Abstract
The consequences of obstructive sleep apnea (OSA) are largely mediated by chronic intermittent hypoxia and sleep fragmentation. The primary molecular domains affected are sympathetic activity, oxidative stress and inflammation. Other affected domains include adipokines, adhesion molecules and molecules that respond to endoplasmic reticulum stress. Changes in molecular domains affected by OSA, assessed in blood and/or urine, can provide a molecular signature for OSA that could potentially be used diagnostically and to predict who is likely to develop different OSA-related comorbidities. High-throughput discovery strategies such as microarrays, assessing changes in gene expression in circulating blood cells, have the potential to find new candidates and pathways thereby expanding the molecular signatures for OSA. More research is needed to fully understand the pathophysiological significance of these molecular signatures and their relationship with OSA comorbidities.
Many OSA subjects are obese, and obesity is an independent risk factor for many comorbidities associated with OSA. Moreover, obesity affects the same molecular pathways as OSA. Thus, a challenge to establishing a molecular signature for OSA is to separate the effects of OSA from obesity. We propose that the optimal strategy is to evaluate the temporal changes in relevant molecular pathways during sleep and, in particular, the alterations from before to after sleep when assessed in blood and/or urine. Such changes will be at least partly a consequence of chronic intermittent hypoxia and sleep fragmentation that occurs during sleep.
Citation:
Arnardottir ES; Mackiewicz M; Gislason T; Teff KL; Pack AI. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. SLEEP 2009;32(4):447–470.
Keywords: Obstructive sleep apnea, molecular mechanisms, chronic intermittent hypoxia, sleep fragmentation, pathophysiology
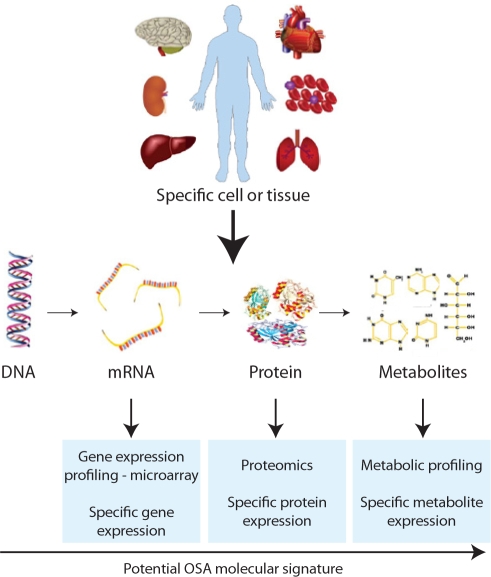
CURRENTLY THERE ARE A LARGE NUMBER OF POWERFUL TECHNIQUES TO ADDRESS MOLECULAR SIGNATURES OF DISEASE. A MOLECULAR SIGNATURE can be described as a pattern of gene or protein expressions in cells or tissues related to a disease state.1–4 The vision of evaluating molecular signatures is that they can be used clinically in a number of ways. They may provide diagnostic information, i.e., allow identification of the presence of a disease from a simple blood, urine or even saliva test.1–4 They may also provide prognostic information, e.g., detecting who with a particular disorder will develop a particular outcome or respond to a particular therapy.5–11 Approaches include the following: a) assessment of changes in gene expression or protein expression of specific molecules in populations of cells or tissue12–17; b) evaluation of the response of relevant cells to in vitro challenges18–21; c) discovery strategies, such as gene expression profiling, using mRNA extracted from a population of cells or tissue to study changes in expression of a large number of genes with known and unknown function using microarrays1,2,5,22–28; d) proteomics to assess changes in a large number of proteins, including post-translational modifications2,4,29–37; and e) global metabolic profiling to discover the differences in a large number of metabolites in cells or tissues due to specific conditions (metabolomics) or as a response to toxic chemicals, drugs, or disease (metabonomics) (Figure 1).38–42
Figure 1.
Possible ways to assess molecular signatures in specific cells or tissues.
Implicit in the approach of molecular signature are 2 concepts. First, a disorder with a physiological level of description that might appear uniform has distinct endophenotypes when molecular signatures are determined. Second, there will be variation between individuals in the response to a particular disorder or challenge. This inter-individual difference is what underlies the concept of personalized medicine.43,44 These differences in response may, at least in part, reflect genetic variants between individuals.44–46
Obstructive sleep apnea (OSA) is, we propose, a condition very amenable to this approach. The physiological measure of OSA is the apnea-hypopnea index (AHI). There is, however, substantial variation both in the nature of the breathing events during sleep and in the consequences of such events in different individuals.47–49 For example, OSA is often associated with sleepiness,50–59 hypertension,60–63 and cardiovascular disease.64–68 However, a large number of OSA patients are not sleepy,49,69 and only about half of OSA patients get hypertension,63 which raises the question: Why do some subjects with OSA develop hypertension and sleepiness while others do not?
The obstructive events during sleep result in changes in molecular processes that can be assessed, thus providing a potential molecular signature for the presence and consequences of OSA. The major current use of this approach is to measure circulating levels of specific biomarkers such as C-reactive protein (CRP) and interleukin-6 (IL-6).70–75 Newer approaches, such as gene expression profiling and proteomics, are beginning to be applied.24,31,32,76 In this perspective we describe current data and indicate areas of opportunity. We propose that assessing changes in relevant biomarkers in blood overnight, for example, comparing levels before and after sleep (or an overnight urine test) assessing specific molecules as well as global gene and protein expression, is feasible and likely will be used in the future to assess OSA severity and inter-individual differences in the effects of the disorder. This approach has the potential to transform the practice of medicine in this area by providing diagnostic information as well as showing who will likely benefit from therapy. To fully understand the pathophysiological significance of these molecular signatures, and their relationship with OSA comorbidities, more research in this field related to both OSA and its consequences is needed.
OSA: PATHOGENETIC MECHANISMS AND CONSEQUENCES
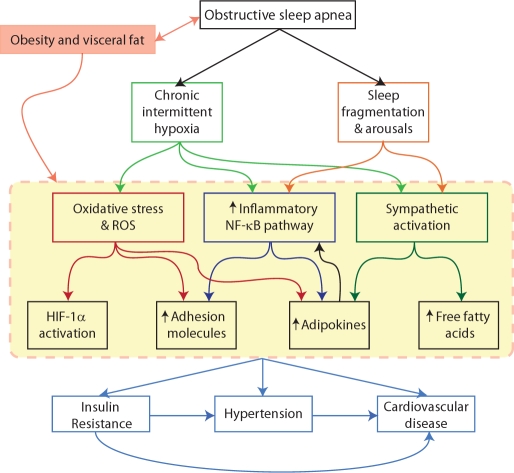
OSA is a common condition with a number of adverse consequences.47,48,77,78 During sleep, individuals with OSA have repeated episodes of declines in breathing (hypopneas) or cessation of breathing (apneas) due to upper airway obstructions. These obstructions result in the following: interruption of sleep with frequent arousals (sleep fragmentation); loss of REM sleep and slow wave sleep (stage 3-4); repetitive decreases in oxygen saturation with rapid reoxygenation causing cyclical deoxygenation/reoxygenation; and repeated changes in intrathoracic pressure and episodic hypercapnia (Figure 2).47,48,78 A particular advantage in studying this common condition is that there is a safe, effective therapy—nasal continuous positive airway pressure (CPAP)—that can quickly reverse the occurrence of sleep disordered breathing events,79–81 and the majority of patients show reasonable compliance (device use > 4 hours per night).82,83
Figure 2.
A schematic illustrating the pathogenetic mechanisms for the consequences of obstructive sleep apnea (OSA) that indicate the areas for potential molecular signatures for the disorder.
One of the important questions in a quest to find molecular signatures of a disease such as OSA is which cells or tissue to choose for measurement. Blood, due to its interaction with all organ systems and tissues in the body, its diverse physiological roles as well as being an easily accessible tissue, is an attractive option.2 Blood is also accepted as a surrogate tissue for conditions where the target tissue is inaccessible (liver, lung).2,3 Other surrogate tissues that can potentially be used include urine, saliva,3 and breath condensate.84 Changes in relevant molecular pathways in blood, urine, and breath condensate have been shown in OSA in various animal and human studies.15,18,31,32,85–91
Chronic Intermittent Hypoxia
Much attention on the effects of OSA has focused on the role of chronic intermittent hypoxia (CIH).92–95 This can be reproduced in animal models, e.g., in rats96–107 and mice,108–119 in which a specific pattern of repetitive deoxygenation/reoxygenation can be produced so that causality can be established. CIH can also be produced in cell culture systems to investigate fundamental mechanisms.120–123
CIH in mice/rats has been shown to lead to a large number of adverse effects: increased sympathetic activity and hypertension97,98,107,119,124; increased catecholamine levels105,107,113; liver dysfunction116; learning deficits99–101,103,104,106 with associated damage to cortical and hippocampal neurons100,101,103,104,112; persistent hypersomnolence with oxidative damage to wake-active neurons108,109,111,118; insulin resistance110,115; atherosclerosis when combined with a high-fat diet116; and vascular remodeling.114,119 The activation of the proinflammatory transcription factor nuclear factor κB (NF-κB) pathways has been shown in response to CIH.15 There is also activation of the transcriptional factor hypoxia induction factor-1 (HIF-1α), a key factor in oxygen homeostasis, which causes direct activation of > 50 downstream molecules such as erythropoietin (EPO)95 in response to CIH in animal and cell models.113,125–128 Activation of HIF-1α plays an important role in the sympathetic response, increased blood pressure and increased triglyceride levels in animal models.113,127,128 In a recent paper it has been shown that NF-κB is a key transcriptional activator of HIF-1α, linking the proinflammatory and hypoxic response pathways together.129 Some cell model systems have shown preferential activation of NF-κB over HIF-1α activation121,122 and one study in rat carotid body showed increased expression of HIF-2α and HIF-3α in response to CIH.130
In studies of chronic intermittent hypoxia, the oxygen levels that the animal models and cell cultures are usually exposed to are more severe than the degree of hypoxia typically found in most human subjects with OSA.97,98,112,124 Thus, the generalizability of the findings needs to be questioned. The level of oxygen saturation in tissue is usually not reported in rodent studies.100,102,106,112,115 Another difference between patients with OSA and studies in animal and cell models is duration of the insult. Human subjects with OSA may suffer over a long period of time from this condition before they seek medical assistance and are diagnosed.131 However, in animal or cell models CIH is usually administered for days or weeks.105,113,122,132 Some investigators have begun to address these issues by measuring the levels of oxygen saturation in tissue and produce less severe hypoxia within each CIH cycle to better simulate the hypoxia levels found in OSA patients in their models.111,118 A study with longer duration of insult in mice, i.e., 6 months, has also recently been reported.116
The effect of intermittent hypoxia has also been assessed in humans. Studies looking at the effects of isocapnic (iso-CO2) hypoxia for 5 minutes (breathing in 10% O2) in awake healthy human subjects showed that isocapnic hypoxia causes increased sympathetic activation, increase in blood pressure and hyperventilation through peripheral chemoreceptor stimulation.133,134 This effect is strongly enhanced by a voluntary apnea at the end of the 5 minute period of hypoxia.133 Moreover, administration of 100% oxygen to OSA patients during obstructive events diminishes the sympathetic response to apneas.135 These studies therefore support the findings from animal models that short duration hypoxia leads to increased sympathetic activation.
Cyclical hypoxia with reoxygenation is thought to be like repeated ischemia-reperfusion damage with increased reactive oxygen species (ROS) production during the restoration of oxygen as occurs in an ischemic region when blood flow is restored (ischemia reperfusion).78,92,136 Thus, OSA is an oxidative stress disorder. CIH and ROS are the central focus of a model developed by Lavie about the pathogenesis of OSA comorbidities.92,137 According to this model, increased ROS production will lead to activation of NF-κB and hence increased expression of a number of downstream NF-κB target genes, e.g., proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-6 as well as adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1).92,137 Increases in inflammatory cytokines and adhesion molecules are proposed to lead to activation of various cells such as monocytes, lymphocytes and endothelial cells leading to endothelial dysfunction and cardiovascular disease.92,137 The evidence supporting this model, both directly and indirectly, comes from studies in both animal models (as described above) and human studies, as will be described below.
Sleep Fragmentation
CIH is not the only physiological challenge that occurs during apneic events; there is also the challenge of sleep fragmentation with repeated arousals. Each of these arousals is associated with a burst of sympathetic activity138–140 and cardiac changes including surges in blood pressure.141,142 Repetitive arousals lead to elevated cortisol and lipid levels,143 increased metabolism,144 and neurobehavioral deficits as a consequence of sleep fragmentation.144,145 The role of sleep fragmentation has, however, not received as much attention as chronic intermittent hypoxia. We do know that short sleep duration and chronic partial sleep deprivation over several days are associated with increased risk of hypertension, weight gain, insulin resistance and type 2 diabetes.146–150 Chronic partial sleep deprivation is also associated with neurobehavioral deficits151–154 and increased levels of inflammatory markers such as CRP,155 IL-6,156 and TNF-α.156 Even partial sleep deprivation for a single night has been shown to cause increased mRNA expression for IL-6 and TNF-α in circulatory white cells.157
Intrathoracic Pressure Changes
The effect of recurrent changes in intrathoracic pressure has been little studied and is often neglected in models of the pathogenetic mechanisms for the consequences of OSA.
The recurring obstructive apneas, which occur with forceful inspiratory effort against an occluded airway, cause significant decreases in intrathoracic pressure.158 The molecular signature for intrathoracic pressure changes may be atrial natriuretic peptide (ANP), a volume-regulating hormone that functions to decrease the volume within the vascular system as a result of fluid overload.159 The intrathoracic pressure changes in OSA may cause increased venous dilation of the right atrium causing a false signal of fluid overload in the heart and hence increased atrial release of ANP.159 CIH may, however, also cause pulmonary vasoconstriction, leading to right atrial dilation and increased ANP release.160
In support of this hypothesis, ANP has been found to be increased in OSA subjects161,162 and decreased with CPAP treatment.161,163–165 ANP increases excretion of both urine and sodium-causing nocturia, which occurs commonly in OSA patients.159 The increase in ANP has been associated with either the intrathoracic pressure changes163 or intermittent hypoxia.163,166 Other studies have, however, questioned the role of OSA in ANP secretion and have found no significant elevation of ANP levels in patients with OSA.167–169 The short half-life of ANP (2-3 minutes) may be a factor in these negative results.170,171
Subjects with what has been called the upper airway resistance syndrome (UARS) show increased upper airway resistance and changes in intrathoracic pressure during sleep, which is usually followed by arousals but occurs without any significant hypoxia.172 Studies on patients with UARS show that inspiratory efforts causing intrathoracic pressure changes are correlated with both systolic and diastolic blood pressure increases as well as an increased prevalence of hypertension.173 However, in a pig model, central apneas, which are characterized by no respiratory effort or intrathoracic pressure changes, caused more changes in mean arterial pressure, systemic vascular resistance and cardiac output than obstructive apneas.174 Another study found similar systemic blood pressure response to obstructive and nonobstructive apneas in an anesthetized primate model.175
A sleep apnea model in rats has been recently described where upper airway collapse and reopening is induced by subjecting rats to recurrent positive and negative pressures by means of a nasal mask valve.176 Overexpression of inflammatory biomarkers, such as TNF-α, was found in the larynx and soft palate tissue in response to these recurrent pressure changes over a period of 5 hours.176 However, the blood oxygen levels were not reported, and the potential effect of intermittent hypoxia therefore not addressed. Interestingly, snoring-like vibrations applied short-term have also been found to produce similar inflammatory changes in upper airway tissue,177 supporting the hypothesis that mechanical stimuli may cause inflammatory changes in the upper airway.
