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV.37 Individuals meeting any of the following criteria were excluded: nursing or pregnant women; current medical condition or history of neurologic illness; axis I psychiatric diagnosis other than abuse or dependence on nicotine, cannabis, or MDMA; recent (within 30 days of MDMA administration) prescription of a CYP2D6 or CYP3A4 inhibitor or CYP3A4 inducer (with reconsideration 30 days after the individual voluntarily stopped use); systolic blood pressure (BP) greater than 135 mmHg, diastolic BP greater than 85 mmHg, or heart rate greater than 100 beats/min after 5 minutes at rest; total cholesterol greater than 250 mg/dL if older than 30 years; hemoglobin less than 12.5 g/100 mL (male) or less than 12 g/100 mL (female); clinically significant abnormal electrocardiogram; and serum transaminase levels greater than three times normal.
Study Design
Participants had two options for study participation while residing on the closed research unit of the NIDA/IRP: a single 23-day continuous stay encompassing all three dosing sessions or three separate stays at least 1 week apart completed in 1 year. Participants remained on the unit for 2 to 7 days after each dose. Participants were required to enter the inpatient unit at least 12 hours before dosing. On admission, staff completed a brief nursing assessment. Separate stay participants were examined at each stay to ensure continued study eligibility. Urine was screened for benzodiazepines, cocaine, amphetamines, cannabis, opiates, phencyclidine (PCP), and barbiturates with a Triage® 7 Drugs of Abuse panel (Biosite, Inc., San Diego, CA). Negative results were required for all drug classes except amphetamines and cannabis for the dosing session to proceed. The next morning, participants were encouraged to eat a light breakfast before dosing. Females were administered a urine pregnancy test. After collection of baseline measures, biologic specimens, and a 12-lead electrocardiogram, participants orally ingested one of three doses: 0 (placebo), 1.0 mg/kg (low), or 1.6 mg/kg (high) MDMA (Lipomed, Arlesheim, Switzerland) while seated in a quiet room. Active drug was prepared as the hydrochloride salt; placebo contained only lactose. For safety purposes, there was a maximum absolute dose limit of 150 mg MDMA. Two participants whose weight exceeded 93.75 kg, one male and one female, received this maximum dose. Dosing sessions were separated by a minimum of 1 week to enable determination of MDMA and metabolite detection times and t1/2. After MDMA or placebo administration, participants remained seated and were monitored by medical staff for 3 hours or until systolic BP, diastolic BP, and heart rate returned to within 20% of predose levels (or heart rate to <95 beats/min), whichever occurred later. Biologic specimen collection, physiological measurements, and subjective evaluations continued for 47 to 167 hours after dosing.
Blood Collection
Whole blood was collected in a sodium heparin Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) at -0.25, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4.0, 4.5, 5.0, 7.0, 9.0, 11, 13, 15, 23, 29, 34, 39, 47, 71, 95, 119, and 143 hours after each dose. Predose through 47-hour specimens were collected from all participants; the exact number of later collections depended on the length of residential stay. Specimens were stored on ice immediately after collection, centrifuged, and plasma-separated within 2 hours. Plasma specimens were frozen at -20°C until analysis.
Plasma Analysis
Plasma specimens were simultaneously analyzed for MDMA, MDA, HMMA, and HMA according to a fully validated procedure.38 Briefly, internal standard solution (MDMA-d5, MDA-d5, and pholedrine) was added to 1 mL of participant plasma, calibrator, or quality control solution. Acidic hydrolysis was performed by addition of 1 mL 0.5 M hydrochloric acid and incubation at 100°C for 40 minutes. After tubes cooled, pH was adjusted to 6 with 1 mL 0.1 M phosphate buffer (pH 6) and 50 μL 10 M sodium hydroxide. Samples were vortexed and centrifuged before solid phase extraction with conditioned Styre Screen (United Chemical Technologies, Bristol, PA) columns. Columns were washed with H2O, 0.1 M acetic acid and methanol before elution of MDMA and metabolites with a fresh mixture of ethyl acetate:isopropanol:ammonium hydroxide (90:6:4). Acidified methanol (15 μL) was added to the eluate before evaporation. Extracts were reconstituted with 100 μL 0.5 M triethylamine in heptane. Ten microliters heptafluorobutyric acid anhydride was added and tubes incubated at 60°C to derivatize HMMA and HMA. A back extraction was performed by addition of 200 μL Tris buffer to cooled tubes. Tubes were vortexed and centrifuged with the top organic layer removed and pipetted into an autosampler vial.
Three microliters derivatized extract were analyzed by two-dimensional gas chromatography/electron impact mass spectrometry (2D-GC/EI-MS) operated in selected ion monitoring mode. 2D-GC/EI-MS parameters are presented in detail in Kolbrich et al.38 The linear dynamic range for each analyte was 1 to 100 ng/mL MDA, 2.5 to 100 ng/mL HMA, and 2.5 to 400 ng/mL MDMA and HMMA. Method accuracy was greater than 80%. The greatest coefficient of variation for an intraassay batch was 8.4%, whereas coefficients of variation for interassay imprecision were 6.7% or less. Specimens with concentrations greater than the upper limit of quantification (LOQ) were diluted with phosphate buffer (pH 6.0) and reextracted.
Pharmacokinetic and Statistical Analyses
Noncompartmental models (WinNonlin v 5.2; Pharsight Corp., Mountain View, CA) were used to calculate t1/2, area-under-the-curve from 0 to infinity (AUC∞), apparent volume of distribution after oral administration (Vd/F, where F is bioavailability), and apparent total body clearance of the drug from plasma (CL/F). Three or more time points were included in the calculation of t1/2.AUC∞ was calculated by extrapolating AUC from time of dosing to infinity based on the last observed concentration and the first-order rate constant (λZ) associated with the terminal (log-linear) portion of the curve (estimated by linear regression of time versus log concentration):
Statistical analyses were performed using SPSS v 14.0 for Windows (SPSS, Inc., Chicago, IL). For within-subject comparisons, paired t tests were used to evaluate variables with Gaussian data distribution and nonparametric Wilcoxon signed rank tests were used for variables with non-Gaussian data distribution. Vd/F, CL/F, MDMA and MDA AUC∞, MDMA, MDA, and HMA peak analyte concentrations (Cmax), HMMA time to reach Cmax (Tmax), and metabolite ratios involving HMA were normally distributed and evaluated by paired t tests. High-dose HMMA to MDMA AUC∞ and low-dose HMMA to MDMA Cmax and Tmax comparisons also displayed Gaussian data distribution and were evaluated by paired t tests. All other within-subject comparisons had at least one variable with non-Gaussian data distribution and were evaluated with the Wilcoxon signed rank test. For between-group gender comparisons, independent-samples t tests and the nonparametric Mann-Whitney U test were used for the relevant variables. The following variables were normally distributed and evaluated with independent-samples t tests: MDMA and MDA low- and high-dose t1/2; HMMA low-dose t1/2; MDMA, MDA, and HMMA high-dose AUC∞;MDA low-dose AUC∞; HMMA and MDMA low- and high-dose Tmax; MDMAVd/F and CL/F; and all Cmax comparisons except HMMA high dose. All other gender comparisons used the Mann-Whitney U test. Statistical comparisons between analytes or doses were always performed between matched participants with all relevant data points. Descriptive statistics, eg, mean, standard deviation (SD) included results from all participants. Statistical comparisons were considered significantly different if P < 0.05 (two-tailed). As a result of smaller sample sizes, standard errors (SEs) are reported for gender data. All other results are expressed as mean ± SD, unless otherwise noted.
RESULTS AND DISCUSSION
Participants
Seventeen healthy volunteers met eligibility criteria: seven black males, six black females, two white males, one white female, and one Hispanic male of unknown race. Mean ± SD age and weight were 21.5 ± 2.5 years (range, 18-27 years) and 76.7 ± 17.8 kg (range, 43.2-105.7 kg), respectively. There was no significant gender difference in mean ± SE age (females 22.6 ± 1.1 years, males 20.7 ± 0.6 years, P = 0.13) or weight (females 66.7 ± 8.2 kg, males 75.3 ± 4.5 kg, P = 0.345). Black participants had a mean ± SD age of 22.2 ± 2.4 years and mean weight of 73.5 ± 16.9 kg.
Four participants completed the study in one stay with 7 to 10 days between drug administration sessions. Thirteen participants were discharged after each session and readmitted later for the next session. The mean ± SD interval (range) between unit discharge and readmission was 17.0 ± 18.2 (3-89) days. All three sessions were completed within a mean of 40.4 ± 27.8 (14-113) days. The median number of hours for blood collection was 143 hours (mean ± SD 120.5 ± 39.3 hours; range, 47-146 hours).
Time of First Detection
MDMA was detected at 15 minutes in less than 25% of participants’ plasma and in all participants 30 minutes after each dose. Time of first HMMA detection was similar to that of MDMA. MDA was detected at 30 minutes in 34% of participants and at 45 minutes in 75%; by 1.25 hours, all participants’ plasma was MDA-positive after both doses. HMA displayed variable first detection times of 1 to 7 hours after the low and 2 to 9 hours after the high dose. Approximately 50% of specimens were HMA-positive at 3 hours.
Other published studies of controlled administration of 75 to 150 mg oral MDMA report similar times for onset of drug detection.6,21 For example, in two subjects administered 1.5 mg/kg MDMA, MDMA was detected at 15 and 30 minutes and MDA at 1.5 and 2.25 hours after dosing.39 Differences in first detection times between studies could be the result of differences in plasma collection time points, LOQ achieved with individual analytical methods, and variation in oral absorption between individuals.
Time of Last Detection
Plasma specimens from all participants remained MDMA-positive at 23 hours with mean concentrations of 19.5 ± 12.4 and 44.1 ± 12.9 ng/mL after the 1.0- and 1.6-mg/kg doses, respectively. After the low dose, plasma specimens from greater than 50% of participants were still positive for MDMA at 39 hours; by 47 hours, four of 17 (23.5%) participants’ plasma contained 3.2 to 22.8 ng/mL MDMA. Of subjects who resided on the research unit for longer than 47 hours, plasma from only one individual remained positive at 71 and 95 hours at concentrations of 10.4 and 5.1 ng/mL MDMA, respectively. By 119 hours, MDMA was below the LOQ in all low-dose specimens. After the high dose, plasma specimens from 82% of participants were MDMA-positive (3.2 to 15.7 ng/mL) at 47 hours. Plasma from one participant with a residential stay longer than 47 hours remained MDMA-positive (2.7 ng/mL) at 71 hours.
HMMA had the longest detection window of the four analytes. Plasma specimens from all participants except one were positive for HMMA at 47 hours after the 1.0-mg/kg dose with a mean concentration of 4.8 ± 1.4 ng/mL. All 47-hour plasma specimens were positive for HMMA after the high dose with a mean concentration of 9.5 ± 3.9 ng/mL. Of participants with a 71-hour plasma collection, 14% and 67% were positive for HMMA after the low and high doses, respectively, at concentrations ranging from 2.6 to 5.2 ng/mL. HMMA was quantifiable in one participant’s plasma (2.8 ng/mL) 95 hours after the high dose, but was less than LOQ by 119 hours.
At 23 hours, all participants’ plasma was MDA-positive at concentrations ranging from 1.6 to 12.0 ng/mL. Positivity decreased more rapidly after the low dose; by 47 hours, 24% of specimens were positive after the low (1.2 to 2.3 ng/mL) and 82% after the high (1.1 to 4.5 ng/mL) doses. One plasma specimen remained positive 71 hours after the low dose (1.4 ng/mL) and one from a different participant after the high dose (1.0 ng/mL).
HMA never exceeded the LOQ in three participants’ plasma after the low and one after the high dose. At 23 hours after the low and high doses, 79% and 100% of plasma specimens, respectively, were HMA-positive. By 47 hours, all plasma specimens were negative after the low dose, whereas plasma from two participants remained positive at 2.8 and 3.5 ng/mL at 47 hours after the high dose; both participants’ plasma specimens were negative at 71 hours.
Detection windows for MDMA, HMMA, and MDA are longer than previously reported, presumably because this study extended plasma collections beyond 48 hours. These data demonstrate that the most prevalent analytes for documenting MDMA exposure with the longest detection window were MDMA or HMMA; however, detection of HMMA requires a hydrolysis step during plasma analysis.
Peak Concentration, Time to Reach Peak Concentration, and Area-Under-the-Curve From Zero to Infinity
Mean MDMA Cmax were attained 2.4 hours postdose (Table 1; Fig. 2). Intersubject variability in Cmax is shown in Figure 3. Mean MDMA Cmax was significantly higher after the 1.6-mg/kg dose (n = 17, P < 0.001); no significant difference in Tmax was observed (n = 17, P = 0.78). Mean AUC∞ was significantly higher (n = 17, P < 0.001) after the high MDMA dose. Although the high MDMA dose was only 1.6 times the low dose, high-dose AUC∞ was 1.9 times the low dose, indicating nonlinearity in the dose-concentration response.
TABLE 1.
Mean ± Standard Deviation (SD) or Standard Error (SE), Median and Range of Maximum Plasma Concentrations (Cmax), Time to Reach Maximum Plasma Concentrations (Tmax), and Area Under the Plasma Concentration-time Curve from Time Zero to ∞ (AUC∞) for 3,4-methylenedioxymethamphetamine (MDMA), 4-hydroxy-3-methoxymethamphetamine (HMMA), 3,4-methylenedioxyamphetamine (MDA), and 4-hydroxy-3-methoxy-amphetamine (HMA) After Controlled Administration of Low- (1.0 mg/kg) and High- (1.6 mg/kg) Dose MDMA
| MDMA |
HMMA |
||||
|---|---|---|---|---|---|
| Low | High | Low | High | ||
| Cmax (ng/mL) | Overall | (n = 17) | (n = 17) | (n = 16)* | (n = 17) |
| Mean ± SD | 162.9 ± 39.8 | 291.8 ± 76.5 | 171.9 ± 79.5 | 173.5 ± 66.3 | |
| Median | 148.5 | 275.8 | 176.1 | 177.8 | |
| Range | 115.4-248.8 | 190.0-465.3 | 36.7-312.7 | 20.0-318.1 | |
| Females | (n = 7) | (n = 7) | (n = 6) | (n = 7) | |
| Mean ± SE | 188.3 ± 15.3 | 327.1 ± 36.9 | 99.9 ± 21.0 | 139.7 ± 25.1 | |