These results collectively suggest that intrathoracic pressure changes are not a major contributor to the systemic changes in patients with OSA, but potentially a cause for upper airway inflammation. This pathogenetic mechanism requires further investigation.
CLINICAL EVIDENCE FOR THE MOLECULAR AND PATHOLOGICAL CONSEQUENCES OF OSA
Primary Molecular Domains Affected by OSA
Sleep fragmentation138–140 and CIH96–98,135 lead to increased sympathetic activity. Sleep fragmentation and CIH also cause an inflammatory response, and CIH causes oxidative stress. This suggests that the molecular signature for OSA will most likely be found using measures of the following: sympathetic activation, oxidative stress and inflammation. Described below is the evidence for each of these domains and potential biomarkers for each domain.
What Is the Evidence of Increased Sympathetic Activity in OSA?
OSA patients have increased sympathetic activity measured by microneurography (direct recording of muscle sympathetic nerve activity) compared to controls during the daytime,178,179 that decreases with CPAP treatment.180–182 Plasma and urine norepinephrine levels which reflect systemic sympathetic neuronal activation,183 are increased in OSA patients compared to controls,184,185 but this is not shown in all studies.179 Many studies have also shown that CPAP treatment reduces noradrenaline levels.182,186–191 The results for epinephrine levels, which reflect adrenomedullary hormonal activation,183 are less convincing, as levels were similar in OSA subjects compared to well-matched controls in 2 studies.184,185 Some studies have found a small decrease with CPAP,192,193 but not all.191 Withdrawal of CPAP for one night does not result in a significant increase in norepinenephrine levels,194 but withdrawal for one week significantly increases urinary norepinephrine levels during the daytime but not the nighttime.195
Factors influencing the decrease in sympathetic activity with CPAP treatment include CPAP compliance,180 length of treatment,181 and whether subjects are initially normotensive or hypertensive.187 The increase in blood pressure found in many untreated OSA patients is considered, at least partly, a result of their high sympathetic activity.196,197
Increased sympathetic activation, such as occurs in OSA, leads to enhanced lipolysis with release of free fatty acids (FFAs), particularly from visceral fat cells.198–200 Elevated FFAs then activate inflammatory pathways,201,202 and act as mediators of insulin resistance.203,204 Interestingly, to date, little research has been done on FFAs in OSA. One recent study found that FFA levels, body mass index (BMI) and AHI were independently associated with insulin resistance in OSA patients but visceral and subcutaneous fat were not.205 As increased sympathetic activity, inflammation and insulin resistance are all associated with OSA, we propose that elevated FFAs likely have a role in the pathogenesis of OSA consequences. This role remains to be determined.
What is the Evidence That There Is Oxidative Stress in OSA?
The concepts described would posit that there should be evidence of oxidative stress in patients with OSA due to the CIH. A number of studies support this hypothesis.18,19,86,90,91,206–209 Oxidative stress is, however, difficult to assess.210–212 Many of the purported measures such as the commonly used thiobarbituric acid reactive substances (TBARS) are nonspecific and were not validated as reliable measures in a multi-institutional study in a rat model designed to assess reliability of methods to assess oxidative stress.213 Thus, current data need to be interpreted with caution, particularly since many studies have small sample sizes and lack appropriate controls.
Increases in the following purported measures of oxidative stress have been reported in OSA, usually compared to controls and in some studies consequent decreases with CPAP: plasma, exhaled breath condensate and nighttime urinary 8-isoprostane levels as measured with an enzyme immunoassay90,91,209; plasma levels of malondialdehyde (MDA)86; urinary o,o-dityrosine86; plasma levels of TBARS206,207; urine levels of 8-hydroxy- 2'-deoxyguanosine (8-OhdG), a marker of DNA oxidation208; reactive oxygen species (ROS) production in specific subpopulations of monocytes, granulocytes, and neutrophils upon in vitro stimulation.18,19 However, studies showing no oxidative stress in OSA are also reported.214–216 Many of the studies performed have either used poorly matched control groups206–208 or used measures that are controversial regarding their ability to assess oxidative stress.206,207,216 One study which assessed the reliability of many different oxidative stress measurements in a small group of OSA subjects found that only plasma MDA and urine o,o-dityrosine were appropriate measurements of oxidative stress86 (This study did not measure 8-isoprostanes.) Given the non-reliability of many of the measures of oxidation, as revealed by recent studies,86,213 one needs to consider which measure is optimal to assess oxidative changes.
From the many candidates available, the best currently available biomarker for oxidative stress, and the one accepted in the field for in vivo studies,210–213 is 8-isoprostane (8-iso-PGF2α), a marker of lipid peroxidation, measured by gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI-MS).210–213 Using immunoassays as has been done in earlier OSA research90,91,209,214 is more cost-effective but information regarding their precision and accuracy is still largely lacking.210,212 Hence, data obtained with this methodology may not be reliable. The use of 8-iso-PGF2α has been shown to be a good biomarker for oxidative stress for the following reasons: in vivo formation increases as a function of oxidative stress212,213; it is relatively stable in isolated samples of serum and urine210,212,217; and it is not influenced by the lipid content of diet.212 Isoprostanes in blood may occur as free fatty acids or esterified to phospholipids or lipoproteins. Therefore it is important to distinguish between the free and esterified isoprostanes.212,213 However, both methods seem to give similar results.213 In plasma the half-life of isoprostanes is very short (1-4 minutes),218 which makes it important to measure its concentration during the night, not simply in the morning after a night of sleep disordered breathing.219 In contrast, isoprostane metabolites in urine are more stable and have been found to be an accurate way to measure oxidative stress in humans.220 They can still be measured 4 hours after exposure.218 Therefore, the cumulative levels of isoprostanes found in urine after a night of sleep disordered breathing should be measurable in the morning.
Thus, we propose, based on the available data, that the gold standard method for assessing oxidative stress in OSA is measuring 8-isoprostane levels by GC/NICI-MS in urine samples after the sleep period or in plasma during sleep.
What is the Evidence that OSA Increases the Inflammatory State?
Based on the overall oxidative stress model described above,92,137 increased ROS production should cause increased expression of inflammatory cytokines through activation of NF-κB in OSA patients. However, this is only one postulated model. As stated above, visceral fat and increased sympathetic activity can increase free fatty acid levels which cause an increase in inflammatory cytokines in the absence of ROS.198–202
Much of the focus on inflammation in OSA research has been on the markers TNF-α, IL-6, and CRP. Most studies show the following: higher levels of TNF-α and/or IL-6 and/or CRP in plasma of subjects with OSA compared to BMI-matched controls,71,74,85,90,221–227 and reductions in these inflammatory markers with CPAP therapy when assessed post-sleep.85,209,223,224 There are, however, also negative studies showing no difference in TNF-α, IL-6, and CRP levels in OSA patients versus matched controls and after CPAP therapy.73,75,85,228–232 Obesity is the primary factor affecting the levels of the inflammatory markers in many of these negative studies,73,228,229,231 illustrating the confounding effect of obesity on OSA research and the importance of having well-matched controls. (This issue of confounding by obesity is discussed more fully below.) These differences between studies support our concept of inter-individual differences and heterogeneity of responses in OSA patients that we also describe more fully below.
Other inflammatory effects such as systemic increases in interleukin-8 (IL-8), granulocyte chemotactic protein-2 (GCP-2), monocyte chemotactic protein-1 (MCP-1) levels, and inflammatory cell infiltration in the upper airway have been shown in OSA subjects.85,233–235 Whether the inflammatory changes in the upper airway occur because of hypoxia, snoring, the mechanical stress of recurrent pressure changes or systemic inflammation or all of the above remains to be answered.236 Moreover, whether at least some of the systemic increases in inflammatory biomarkers are due to a spill-over effect from the upper airway remains unknown.
Given available evidence on the effects of OSA, the inflammatory markers with the strongest rationale to study further are TNF-α and IL-6. Assessment of each of these has particular challenges. TNF-α may be useful to study dynamic changes. Rapid increase in TNF-α in plasma following apneic events has been described.88 TNF-α is, however, very sensitive to handling and the blood needs to be processed and frozen immediately for accurate measurements of its levels.237,238 This procedure should, of course, be applied to all measured biomarkers to avoid artificial changes in their levels. The challenge for IL-6 is that its levels can be artificially elevated over time when blood is obtained from an indwelling intravenous line due to local production by the endothelium.239–242 This does not apply to TNF-α.242
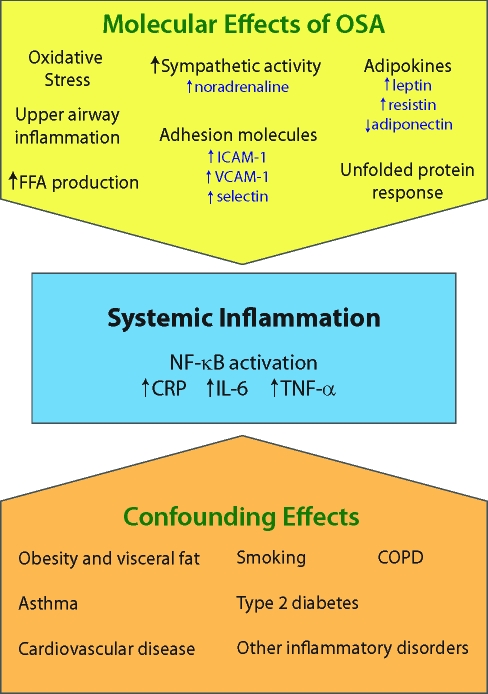
Systemic inflammation is, however, also produced by diseases other than obstructive sleep apnea. Thus, there are a number of confounding effects that need to be considered in assessing these biomarkers (Figure 3). This aspect is discussed more fully below.
Figure 3.
Possible pathways for systemic inflammation in OSA subjects. Displayed are both the different molecular pathways that can occur in OSA and confounding effects of other diseases and lifestyle choices.
Secondary Domains Affected by OSA
The three domains described above represent, in our opinion, the key variables that are affected by the pathogenetic processes in OSA. However, other domains are also affected, such as adipokines,243–259 adhesion molecules,260,261 and possibly stress in the endoplasmic reticulum (ER).111 We now describe evidence for each of these.
What is the Evidence for Changes in Adipokines?
White adipose tissue (WAT) is a metabolically active tissue that produces over 50 molecules termed adipokines with various functions.262,263 Oxidative stress,243–248,264–266 inflammation,243,249–257,264,266–271 and sympathetic activation259,272 can all affect the expression of adipokines. Therefore OSA potentially affects adipokine levels. A more detailed discussion of the function of the more investigated adipokines now follows.
Leptin:
Leptin is a pleiotropic cytokine with a circadian rhythm in expression, which is produced mainly by WAT and has a regulatory role in body adiposity with high levels acting as a satiety signal.273–277 Leptin also has an immunomodulatory role278–281 and is both activated by proinflammatory mediators249–251 and works as a stimulant of proinflammatory cytokine production such as IL-6 and TNF-α.282 Leptin levels are increased in obesity,273 but due to a central leptin resistance that occurs in obesity, it fails its regulatory role in reducing adiposity.277,283 However, despite the metabolic leptin resistance, high leptin levels still induce sympathetic activation of tissues such as the heart and the adrenal glands,283 which may contribute to the low-grade systemic inflammation and development of hypertension in obesity.283–285 Leptin acts as an independent risk factor for cardiovascular disease.286–288 Hyperleptinemia and hyperinsulinemia are both commonly found in obesity289; insulin acts as a stimulant of leptin production,290,291 and the resistance to both leptin and insulin due to overfeeding occurs quickly and simultaneously.292 Paradoxically to other leptin functions, leptin is considered anti-diabetic and can hinder the effects of insulin on lipid oxidation and production.293–295
OSA patients have been found to have higher leptin70,185,232,296,297 measured in the morning than BMI-matched controls in all but one study looking at obese subjects only.298 There is, however, a potential difference in visceral fat volume despite the BMI matching (discussed more fully below).232 CPAP therapy reduces leptin levels in OSA patients,70,298–302 but the reduction is more pronounced in non-obese than obese patients,70,298,301 and one study found no change in obese OSA subjects.298 In obese OSA patients with type 2 diabetes only a trend for a decrease in leptin level was found after 3 months on CPAP and the change was not significant.303
Adiponectin:
Adiponectin, produced by mature adipocytes,304,305 increases oxidation of fatty acids306 and has inhibitory effects on glucose synthesis by the liver.307 Unlike leptin, adiponectin shows no circadian rhythm but has some apparently random fluctuations in levels across the day and night.273 Despite being produced by adipocytes, adiponectin levels decrease with increased fat stores,258 and a decrease in adiponectin levels is associated with obesity, cardiovascular disease, insulin resistance and type 2 diabetes.258,273,308–312 It has an anti-inflammatory function, as it inhibits NF-κB activation and hence production of IL-6 and TNF-α.313–315 It also induces production of the anti-inflammatory interleukin-10 (IL-10).316 Oxidative stress, TNF-α, and IL-6 all inhibit adiponectin production,247,248,252,253,258 hence potentiating their effects.
Adiponectin levels have been shown to be lower both in the morning and evening in OSA patients compared to BMI-matched controls.308,317,318 However, other small studies have found increased adiponectin levels in the evening in OSA patients319 and no difference in the morning from controls.185 One study found a nocturnal decrease in adiponectin levels in severe OSA, which was not found in controls or milder OSA.318 Results from studies looking at the effect of CPAP therapy on adiponectin levels are also conflicting. Two studies found no immediate changes in adiponectin levels in the morning with CPAP,308,320 while one study found a decrease after 2 days on CPAP (time of day of measurement is unclear),321 and another found a reduction in a nocturnal decrease of adiponectin after one night of CPAP.318 Two small studies have also given conflicting results about long-term effects of therapy. One found an increase in adiponectin levels after long-term CPAP therapy320 but the other no change from baseline levels.321 Finally, a larger study that measured visceral and subcutaneous abdominal fat (by computerized tomography) in untreated OSA patients found no relationship between morning adiponectin levels and OSA severity, but did find a relationship with visceral fat area and body weight.205
Therefore the question whether adiponectin has a role in the pathogenetic consequences of OSA or whether its levels are affected by OSA remains unclear. Further studies assessing both abdominal fat volume and adiponectin levels across the day and night in OSA patients, when untreated and on CPAP, are needed to address this issue as OSA possibly affects the usually random secretion of adiponectin273 and causes it to decrease across the night.318
Resistin:
Resistin is almost exclusively expressed in WAT in murine models322 but is expressed in high levels in other tissues such as bone marrow and macrophages in humans and associated with the immune system.323–327 Resistin has been implicated in insulin resistance based on research in rodent models,322,328–330 but the data from human studies are controversial.310,331,332 Resistin appears to have a role in inflammation333,334 and has been shown to be both activated by, and cause activation of, IL-6 and TNF-α through the NF-κB pathway in peripheral blood mononuclear cells.254,255 In adipocytes, however, IL-6, TNF-α and epinephrine either have no effect on or downregulate resistin production.256,259,335 Antioxidant treatment causes decreased resistin levels in serum,246 indicating that oxidative stress has some regulatory role in resistin production. Resistin has been shown to induce the production of adhesion molecules and be increased in patients with coronary artery disease,336,337 suggesting that it has a role in atherogenesis.
Two small studies to date have looked at the relationship between resistin and OSA in adults. One study looking at obese subjects with severe OSA found that resistin levels were related to obesity, inflammation and atherogenesis, not to OSA, and did not change with CPAP.338 The other study looked at less obese OSA subjects with different disease severity and controls. They found that resistin levels and inflammation levels were related but also found increased resistin levels with increased OSA severity and a reduction with CPAP.339 A study in children with OSA found no relationship between resistin levels and OSA.340 However, since inflammatory cytokines affect resistin levels and vice versa, and resistin has a potential role in atherogenesis, further studies are needed to address whether OSA alters resistin levels. This relationship between resistin and OSA is potentially dependent on OSA severity and obesity levels.