| Median | 205.8 | 286.2 | 112.2 | 148.6 | |
| Range | 136.3-248.8 | 190.0-465.3 | 36.7-170.1 | 20.0-210.2 | |
| Males | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| Mean ± SE | 145.1 ± 9.3 | 267.1 ± 15.5 | 215.1 ± 18.8 | 197.2 ± 18.3 | |
| Median | 138.3 | 260.3 | 199.1 | 179.0 | |
| Range | 115.4-218.1 | 206.7-368.8 | 133.7-312.7 | 145.3-318.1 | |
| Blacks | (n = 13) | (n = 13) | (n = 12) | (n = 13) | |
| Mean ± SD | 167.2 ± 43.7 | 300.8 ± 85.2 | 176.0 ± 79.1 | 179.2 ± 71.9 | |
| Median | 145.5 | 283.3 | 176.1 | 177.8 | |
| Range | 115.4-248.8 | 190.0-465.3 | 36.7-312.7 | 20.0-318.1 | |
| Tmax (h) | Overall | (n = 17) | (n = 17) | (n = 16)* | (n = 17) |
| Mean ± SD | 2.4 ± 0.6 | 2.4 ± 0.7 | 1.8 ± 0.7 | 1.9 ± 0.5 | |
| Median | 2.3 | 2.3 | 1.8 | 2.0 | |
| Range | 1.8-3.5 | 1.5-4.0 | 1.3-3.5 | 1.1-2.8 | |
| AUC∞(h·ng/mL) | Overall | (n = 17) | (n = 17) | (n = 16)* | (n = 17) |
| Mean ± SD | 1833.2 ± 840.9 | 3485.3 ± 760.1 | 1839.2 ± 502.9 | 2354.4 ± 670.1 | |
| Median | 1670.6 | 3513.2 | 1843.4 | 2335.7 | |
| Range | 1056.8-4680.6 | 2223.0-4992.9 | 788.6-2740.0 | 458.6-3239.0 | |
| Females | (n = 7) | (n = 7) | (n = 6) | (n = 7) | |
| Mean ± SE | 2410.0 ± 391.4 | 3901.6 ± 297.7 | 1398.3 ± 176.5 | 1938.3 ± 301.9 | |
| Median | 2043.7 | 3866.7 | 1645.6 | 2244.2 | |
| Range | 1712.5-4680.6 | 2589.9-4992.9 | 788.6-1743.8 | 458.6-2950.2 | |
| Males | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |
| Mean ± SE | 1429.4 ± 101.1 | 3193.9 ± 196.1 | 2103.7 ± 105.0 | 2645.6 ± 120.0 | |
| Median | 1407.3 | 3153.5 | 2080.3 | 2592.3 | |
| Range | 1056.8-2122.9 | 2223.0-4249.8 | 1661.2 -2740.0 | 2001.8-3239.0 | |
| Blacks | (n = 13) | (n = 13) | (n = 12) | (n = 13) | |
| Mean ± SD | 1891.7 ± 929.1 | 3457.6 ± 834.5 | 1826.3 ± 429.6 | 2333.0 ± 682.8 | |
| Median | 1712.5 | 3486.6 | 1824.3 | 2313.2 | |
| Range | 1089.0-4680.6 | 2223.0-4992.9 | 788.6-2585.7 | 458.6-3239.0 | |
| Cmax (ng/mL) | Overall | (n = 17) | (n = 17) | (n = 13)† | (n = 16)‡ |
| Mean ± SD | 8.4 ± 2.1 | 13.8 ± 3.8 | 3.5 ± 0.4 | 3.9 ± 0.9 | |
| Median | 7.6 | 13.7 | 3.4 | 3.6 | |
| Range | 5.6-14.2 | 6.0-22.3 | 2.9-4.2 | 3.1-6.1 | |
| Females | (n = 7) | (n = 7) | (n = 4) | (n = 6) | |
| Mean ± SE | 9.6 ± 1.0 | 14.0 ± 2.1 | 3.6 ± 0.2 | 4.1 ± 0.4 | |
| Median | 8.8 | 13.5 | 3.4 | 4.0 | |
| Range | 6.6-14.2 | 6.0-22.3 | 3.3-14.2 | 3.2-6.1 | |
| Males | (n = 10) | (n = 10) | (n = 9) | (n = 10) | |
| Mean ± SE | 7.6 ± 0.4 | 13.7 ± 0.7 | 3.4 ± 0.1 | 3.7 ± 0.2 | |
| Median | 7.4 | 13.7 | 3.3 | 3.5 | |
| Range | 5.6-9.8 | 9.9-17.0 | 2.9-4.1 | 3.1-5.5 | |
| Blacks | (n = 13) | (n = 13) | (n = 10) | (n = 12) | |
| Mean ± SD | 8.6 ± 2.3 | 13.9 ± 4.0 | 3.4 ± 0.4 | 3.8 ± 0.9 | |
| Median | 8.2 | 13.7 | 3.3 | 3.4 | |
| Range | 5.6-14.2 | 6.0-22.3 | 2.9-4.2 | 3.1-6.1 | |
| Tmax (h) | (n = 17) | (n = 17) | (n = 13)† | (n = 16)‡ | |
| Mean ± SD | 7.5 ± 1.7 | 7.6 ± 2.6 | 10.6 ± 2.6 | 15.1 ± 7.9 | |
| Median | 7.1 | 7.1 | 11.0 | 12.0 | |
| Range | 4.7-11.0 | 5.0-15.0 | 5.0-13.1 | 5.0-34.0 | |
| AUC∞(h·ng/mL) | Overall | (n = 17) | (n = 17) | (n = 12)†§ | (n = 12)‡§ |
| Mean ± SD | 188.2 ± 54.4 | 352.2 ± 111.8 | 322.9 ± 169.4 | 603.0 ± 695.7 | |
| Median | 189.4 | 332.6 | 257.5 | 306.2 | |
| Range | 102.6-326.4 | 209.2-680.9 | 121.6-686.2 | 173.2-2618.7 | |
| Females | (n = 7) | (n = 7) | (n = 4) | (n = 6) | |
| Mean ± SE | 233.3 ± 17.2 | 375.10 ± 55.8 | 293.8 ± 67.8 | 339.9 ± 128.5 | |
| Median | 215.1 | 332.6 | 238.6 | 189.3 | |
| Range | 191.5-326.4 | 233.4-680.9 | 202.7-495.5 | 173.2-969.5 | |
| Males | (n = 10) | (n = 10) | (n = 9) | (n = 6) | |
| Mean ± SE | 156.6 ± 10.9 | 336.2 ± 26.5 | 321.7 ± 61.6 | 866.0 ± 365.1 | |
| Median | 152.1 | 327.7 | 270.6 | 614.0 | |
| Range | 102.6-218.3 | 209.2-528.4 | 121.6-686.2 | 215.0-2618.7 | |
| Blacks | (n = 13) | (n = 13) | (n = 10) | (n = 8) | |
| Mean ± SD | 187.9 ± 60.0 | 339.6 ± 116.1 | 343.7 ± 175.7 | 699.4 ± 837.4 | |
| Median | 189.4 | 318.2 | 262.8 | 306.2 | |
| Range | 102.6-326.4 | 209.2-680.9 | 195.3-686.2 | 173.2-2618.7 | |
One plasma concentration unavailable at/near peak for one participant, data not included in calculations.
HMA concentration less than limit of quantification (LOQ) during entire time course for three participants; one plasma concentration unavailable at/near peak for one participant, data not included in calculations.
HMA concentration less than LOQ during entire time course for one participant.
Terminal elimination slope near zero, unable to calculate AUC∞ for one participant (low dose) and four participants (high dose).
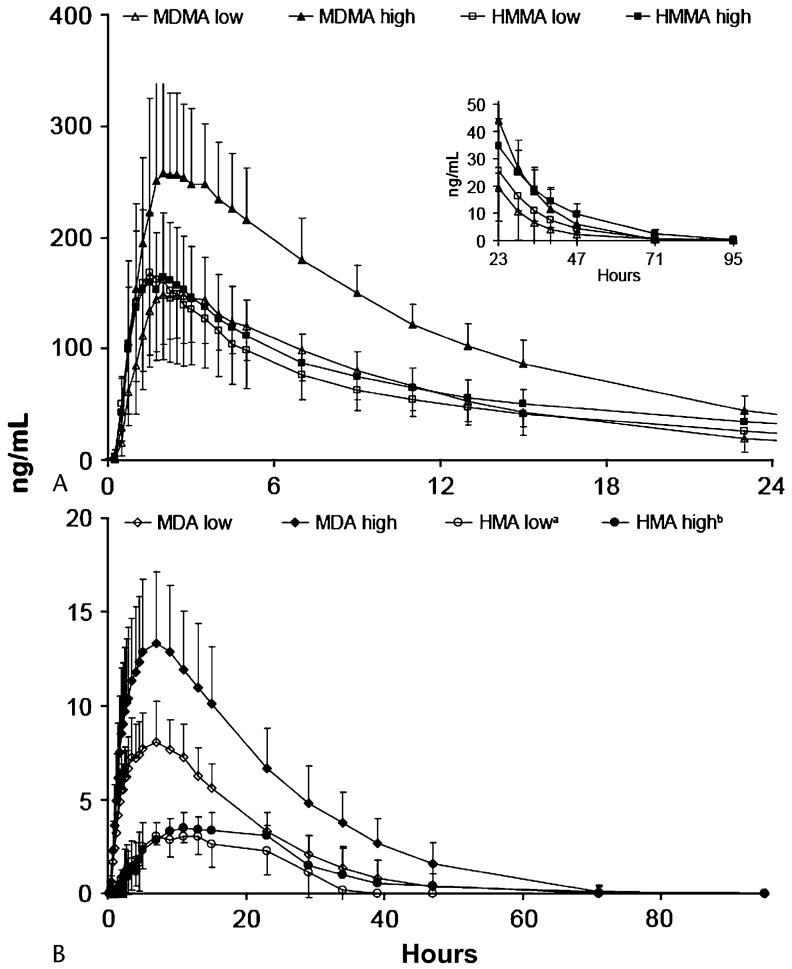
FIGURE 2.
Mean ± standard deviation concentrations of (A) 3,4-methylenedioxymethamphetamine (MDMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) and (B) 3,4-methylenedioxyamphetamine (MDA) and 4-hydroxy-3-methoxyamphetamine (HMA) after administration of 1.0 (low) and 1.6 (high) mg/kg oral MDMA to 17 volunteers. Note: Plasma concentrations of 0 occurring at the beginning and end of the time course were included in mean calculations. If concentrations were less than limit of quantification (LOQ) during the entire time course, the participant was excluded from calculations for that analyte and dose only. an = 14, because HMA concentrations were always less than LOQ in plasma specimens from three participants. bn = 16, because HMA concentrations were always less than LOQ in plasma specimens from one participant.
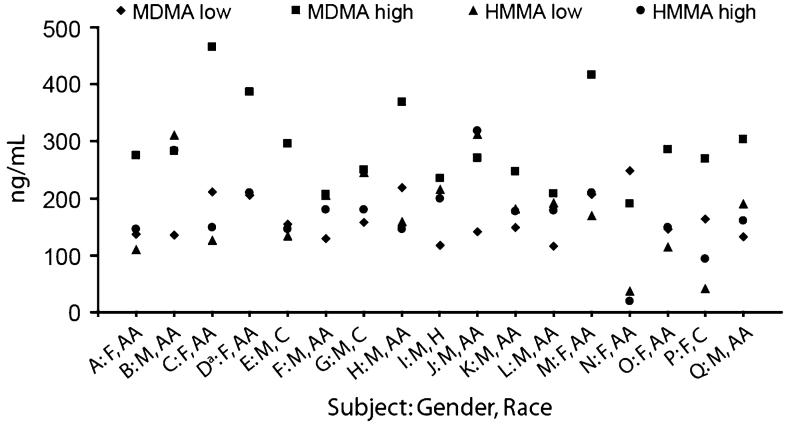
FIGURE 3.
Individual 4-hydroxy-3-methoxymethamphetamine (HMMA) and 3,4-methylenedioxymethamphetamine (MDMA) maximum plasma concentrations (Cmax) after low- (1.0 mg/kg) and high- (1.6 mg/kg) dose oral MDMA administration with participants’ gender and race identified. F, female; AA, African-American; M, male; C, Caucasian; H, Hispanic. aNo HMMA low-dose Cmax as a result of unavailable plasma concentration at/near peak.
Mean HMMA Cmax after the low and high doses were nearly identical (Table 1), although intersubject dose response varied (Fig. 3). In 11 of 16 individuals, HMMA low- and high-dose Cmax were within ± 20%. For the other five participants, three had higher HMMA plasma concentrations after the 1.6-mg/kg dose and two after the 1.0-mg/kg dose. There was no significant difference in mean Tmax between doses (n = 16, P = 0.48) with peak HMMA concentrations occurring approximately 1.8 hours after drug administration. Although mean Cmax were not significantly different (P = 0.96), mean AUC∞ was significantly higher after the 1.6-mg/kg dose (n = 16, P = 0.001). Mean high dose AUC∞ was only 1.3 times low-dose AUC∞.
Mean and individual HMMA and MDMA plasma data are presented in Figures 2 and 3. After 1.0 mg/kg MDMA (n =16), HMMA and MDMA mean Cmax (P = 0.66) and AUC∞ (P = 0.50) were not significantly different, although mean HMMA Tmax occurred significantly earlier (n = 16, P = 0.02). After the 1.6-mg/kg dose, MDMA mean Cmax (P = 0.001) and AUC∞ (P < 0.001) were significantly higher than those of HMMA, whereas there was no significant difference in Tmax (n = 17, P = 0.08).
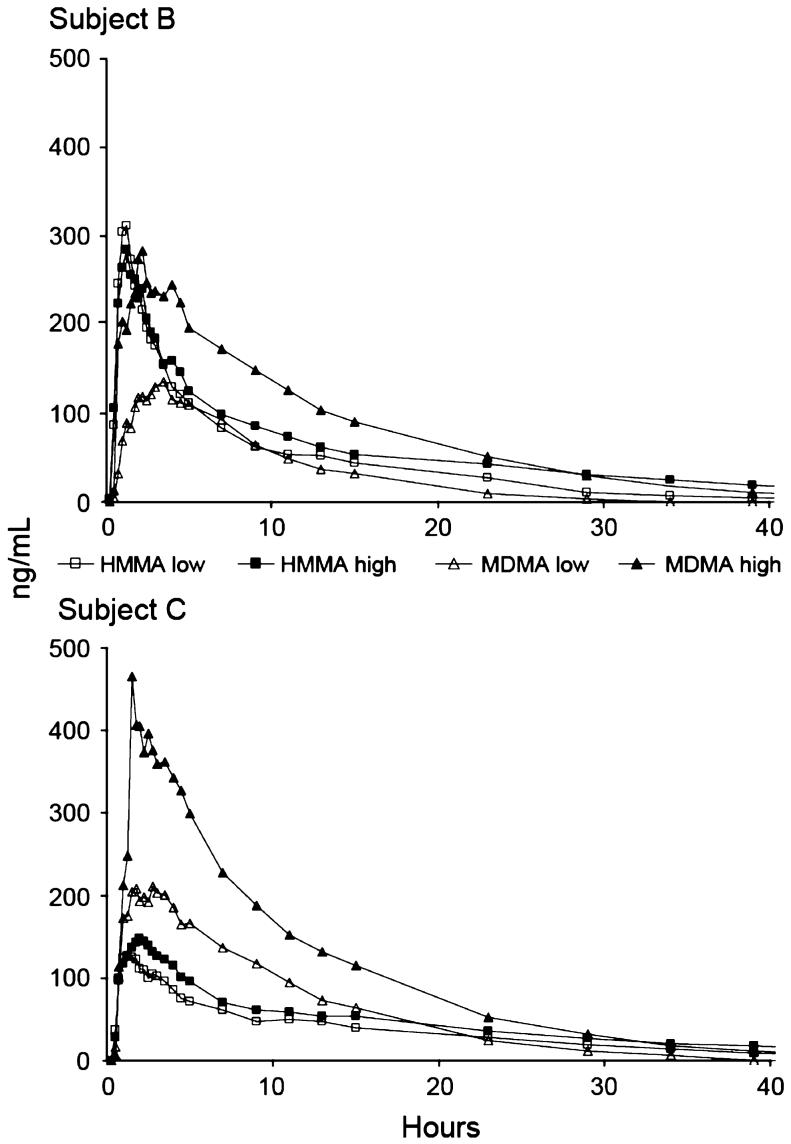
Plasma profiles showed high intersubject variation. Figure 4 displays the time course of HMMA and MDMA plasma concentrations in one male and one female participant; subjects had similar weights, 80 and 81 kg, respectively, and therefore, received nearly identical absolute doses of approximately 80 and 128 mg MDMA after the low and high doses, respectively. In male subject B, HMMA low and high dose and MDMA high-dose Cmax were clustered around 300 ng/mL; MDMA low-dose Cmax was less than half this value. Female subject C displayed an entirely different profile, with 210.8 and 465.3 ng/mL low- and high-dose MDMA Cmax, and similar HMMA low- and high-dose Cmax of 126.0 and 148.6 ng/mL, respectively.
FIGURE 4.
Plasma profiles of 3,4-methylenedioxymethamphetamine (MDMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) in two subjects, (B), male and (C), female, administered approximately 80 (low) and 130 (high) mg MDMA.
Mean MDA Cmax were reached at approximately 7.5 hours after dosing (Table 1). Cmax and AUC∞ were significantly higher after the high dose (n = 17, P < 0.001 for both comparisons), whereas no significant differences in Tmax were observed (n = 17, P = 0.25). The mean HMA Cmax after the high dose was significantly greater (n = 13; P = 0.02) than after the low dose (Table 1). The highest plasma HMA concentrations of 4.2 (low) and 6.1 (high) ng/mL were from the same individual. There was no significant difference in mean HMA Tmax (n = 13, P = 0.25) or AUC∞ (n = 9, P = 0.14) as a result of high intra- and intersubject variability. Mean AUC∞ ratios for MDA/MDMA and HMA/MDMA remained constant between doses at 10% and 17%, respectively.
The absolute MDMA doses administered in this study were 43 to 106 mg (1.0-mg/kg dose) and 69 to 150 mg (1.6-mg/kg dose), which are similar to common recreational doses.18,40-42 The resulting MDMA and MDA Cmax (Table 1; Fig. 3) were in the range of peak concentrations reported in other studies after oral administration of 47.5 to 150 mg MDMA.6,17,18,32,33,39,43-48 Concentrations of hydroxylated metabolites after MDMA administration have been evaluated less frequently. Mean HMMA Cmax after MDMA doses of 50 mg (n = 2),18 100 mg (n = 86; n=745), and 150 mg (n = 2)18 have been published. One individual’s HMMA Cmax was reported after a 75-mg MDMA dose and one different individual’s Cmax was available after a 125-mg dose.21 Current HMMA Cmax (Table 1; Fig. 3) after doses of 43 to 150 mg MDMA were in the range of Cmax achieved after doses of 50 to 150 mg in these earlier studies. The only HMA plasma Cmax data previously published were from a single 100-mg MDMA administration to eight subjects6; mean HMA Cmax was 7.5 ± 4.0 ng/mL. The highest HMA Cmax in the current study was 6.1 ng/mL with mean values of less than 4 ng/mL (Table 1). Differences may be attributable to the hydrolysis method used to break glucuronide and sulfate bonds. Acidic hydrolysis was selected in the current study because of its speed, low cost, and reported efficiency over basic and enzymatic hydrolysis.39,49 The previous study used enzymatic hydrolysis using β-glucuronidase from Helix pomatia.19
Only one participant (N) had MDMA and MDA concentrations and AUC∞ higher after the low than after the high dose. HMMA concentrations showed the same trend and were the lowest HMMA Cmax after each dose with concentrations 36.7 ng/mL or less. These unusual pharmacokinetic data are difficult to explain simply by a slow metabolism rate.