Other Newly Discovered Adipokines:
Other adipokines, recently discovered, include visfatin, apelin, vaspin, and hepcidin.262 These adipokines have not yet been studied in the context of OSA but hepcidin has been suggested as a marker of OSA.341
Studies looking at the relationship between OSA, obesity and these adipokines are of interest as they are regulated by many of the same molecular processes as are found in OSA pathophysiology (e.g., hypoxia and inflammation).243–245,252,264–272 This is a future direction for research.
What is the Evidence of Increased Adhesion Molecules in OSA?
The release of cellular adhesion molecules, which promote adhesion of circulating leukocytes to endothelial cells, is potentially one of the first steps in the pathogenesis of atherosclerosis.342 The release of cellular adhesion molecules can be stimulated by inflammatory cytokines, oxidative stress as well as other mechanisms such as lipopolysaccharide. 260,261,343–346
A variety of cellular adhesion markers, such as ICAM-1, vascular adhesion molecule-1 (VCAM-1), E-selectin, and L-selectin, have been shown to be elevated in OSA compared to controls.72,227,347–350 However, not all studies used well-matched controls,227,347,348 and none matched for visceral fat. More convincing is the demonstration that adhesion molecules are reduced with CPAP treatment.347,349,351
What is the Evidence of Changes in the Unfolded Protein Response in OSA
Stress in the endoplasmic reticulum (ER), caused by a disruption of protein folding and buildup of unfolded proteins in the ER,352 occurs in response to stressors such as hypoxia353,354 and cholesterol loading355 and in conditions such as obesity356,357 and type 2 diabetes.352,358 ER stress causes the unfolded protein response (UPR), an adaptive response which increases the upregulation of ER chaperones and downregulates protein translation in order to reestablish normal function of the ER.352 The UPR has been shown to occur in the liver of obese mouse models356 and fat of obese subjects (not in lean).359 UPR has a role in initiating insulin resistance in obesity,356 as well as in promoting atherosclerosis355 and possibly inflammation.359
Increased expression of the prototypical molecular chaperone termed binding immunoglobulin protein (BiP, also known as GRP78), phosphorylation of PKR-like ER kinase (PERK) and the eukaryotic initiation factor 2-α (eIF2α) can be used as molecular signals that indicate activation of the UPR.352,358,360
A recent study provides support for the presence of ER stress in OSA as CIH in mice leads to increased phosphorylation of PERK and other changes signalling upregulation of the UPR in motoneurons.361 Total sleep deprivation has also been found to activate the UPR in the brain of different animal models.25,362–367 Research on the UPR in OSA is of interest, and it is conceivable that it will be activated across the sleep period in circulating cells.
POTENTIAL CONFOUNDING EFFECTS ON THE MOLECULAR SIGNATURES OF OSA
Confounding Effects of Obesity
Obesity, in particular central obesity, is the most important risk factor for OSA.69,368–374 OSA with complaints of excessive sleepiness affects 4% of middle-aged males and 2% of middle-aged females,69 but among obese subjects, these percentages are much higher.69,371,372,375–378 OSA prevalence in morbidly obese subjects requiring bariatric surgery has been found to be between 40% and 94%.372,375–378
Both obesity and OSA have been shown to be independent risk factors for insulin resistance, hypertension, and cardiovascular disease (for reviews on OSA, see47,48,78; for reviews on obesity, see379–381). Insulin resistance is also an independent risk factor for hypertension and cardiovascular disease.382
Intervention studies based on treating subjects with OSA with CPAP have shown improvements in insulin resistance303,383,384 and hypertension.385–390 An observational study found improvement in glucose control in obese type 2 diabetics with OSA treated with CPAP,391 but a randomized trial of CPAP therapy in very obese type 2 diabetic OSA patients found no improvements in glucose control with CPAP.392 Meta-analysis of treatment trials suggests that the effects of treatment of OSA on blood pressure are modest.386
Because OSA and obesity commonly coexist and have been shown to have similar clinical consequences, it is important to consider their relative roles in causing adverse clinical consequences; it is also important to delineate the relative importance of shared common pathways and whether there are unique pathways related to OSA that mediate clinical consequences. The key pathogenetic mechanisms resulting from OSA, i.e., oxidative stress, inflammation and sympathetic activation, also occur in obesity,393–398 and have a role in insulin resistance.399–406 Thus, at this mechanistic level both obesity and OSA affect the same processes, and their relative roles in oxidative burden, the inflammatory state (see Figure 3), and sympathetic activation need to be assessed. Unfortunately, the large literature on obesity simply ignores this issue. In a recent meta-analysis of studies demonstrating a link between obesity and cardiovascular disease published in Lancet,407 sleep disordered breathing was not assessed in a single study, which, from a scientific perspective, is an important omission. This is an important question to address, since there is a safe, effective therapy available for OSA (i.e., CPAP).79,81 CPAP treatment of OSA in obese individuals has the potential to alter the cardiovascular consequences of obesity.
A critical issue is how to determine the relative role of OSA. One commonly used strategy is to assess differences between OSA patients and controls without OSA but matched for BMI. Matching for BMI is likely, however, not to be sufficient. Abdominal visceral fat has been shown to be a stronger risk factor than other fat tissue for adverse health consequences and is associated more strongly with hypertension, insulin resistance, diabetes, the metabolic syndrome than other fat deposits.408–412 Further evidence that visceral fat rather than other fat deposits is causal (not only associative) for insulin resistance is, however, needed.413 Visceral fat is also a risk factor for OSA and has been shown to be increased in OSA patients compared to BMI-matched controls.232,368,414 Visceral fat, therefore, plays an important part in understanding of OSA pathophysiology moving forward and needs to be directly assessed in studies of OSA.
An alternative and more powerful strategy to separate the effects of obesity and OSA is to use a within-subject design, i.e., assess differences before and after effective CPAP therapy. This strategy has been used in multiple studies.19,85,91,189,206,209,223,224,320,385,389,391,392,415 There are, however, problems with this strategy. First, there are data to indicate that successful CPAP therapy reduces the amount of visceral fat,299,302 albeit by a small amount (8% to 16% over a period of 3-6 months). Thus, changes with CPAP treatment could be due, at least in part, to reductions in visceral fat mass. Moreover, in patients with OSA who are effectively treated with CPAP, there could be irreversible effects of OSA. OSA is a chronic, slowly progressive disorder,371 and it can be present for years before it is diagnosed.131,376,416 Residual sleepiness in OSA has been described,417 i.e., persistent sleepiness even on effective CPAP therapy. This effect might be mediated, at least in part, by oxidative damage to wake-active neurons.108,109,111,118 There could also be vascular wall remodeling that occurs during the years with untreated OSA.119 Currently the magnitude of irreversible effects of untreated OSA is largely unknown. Studies are needed to estimate the reversible effects of OSA (differences pre- to post-CPAP in effectively treated individuals) and irreversible effects of OSA, i.e., estimate the difference between patients with OSA after effective treatment when compared to controls with similar levels of visceral fat but not with OSA.
Since both free fatty acids and proinflammatory cytokines are produced by visceral fat,200,397,418 it is likely that for equivalent degrees of OSA, there will be an enhanced production of these biomarkers in obese subjects as a consequence of OSA in comparison to lean subjects with OSA. Since obesity in the absence of OSA can lead to production of these biomarkers, we would anticipate that obese individuals with OSA will have higher levels of biomarkers even after effective treatment of OSA than lean subjects. Comparison of biomarkers in individuals with different degrees of visceral fat, all of whom are effectively treated for OSA, will provide the much needed estimate of the effect of obesity per se. In such studies, waist circumference can be used, since waist circumference is increasingly recognized to be the best proxy for the degree of visceral adiposity.419,420 But direct measurement of both visceral and subcutaneous fat distribution would add more definitive information, as waist circumference cannot distinguish between subcutaneous and visceral fat. A study by Vgontzas et al232 emphasizes the importance of matching for visceral fat between groups in studies of OSA. In this study, plasma levels of IL-6, TNF-α, and leptin in the evening and morning were measured. Obese OSA patients were found to have the highest levels of the 3 biomarkers, obese controls without OSA intermediate levels, and lean controls the lowest.232 However, the OSA patients also had more visceral fat than the obese controls, confounding the results.232 Thus, directly assessing visceral fat is required to answer these questions.
Other Coexisting Conditions
Systemic inflammation, a key aspect of pathological mechanisms for the consequences of OSA, also occurs in cardiovascular disease,421–425 type 2 diabetes,426,427 asthma428,429 and smoking,430–432 all of which are commonly found in patients with OSA (see Figure 3).
Oxidative stress is another key element in OSA but is also increased with type 2 diabetes,433–435 smoking,432,436 cardiovascular disease,424,437 age,438 and joint diseases.439
Sympathetic activation has also been shown to be increased in cardiovascular disease,421,422 the metabolic syndrome,394,421,440 and insulin resistance440—all conditions commonly found in OSA patients.67,441–443
Thus, establishing the actual role of OSA is challenging, and will require study designs that deal with the confounding effect of obesity and the coexistence of other conditions that affect the same mechanisms as OSA does.
INTER-INDIVIDUAL DIFFERENCES IN OSA
Variability in the Nature of OSA
Differences between individuals with OSA may be directly related to the nature of their sleep disordered breathing events. Some individuals with OSA have marked sleep fragmentation with little, if any, intermittent hypoxia, while others may show marked intermittent hypoxia with lesser degrees of fragmentation. Within an individual the magnitude of hypoxia and arousals may vary across the sleep period. We propose the need for studies that capture this source of variability by recruiting subjects from both extremes of the spectrum (with regard to severity of intermittent hypoxia and number of arousals) and assess associations between changes in biomarkers and the nature of the sleep disordered breathing events both between and within individuals.
Heterogeneity in the Biological Response
The change in molecular pathways between different individuals for identical degrees of sleep disordered breathing is also unlikely to be the same. There will likely be inter-individual differences in response. Such inter-individual differences might be seen as a challenge but represent, in our view, another opportunity. We know that many individuals with OSA do not have excessive sleepiness,69 and only about 50% of OSA subjects develop hypertension.63 The question is why—why do some subjects with OSA develop hypertension and cardiovascular disease while others do not? One possibility is that individuals who develop hypertension and cardiovascular disease have increased pathogenetic responses or decreased adaptive response for equivalent degrees of OSA.
Such heterogeneity in response might be related to differences in protective mechanisms. Individuals vary in their antioxidant ability, which is complex, as it involves dietary factors and multiple intrinsic antioxidant systems.444,445 Candidates for measurement are numerous, including antioxidant enzymes (such as superoxide dismutates and glutathione peroxidases), non-enzymatic antioxidants (such as glutathione, bilirubin, and vitamins C and E), and melatonin, which has a role in the regulation of antioxidant enzyme activity and expression.445,446 Measurements of total antioxidant capacity that are meant to functionally assess the total antioxidants in plasma or serum are also used but the reliability of these assays and what they actually measure is questionable.447
The question whether there is a change in antioxidant levels in OSA patients remains unanswered. The studies done so far have been relatively small, used various methods for assessing them and have contradictory results.24,86,207,216,415,448,449
Levels of anti-inflammatory molecules such as IL-10 can also vary between individuals.6,450 IL-10 has a primary role in restricting inflammatory responses451 and has a role in protecting against atherosclerosis,452,453 insulin resistance,6,454 and type 2 diabetes.450 Thus, individuals with OSA and comorbidities may have lower IL-10 levels than those without comorbidities.
IL-10 levels have been reported to be lower in non-obese children with OSA than controls and increased after tonsillectomy and adenoidectomy in children with OSA.455 IL-10 expression in T cells has been negatively correlated with the severity of OSA.456 One study showed that IL-10 levels were decreased in the evening in OSA patients compared to ill-matched controls88 but another study with better controls found no difference.85
Adaptive responses that affect sympathetic activation are also potentially heterogenous between individuals with OSA. Likely candidates include differential downregulation of adrenergic receptors457 and differences in norepinephrine clearance rates,458 both of which act to diminish the stimulatory response and the increased sympathetic activity in OSA.
Differences between those with OSA with comorbidities and those without could be genetic in origin. There is now a large literature on genes conferring risk for hypertension, insulin resistance and CV disease.459–463 Polymorphisms in genes affecting oxidative stress, inflammation and sympathetic activity464–466 are also of high interest. There is a limited but growing number of candidate gene studies addressing the question of gene variants and their pathophysiological effect in the OSA population.467–475 More studies in this field are clearly needed, preferentially using more high-throughput technology such as genome-wide association, custom genotyping arrays such as the new cardiovascular chip to look for single nucleotide polymorphisms associated with increased risk for comorbidities in individuals with OSA, copy number variations as well as gene-gene and gene-environment interactions.476–478
THE CONCEPT OF A TEMPORAL MOLECULAR SIGNATURE FOR OSA
One approach to understanding the clinical variability in the consequences of OSA in different subjects is to evaluate the molecular signatures of the disorder.2,43,44 There are certain aspects that we believe make OSA unique: a) the pathogenetic events occur in a temporally controlled fashion, i.e., during sleep; and b) the nature of the pathogenetic events can be characterized, e.g., breathing cessations, sleep fragmentation, hypoxia, cyclical deoxygenation/reoxygenation, etc. These concepts would argue that if we are to develop a molecular signature of OSA we should focus on the temporal changes taking place across the sleep period and relate these to the postulated pathogenetic events. Such temporal changes during sleep might, of course, in part, be related to circadian factors and/or alterations in sleep stages. There is, for example, circadian control of molecules (such as melatonin) and functions (such as body temperature)274,479–481 and a sleep-stage specific regulation of others, such as plasma catecholamine concentration,482 heart rate variability,483 thermoregulation, and sweating.484 To initially evaluate this source of temporal variability it will be necessary to study OSA subjects before and while on an effective therapy with CPAP as well as matched controls with no OSA. The changes in sleep stages usually seen with successful CPAP treatment485,486 are harder to control for but can at least be addressed as covariates in statistical models of larger studies, as there are substantial inter-individual differences in the sleep stage architecture in untreated OSA patients and changes with treatment.82,487
Thus, we believe that the optimal molecular signature for OSA is likely to be change in relevant biomarkers across the sleep period; that is to measure the change from pre-sleep to post-sleep. An advantage of assessing an overnight change, instead of a single time point, is that many of the molecular mechanisms activated by OSA are shared by other common conditions (as discussed earlier), particularly obesity,393–398 cardiovascular disease,423–425,437 and type 2 diabetes.426,427,433–435 Assessment of biomarkers at a single time point will be confounded by these conditions, whereas overnight changes due to the challenge of OSA will give, in our view, a clearer picture of the contribution of OSA.
Given the postulated relationship between breathing events during sleep and the molecular process we are assessing, there is a strong rationale for the overnight change to be an ideal molecular signature for OSA. Previous data to support this assertion are, however, limited since most studies have simply measured biomarkers in a single sample in the morning, i.e., post-sleep. Available data from studies looking at overnight change in OSA, which are scant, support this hypothesis that studying change across the sleep period will give more information than a single sample: a) progressive increase in the oxidative stress marker MDA across the night in patients with OSA that correlates with the percentage of time with SaO2 < 85%86; b) an almost 3-fold increase in plasma TNF-α levels after the first hypoxic apneic event of the night with oxygen saturation below 85% in OSA patients compared to pre-sleep levels88; c) 24-hour measurements of erythropoietin that showed attenuation in oscillation and changed peak values from untreated to CPAP treated subjects;488 and d) acute changes in sympathetic activity and blood pressure during apneas.196
Our postulate that overnight measurements of change in biomarkers will provide superior diagnostic ability for OSA than post-sleep measurements only is that it addresses the problems of coexisting conditions such as obesity. It takes advantage of the temporal nature of OSA. This postulate needs to be tested further, as there are limited studies addressing this issue to date and more research is clearly needed. We summarize in Table 1 what we propose are the advantages of overnight measures (repeated samples) compared to assessments at a single time-point.
Table 1.
A Comparison of Biomarker Assessment at a Single Time-point Versus Overnight Measurement
| Single Time-Point | Overnight Measurement (repeated) | |
|---|---|---|
| Time of Measurement | Generally in the morning following OSA events | During OSA events and at different sleep stages |
| Circadian rhythm detection | No | Shows changes in amplitude and shifts in circadian rhythm |
| Assessment of dynamic changes during sleep | No | Detects rapid changes in biomarkers (e.g., TNF-α, 8-isoprostanes) |
| Assessment of confounders | Limited | Shows acute effects of OSA vs. combined chronic effects of OSA and comorbidities |
| Reliability of measurement | Single sample more affected by measurement errors and biological variations | Multiple samples less affected by measurement errors and biological variations |
COMBINING A TEMPORAL STRATEGY WITH NEWER APPROACHES
Gene Expression Profiling
Microarrays are increasingly being used to evaluate potential molecular signatures of disease.23,489,490 They offer the advantage of providing a broad unbiased approach to study changes in gene expression of thousands of genes simultaneously and the ability to discover new pathways and molecules affected by a disease such as OSA.1,2 The main disadvantage is the cost of this high-throughput technology.
Whether looking at the gene expression of specific genes or using a microarray approach, investigators need to be aware of the sensitivity of mRNA to storage and handling. mRNA expression continues to change after blood or other tissue is extracted, if the tissue is not immediately suspended in chemicals to kill the cells and the tissue is kept frozen.491–498 For example, after 4 hours of storage at room temperature of whole blood with EDTA, there is a 10- to 100-fold increase in expression of IL-8, c-myc, and c-fos.491 Care must also be taken with the statistical analysis of microarray data, as well as with other high-throughput methods, such as proteomics, metabolomics, and lipidomics, because of the multiple comparison issue. Complex statistical analyses have been designed for this purpose.499–506
To date, there have been 2 studies looking at gene expression profiling in OSA patients: one in adult patients24 and one in non-obese children with OSA.76 In the study in adults, gene expression was measured both before and after sleep, albeit in a small sample (4 OSA patients, 4 controls), therefore allowing for the assessment of overnight changes in gene expression. In OSA patients, but not controls, overnight upregulation of expression of oxidative stress responsive genes such as the antioxidant enzymes superoxidase dismutase 2 (SOD2) and catalase were found as well as downregulation of other genes, such as superoxidase dismutase 1 (SOD1).24 Overnight changes in OSA patients, but not controls, were also found in genes that modulate the cell cycle.24 In the study on pediatric OSA,76 gene expression changes in leukocytes were assessed at a single time point (post-sleep) in 20 non-obese children with OSA and 20 well-matched controls. Differences between these groups were found in a number of genes involved in various biological pathways, including genes such as interleukin-27 in the inflammatory pathway. These studies show the promise of this approach, but need to be done in a larger number of subjects to assess inter-individual differences in response (see above). As argued earlier, temporal changes in gene expression across the sleep period will, we propose, provide the most information.
Proteomics
Proteomics is a discovery strategy, similar to gene expression profiling, that examines expression of proteins and post-translational modifications of thousands of proteins simultaneously.4,36,37,507,508 The benefits of proteomics is to obtain information regarding changes in protein quantities, post-translational modifications, and protein-protein interactions.509 This information is needed to understand the true molecular phenotype of a disease since knowledge on gene variants, and changes in gene expression levels, may not translate into actual changes in protein.510
Proteomics is more complicated than genomics and the state of the technology is not as developed as microarrays. Major issues with proteomics are the sheer number of proteins compared to genes (100-fold increase) and mRNA (10-fold increase), the huge difference in protein concentrations of the dynamic range of 1010 as well as the myriad post-translational modifications that alter the protein signatures of samples (see 510). The most employed proteomics technologies today include 2-dimensional gel electrophoresis and/or HPLC coupled to different mass spectrometry approaches. A discussion on the advantages and disadvantages of the different technologies is beyond the scope of this paper but for an excellent review see Gulcicek.510
An important issue with proteomics is that of protein abundance; abundant proteins tend to be overrepresented in studies, while less abundant proteins are often not detected. This is especially important/true in plasma proteomics, in which 99% of plasma is comprised of 22 highly abundant proteins, and most of the clinically interesting biomarkers are found at much lower levels.509,511 Immunoaffinity depletion of samples as well as the use of fluorescent dyes with greater dynamic ranges are being used to address this problem. Proteomics in blood can be done either on plasma or serum. The use of plasma is considered superior to serum for at least certain proteome measurements, as approximately 40% of signals found in serum are not found in plasma because of ex vivo generation during clotting.512 Urine proteomics is also considered a promising method for new biomarker discoveries in relation to various systemic diseases.4 Urine analysis has the potential to find changes in circulating proteins and peptides that are small enough to pass through the glomerular filter; it is therefore not isolated to diseases related to the kidneys but all diseases that have an effect on circulating molecules.4
Only two studies have been published looking at proteomic patterns in relation to OSA. Both studies come from the same research group investigating OSA in children.31,32 One study assessed the serum proteomics patterns associated with OSA in children and found that 3 proteins differed in expression between children with OSA and those with snoring only. Assessment of the proteins could predict OSA diagnosis with a sensitivity of 93% and specificity of 90%.32 One of the 3 proteins was identified as osteocalcin, a precursor for a γ-carboxyglutamic acid-containing protein that has been used as a biomarker for growth retardation,32 an important consequence of OSA in children.513 The other study assessed the difference in first-morning urine samples between children with OSA and controls and found significant differences in the expression of 2 proteins: gelsolin (severs actin filaments when activated) and perlecan (a heparin sulfate proteoglycan), between the 2 groups.31 These interesting findings need to be replicated by other studies.
Metabolic Profiling
Metabolic profiling, the global analysis of metabolites found in a tissue or cell under specific conditions (also known as metabolomics) and the response of a tissue or cell to a disease or drug toxicity (also known as metabonomics),41,42,514–516 is still in its infancy and currently has its share of technical problems.42,517,518 Metabolic profiling, however, has the potential to shed further light on the molecular signature of diseases such as OSA in conjunction with other high-throughput techniques.
No studies using this approach have been done in the field of OSA but studies looking at diabetes39,40,519 and cardiovascular disease38,519,520 show initial promising results. This will likely be a topic of more studies in the future.
Investigating a Cellular Window
Measuring circulating biomarkers in plasma or assessment of molecules in urine can be used to estimate the changes in biomarkers due to OSA. Another approach is to assess changes in specific cell populations in blood. This provides a more cellular approach to the quest of finding biomarkers for OSA, and gives researchers the opportunity to look at intracellular mechanisms without employing invasive measures to look at affected organs. Such cellular approaches have already been applied successfully to study the effects of obesity and cardiovascular disease, hypoxia and seizures.13,14,16,20,23,27,521 While earlier studies using this approach often used all white blood cells to study changes,14,16,23,521 there is, not surprisingly, a heterogeneity in response of different cell types.27 Therefore studies have started focusing on changes in specific subpopulations of leukocytes, such as monocytes.20,224,489,522 Recruitment of circulating monocytes into adipose tissue523 and inflamed intima524,525 has been implicated in insulin resistance/type 2 diabetes and atherosclerosis, respectively, both of which are considered comorbidities of OSA.48,78 Monocytes have also been shown to be affected specifically by OSA, e.g., by NF-κB activation,21 increased production of TNF-α,21,224 and IL-6.223 Other subpopulations of leukocytes such as lymphocytes have also been examined in patients with OSA.456,526–528 Different subpopulations of T lymphocytes are activated and more cytotoxic in OSA patients.456,526,527 Lymphocytes also have less repair capacity for DNA damage in OSA patients.528 Therefore, studying molecular changes in different subpopulations of circulating cells provides additional opportunities for establishing molecular signatures for OSA.
CONCLUSION
Throughout this review and perspective, we have provided information to support our assertion that there are major opportunities to establish molecular signatures for OSA. These opportunities include both hypothesis-driven studies on specific pathways and use of broad discovery strategies such as expression profiling, proteomics, and metabolomics. Investigating changes in gene or protein expression in specific circulating cells will likely provide the clearest signatures.
Part of the challenge for establishing a molecular signature for OSA is that obesity, which is commonly associated with OSA, leads to activation of the same pathways as does OSA. This suggests that we need approaches to separate the effects of OSA from that of obesity per se. Examining the overnight change in relevant biomarkers across the sleep period is, we propose, the optimal strategy to do so.
It is conceivable that since changes in molecules in key pathways across the sleep period will be the consequence of the aberrant breathing events, considerable information about these events can be obtained from simple blood and/or urine tests, without the need for overnight recording of many physiological variables. It is likely that the changes across the sleep period will reflect the degree of free radical production, as a consequence of cyclical intermittent hypoxia, and the degree of hypoxia potentially assessed by looking at HIF-1α expression in circulating cells as well as levels of proteins whose level is affected by HIF-1α.
It is conceivable that establishing molecular signatures for OSA will provide not only diagnostic information, but also prognostic information, i.e., which person with OSA is likely to develop specific consequences. We need to focus on inter-individual differences within the OSA population and the heterogeneity in response to the disease between individuals. It is important to evaluate the sources of this inter-individual variability. Developing and validating molecular signatures for OSA will be a major part of this effort. It will complement genetic studies to make the goal of personalized sleep medicine a reality.
The biomarkers studied in the OSA field to date, are neither sensitive nor specific enough to be a molecular signature for OSA since changes in levels of the biomarkers studied are also found in many of the comorbidities of OSA. One of the major thrusts of this review is to urge investigators to collect data in a manner that allows us to evaluate what happens temporally to biomarkers across the sleep period, by sampling at times that are more specific to elucidating differences due to OSA. These studies are not at present at the stage of clinical application but the experimental approaches outlined here are likely to lead in the future to new approaches to diagnose as well as providing prognostic information. The future clinical utility in routine practice will of course be determined by the results of such studies.
Thus, much investigation remains to be done. We now have the tools to study candidate pathways and also take broader unbiased approaches. When combined with assessment of temporal change across the sleep period, we can fully address whether, as we propose, measuring molecular signatures of OSA will provide critical new and relevant information for diagnosis and management of patients with obstructive sleep apnea.
ACKNOWLEDGMENTS
This work was supported by NIH grants HL72067 and HL60287 and the Eimskip Fund of the University of Iceland. We are grateful to Dr. Nirinjini Naidoo for discussions and editing of the manuscript as well as Mr. Daniel Barrett and Ms. Jennifer Montoya for help in preparation of the manuscript.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Allan Pack is the John L. Miclot Professor of Medicine. Funds for this position have been provided by Phillips Respironics. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kittleson MM, Hare JM. Molecular signature analysis: using the myocardial transcriptome as a biomarker in cardiovascular disease. Trends Cardiovasc Med. 2005;15:130–8. doi: 10.1016/j.tcm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Mohr S, Liew CC. The peripheral-blood transcriptome: new insights into disease and risk assessment. Trends Mol Med. 2007;13:422–32. doi: 10.1016/j.molmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Rockett JC, Burczynski ME, Fornace AJ, Herrmann PC, Krawetz SA, Dix DJ. Surrogate tissue analysis: monitoring toxicant exposure and health status of inaccessible tissues through the analysis of accessible tissues and cells. Toxicol Appl Pharmacol. 2004;194:189–99. doi: 10.1016/j.taap.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. CMAJ. 2007;177:361–8. doi: 10.1503/cmaj.061590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakonarson H, Bjornsdottir US, Halapi E, et al. Profiling of genes expressed in peripheral blood mononuclear cells predicts glucocorticoid sensitivity in asthma patients. Proc Natl Acad Sci U S A. 2005;102:14789–94. doi: 10.1073/pnas.0409904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarpelli D, Cardellini M, Andreozzi F, et al. Variants of the interleukin-10 promoter gene are associated with obesity and insulin resistance but not type 2 diabetes in caucasian italian subjects. Diabetes. 2006;55:1529–33. doi: 10.2337/db06-0047. [DOI] [PubMed] [Google Scholar]
- 7.Lemaitre RN, Heckbert SR, Sotoodehnia N, et al. beta1- and beta2-Adrenergic receptor gene variation, beta-blocker use and risk of myocardial infarction and stroke. Am J Hypertens. 2008;21:290–6. doi: 10.1038/ajh.2007.71. [DOI] [PubMed] [Google Scholar]
- 8.Shearman AM, Cooper JA, Kotwinski PJ, et al. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res. 2006;98:590–2. doi: 10.1161/01.RES.0000210578.62102.a6. [DOI] [PubMed] [Google Scholar]
- 9.Bohen SP, Troyanskaya OG, Alter O, et al. Variation in gene expression patterns in follicular lymphoma and the response to rituximab. Proc Natl Acad Sci U S A. 2003;100:1926–30. doi: 10.1073/pnas.0437875100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 11.Kittleson MM, Minhas KM, Irizarry RA, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21:299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 12.Tsiotra PC, Tsigos C, Yfanti E, et al. Visfatin, TNF-alpha and IL-6 mRNA expression is increased in mononuclear cells from type 2 diabetic women. Horm Metab Res. 2007;39:758–63. doi: 10.1055/s-2007-990288. [DOI] [PubMed] [Google Scholar]
- 13.Lupattelli G, Marchesi S, Ronti T, et al. Endothelial dysfunction in vivo is related to monocyte resistin mRNA expression. J Clin Pharm Ther. 2007;32:373–9. doi: 10.1111/j.1365-2710.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 14.de Mello VD, Kolehmainen M, Schwab U, et al. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism. 2008;57:192–9. doi: 10.1016/j.metabol.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–6. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–71. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 17.Yndestad A, Holm AM, Muller F, et al. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res. 2003;60:141–6. doi: 10.1016/s0008-6363(03)00362-6. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 19.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 20.Foldes G, von Haehling S, Okonko DO, Jankowska EA, Poole-Wilson PA, Anker SD. Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol. 2008;124:80–5. doi: 10.1016/j.ijcard.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi M, Tamaki S, Tomoda K, et al. Evidence for activation of nuclear factor kappaB in obstructive sleep apnea. Sleep Breath. 2006;10:189–93. doi: 10.1007/s11325-006-0074-x. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Lu A, Aronow BJ, Wagner KR, Sharp FR. Genomic responses of the brain to ischemic stroke, intracerebral haemorrhage, kainate seizures, hypoglycemia, and hypoxia. Eur J Neurosci. 2002;15:1937–52. doi: 10.1046/j.1460-9568.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Lu A, Aronow BJ, Sharp FR. Blood genomic responses differ after stroke, seizures, hypoglycemia, and hypoxia: blood genomic fingerprints of disease. Ann Neurol. 2001;50:699–707. doi: 10.1002/ana.10042. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann MS, Singh P, Wolk R, Romero-Corral A, Raghavakaimal S, Somers VK. Microarray studies of genomic oxidative stress and cell cycle responses in obstructive sleep apnea. Antioxid Redox Signal. 2007;9:661–9. doi: 10.1089/ars.2007.1589. [DOI] [PubMed] [Google Scholar]
- 25.Mackiewicz M, Shockley KR, Romer MA, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–57. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman JE, Rizzo W, Shockley KR, et al. Multiple mechanisms limit the duration of wakefulness in Drosophila brain. Physiol Genomics. 2006;27:337–50. doi: 10.1152/physiolgenomics.00030.2006. [DOI] [PubMed] [Google Scholar]