MDMA, HMMA, and MDA Tmax in the current study (Table 1) are consistent with published data.6,17,18,32,33,39,44-48,50 HMA Tmax occurred later than previously reported,6 probably as a result of the extended plasma collection times in this study. HMA Tmax exhibited great variability (Table 1), likely a result of HMA concentrations remaining near the LOQ during the entire plasma time course. Although mean Tmax was consistent for each analyte across doses, HMMA Tmax occurred significantly earlier than MDMA Tmax after the low dose. Mean MDMA Tmax was the same or earlier than HMMA Tmax in all but one previously published study.47 The reason for the discrepancy between studies is unclear. Variable absorption after oral drug administration or differences in plasma collection time points may play a role. However, evidence for rapid first-pass metabolism of MDMA comes from a Tmax of 1 to 1.2 hours for HHMA, the metabolic precursor of HMMA, as reported in earlier studies.17,45
Metabolite Ratios
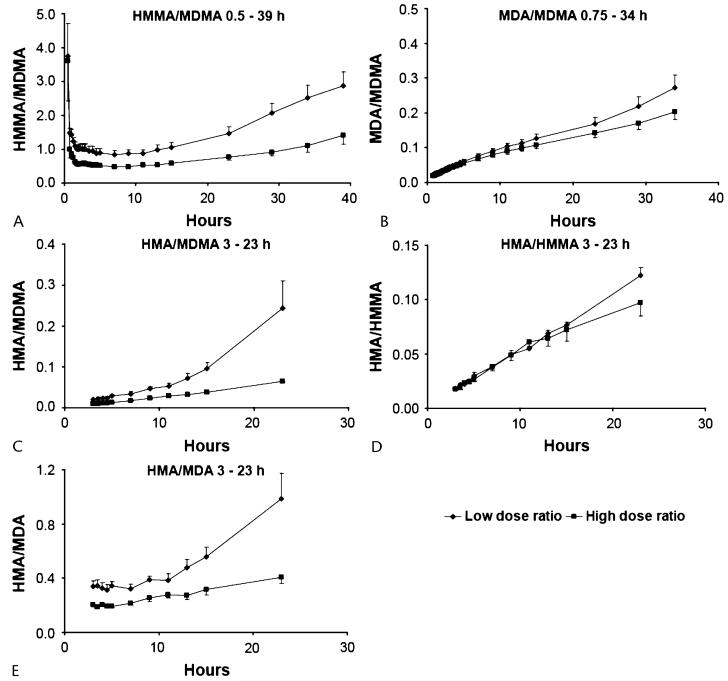
Mean HMMA/MDMA ratios were higher (P < 0.001) after the 1.0-mg/kg than after the 1.6-mg/kg dose. After both doses, ratios were larger at the beginning and end of the plasma time course (Fig. 5A) when HMMA concentrations were higher than MDMA concentrations. There was high inter-subject variability with individual ratios ranging from 0.06 to 22.9.
FIGURE 5.
Metabolite ratios after administration of 1.0- (low) and 1.6- (high) mg/kg 3,4-methylenedioxymethamphetamine (MDMA) to healthy volunteers. (A) 4-hydroxy-3-methoxymethamphetamine (HMMA)/MDMA; (B) 3,4-methylenedioxyamphetamine (MDA)/MDMA; (C) 4-hydroxy-3-methoxyamphetamine (HMA)/MDMA; (D) HMA/HMMA; and e) HMA/MDA.
Low- and high-dose mean MDA/MDMA ratios increased linearly and similarly from 0.75 to approximately 23 hours, when low-dose ratios began to increase more quickly (Fig. 5B). The largest ratio was 0.47, 39 hours after the low dose.
Mean HMA/MDMA and HMA/MDA ratios were greater after the low dose (P = 0.04 and P = 0.003, respectively; Figs. 5C and E). The low-dose ratio slope began a steeper ascent starting approximately 11 hours after dosing. There was no significant difference between doses for the HMA/HMMA ratio (P = 0.10; Fig. 5D).
Half-Lives, Volume of Distribution, and Total Body Clearance of the Drug From Plasma
There was no significant difference between doses in mean MDMAVd/F (P = 0.78) (Table 2). Clearance (CL/F) was significantly faster after the low dose (P = 0.004) (Table 2). Mean Vd/F was similar to values previously reported after chiral and achiral plasma analyses.20,23 Total clearance rates were significantly lower after the high dose (P = 0.004; Table 2), consistent with a previous study that suggested an impairment in MDMA hepatic clearance, because renal clearance was constant, whereas total and nonrenal clearance generally decreased as dose increased.18
TABLE 2.
Individual, Mean 6 Standard Deviation (SD), and Median Apparent Volumes of Distribution (Vd/F) and Clearance (CL/F) of 3,4-methylenedioxymethamphetamine (MDMA) After 1.0 Mg/kg (low) and 1.6 Mg/kg (high) Oral Doses of MDMA
| Vd/F (L/kg) |
Cl/F (L/h/kg) |
|||||
|---|---|---|---|---|---|---|
| Participant | Gender | Race | Low | High | Low | High |
| A | Female | AA | 6.9 | 7.0 | 0.56 | 0.62 |
| B | Male | AA | 5.2 | 4.9 | 0.75 | 0.46 |
| C | Female | AA | 3.5 | 3.4 | 0.41 | 0.35 |
| D | Female | AA | 4.6 | 4.6 | 0.58 | 0.32 |
| E | Male | C | 5.3 | 4.7 | 0.60 | 0.38 |
| F | Male | AA | 5.4 | 4.5 | 0.92 | 0.66 |
| G | Male | C | 6.4 | 6.3 | 0.64 | 0.46 |
| H | Male | AA | 3.9 | 4.2 | 0.47 | 0.42 |
| I | Male | U | 6.3 | 5.5 | 0.95 | 0.54 |
| J | Male | AA | 4.9 | 4.7 | 0.66 | 0.55 |
| K | Male | AA | 6.7 | 5.6 | 0.69 | 0.54 |
| L | Male | AA | 5.7 | 6.6 | 0.73 | 0.72 |
| M | Male | AA | 4.2 | 4.1 | 0.49 | 0.38 |
| N | Female | AA | 5.6 | 8.8 | 0.21 | 0.46 |
| O | Female | AA | 7.2 | 6.2 | 0.52 | 0.41 |
| P | Female | C | 6.0 | 6.8 | 0.44 | 0.45 |
| Q | Male | AA | 5.9 | 4.8 | 0.88 | 0.48 |
| Mean ± SD | 5.5 ± 1.1 | 5.5 ± 1.3 | 0.62 ± 0.19 | 0.48 ± 0.11 | ||
| Median | 5.6 | 4.9 | 0.60 | 0.46 | ||
AA, African-American; C, Caucasian; U, unknown race, Hispanic ethnicity.
Vd/F and CL/F results are consistent with the significantly longer mean MDMA t1/2 (P = 0.009) observed after the high dose (Table 3). MDA t1/2 was also significantly longer after the high dose (P = 0.01; Table 3). HMA t1/2 did not differ significantly between doses (P = 0.89; Table 3).
TABLE 3.