- 27.Du X, Tang Y, Xu H, et al. Genomic profiles for human peripheral blood T cells, B cells, natural killer cells, monocytes, and polymorphonuclear cells: comparisons to ischemic stroke, migraine, and Tourette syndrome. Genomics. 2006;87:693–703. doi: 10.1016/j.ygeno.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Ardigo D, Gaillard CA, Braam B. Application of leukocyte transcriptomes to assess systemic consequences of risk factors for cardiovascular disease. Clin Chem Lab Med. 2007;45:1109–20. doi: 10.1515/CCLM.2007.261. [DOI] [PubMed] [Google Scholar]
- 29.Basheer R, Brown R, Ramesh V, Begum S, McCarley RW. Sleep deprivation-induced protein changes in basal forebrain: implications for synaptic plasticity. J Neurosci Res. 2005;82:650–8. doi: 10.1002/jnr.20675. [DOI] [PubMed] [Google Scholar]
- 30.Klein JB, Gozal D, Pierce WM, et al. Proteomic identification of a novel protein regulated in CA1 and CA3 hippocampal regions during intermittent hypoxia. Respir Physiol Neurobiol. 2003;136:91–103. doi: 10.1016/s1569-9048(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 31.Krishna J, Shah ZA, Merchant M, Klein JB, Gozal D. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med. 2006;7:221–7. doi: 10.1016/j.sleep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Shah ZA, Jortani SA, Tauman R, Valdes R, Jr, Gozal D. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatr Res. 2006;59:466–70. doi: 10.1203/01.pdr.0000198817.35627.fc. [DOI] [PubMed] [Google Scholar]
- 33.Koopmann J, Zhang Z, White N, et al. Serum diagnosis of pancreatic adenocarcinoma using surface-enhanced laser desorption and ionization mass spectrometry. Clin Cancer Res. 2004;10:860–8. doi: 10.1158/1078-0432.ccr-1167-3. [DOI] [PubMed] [Google Scholar]
- 34.Kang X, Xu Y, Wu X, et al. Proteomic fingerprints for potential application to early diagnosis of severe acute respiratory syndrome. Clin Chem. 2005;51:56–64. doi: 10.1373/clinchem.2004.032458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlyk AC, Ferber M, Shah A, Pack AI, Naidoo N. Proteomic analysis of the effects and interactions of sleep deprivation and aging in mouse cerebral cortex. J Neurochem. 2007;103:2301–13. doi: 10.1111/j.1471-4159.2007.04949.x. [DOI] [PubMed] [Google Scholar]
- 36.Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006;5:1760–71. doi: 10.1074/mcp.R600004-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Meyer HE, Stuhler K. High-performance proteomics as a tool in biomarker discovery. Proteomics. 2007;7(Suppl 1):18–26. doi: 10.1002/pmic.200700183. [DOI] [PubMed] [Google Scholar]
- 38.Sabatine MS, Liu E, Morrow DA, et al. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation. 2005;112:3868–75. doi: 10.1161/CIRCULATIONAHA.105.569137. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Kong H, Guan Y, et al. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal Chem. 2005;77:4108–16. doi: 10.1021/ac0481001. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Xu G, Hong Q, et al. Discrimination of Type 2 diabetic patients from healthy controls by using metabonomics method based on their serum fatty acid profiles. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;813:53–8. doi: 10.1016/j.jchromb.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Schnackenberg LK, Beger RD. Monitoring the health to disease continuum with global metabolic profiling and systems biology. Pharmacogenomics. 2006;7:1077–86. doi: 10.2217/14622416.7.7.1077. [DOI] [PubMed] [Google Scholar]
- 42.Weckwerth W, Morgenthal K. Metabolomics: from pattern recognition to biological interpretation. Drug Discov Today. 2005;10:1551–8. doi: 10.1016/S1359-6446(05)03609-3. [DOI] [PubMed] [Google Scholar]
- 43.Bell J. Predicting disease using genomics. Nature. 2004;429:453–6. doi: 10.1038/nature02624. [DOI] [PubMed] [Google Scholar]
- 44.Ginsburg GS, Donahue MP, Newby LK. Prospects for personalized cardiovascular medicine: the impact of genomics. J Am Coll Cardiol. 2005;46:1615–27. doi: 10.1016/j.jacc.2005.06.075. [DOI] [PubMed] [Google Scholar]
- 45.Lango H, Weedon MN. What will whole genome searches for susceptibility genes for common complex disease offer to clinical practice? J Intern Med. 2008;263:16–27. doi: 10.1111/j.1365-2796.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 46.Arnett DK, Baird AE, Barkley RA, et al. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2007;115:2878–901. doi: 10.1161/CIRCULATIONAHA.107.183679. [DOI] [PubMed] [Google Scholar]
- 47.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–97. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 48.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173:7–15. doi: 10.1164/rccm.200509-1478OE. [DOI] [PubMed] [Google Scholar]
- 49.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 50.Thorpy MJ. The clinical use of the Multiple Sleep Latency Test. The Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1992;15:268–76. doi: 10.1093/sleep/15.3.268. [DOI] [PubMed] [Google Scholar]
- 51.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 52.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 53.Doghramji K, Mitler MM, Sangal RB, et al. A normative study of the maintenance of wakefulness test (MWT) Electroencephalogr Clin Neurophysiol. 1997;103:554–62. doi: 10.1016/s0013-4694(97)00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 55.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–5. [Google Scholar]
- 56.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 57.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 58.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 59.Kaida K, Takahashi M, Akerstedt T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117:1574–81. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 61.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–95. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 63.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 64.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 65.Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–84. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 66.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 67.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 68.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 69.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 70.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 71.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 72.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121:1541–7. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 73.Barcelo A, Barbe F, Llompart E, et al. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117:118–21. doi: 10.1016/j.amjmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 74.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 75.Imagawa S, Yamaguchi Y, Ogawa K, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration. 2004;71:24–9. doi: 10.1159/000075645. [DOI] [PubMed] [Google Scholar]
- 76.Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med. 2008 Feb 6; doi: 10.1016/j.sleep.2007.11.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 78.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 79.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 80.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 81.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD001106.pub2. CD001106. [DOI] [PubMed] [Google Scholar]
- 82.Meurice JC, Dore P, Paquereau J, et al. Predictive factors of long-term compliance with nasal continuous positive airway pressure treatment in sleep apnea syndrome. Chest. 1994;105:429–33. doi: 10.1378/chest.105.2.429. [DOI] [PubMed] [Google Scholar]
- 83.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Effros RM, Dunning MB, 3rd, Biller J, Shaker R. The promise and perils of exhaled breath condensates. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1073–80. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 85.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 86.Jordan W, Cohrs S, Degner D, et al. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm. 2006;113:239–54. doi: 10.1007/s00702-005-0316-2. [DOI] [PubMed] [Google Scholar]
- 87.Chin K, Ohi M, Shimizu K, Nakamura T, Miyaoka F, Mishima M. Increase in bilirubin levels of patients with obstructive sleep apnea in the morning--a possible explanation of induced heme oxygenase-1. Sleep. 2001;24:218–23. doi: 10.1093/sleep/24.2.218. [DOI] [PubMed] [Google Scholar]
- 88.Alberti A, Sarchielli P, Gallinella E, Floridi A, Mazzotta G, Gallai V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–11. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 89.Sonka K, Kelemen J, Kemlink D, et al. Evening and morning plasma levels of protein S100B in patients with obstructive sleep apnea. Neuro Endocrinol Lett. 2007;28:575–9. [PubMed] [Google Scholar]
- 90.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–7. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 91.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 92.Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin. 2005;23:1059–75. doi: 10.1016/j.ncl.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Chiang AA. Obstructive sleep apnea and chronic intermittent hypoxia: a review. Chin J Physiol. 2006;49:234–43. [PubMed] [Google Scholar]
- 94.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 95.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–6. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 96.Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–61. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 97.Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–84. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 98.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 99.Gozal D, Row BW, Kheirandish L, et al. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem. 2003;86:1545–52. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 100.Li RC, Row BW, Gozal E, et al. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med. 2003;168:469–75. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- 101.Row BW, Goldbart A, Gozal E, Gozal D. Spatial pre-training attenuates hippocampal impairments in rats exposed to intermittent hypoxia. Neurosci Lett. 2003;339:67–71. doi: 10.1016/s0304-3940(02)01459-3. [DOI] [PubMed] [Google Scholar]
- 102.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med. 2005;172:915–20. doi: 10.1164/rccm.200504-560OC. [DOI] [PubMed] [Google Scholar]
- 103.Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E-deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–7. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- 104.Goldbart AD, Row BW, Kheirandish-Gozal L, Cheng Y, Brittian KR, Gozal D. High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res. 2006;1090:190–6. doi: 10.1016/j.brainres.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 105.Kumar GK, Rai V, Sharma SD, et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–39. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res. 2007;177:308–14. doi: 10.1016/j.bbr.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol. 2007;92:79–85. doi: 10.1113/expphysiol.2006.035501. [DOI] [PubMed] [Google Scholar]
- 108.Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med. 2005;171:1414–20. doi: 10.1164/rccm.200411-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhan G, Serrano F, Fenik P, et al. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–9. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Polotsky VY, Li J, Punjabi NM, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol (Lond) 2003;552:253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 112.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 113.Peng YJ, Yuan G, Ramakrishnan D, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Phillips SA, Olson EB, Lombard JH, Morgan BJ. Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. J Appl Physiol. 2006;100:1117–23. doi: 10.1152/japplphysiol.00994.2005. [DOI] [PubMed] [Google Scholar]
- 115.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–7. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Savransky V, Bevans S, Nanayakkara A, et al. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–7. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 117.Yokoe T, Alonso LC, Romano LC, et al. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol. 2008;586:899–911. doi: 10.1113/jphysiol.2007.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y, Fenik P, Zhan G, et al. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci. 2007;27:10060–71. doi: 10.1523/JNEUROSCI.0857-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dematteis M, Julien C, Guillermet C, et al. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177:227–35. doi: 10.1164/rccm.200702-238OC. [DOI] [PubMed] [Google Scholar]
- 120.Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557:773–83. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 122.Ryan S, McNicholas WT, Taylor CT. A critical role for p38 map kinase in NF-kappaB signaling during intermittent hypoxia/reoxygenation. Biochem Biophys Res Commun. 2007;355:728–33. doi: 10.1016/j.bbrc.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 123.Shan X, Chi L, Ke Y, et al. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis. 2007;28:206–15. doi: 10.1016/j.nbd.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992;20:612–9. doi: 10.1161/01.hyp.20.5.612. [DOI] [PubMed] [Google Scholar]
- 125.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–8. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 126.Toffoli S, Feron O, Raes M, Michiels C. Intermittent hypoxia changes HIF-1alpha phosphorylation pattern in endothelial cells: unravelling of a new PKA-dependent regulation of HIF-1alpha. Biochim Biophys Acta. 2007;1773:1558–71. doi: 10.1016/j.bbamcr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 127.Li J, Bosch-Marce M, Nanayakkara A, et al. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics. 2006;25:450–7. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 128.Belaidi E, Beguin PC, Levy P, Ribuot C, Godin-Ribuot D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00715.2007. [DOI] [PubMed] [Google Scholar]
- 129.Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lam SY, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxia-inducible factor-1alpha, −2alpha and −3alpha in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23:271–80. doi: 10.14670/HH-23.271. [DOI] [PubMed] [Google Scholar]
- 131.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 132.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 133.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–6. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 134.Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol. 1989;67:2095–100. doi: 10.1152/jappl.1989.67.5.2095. [DOI] [PubMed] [Google Scholar]
- 135.Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–8. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- 136.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 137.Lavie L. Obstructive sleep apnoea syndrome - an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 138.Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol. 1995;79:151–62. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- 139.Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 140.Blasi A, Jo JA, Valladares E, Juarez R, Baydur A, Khoo MC. Autonomic cardiovascular control following transient arousal from sleep: a time-varying closed-loop model. IEEE Trans Biomed Eng. 2006;53:74–82. doi: 10.1109/TBME.2005.859789. [DOI] [PubMed] [Google Scholar]
- 141.Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 142.Nalivaiko E, Catcheside PG, Adams A, Jordan AS, Eckert DJ, McEvoy RD. Cardiac changes during arousals from non-REM sleep in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1320–7. doi: 10.1152/ajpregu.00642.2006. [DOI] [PubMed] [Google Scholar]
- 143.Ekstedt M, Akerstedt T, Soderstrom M. Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom Med. 2004;66:925–31. doi: 10.1097/01.psy.0000145821.25453.f7. [DOI] [PubMed] [Google Scholar]
- 144.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71:1112–8. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 145.Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 146.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 147.Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–72. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- 148.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 149.Yu Y, Lu BS, Wang B, et al. Short sleep duration and adiposity in Chinese adolescents. Sleep. 2007;30:1688–97. doi: 10.1093/sleep/30.12.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 151.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–7. [PubMed] [Google Scholar]
- 152.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 153.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 154.Casement MD, Broussard JL, Mullington JM, Press DZ. The contribution of sleep to improvements in working memory scanning speed: a study of prolonged sleep restriction. Biol Psychol. 2006;72:208–12. doi: 10.1016/j.biopsycho.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 156.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 157.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 158.Jain V. Clinical perspective of obstructive sleep apnea-induced cardiovascular complications. Antioxid Redox Signal. 2007;9:701–10. doi: 10.1089/ars.2007.1558. [DOI] [PubMed] [Google Scholar]
- 159.Umlauf MG, Chasens ER. Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev. 2003;7:403–11. doi: 10.1053/smrv.2002.0273. [DOI] [PubMed] [Google Scholar]
- 160.Lew RA, Baertschi AJ. Mechanisms of hypoxia-induced atrial natriuretic factor release from rat hearts. Am J Physiol. 1989;257:H147–56. doi: 10.1152/ajpheart.1989.257.1.H147. [DOI] [PubMed] [Google Scholar]
- 161.Lin CC, Tsan KW, Lin CY. Plasma levels of atrial natriuretic factor in moderate to severe obstructive sleep apnea syndrome. Sleep. 1993;16:37–9. doi: 10.1093/sleep/16.1.37. [DOI] [PubMed] [Google Scholar]
- 162.Umlauf MG, Chasens ER, Greevy RA, Arnold J, Burgio KL, Pillion DJ. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep. 2004;27:139–44. doi: 10.1093/sleep/27.1.139. [DOI] [PubMed] [Google Scholar]
- 163.Krieger J, Follenius M, Sforza E, Brandenberger G, Peter JD. Effects of treatment with nasal continuous positive airway pressure on atrial natriuretic peptide and arginine vasopressin release during sleep in patients with obstructive sleep apnoea. Clin Sci (Lond) 1991;80:443–9. doi: 10.1042/cs0800443. [DOI] [PubMed] [Google Scholar]
- 164.Krieger J, Laks L, Wilcox I, et al. Atrial natriuretic peptide release during sleep in patients with obstructive sleep apnoea before and during treatment with nasal continuous positive airway pressure. Clin Sci (Lond) 1989;77:407–11. doi: 10.1042/cs0770407. [DOI] [PubMed] [Google Scholar]
- 165.Kita H, Ohi M, Chin K, et al. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7:199–207. doi: 10.1046/j.1365-2869.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 166.Yalkut D, Lee LY, Grider J, Jorgensen M, Jackson B, Ott C. Mechanism of atrial natriuretic peptide release with increased inspiratory resistance. J Lab Clin Med. 1996;128:322–8. doi: 10.1016/s0022-2143(96)90034-7. [DOI] [PubMed] [Google Scholar]
- 167.Schafer H, Ehlenz K, Ewig S, et al. Atrial natriuretic peptide levels and pulmonary artery pressure awake, at exercise and asleep in obstructive sleep apnoea syndrome. J Sleep Res. 1999;8:205–10. doi: 10.1046/j.1365-2869.1999.00151.x. [DOI] [PubMed] [Google Scholar]
- 168.Patwardhan AA, Larson MG, Levy D, et al. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29:1301–6. doi: 10.1093/sleep/29.10.1301. [DOI] [PubMed] [Google Scholar]
- 169.Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Increased nocturnal sodium excretion in obstructive sleep apnoea. Relation to nocturnal change in diastolic blood pressure. Scand J Clin Lab Invest. 2008;68:11–21. doi: 10.1080/00365510701352020. [DOI] [PubMed] [Google Scholar]
- 170.Kimura K, Yamaguchi Y, Horii M, et al. ANP is cleared much faster than BNP in patients with congestive heart failure. Eur J Clin Pharmacol. 2007;63:699–702. doi: 10.1007/s00228-007-0309-1. [DOI] [PubMed] [Google Scholar]
- 171.Yandle TG, Richards AM, Nicholls MG, Cuneo R, Espiner EA, Livesey JH. Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. Life Sci. 1986;38:1827–33. doi: 10.1016/0024-3205(86)90137-2. [DOI] [PubMed] [Google Scholar]
- 172.Exar EN, Collop NA. The upper airway resistance syndrome. Chest. 1999;115:1127–39. doi: 10.1378/chest.115.4.1127. [DOI] [PubMed] [Google Scholar]
- 173.Guilleminault C, Stoohs R, Shiomi T, Kushida C, Schnittger I. Upper airway resistance syndrome, nocturnal blood pressure monitoring, and borderline hypertension. Chest. 1996;109:901–8. doi: 10.1378/chest.109.4.901. [DOI] [PubMed] [Google Scholar]
- 174.Chen L, Scharf SM. Comparative hemodynamic effects of periodic obstructive and simulated central apneas in sedated pigs. J Appl Physiol. 1997;83:485–94. doi: 10.1152/jappl.1997.83.2.485. [DOI] [PubMed] [Google Scholar]
- 175.White SG, Fletcher EC, Miller CC., 3rd Acute systemic blood pressure elevation in obstructive and nonobstructive breath hold in primates. J Appl Physiol. 1995;79:324–30. doi: 10.1152/jappl.1995.79.1.324. [DOI] [PubMed] [Google Scholar]
- 176.Almendros I, Carreras A, Ramirez J, Montserrat JM, Navajas D, Farre R. Upper airway collapse and reopening induce inflammation in a sleep apnoea model. Eur Respir J. 2008;32:399–404. doi: 10.1183/09031936.00161607. [DOI] [PubMed] [Google Scholar]
- 177.Almendros I, Acerbi I, Puig F, Montserrat JM, Navajas D, Farre R. Upper-airway inflammation triggered by vibration in a rat model of snoring. Sleep. 2007;30:225–7. doi: 10.1093/sleep/30.2.225. [DOI] [PubMed] [Google Scholar]
- 178.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 179.Grassi G, Facchini A, Trevano FQ, et al. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–5. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- 180.Waradekar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–8. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 181.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 182.Donadio V, Liguori R, Vetrugno R, et al. Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. J Sleep Res. 2007;16:327–32. doi: 10.1111/j.1365-2869.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- 183.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–11. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 184.Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10:35–44. doi: 10.1093/sleep/10.1.35. [DOI] [PubMed] [Google Scholar]
- 185.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190–5. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 186.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 187.Heitmann J, Ehlenz K, Penzel T, et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur Respir J. 2004;23:255–62. doi: 10.1183/09031936.04.00015604. [DOI] [PubMed] [Google Scholar]
- 188.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 189.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–93. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 190.Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J. 1995;8:222–9. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 191.Minemura H, Akashiba T, Yamamoto H, Akahoshi T, Kosaka N, Horie T. Acute effects of nasal continuous positive airway pressure on 24-hour blood pressure and catecholamines in patients with obstructive sleep apnea. Intern Med. 1998;37:1009–13. doi: 10.2169/internalmedicine.37.1009. [DOI] [PubMed] [Google Scholar]
- 192.Sukegawa M, Noda A, Sugiura T, et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24-hour urinary catecholamines. Clin Cardiol. 2005;28:519–22. doi: 10.1002/clc.4960281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103:722–7. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- 194.Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sullivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep. 1996;19:774–82. doi: 10.1093/sleep/19.10.774. [DOI] [PubMed] [Google Scholar]
- 195.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 196.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wieber SJ. The cardiac consequences of the obstructive sleep apnea-hypopnea syndrome. Mt Sinai J Med. 2005;72:10–2. [PubMed] [Google Scholar]
- 198.Marcus C, Ehren H, Bolme P, Arner P. Regulation of lipolysis during the neonatal period. Importance of thyrotropin. J Clin Invest. 1988;82:1793–7. doi: 10.1172/JCI113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hucking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111:257–64. doi: 10.1172/JCI14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35:177–93. [PubMed] [Google Scholar]
- 201.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 202.Nguyen MT, Satoh H, Favelyukis S, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–71. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 203.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292:E1590–8. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 204.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–6. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 205.Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol (Oxf) 2006;64:12–9. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 206.Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16:644–7. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- 207.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–8. [PubMed] [Google Scholar]
- 208.Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–9. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 209.Minoguchi K, Yokoe T, Tanaka A, et al. Association between lipid peroxidation and inflammation in obstructive sleep apnoea. Eur Respir J. 2006;28:378–85. doi: 10.1183/09031936.06.00084905. [DOI] [PubMed] [Google Scholar]
- 210.Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med. 2002;166:S25–30. doi: 10.1164/rccm.2206011. [DOI] [PubMed] [Google Scholar]
- 211.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 213.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 214.Svatikova A, Wolk R, Lerman LO, et al. Oxidative stress in obstructive sleep apnoea. Eur Heart J. 2005;26:2435–9. doi: 10.1093/eurheartj/ehi440. [DOI] [PubMed] [Google Scholar]
- 215.Ozturk L, Mansour B, Yuksel M, Yalcin AS, Celikoglu F, Gokhan N. Lipid peroxidation and osmotic fragility of red blood cells in sleep-apnea patients. Clin Chim Acta. 2003;332:83–8. doi: 10.1016/s0009-8981(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 216.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep Breath. 2005;9:119–26. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 217.Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J Lipid Res. 2007;48:1607–17. doi: 10.1194/jlr.M700097-JLR200. [DOI] [PubMed] [Google Scholar]
- 218.Basu S. Metabolism of 8-iso-prostaglandin F2alpha. FEBS Lett. 1998;428:32–6. doi: 10.1016/s0014-5793(98)00481-5. [DOI] [PubMed] [Google Scholar]
- 219.Griffiths HR, Moller L, Bartosz G, et al. Biomarkers. Mol Aspects Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 220.Morrow JD, Zackert WE, Yang JP, et al. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269:326–31. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 221.Liu H, Liu J, Xiong S, Shen G, Zhang Z, Xu Y. The change of interleukin-6 and tumor necrosis factor in patients with obstructive sleep apnea syndrome. J Tongji Med Univ. 2000;20:200–2. doi: 10.1007/BF02886988. [DOI] [PubMed] [Google Scholar]
- 222.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 223.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 224.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 225.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 226.Kobayashi K, Nishimura Y, Shimada T, et al. Effect of continuous positive airway pressure on soluble CD40 ligand in patients with obstructive sleep apnea syndrome. Chest. 2006;129:632–7. doi: 10.1378/chest.129.3.632. [DOI] [PubMed] [Google Scholar]
- 227.Bravo Mde L, Serpero LD, Barcelo A, Barbe F, Agusti A, Gozal D. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath. 2007;11:177–85. doi: 10.1007/s11325-007-0100-7. [DOI] [PubMed] [Google Scholar]
- 228.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27:1507–11. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 229.Sharma SK, Mishra HK, Sharma H, et al. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep Med. 2008;9:149–56. doi: 10.1016/j.sleep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 230.Roytblat L, Rachinsky M, Fisher A, et al. Raised interleukin-6 levels in obese patients. Obes Res. 2000;8:673–5. doi: 10.1038/oby.2000.86. [DOI] [PubMed] [Google Scholar]
- 231.Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166:1725–31. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- 232.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 233.Hayashi M, Fujimoto K, Urushibata K, Takamizawa A, Kinoshita O, Kubo K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology. 2006;11:24–31. doi: 10.1111/j.1440-1843.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 234.Paulsen FP, Steven P, Tsokos M, et al. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2002;166:501–9. doi: 10.1164/rccm.2109099. [DOI] [PubMed] [Google Scholar]
- 235.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 236.Sabato R, Guido P, Salerno FG, Resta O, Spanevello A, Barbaro MP. Airway inflammation in patients affected by obstructive sleep apnea. Monaldi Arch Chest Dis. 2006;65:102–5. doi: 10.4081/monaldi.2006.572. [DOI] [PubMed] [Google Scholar]
- 237.Exley AR, Cohen J. Optimal collection of blood samples for the measurement of tumor necrosis factor alpha. Cytokine. 1990;2:353–6. doi: 10.1016/1043-4666(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 238.Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine. 2000;12:1712–6. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- 239.Seiler W, Muller H, Hiemke C. Interleukin-6 in plasma collected with an indwelling cannula reflects local, not systemic, concentrations. Clin Chem. 1994;40:1778–9. [PubMed] [Google Scholar]
- 240.Gudmundsson A, Ershler WB, Goodman B, Lent SJ, Barczi S, Carnes M. Serum concentrations of interleukin-6 are increased when sampled through an indwelling venous catheter. Clin Chem. 1997;43:2199–201. [PubMed] [Google Scholar]
- 241.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmacher T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–31. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 242.Haack M, Reichenberg A, Kraus T, Schuld A, Yirmiya R, Pollmacher T. Effects of an intravenous catheter on the local production of cytokines and soluble cytokine receptors in healthy men. Cytokine. 2000;12:694–8. doi: 10.1006/cyto.1999.0665. [DOI] [PubMed] [Google Scholar]
- 243.Bekri S, Gual P, Anty R, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–96. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 244.Bae SK, Kim SR, Kim JG, et al. Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1. FEBS Lett. 2006;580:4105–13. doi: 10.1016/j.febslet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 245.Segawa K, Fukuhara A, Hosogai N, et al. Visfatin in adipocytes is upregulated by hypoxia through HIF1alpha-dependent mechanism. Biochem Biophys Res Commun. 2006;349:875–82. doi: 10.1016/j.bbrc.2006.07.083. [DOI] [PubMed] [Google Scholar]
- 246.Bo S, Ciccone G, Durazzo M, et al. Efficacy of antioxidant treatment in reducing resistin serum levels: a randomized study. PLoS Clin Trials. 2007;2:e17. doi: 10.1371/journal.pctr.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kamigaki M, Sakaue S, Tsujino I, et al. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339:624–32. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 248.Soares AF, Guichardant M, Cozzone D, et al. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38:882–9. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 249.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Grunfeld C, Zhao C, Fuller J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–7. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–8. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 252.Hector J, Schwarzloh B, Goehring J, et al. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39:250–5. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 253.Fasshauer M, Kralisch S, Klier M, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–50. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 254.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 256.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Tumor necrosis factor alpha is a negative regulator of resistin gene expression and secretion in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2001;288:1027–31. doi: 10.1006/bbrc.2001.5874. [DOI] [PubMed] [Google Scholar]
- 257.Nowell MA, Richards PJ, Fielding CA, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–95. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 258.Bruun JM, Lihn AS, Verdich C, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527–33. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 259.Shojima N, Sakoda H, Ogihara T, et al. Humoral regulation of resistin expression in 3T3-L1 and mouse adipose cells. Diabetes. 2002;51:1737–44. doi: 10.2337/diabetes.51.6.1737. [DOI] [PubMed] [Google Scholar]
- 260.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 261.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB. Int J Mol Med. 1999;4:223–30. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 262.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–25. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 263.Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin--the classical, resistin--the controversical, adiponectin--the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19:525–46. doi: 10.1016/j.beem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 264.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hashimoto T, Kihara M, Imai N, et al. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol. 2007;171:1705–12. doi: 10.2353/ajpath.2007.070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kim SR, Bae YH, Bae SK, et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim Biophys Acta. 2008;1783:886–95. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 267.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 269.Hida K, Wada J, Eguchi J, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005;102:10610–5. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Daviaud D, Boucher J, Gesta S, et al. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20:1528–30. doi: 10.1096/fj.05-5243fje. [DOI] [PubMed] [Google Scholar]
- 271.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 272.Wen Y, Wang HW, Wu J, Lu HL, Hu XF, Cianflone K. Effects of fatty acid regulation on visfatin gene expression in adipocytes. Chin Med J (Engl) 2006;119:1701–8. [PubMed] [Google Scholar]
- 273.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–9. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Alam I, Lewis K, Stephens JW, Baxter JN. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev. 2006;8:119–27. doi: 10.1111/j.1467-789X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 277.Otero M, Lago R, Gomez R, et al. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 2006;45:944–50. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 278.Sanna V, Di Giacomo A, La Cava A, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–50. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–52. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 281.Raso GM, Pacilio M, Esposito E, Coppola A, Di Carlo R, Meli R. Leptin potentiates IFN-gamma-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A.1. Br J Pharmacol. 2002;137:799–804. doi: 10.1038/sj.bjp.0704903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–9. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 283.Bravo PE, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and hypertension in obesity. Vasc Health Risk Manag. 2006;2:163–9. doi: 10.2147/vhrm.2006.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–31. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 285.Bullo M, Garcia-Lorda P, Salas-Salvado J. Plasma soluble tumor necrosis factor alpha receptors and leptin levels in normal-weight and obese women: effect of adiposity and diabetes. Eur J Endocrinol. 2002;146:325–31. doi: 10.1530/eje.0.1460325. [DOI] [PubMed] [Google Scholar]
- 286.Ciccone M, Vettor R, Pannacciulli N, et al. Plasma leptin is independently associated with the intima-media thickness of the common carotid artery. Int J Obes Relat Metab Disord. 2001;25:805–10. doi: 10.1038/sj.ijo.0801623. [DOI] [PubMed] [Google Scholar]