Individual, Mean ± Standard Deviation (SD), and Median Plasma Elimination Half-lives (t1/2) of 3,4-methylenedioxymethamphetamine (MDMA) and Metabolites After a 1.0- (low) and 1.6- (high) mg/kg MDMA Dose
| Elimination Half-Life (Hours) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDMA |
HMMA |
MDA |
HMA |
|||||||||||||
| Low |
High |
Low |
High |
Low |
High |
Low |
High |
|||||||||
| Participant | t1/2 | n | t1/2 | n | t1/2 | n | t1/2 | n | t1/2 | n | t1/2 | n | t1/2 | n | t1/2 | n |
| A | 8.5 | 6 | 7.8 | 7 | 12.3 | 6 | 11.8 | 7 | 14.6 | 7 | 11.6 | 7 | 111.4 | 3 | 219.8 | 3 |
| B | 4.8 | 4 | 7.5 | 7 | 7.7 | 7 | 13.5 | 8 | 7.2 | 3 | 11.2 | 5 | 37.4 | 4 | 202.7 | 4 |
| C | 5.9 | 5 | 6.8 | 7 | 10.9 | 7 | 14.4 | 7 | 9.4 | 5 | 10.6 | 6 | 38.4 | 4 | 26.3 | 4 |
| D | 5.4 | 5 | 10.0 | 7 | 12.4 | 8 | 17.6 | 9 | 8.1 | 5 | 15.4 | 7 | 35.5 | 4 | 39.9 | 5 |
| E | 6.1 | 6 | 8.7 | 7 | 9.7 | 7 | 14.9 | 7 | 8.0 | 4 | 12.2 | 5 | 49.6 | 3 | 25.2 | 4 |
| F | 4.1 | 3 | 4.7 | 5 | 7.0 | 6 | 8.1 | 7 | 6.1 | 3 | 7.1 | 5 | 61.9 | 3 | * | |
| G | 6.9 | 6 | 9.6 | 6 | 10.6 | 7 | 13.6 | 8 | 12.4 | 6 | 14.7 | 5 | 20.1 | 3 | 65.0 | 6 |
| H | 5.7 | 6 | 6.9 | 6 | 9.8 | 7 | 13.6 | 8 | 9.3 | 5 | 9.2 | 5 | 37.9 | 3 | * | |
| I | 4.6 | 3 | 7.1 | 7 | 7.8 | 7 | 12.5 | 8 | 8.4 | 4 | 10.0 | 5 | 43.3 | 4 | 165.6 | 4 |
| J | 5.1 | 5 | 6.0 | 6 | 8.0 | 7 | 11.7 | 8 | 9.4 | 4 | 10.3 | 5 | 135.7 | 4 | * | |
| K | 6.7 | 6 | 7.3 | 6 | 10.6 | 7 | 13.6 | 8 | 9.7 | 4 | 11.0 | 6 | 55.7 | 3 | 56.4 | 4 |
| L | 5.5 | 5 | 6.3 | 5 | 9.4 | 7 | 10.0 | 7 | 7.2 | 3 | 8.8 | 4 | 147.1 | 3 | * | |
| M | 5.9 | 6 | 7.5 | 7 | 9.7 | 7 | 11.6 | 7 | 8.7 | 5 | 12.3 | 7 | 47.8 | 3 | 27.3 | 3 |
| N | 18.3 | 9 | 13.3 | 6 | 30.2 | 6 | 19.2 | 7 | 23.0 | 8 | 21.4 | 5 | * | * | ||
| O | 9.6 | 7 | 10.4 | 6 | 16.1 | 8 | 16.9 | 8 | 15.4 | 6 | 17.5 | 5 | * | 30.6 | 3 | |
| P | 9.4 | 7 | 10.6 | 7 | 15.4 | 7 | 12.2 | 7 | 15.1 | 7 | 16.8 | 6 | * | 41.1 | 3 | |
| Q | 4.6 | 4 | 6.9 | 7 | 7.6 | 7 | 12.7 | 8 | 7.8 | 3 | 9.2 | 5 | * | 568.1 | 5 | |
| Mean ± SD | 6.9 ± 3.4 | 8.1 ± 2.1 | 11.5 ± 5.5 | 13.4 ± 2.7 | 10.6 ± 4.3 | 12.3 ± 3.7 | 63.2 ± 40.9 | 122.3 ± 157.7 | ||||||||
| Median | 5.9 | 7.5 | 9.8 | 13.5 | 9.3 | 11.2 | 47.8 | 48.8 | ||||||||
All plasma concentrations were less than limit of quantification or elimination half-life was unable to be calculated as a result of a slope close to zero.
HMMA, 4-hydroxy-3-methoxymethamphetamine; MDA, 3,4-methylenedioxyamphetamine; HMA, 4-hydroxy-3-methoxyamphetamine; n, number of data points for t1/2 calculation.
Mean MDMA t1/2 results are similar to those reported in smaller studies administering 75, 100, or 125 mg MDMA.6,17,18,21,23,33,45,47 Half-lives resulting from chiral and achiral plasma analyses after 40 and 50 mg oral MDMA to male volunteers18,20,50 were shorter than in participants receiving similar doses in this study. All three subjects receiving 40 to 50 mg MDMA were females weighing less than 50 kg. The difference in t1/2 could be the result of the gender difference in subjects (females had a significantly slower MDMA clearance rate after the low dose compared with males in this study; P = 0.001) to differences in body weight. Mean HMMA t1/2 after 1.0 mg/kg was in the upper range of previously published values,6,17,45,47 whereas mean MDA t1/2 were shorter than those previously published.18,23,32,33 The earliest time point included in this study for MDA t1/2 determinations was 13 hours. The majority of previous studies determined MDA t1/2 with only 10- and 24- hour plasma collections after the peak, potentially resulting in an inaccurate estimation of MDA t1/2. Mean HMA t1/2 (Table 3) was substantially greater than the value of 37.4 hours reported after a 100-mg MDMA dose.6
Gender
This study is the first to report MDMA and metabolite concentrations for females after controlled MDMA administration. Similar gender patterns were observed for AUC∞ and Cmax: mean MDMA and MDA Cmax were greater in female than male subjects; the reverse was observed for HMMA (Table 1). Tmax did not significantly differ by gender for any analyte (Table 1). Gender differences in MDMA and MDA AUC∞ and Cmax were significant after the low dose only (MDMA: AUC∞, P = 0.002; Cmax, P = 0.02; MDA: AUC∞, P = 0.001; Cmax, P = 0.05). Mean MDMA AUC∞ after the high dose showed a trend toward significance (P = 0.06). Mean HMMA AUC∞ was significantly greater in male plasma specimens after both the 1.0- and 1.6-mg/kg doses (low, P = 0.002, six females, 10 males; high, P = 0.03), whereas mean Cmax reached significance only after the 1.0-mg/kg dose (P = 0.002, six females, 10 males). No significant gender differences were observed for mean HMA Cmax and AUC∞. In general, female subjects had longer t1/2 than male subjects (Table 3). Significantly longer mean t1/2 were observed in females for MDMA after the high dose (P = 0.02), HMMA after the low dose (P = 0.002), and MDA after both doses (low, P = 0.05; high, P = 0.005). Trends toward significance were observed for mean low-dose MDMA (P = 0.08) and high-dose HMMA (P = 0.07) t1/2.
Among participants who resided on the research unit longer than 47 hours, only female participants’ plasma remained MDMA-positive more than 2 days postdose, although their absolute MDMA doses tended to be smaller because of lighter body weight. MDMA is a lipophilic drug, so the longer residence time in females could be related to their greater adipose tissue mass, although Vd/F in males and females was similar with mean values ranging from 5.2 to 5.8 L/kg for both genders at both doses. Mean MDMA clearance results are consistent with longer t1/2 in females with a significantly slower mean rate (P = 0.001) after the low dose and a nearly significant slower mean rate (P = 0.08) after the high dose. In general, renal processes, including clearance of drugs metabolized by CYP2D6, are faster in males.51 This may help to explain higher concentrations of HMMA in males, but leaves unexplained higher concentrations of MDA in females.
These observed gender differences in MDMA pharmacokinetics are not confounded by body weight because dosing was based on milligrams per kilograms. They should be interpreted cautiously because of the small sample sizes.
Race/Ethnicity
The influence of race/ethnicity on MDMA pharmacokinetics has not been well studied. This study included a substantial number of black subjects, a population group whose MDMA use has increased over the past decade.52 Thus, evaluation of MDMA pharmacokinetics in this group has public health significance. Racial subgroup comparisons could not be performed within this study because of the small sample sizes. However, mean plasma pharmacokinetic data after administration of 75 and 125 mg MDMA to eight European males are available for comparison.21 Their mean weight was 74.4 kg, similar to the 76.0-kg mean weight of the seven black males in the current study. Because our participants were dosed based on weight, MDMA dosages ranged from 58 to 106 mg (mean ± SE: 76.3 ± 5.6) after the low dose and 97 to 150 mg (mean ± SE: 118.8 ± 6.1) after the high dose. Mean MDMA Cmax after the low and high doses were higher by 22% and 21%, respectively, in blacks than in Europeans; mean MDA concentrations were higher by 9.3% and 1.4%, respectively; mean MDMA AUC were higher by 19% and 8%, respectively. Mean MDMA and MDA Tmax in blacks were always ≥ mean Tmax than of Europeans. Mean MDMA t1/2 were similar in the two racial groups; mean MDA t1/2 were shorter in blacks by 35% after the low and 131% after the high dose. These racial differences should be interpreted cautiously because of methodological differences between the studies, including more frequent and extended sampling in the current study and different analytical methods.