- 287.Schafer K, Halle M, Goeschen C, et al. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vasc Biol. 2004;24:112–7. doi: 10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- 288.Paolisso G, Tagliamonte MR, Galderisi M, et al. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047–52. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 289.de Courten M, Zimmet P, Hodge A, et al. Hyperleptinaemia: the missing link in the, metabolic syndrome? Diabet Med. 1997;14:200–8. doi: 10.1002/(SICI)1096-9136(199703)14:3<200::AID-DIA336>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 290.Wabitsch M, Jensen PB, Blum WF, et al. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–8. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- 291.Russell CD, Petersen RN, Rao SP, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998;275:E507–15. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- 292.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50:2786–91. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- 293.Muller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem. 1997;272:10585–93. doi: 10.1074/jbc.272.16.10585. [DOI] [PubMed] [Google Scholar]
- 294.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–92. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 295.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 296.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–7. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 297.Tatsumi K, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, Kuriyama T. Sleep oxygen desaturation and circulating leptin in obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:716–21. doi: 10.1378/chest.127.3.716. [DOI] [PubMed] [Google Scholar]
- 298.Barcelo A, Barbe F, Llompart E, et al. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171:183–7. doi: 10.1164/rccm.200405-579OC. [DOI] [PubMed] [Google Scholar]
- 299.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 300.Shimizu K, Chin K, Nakamura T, et al. Plasma leptin levels and cardiac sympathetic function in patients with obstructive sleep apnoea-hypopnoea syndrome. Thorax. 2002;57:429–34. doi: 10.1136/thorax.57.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–7. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 302.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–87. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 303.Harsch IA, Schahin SP, Bruckner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–9. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 304.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 305.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 306.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 308.Masserini B, Morpurgo PS, Donadio F, et al. Reduced levels of adiponectin in sleep apnea syndrome. J Endocrinol Invest. 2006;29:700–5. doi: 10.1007/BF03344179. [DOI] [PubMed] [Google Scholar]
- 309.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 310.Shin MJ, Lee JH, Jang Y, et al. Insulin resistance, adipokines, and oxidative stress in nondiabetic, hypercholesterolemic patients: leptin as an 8-epi-prostaglandin F2alpha determinant. Metabolism. 2006;55:918–22. doi: 10.1016/j.metabol.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 311.Tan KC, Xu A, Chow WS, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–9. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 312.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 313.Masaki T, Chiba S, Tatsukawa H, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–84. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 314.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–5. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 315.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 316.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 317.Zhang XL, Yin KS, Wang H, Su S. Serum adiponectin levels in adult male patients with obstructive sleep apnea hypopnea syndrome. Respiration. 2006;73:73–7. doi: 10.1159/000088690. [DOI] [PubMed] [Google Scholar]
- 318.Nakagawa Y, Kishida K, Kihara S, et al. Nocturnal reduction in circulating adiponectin concentrations related to hypoxic stress in severe obstructive sleep apnea-hypopnea syndrome. Am J Physiol Endocrinol Metab. 2008;294:E778–84. doi: 10.1152/ajpendo.00709.2007. [DOI] [PubMed] [Google Scholar]
- 319.Wolk R, Svatikova A, Nelson CA, et al. Plasma levels of adiponectin, a novel adipocyte-derived hormone, in sleep apnea. Obes Res. 2005;13:186–90. doi: 10.1038/oby.2005.24. [DOI] [PubMed] [Google Scholar]
- 320.Zhang XL, Yin KS, Li C, Jia EZ, Li YQ, Gao ZF. Effect of continuous positive airway pressure treatment on serum adiponectin level and mean arterial pressure in male patients with obstructive sleep apnea syndrome. Chin Med J (Engl) 2007;120:1477–81. [PubMed] [Google Scholar]
- 321.Harsch IA, Wallaschofski H, Koebnick C, et al. Adiponectin in patients with obstructive sleep apnea syndrome: course and physiological relevance. Respiration. 2004;71:580–6. doi: 10.1159/000081758. [DOI] [PubMed] [Google Scholar]
- 322.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 323.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 324.Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun. 2003;300:674–8. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 325.Yura S, Sagawa N, Itoh H, et al. Resistin is expressed in the human placenta. J Clin Endocrinol Metab. 2003;88:1394–7. doi: 10.1210/jc.2002-011926. [DOI] [PubMed] [Google Scholar]
- 326.Minn AH, Patterson NB, Pack S, et al. Resistin is expressed in pancreatic islets. Biochem Biophys Res Commun. 2003;310:641–5. doi: 10.1016/j.bbrc.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 327.Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–9. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 328.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–30. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Moon B, Kwan JJ, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab. 2003;285:E106–15. doi: 10.1152/ajpendo.00457.2002. [DOI] [PubMed] [Google Scholar]
- 330.Palanivel R, Maida A, Liu Y, Sweeney G. Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia. 2006;49:183–90. doi: 10.1007/s00125-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 331.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–9. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 332.Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 333.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450–7. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 334.Senolt L, Housa D, Vernerova Z, et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis. 2007;66:458–63. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rajala MW, Lin Y, Ranalletta M, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002;16:1920–30. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- 336.Burnett MS, Lee CW, Kinnaird TD, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–8. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 337.Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–40. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 338.Harsch IA, Koebnick C, Wallaschofski H, et al. Resistin levels in patients with obstructive sleep apnoea syndrome--the link to subclinical inflammation? Med Sci Monit. 2004;10:CR510–5. [PubMed] [Google Scholar]
- 339.Yamamoto Y, Fujiuchi S, Hiramatsu M, et al. Resistin is closely related to systemic inflammation in obstructive sleep apnea. Respiration. 2008;76:377–85. doi: 10.1159/000141866. [DOI] [PubMed] [Google Scholar]
- 340.Tauman R, Serpero LD, Capdevila OS, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30:443–9. doi: 10.1093/sleep/30.4.443. [DOI] [PubMed] [Google Scholar]
- 341.Kanbay A, Hasanoglu HC. A new prognostic marker for obstructive sleep apnea: hepcidin. Med Hypotheses. 2007;69:1381–2. doi: 10.1016/j.mehy.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 342.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 343.Sawa Y, Sugimoto Y, Ueki T, et al. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem. 2007;55:721–33. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- 344.Sawa Y, Ueki T, Hata M, et al. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem. 2008;56:97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kalogeris TJ, Kevil CG, Laroux FS, Coe LL, Phifer TJ, Alexander JS. Differential monocyte adhesion and adhesion molecule expression in venous and arterial endothelial cells. Am J Physiol. 1999;276:L9–L19. doi: 10.1152/ajplung.1999.276.1.L9. [DOI] [PubMed] [Google Scholar]
- 346.Elangbam CS, Qualls CW, Jr, Dahlgren RR. Cell adhesion molecules--update. Vet Pathol. 1997;34:61–73. doi: 10.1177/030098589703400113. [DOI] [PubMed] [Google Scholar]
- 347.Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 348.Ohga E, Nagase T, Tomita T, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87:10–4. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 349.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 350.Ursavas A, Karadag M, Rodoplu E, Yilmaztepe A, Oral HB, Gozu RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration. 2007;74:525–32. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- 351.Chin K, Nakamura T, Shimizu K, Mishima M, Miyasaka M, Ohi M. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 352.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 353.Magagnin MG, Sergeant K, van den Beucken T, et al. Proteomic analysis of gene expression following hypoxia and reoxygenation reveals proteins involved in the recovery from endoplasmic reticulum and oxidative stress. Radiother Oncol. 2007;83:340–5. doi: 10.1016/j.radonc.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 354.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–82. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 355.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 356.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 357.Boden G, Duan X, Homko C, et al. Increase in Endoplasmic Reticulum (ER) Stress Related Proteins and Genes in Adipose Tissue of Obese, Insulin Resistant Individuals. Diabetes. 2008;57:2438–44. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–8. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 359.Boden G, Duan X, Homko C, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–44. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 361.Zhu Y, Fenik P, Zhan G, Sanfillipo-Cohn B, Naidoo N, Veasey SC. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci. 2008;28:2168–78. doi: 10.1523/JNEUROSCI.5232-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI. A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep. 2007;30:557–65. doi: 10.1093/sleep/30.5.557. [DOI] [PubMed] [Google Scholar]
- 363.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–7. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 364.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 365.Jones S, Pfister-Genskow M, Benca RM, Cirelli C. Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J Neurochem. 2008;105:46–62. doi: 10.1111/j.1471-4159.2007.05089.x. [DOI] [PubMed] [Google Scholar]
- 366.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 367.Terao A, Wisor JP, Peyron C, et al. Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip study. Neuroscience. 2006;137:593–605. doi: 10.1016/j.neuroscience.2005.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–8. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 369.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- 370.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 371.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 372.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 373.Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J. 1992;5:377–81. [PubMed] [Google Scholar]
- 374.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107:362–6. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 375.Catheline JM, Bihan H, Le Quang T, et al. Preoperative cardiac and pulmonary assessment in bariatric surgery. Obes Surg. 2008;18:271–7. doi: 10.1007/s11695-007-9329-2. [DOI] [PubMed] [Google Scholar]
- 376.Hallowell PT, Stellato TA, Schuster M, et al. Potentially life-threatening sleep apnea is unrecognized without aggressive evaluation. Am J Surg. 2007;193:364–7. doi: 10.1016/j.amjsurg.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 377.Lee YH, Johan A, Wong KK, Edwards N, Sullivan C. Prevalence and risk factors for obstructive sleep apnea in a multiethnic population of patients presenting for bariatric surgery in Singapore. Sleep Med. 2008 Mar 31; doi: 10.1016/j.sleep.2008.01.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 378.Daltro C, Gregorio PB, Alves E, et al. Prevalence and severity of sleep apnea in a group of morbidly obese patients. Obes Surg. 2007;17:809–14. doi: 10.1007/s11695-007-9147-6. [DOI] [PubMed] [Google Scholar]
- 379.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12–8. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 380.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 381.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 382.McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab. 2001;86:713–8. doi: 10.1210/jcem.86.2.7202. [DOI] [PubMed] [Google Scholar]
- 383.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 384.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 385.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 386.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 387.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 388.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 389.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 390.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–7. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 391.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 392.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Eikelis N, Esler M. The neurobiology of human obesity. Exp Physiol. 2005;90:673–82. doi: 10.1113/expphysiol.2005.031385. [DOI] [PubMed] [Google Scholar]
- 394.Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–52. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- 395.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 396.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–39. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 397.Clement K, Langin D. Regulation of inflammation-related genes in human adipose tissue. J Intern Med. 2007;262:422–30. doi: 10.1111/j.1365-2796.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 398.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–6. doi: 10.1016/j.amjmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 399.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 400.Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960–5. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 401.Bitar MS, Al-Saleh E, Al-Mulla F. Oxidative stress--mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci. 2005;77:2552–73. doi: 10.1016/j.lfs.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 402.Ge X, Yu Q, Qi W, Shi X, Zhai Q. Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic Res. 2008;42:582–91. doi: 10.1080/10715760802158448. [DOI] [PubMed] [Google Scholar]
- 403.Ogihara T, Asano T, Katagiri H, et al. Oxidative stress induces insulin resistance by activating the nuclear factor-kappa B pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia. 2004;47:794–805. doi: 10.1007/s00125-004-1391-x. [DOI] [PubMed] [Google Scholar]
- 404.Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21:618–23. doi: 10.1161/01.hyp.21.5.618. [DOI] [PubMed] [Google Scholar]
- 405.Lembo G, Rendina V, Iaccarino G, Lamenza F, Volpe M, Trimarco B. Insulin reduces reflex forearm sympathetic vasoconstriction in healthy humans. Hypertension. 1993;21:1015–9. doi: 10.1161/01.hyp.21.6.1015. [DOI] [PubMed] [Google Scholar]
- 406.Mooney RA, Senn J, Cameron S, et al. Suppressors of cytokine signaling-1 and −6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance. J Biol Chem. 2001;276:25889–93. doi: 10.1074/jbc.M010579200. [DOI] [PubMed] [Google Scholar]
- 407.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 408.Kim SK, Kim HJ, Hur KY, et al. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004;79:593–9. doi: 10.1093/ajcn/79.4.593. [DOI] [PubMed] [Google Scholar]
- 409.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 410.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 411.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28:1018–25. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 412.Pascot A, Despres JP, Lemieux I, et al. Contribution of visceral obesity to the deterioration of the metabolic risk profile in men with impaired glucose tolerance. Diabetologia. 2000;43:1126–35. doi: 10.1007/s001250051503. [DOI] [PubMed] [Google Scholar]
- 413.Frayn KN. Visceral fat and insulin resistance--causative or correlative? Br J Nutr. 2000;83(Suppl 1):S71–7. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- 414.Carmelli D, Swan GE, Bliwise DL. Relationship of 30-year changes in obesity to sleep-disordered breathing in the Western Collaborative Group Study. Obes Res. 2000;8:632–7. doi: 10.1038/oby.2000.81. [DOI] [PubMed] [Google Scholar]
- 415.Barcelo A, Barbe F, de la Pena M, et al. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J. 2006;27:756–60. doi: 10.1183/09031936.06.00067605. [DOI] [PubMed] [Google Scholar]
- 416.Jurado-Gamez B, Martin-Malo A, Alvarez-Lara MA, Munoz L, Cosano A, Aljama P. Sleep disorders are underdiagnosed in patients on maintenance hemodialysis. Nephron Clin Pract. 2007;105:c35–42. doi: 10.1159/000096982. [DOI] [PubMed] [Google Scholar]
- 417.Santamaria J, Iranzo A, Ma Montserrat J, de Pablo J. Persistent sleepiness in CPAP treated obstructive sleep apnea patients: evaluation and treatment. Sleep Med Rev. 2007;11:195–207. doi: 10.1016/j.smrv.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 418.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 419.Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–9. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 420.Despres JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–9. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 421.Sinski M, Lewandowski J, Abramczyk P, Narkiewicz K, Gaciong Z. Why study sympathetic nervous system? J Physiol Pharmacol. 2006;57(Suppl 11):79–92. [PubMed] [Google Scholar]
- 422.Watson AM, Hood SG, May CN. Mechanisms of sympathetic activation in heart failure. Clin Exp Pharmacol Physiol. 2006;33:1269–74. doi: 10.1111/j.1440-1681.2006.04523.x. [DOI] [PubMed] [Google Scholar]
- 423.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 424.Das UN. Hypertension as a low-grade systemic inflammatory condition that has its origins in the perinatal period. J Assoc Physicians India. 2006;54:133–42. [PubMed] [Google Scholar]
- 425.Di Napoli M, Papa F. Inflammation, blood pressure, and stroke: an opportunity to target primary prevention? Curr Hypertens Rep. 2005;7:44–51. doi: 10.1007/s11906-005-0054-8. [DOI] [PubMed] [Google Scholar]
- 426.Pietropaolo M, Barinas-Mitchell E, Kuller LH. The heterogeneity of diabetes: unraveling a dispute: is systemic inflammation related to islet autoimmunity? Diabetes. 2007;56:1189–97. doi: 10.2337/db06-0880. [DOI] [PubMed] [Google Scholar]
- 427.Duncan BB, Schmidt MI. The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther. 2006;8:7–17. doi: 10.1089/dia.2006.8.7. [DOI] [PubMed] [Google Scholar]
- 428.Wu TL, Chang PY, Tsao KC, Sun CF, Wu LL, Wu JT. A panel of multiple markers associated with chronic systemic inflammation and the risk of atherogenesis is detectable in asthma and chronic obstructive pulmonary disease. J Clin Lab Anal. 2007;21:367–71. doi: 10.1002/jcla.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Silvestri M, Bontempelli M, Giacomelli M, et al. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthma: markers of systemic inflammation? Clin Exp Allergy. 2006;36:1373–81. doi: 10.1111/j.1365-2222.2006.02502.x. [DOI] [PubMed] [Google Scholar]
- 430.Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 431.Andrews JO, Tingen MS. The effect of smoking, smoking cessation, and passive smoke exposure on common laboratory values in clinical settings: a review of the evidence. Crit Care Nurs Clin North Am. 2006;18:63–9. xii. doi: 10.1016/j.ccell.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 432.van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–21. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–9. doi: 10.1515/CCLM.2003.177. [DOI] [PubMed] [Google Scholar]
- 434.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 435.Kaneto H, Matsuoka TA, Katakami N, et al. Oxidative Stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr Mol Med. 2007;7:674–86. doi: 10.2174/156652407782564408. [DOI] [PubMed] [Google Scholar]
- 436.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–66. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 437.Schleicher E, Friess U. Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl. 2007:S17–26. doi: 10.1038/sj.ki.5002382. [DOI] [PubMed] [Google Scholar]
- 438.Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006;127:436–43. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 439.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–9. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 440.Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–20. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- 441.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 442.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467–72. [PMC free article] [PubMed] [Google Scholar]
- 443.Jordan W, Berger C, Cohrs S, et al. CPAP-therapy effectively lowers serum homocysteine in obstructive sleep apnea syndrome. J Neural Transm. 2004;111:683–9. doi: 10.1007/s00702-004-0130-2. [DOI] [PubMed] [Google Scholar]
- 444.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profiles of antioxidants in human plasma. Free Radic Biol Med. 2001;30:456–62. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 445.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 446.Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 447.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309–15. [PubMed] [Google Scholar]
- 448.Hernandez C, Abreu J, Abreu P, Castro A, Jimenez A. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J. 2007;30:496–500. doi: 10.1183/09031936.00051906. [DOI] [PubMed] [Google Scholar]
- 449.Ulfberg J, Micic S, Strom J. Afternoon serum-melatonin in sleep disordered breathing. J Intern Med. 1998;244:163–8. doi: 10.1046/j.1365-2796.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- 450.van Exel E, Gussekloo J, de Craen AJ, Frolich M, Bootsma-Van Der Wiel A, Westendorp RG. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: the Leiden 85-Plus Study. Diabetes. 2002;51:1088–92. doi: 10.2337/diabetes.51.4.1088. [DOI] [PubMed] [Google Scholar]
- 451.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 452.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 453.Pinderski Oslund LJ, Hedrick CC, Olvera T, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–53. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 454.Kim HJ, Higashimori T, Park SY, et al. Differential effects of interleukin-6 and −10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–7. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 455.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;62:489–94. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–50. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 457.Grote L, Kraiczi H, Hedner J. Reduced alpha- and beta(2)-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:1480–7. doi: 10.1164/ajrccm.162.4.9912028. [DOI] [PubMed] [Google Scholar]
- 458.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 459.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–97. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–5. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Wang SS, Schadt EE, Wang H, et al. Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ Res. 2007;101:e11–30. doi: 10.1161/CIRCRESAHA.107.152975. [DOI] [PubMed] [Google Scholar]
- 463.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–41. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Zhu H, Poole J, Lu Y, et al. Sympathetic nervous system, genes and human essential hypertension. Curr Neurovasc Res. 2005;2:303–17. doi: 10.2174/156720205774322575. [DOI] [PubMed] [Google Scholar]
- 465.Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1332–40. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- 466.Olivieri F, Antonicelli R, Cardelli M, et al. Genetic polymorphisms of inflammatory cytokines and myocardial infarction in the elderly. Mech Ageing Dev. 2006;127:552–9. doi: 10.1016/j.mad.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 467.Borgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27:121–7. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 468.Bostrom KB, Hedner J, Melander O, et al. Interaction between the angiotensin-converting enzyme gene insertion/deletion polymorphism and obstructive sleep apnoea as a mechanism for hypertension. J Hypertens. 2007;25:779–83. doi: 10.1097/HJH.0b013e328017f6d5. [DOI] [PubMed] [Google Scholar]
- 469.Lavie L, Lotan R, Hochberg I, Herer P, Lavie P, Levy AP. Haptoglobin polymorphism is a risk factor for cardiovascular disease in patients with obstructive sleep apnea syndrome. Sleep. 2003;26:592–5. doi: 10.1093/sleep/26.5.592. [DOI] [PubMed] [Google Scholar]
- 470.Lin L, Finn L, Zhang J, Young T, Mignot E. Angiotensin-converting enzyme, sleep-disordered breathing, and hypertension. Am J Respir Crit Care Med. 2004;170:1349–53. doi: 10.1164/rccm.200405-616OC. [DOI] [PubMed] [Google Scholar]
- 471.Patel SR, Larkin EK, Mignot E, Lin L, Redline S. The association of angiotensin converting enzyme (ACE) polymorphisms with sleep apnea and hypertension. Sleep. 2007;30:531–3. doi: 10.1093/sleep/30.4.531. [DOI] [PubMed] [Google Scholar]
- 472.Barcelo A, Llompart E, Barbe F, Morla M, Vila M, Agusti AG. Plasminogen activator inhibitor-I (PAI-I) polymorphisms in patients with obstructive sleep apnoea. Respir Med. 2002;96:193–6. doi: 10.1053/rmed.2001.1239. [DOI] [PubMed] [Google Scholar]
- 473.Bayazit YA, Yilmaz M, Erdal E, et al. Role of nitric oxide synthase gene intron 4 and exon 7 polymorphisms in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2008 doi: 10.1007/s00405-008-0763-0. [DOI] [PubMed] [Google Scholar]
- 474.Diefenbach K, Kretschmer K, Bauer S, et al. Endothelin-1 gene variant lys198asn and plasma endothelin level in obstructive sleep apnea. Cardiology. 2008;112:62–68. doi: 10.1159/000137702. [DOI] [PubMed] [Google Scholar]
- 475.Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007;69:243–9. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- 476.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Maresso K, Broeckel U. Genotyping platforms for mass-throughput genotyping with SNPs, including human genome-wide scans. Adv Genet. 2008;60:107–39. doi: 10.1016/S0065-2660(07)00405-1. [DOI] [PubMed] [Google Scholar]
- 478.Morton NE. Into the post-HapMap era. Adv Genet. 2008;60:727–42. doi: 10.1016/S0065-2660(07)00425-7. [DOI] [PubMed] [Google Scholar]
- 479.Uchiyama M, Ishibashi K, Enomoto T, et al. Twenty-four hour profiles of four hormones under constant routine. Psychiatry Clin Neurosci. 1998;52:241–3. doi: 10.1111/j.1440-1819.1998.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 480.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 481.Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8:81–93. doi: 10.1016/S1087-0792(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 482.Rasch B, Dodt C, Molle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32:884–91. doi: 10.1016/j.psyneuen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 483.Mendez M, Bianchi AM, Villantieri O, Cerutti S. Time-varying analysis of the heart rate variability during REM and non REM sleep stages. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3576–9. doi: 10.1109/IEMBS.2006.260067. [DOI] [PubMed] [Google Scholar]
- 484.Bach V, Telliez F, Libert JP. The interaction between sleep and thermoregulation in adults and neonates. Sleep Med Rev. 2002;6:481–92. doi: 10.1053/smrv.2001.0177. [DOI] [PubMed] [Google Scholar]
- 485.Issa FG, Sullivan CE. The immediate effects of nasal continuous positive airway pressure treatment on sleep pattern in patients with obstructive sleep apnea syndrome. Electroencephalogr Clin Neurophysiol. 1986;63:10–7. doi: 10.1016/0013-4694(86)90056-8. [DOI] [PubMed] [Google Scholar]
- 486.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep. 2006;29:564–71. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 487.Aldrich M, Eiser A, Lee M, Shipley JE. Effects of continuous positive airway pressure on phasic events of REM sleep in patients with obstructive sleep apnea. Sleep. 1989;12:413–9. doi: 10.1093/sleep/12.5.413. [DOI] [PubMed] [Google Scholar]
- 488.Cahan C, Decker MJ, Arnold JL, Goldwasser E, Strohl KP. Erythropoietin levels with treatment of obstructive sleep apnea. J Appl Physiol. 1995;79:1278–85. doi: 10.1152/jappl.1995.79.4.1278. [DOI] [PubMed] [Google Scholar]
- 489.Hashimoto S, Morohoshi K, Suzuki T, Matsushima K. Lipopolysaccharide-inducible gene expression profile in human monocytes. Scand J Infect Dis. 2003;35:619–27. [PubMed] [Google Scholar]
- 490.Moore DF, Li H, Jeffries N, et al. Using peripheral blood mononuclear cells to determine a gene expression profile of acute ischemic stroke: a pilot investigation. Circulation. 2005;111:212–21. doi: 10.1161/01.CIR.0000152105.79665.C6. [DOI] [PubMed] [Google Scholar]
- 491.Tanner MA, Berk LS, Felten DL, Blidy AD, Bit SL, Ruff DW. Substantial changes in gene expression level due to the storage temperature and storage duration of human whole blood. Clin Lab Haematol. 2002;24:337–41. doi: 10.1046/j.1365-2257.2002.00474.x. [DOI] [PubMed] [Google Scholar]
- 492.Rainen L, Oelmueller U, Jurgensen S, et al. Stabilization of mRNA expression in whole blood samples. Clin Chem. 2002;48:1883–90. [PubMed] [Google Scholar]
- 493.Debey S, Schoenbeck U, Hellmich M, et al. Comparison of different isolation techniques prior gene expression profiling of blood derived cells: impact on physiological responses, on overall expression and the role of different cell types. Pharmacogenomics J. 2004;4:193–207. doi: 10.1038/sj.tpj.6500240. [DOI] [PubMed] [Google Scholar]
- 494.Ovstebo R, Lande K, Kierulf P, Haug KB. Quantification of relative changes in specific mRNAs from frozen whole blood - methodological considerations and clinical implications. Clin Chem Lab Med. 2007;45:171–6. doi: 10.1515/CCLM.2007.035. [DOI] [PubMed] [Google Scholar]
- 495.Kagedal B, Lindqvist M, Farneback M, Lenner L, Peterson C. Failure of the PAXgene Blood RNA System to maintain mRNA stability in whole blood. Clin Chem Lab Med. 2005;43:1190–2. doi: 10.1515/CCLM.2005.206. [DOI] [PubMed] [Google Scholar]
- 496.Kim SJ, Dix DJ, Thompson KE, et al. Effects of storage, RNA extraction, genechip type, and donor sex on gene expression profiling of human whole blood. Clin Chem. 2007;53:1038–45. doi: 10.1373/clinchem.2006.078436. [DOI] [PubMed] [Google Scholar]
- 497.Mutter GL, Zahrieh D, Liu C, et al. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004;5:88. doi: 10.1186/1471-2164-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Thach DC, Lin B, Walter E, et al. Assessment of two methods for handling blood in collection tubes with RNA stabilizing agent for surveillance of gene expression profiles with high density microarrays. J Immunol Methods. 2003;283:269–79. doi: 10.1016/j.jim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 499.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 500.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 501.Hariharan R. The analysis of microarray data. Pharmacogenomics. 2003;4:477–97. doi: 10.1517/phgs.4.4.477.22744. [DOI] [PubMed] [Google Scholar]
- 502.Mantini D, Petrucci F, Pieragostino D, et al. LIMPIC: a computational method for the separation of protein MALDI-TOF-MS signals from noise. BMC Bioinformatics. 2007;8:101. doi: 10.1186/1471-2105-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Cui X, Hwang JT, Qiu J, Blades NJ, Churchill GA. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics. 2005;6:59–75. doi: 10.1093/biostatistics/kxh018. [DOI] [PubMed] [Google Scholar]
- 504.Kerr MK, Churchill GA. Bootstrapping cluster analysis: assessing the reliability of conclusions from microarray experiments. Proc Natl Acad Sci U S A. 2001;98:8961–5. doi: 10.1073/pnas.161273698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Churchill GA. Fundamentals of experimental design for cDNA microarrays. Nat Genet. 2002;32(Suppl):490–5. doi: 10.1038/ng1031. [DOI] [PubMed] [Google Scholar]
- 506.Kerr MK, Churchill GA. Statistical design and the analysis of gene expression microarray data. Genet Res. 2001;77:123–8. doi: 10.1017/s0016672301005055. [DOI] [PubMed] [Google Scholar]
- 507.Coombes KR, Morris JS, Hu J, Edmonson SR, Baggerly KA. Serum proteomics profiling--a young technology begins to mature. Nat Biotechnol. 2005;23:291–2. doi: 10.1038/nbt0305-291. [DOI] [PubMed] [Google Scholar]
- 508.Conrads TP, Hood BL, Veenstra TD. Sampling and analytical strategies for biomarker discovery using mass spectrometry. Biotechniques. 2006;40:799–805. doi: 10.2144/000112196. [DOI] [PubMed] [Google Scholar]
- 509.Mayr M, Zhang J, Greene AS, Gutterman D, Perloff J, Ping P. Proteomics-based development of biomarkers in cardiovascular disease: mechanistic, clinical, and therapeutic insights. Mol Cell Proteomics. 2006;5:1853–64. doi: 10.1074/mcp.R600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 510.Gulcicek EE, Colangelo CM, McMurray W, et al. Proteomics and the analysis of proteomic data: an overview of current protein-profiling technologies. Curr Protoc Bioinformatics. 2005 doi: 10.1002/0471250953.bi1301s10. Chapter 13:Unit 13 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Lee HJ, Wark AW, Corn RM. Microarray methods for protein biomarker detection. Analyst. 2008;133:975–83. doi: 10.1039/b717527b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Tammen H, Schulte I, Hess R, et al. Peptidomic analysis of human blood specimens: comparison between plasma specimens and serum by differential peptide display. Proteomics. 2005;5:3414–22. doi: 10.1002/pmic.200401219. [DOI] [PubMed] [Google Scholar]
- 513.Soultan Z, Wadowski S, Rao M, Kravath RE. Effect of treating obstructive sleep apnea by tonsillectomy and/or adenoidectomy on obesity in children. Arch Pediatr Adolesc Med. 1999;153:33–7. doi: 10.1001/archpedi.153.1.33. [DOI] [PubMed] [Google Scholar]
- 514.Berliner JA, Zimman A. Future of toxicology--lipidomics, an important emerging area for toxicologists: focus on lipid oxidation products. Chem Res Toxicol. 2007;20:849–53. doi: 10.1021/tx7000652. [DOI] [PubMed] [Google Scholar]
- 515.German JB, Gillies LA, Smilowitz JT, Zivkovic AM, Watkins SM. Lipidomics and lipid profiling in metabolomics. Curr Opin Lipidol. 2007;18:66–71. doi: 10.1097/MOL.0b013e328012d911. [DOI] [PubMed] [Google Scholar]
- 516.Wiest MM, Watkins SM. Biomarker discovery using high-dimensional lipid analysis. Curr Opin Lipidol. 2007;18:181–6. doi: 10.1097/MOL.0b013e3280895d82. [DOI] [PubMed] [Google Scholar]
- 517.Maher AD, Zirah SF, Holmes E, Nicholson JK. Experimental and analytical variation in human urine in 1H NMR spectroscopy-based metabolic phenotyping studies. Anal Chem. 2007;79:5204–11. doi: 10.1021/ac070212f. [DOI] [PubMed] [Google Scholar]
- 518.Wilson ID, Plumb R, Granger J, Major H, Williams R, Lenz EM. HPLC-MS-based methods for the study of metabonomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;817:67–76. doi: 10.1016/j.jchromb.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 519.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry. 2005;44:5234–45. doi: 10.1021/bi047773a. [DOI] [PubMed] [Google Scholar]
- 520.Brindle JT, Antti H, Holmes E, et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–44. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- 521.Patel C, Ghanim H, Ravishankar S, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. 2007;92:4476–9. doi: 10.1210/jc.2007-0778. [DOI] [PubMed] [Google Scholar]
- 522.Ulrich C, Heine GH, Garcia P, et al. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol Dial Transplant. 2006;21:1596–602. doi: 10.1093/ndt/gfl008. [DOI] [PubMed] [Google Scholar]
- 523.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 525.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 526.Dyugovskaya L, Lavie P, Lavie L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am J Respir Crit Care Med. 2003;168:242–9. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- 527.Dyugovskaya L, Lavie P, Hirsh M, Lavie L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur Respir J. 2005;25:820–8. doi: 10.1183/09031936.05.00103204. [DOI] [PubMed] [Google Scholar]
- 528.Kontogianni K, Messini-Nikolaki N, Christou K, Gourgoulianis K, Tsilimigaki S, Piperakis SM. DNA damage and repair capacity in lymphocytes from obstructive sleep apnea patients. Environ Mol Mutagen. 2007;48:722–7. doi: 10.1002/em.20351. [DOI] [PubMed] [Google Scholar]