Nonlinear Pharmacokinetics
This is the first study to report MDMA, HMMA, MDA, and HMA concentrations after administration of multiple MDMA doses to the same individuals (ie, a within-subjects design with respect to MDMA dose). Results support the theory of nonlinear MDMA pharmacokinetics postulated by de la Torre et al.18 They observed nonlinear increases in MDMA and HMMA concentrations after the administration of 50, 100, and 150 mg MDMA; however, a different set of two subjects received each dose (ie, a between-subjects design with respect to MDMA dose) and only one subject’s HMMA data were reported. In a later study, 75 and 125 mg MDMA were administered to eight male subjects.18,21 Although multiple MDMA and MDA pharmacokinetic parameters were reported, the only HMMA data available were Cmax for a single (different) participant after each dose.18
In the current study, mean MDMA Cmax and AUC∞ increased approximately 1.8- and 1.9-fold (versus the expected 1.6) between the low and high doses. Ten of 17 participants had AUC∞ increases greater than twofold higher after the high dose; five of these individuals showed a greater than 2.5-fold increase. Mean HMMA Cmax was unchanged, whereas mean AUC∞ was 1.3 times higher. Change in individual AUC∞ exceeded a 1.4-fold increase for a single participant. Interindividual variations in HMMA dose response did not appear to be related to the amount of drug administered (see Fig. 4). Mean HMMA/MDMA AUC∞ comparisons were 1.0 after the low dose and 0.67 after the high. Mean AUC∞ comparisons of MDA and HMA to MDMA remained constant after both doses, at 0.10 and 0.17, respectively. This suggests that these minor metabolites display the same nonlinear increase as MDMA. HMA/HMMA AUC∞ ratios are not consistent between doses, at 0.17 (low) and 0.26 (high). Because HMMA is a direct precursor of HMA, and metabolism is primarily mediated by CYP1A2, which has not been associated with variation in MDMA metabolism, it is interesting that HMA/MDMA AUC∞ ratios are constant, whereas HMA/HMMA AUC∞ ratios are not. However, these data suggest that HMA is primarily formed through the minor, not major, metabolic pathway of MDMA. Metabolite ratios also support nonlinear pharmacokinetics of MDMA with metabolite/MDMA ratios always significantly lower after the high dose; a linear increase in concentration should result in similar ratios across doses. Although the time course of HMMA/MDMA, HMA/MDMA, and HMA/MDA ratios are initially parallel between doses (Fig. 5), at 15 to 24 hours postdose, the gap between doses widens as the low-dose slope becomes steeper. This may occur because MDMA concentrations have decreased to levels where there is less inhibition of its metabolism.18,53
The predominant theory of nonlinear pharmacokinetics of MDMA and HMMA is mechanism-based inactivation of CYP2D6 by MDMA.53 In vitro data suggest that a metabolic complex formed by the methylenedioxyphenyl ring inhibits O-methylation. Amine groups can also reportedly form these complexes, although the mean MDA/MDMA AUC∞ ratio was constant between doses, making this an unlikely binding site. Saturation of enzymes or interaction of metabolites with enzymes in the metabolic pathway also have been suggested.18
CONCLUSION
Although ecstasy has been associated with a low number of emergency room visits and fatalities,54,55 MDMA is rarely the sole drug ingested. Many substance abusers also use other licit and illicit drugs, notably cannabis.56 In addition, the purity of “street” ecstasy is notoriously poor; it often contains little or no MDMA.41,57,58 Substitutes include other illicit drugs, caffeine, ephedrine, pseudoephedrine, salicylates, dextromethorphan, starch, and lactose. Contaminants from the manufacturing process also may be present. Pharmacokinetic data after controlled drug administration provides a more rigorous scientific framework for interpreting plasma MDMA concentrations collected in clinical and forensic cases. The non-linearity observed in the MDMA dose-concentration relationship was noted within the typical recreational dose range and could be a contributory factor in observed toxicity of MDMA.
This study used a rigorous scientific design to perform an extended pharmacokinetic analysis of MDMA and three metabolites, HMMA, MDA, and HMA, characterizing Cmax, Tmax, AUC∞, detection windows, t1/2, Vd/F, CL/F, and metabolite ratios for up to 143 hours after dosing. Low (1.0 mg/kg) and high (1.6 mg/kg) oral MDMA doses were administered to 17 volunteers of both genders, including, for the first time, black participants. Strengths of this study include measurement of HMMA and HMA concentrations after low and high MDMA doses, more frequent and extended plasma sampling (up to 143 hours post dose versus 48 hours or less in earlier reports), and participants who resided on a closed research unit with 24-hour monitoring to prevent self-administration of MDMA or other drugs. This study provides support for the theory of MDMA nonlinear pharmacokinetics within the range of doses used recreationally and provides preliminary information on gender differences in drug elimination.
ACKNOWLEDGMENTS
The authors thank Kathleen Demuth, Susan Baskin, Cecile Shindell, Janeen Nichels, and John Etter for assistance in experimental sessions.
This research was supported by the Intramural Research Program, National Institutes of Health, National Institute on Drug Abuse.
REFERENCES
- 1.Pentney AR. An exploration of the history and controversies surrounding MDMA and MDA. J Psychoactive Drugs. 2001;33:213–221. doi: 10.1080/02791072.2001.10400568. [DOI] [PubMed] [Google Scholar]
- 2.Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- 3.Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs. 1986;18:319–327. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- 4.Downing J. The psychological and physiological effects of MDMA on normal volunteers. J Psychoactive Drugs. 1986;18:335–340. doi: 10.1080/02791072.1986.10472366. [DOI] [PubMed] [Google Scholar]
- 5.Vollenweider FX, Gamma A, Liechti M, et al. Psychological and cardiovascular effects and short-term sequelae of MDMA (‘ecstasy’) in MDMA-naive healthy volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- 6.de la Torre R, Farre M, Roset PN, et al. Pharmacology of MDMA in humans. Ann N Y Acad Sci. 2000;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 7.Cami J, Farre M, Mas M, et al. Human pharmacology of 3,4-methylenedioxymethamphetamine (‘ecstasy’): psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RK. MDMA. Nonmedical use and intoxication. J Psychoactive Drugs. 1986;18:349–354. doi: 10.1080/02791072.1986.10472368. [DOI] [PubMed] [Google Scholar]
- 10.Lester SJ, Baggott M, Welm S, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- 11.Harris DS, Baggott M, Mendelson JH, et al. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- 12.Elliott SP. MDMA and MDA concentrations in antemortem and postmortem specimens in fatalities following hospital admission. J Anal Toxicol. 2005;29:296–300. doi: 10.1093/jat/29.5.296. [DOI] [PubMed] [Google Scholar]
- 13.Swan MC, Lam D, Giele HP. Intravascular ecstasy: an unusual cause of thigh compartment syndrome. J Trauma. 2006;60:1129–1131. doi: 10.1097/01.ta.0000217247.90726.bd. [DOI] [PubMed] [Google Scholar]
- 14.Moore KA, Mozayani A, Fierro MF, et al. Distribution of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyam-phetamine (MDA) stereoisomers in a fatal poisoning. Forensic Sci Int. 1996;83:111–119. doi: 10.1016/s0379-0738(96)02025-7. [DOI] [PubMed] [Google Scholar]
- 15.Duffy MR, Swart M. Severe ecstasy poisoning in a toddler. Anaesthesia. 2006;61:498–501. doi: 10.1111/j.1365-2044.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraemer T, Maurer HH. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24:277–289. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Segura M, Ortuno J, Farre M, et al. 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol. 2001;14:1203–1208. doi: 10.1021/tx010051p. [DOI] [PubMed] [Google Scholar]
- 18.de la Torre R, Farre M, Ortuno J, et al. Non-linear pharmacoki-netics of MDMA (‘ecstasy’) in humans. J Clin Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortuno J, Pizarro N, Farre M, et al. Quantification of 3,4-methylenedioxymethamphetamine and its metabolites in plasma and urine by gas chromatography with nitrogen-phosphorus detection. J Chromatogr B Analyt Technol Biomed Life Sci. 1999;723:221–232. doi: 10.1016/s0378-4347(98)00506-4. [DOI] [PubMed] [Google Scholar]
- 20.Fallon JK, Kicman AT, Henry JA, et al. Stereospecific analysis and enantiomeric disposition of 3,4-methylenedioxymethamphetamine (ecstasy) in humans. Clin Chem. 1999;45:1058–1069. [PubMed] [Google Scholar]
- 21.Mas M, Farre M, de la Torre R, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- 22.Navarro M, Pichini S, Farre M, et al. Usefulness of saliva for measurement of 3,4-methylenedioxymehamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clin Chem. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 23.de la Torre R, Farre M, Roset PN, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Carmo H, Brulport M, Hermes M, et al. Influence of CYP2D6 polymorphism on 3,4-methylenedioxymethamphetamine (‘ecstasy’) cytotoxicity. Pharmacogenet Genomics. 2006;16:789–799. doi: 10.1097/01.fpc.0000230419.05221.fc. [DOI] [PubMed] [Google Scholar]
- 25.Cai WM, Nikoloff DM, Pan RM, et al. CYP2D6 genetic variation in healthy adults and psychiatric African-American subjects: implications for clinical practice and genetic testing. Pharmacogenomics J. 2006;6:343–350. doi: 10.1038/sj.tpj.6500378. [DOI] [PubMed] [Google Scholar]
- 26.Linder MW, Prough RA, Valdes R., Jr. Pharmacogenetics: a laboratory tool for optimizing therapeutic efficiency. Clin Chem. 1997;43:254–266. [PubMed] [Google Scholar]
- 27.Nebert DW, Dieter MZ. The evolution of drug metabolism. Pharmacology. 2000;61:124–125. doi: 10.1159/000028393. [DOI] [PubMed] [Google Scholar]
- 28.McLeod HL, Fang L, Luo X, et al. Ethnic differences in erythrocyte catechol-O-methyltransferase activity in black and white Americans. J Pharmacol Exp Ther. 1994;270:26–29. [PubMed] [Google Scholar]
- 29.Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 30.Esteban B, O’Shea E, Camarero J, et al. 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology (Berl) 2001;154:251–260. doi: 10.1007/s002130000645. [DOI] [PubMed] [Google Scholar]
- 31.de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Farre M, de la Torre R, Mathuna BO, et al. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berl) 2004;173:364–375. doi: 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- 33.Pizarro N, Farre M, Pujadas M, et al. Stereochemical analysis of 3,4-methylenedioxymethamphetamine and its main metabolites in human samples including the catechol-type metabolite (3,4-dihydroxymethamphetamine) Drug Metab Dispos. 2004;32:1001–1007. [PubMed] [Google Scholar]
- 34.de la Torre R, Farre M, Mathúna BO, et al. MDMA (ecstasy) pharmacokinetics in a CYP2D6 poor metaboliser and in nine CYP2D6 extensive metabolisers. Eur J Clin Pharmacol. 2005;61:551–554. doi: 10.1007/s00228-005-0965-y. [DOI] [PubMed] [Google Scholar]
- 35.Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94:1279–1298. doi: 10.1046/j.1360-0443.1999.94912792.x. [DOI] [PubMed] [Google Scholar]
- 36.Han E, Yang W, Lee J, et al. The prevalence of MDMA/MDA in both hair and urine in drug users. Forensic Sci Int. 2005;152:73–77. doi: 10.1016/j.forsciint.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 38.Kolbrich EA, Lowe RH, Huestis MA. Two-dimensional gas chromatography/electron impact-mass spectrometry with cryofocusing for the sensitive, specific and simultaneous quantification of MDMA, MDA, HMMA, HMA, and MDEA in human plasma. Clin Chem. 2008;54:379–387. doi: 10.1373/clinchem.2007.096800. [DOI] [PubMed] [Google Scholar]
- 39.Helmlin H, Bracher K, Bourquin D, et al. Analysis of 3,4-methylenedioxymethamphetamine (MDMA) and its metabolites in plasma and urine by HPLC-DAD and GC-MS. J Anal Toxicol. 1996;20:432–440. doi: 10.1093/jat/20.6.432. [DOI] [PubMed] [Google Scholar]
- 40.Murphy PN, Fisk JE, Wareing M. Ecstasy—sorting out the facts. Psychologist. 2002:15. [Google Scholar]
- 41.Pham JV, Puzantian T. Ecstasy: dangers and controversies. Pharmacotherapy. 2001;21:1561–1565. doi: 10.1592/phco.21.20.1561.34474. [DOI] [PubMed] [Google Scholar]
- 42.Ropero-Miller JD, Goldberger BA. Recreational drugs: current trends in the 90s. Toxicology. 1998;18:727–746. [PubMed] [Google Scholar]
- 43.Henry JA, Fallon JK, Kicman AT, et al. Low-dose MDMA (‘ecstasy’) induces vasopressin secretion. Lancet. 1998;351:1784. doi: 10.1016/S0140-6736(05)78744-4. [DOI] [PubMed] [Google Scholar]
- 44.Samyn N, De Boeck G, Wood M, et al. Plasma, oral fluid and sweat wipe ecstasy concentrations in controlled and real life conditions. Forensic Sci Int. 2002;128:90–97. doi: 10.1016/s0379-0738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 45.Segura M, Farre M, Pichini S, et al. Contribution of cytochrome P450 2D6 to 3,4-methylenedioxymethamphetamine disposition in humans: use of paroxetine as a metabolic inhibitor probe. Clin Pharmacokinet. 2005;44:649–660. doi: 10.2165/00003088-200544060-00006. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez-Lopez C, Farre M, Roset PN, et al. 3,4-methylenedioxymethamphetamine (Ecstasy) and alcohol interactions in humans: psychomotor performance, subjective effects, and pharmacokinetics. J Pharmacol Exp Ther. 2002;300:236–244. doi: 10.1124/jpet.300.1.236. [DOI] [PubMed] [Google Scholar]
- 47.Pizarro N, Ortuno J, Farre M, et al. Determination of MDMA and its metabolites in blood and urine by gas chromatography-mass spectrometry and analysis of enantiomers by capillary electrophoresis. J Anal Toxicol. 2002;26:157–165. doi: 10.1093/jat/26.3.157. [DOI] [PubMed] [Google Scholar]
- 48.Pacifici R, Zuccaro P, Lopez CH, et al. Acute effects of 3,4-methylenedioxymethamphetamine alone and in combination with ethanol in the immune system in humans. J Pharmacol Exp Ther. 2001;296:207–215. [PubMed] [Google Scholar]
- 49.Pirnay SO, Abraham TT, Lowe RH, et al. Selection and optimization of hydrolysis conditions for the quantification of urinary metabolites of MDMA. J Anal Toxicol. 2006;30:563–569. doi: 10.1093/jat/30.8.563. [DOI] [PubMed] [Google Scholar]
- 50.Verebey K, Alrazi J, Jaffe JH. The complications of ‘ecstasy’ (MDMA) JAMA. 1988;259:1649–1650. doi: 10.1001/jama.259.11.1649. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–121. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 52.Johnston LD, O’Malley PM, Bachman JG, et al. Demographic subgroup trends for various licit and illicit drugs, 1975-2006. Monitoring the Future Occasional Paper No. 67. 2007:1–427. [Google Scholar]
- 53.Heydari A, Yeo KR, Lennard MS, et al. Mechanism-based inactivation of CYP2D6 by methylenedioxymethamphetamine. Drug Metab Dispos. 2004;32:1213–1217. doi: 10.1124/dmd.104.001180. [DOI] [PubMed] [Google Scholar]
- 54.Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to ecstasy use. Case-series of emergency department visits. Swiss Med Wkly. 2005;135:652–657. doi: 10.4414/smw.2005.11231. [DOI] [PubMed] [Google Scholar]
- 55.Hall AP, Henry JA. Acute toxic effects of ’ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- 56.Parrott AC. MDMA in humans: factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J Psychopharmacol. 2006;20:147–63. doi: 10.1177/0269881106063268. [DOI] [PubMed] [Google Scholar]
- 57.Baggott M, Heifets B, Jones RT, et al. Chemical analysis of ecstasy pills. JAMA. 2000;284:2190. doi: 10.1001/jama.284.17.2190. [DOI] [PubMed] [Google Scholar]
- 58.Palhol F, Boyer S, Naulet N, et al. Impurity profiling of seized MDMA tablets by capillary gas chromatography. Annals Bioanalytical Chemistry. 2002;374:274–281. doi: 10.1007/s00216-002-1477-6. [DOI] [PubMed] [Google Scholar]