Abstract
Study objectives:
The injection of cholinergic drugs in the pons has been largely used to induce REM sleep as a useful model to study different processes during this period. In the present study, microinjections of carbachol in the nucleus reticularis pontis oralis (NRPO) were performed to test the hypothesis that eye movements and the behavior of extraocular motoneurons during induced REM sleep do not differ from those during spontaneous REM sleep.
Methods:
Six female adult cats were prepared for chronic recording of eye movements (by means of the search-coil technique) and electroencephalography, electromyography, ponto-geniculo-occipital (PGO) waves at the lateral geniculate nucleus, and identified abducens motoneuron activities after microinjections of the cholinergic agonist carbachol into the NRPO.
Results:
Unilateral microinjections (n = 13) of carbachol in the NRPO induced REM sleep-like periods in which the eyes performed a convergence and downward rotation interrupted by phasic complex rapid eye movements associated to PGO waves. During induced-REM sleep abducens motoneurons lost their tonic activity and eye position codification, but continued codifying eye velocity during the burst of eye movements.
Conclusion:
The present results show that eye movements and the underlying behavior of abducens motoneurons are very similar to those present during natural REM sleep. Thus, microinjection of carbachol seems to activate the structures responsible for the exclusive oculomotor behavior observed during REM sleep, validating this pharmacological model and enabling a more efficient exploration of phasic and tonic phenomena underlying eye movements during REM sleep.
Citation:
Marquez-Ruiz J; Escudero M. Eye movements and abducens motoneuron behavior during cholinergically induced REM sleep. SLEEP 2009;32(4):471–481.
Keywords: Rapid eye movement sleep, oculomotor system, NRPO, abducens, motoneurons
RAPID EYE MOVEMENT (REM) SLEEP IS CHARACTERIZED BY ABSENCE OF ANTIGRAVITY MUSCULAR TONUS AND BY HIGH-FREQUENCY AND LOW-AMPLITUDE electroencephalographic (EEG) activity and phasic high-amplitude spiky potentials—recorded mainly in pontine, geniculate, and occipital areas—named ponto-geniculo-occipital (PGO) waves, which are associated to the occurrence of rapid eye movements and sporadic muscular twitches.1 During recent decades, considerable effort has been made to find neural structures and neurotransmitters underlying these phenomena, demonstrating the important role of pontine cholinergic mechanisms in the generation of the REM sleep state.2–5 Although there are some discrepancies about which sites in the pons are the most effective in REM sleep generation,6 two major areas in the medial pontine reticular formation have been proposed as being involved: the peri-locus coeruleus α, just ventral to the locus coeruleus,7 and the ventral part of the nucleus reticularis pontis oralis (NRPO).8
Cholinergic activation of the NRPO has been used to approach the mechanisms underlying muscular atonia,9–11 cortical and hippocampal activities,12,13 and PGO waves at the lateral geniculate nucleus (LGN).8,14 However, there are no studies on the behavior of the oculomotor system during cholinergically induced REM (i-REM) sleep. Recently, binocular eye position and the underlying activities of abducens (ABD) motoneurons have been precisely described along the spontaneous sleep-wake cycle in cats,15,16 spawning new data on the behavior of this motor system during sleep. Thus, during spontaneous REM sleep, the recording—by means of the search-coil technique—of the position of the eyes shows the existence of a convergence and maintained downward rotation of the eyes. This tonic position of the eyes is broken by high-frequency bursts of complex rapid eye movements during REM sleep15 that coincide with the occurrence of PGO waves at the LGN.15,17–20 Each rapid eye movement during REM sleep comprises 2 consecutive rotations in opposite directions, in which abducting movements are faster than adducting ones.15 The extracellular recording of ABD motoneurons shows a strong decrease in their tonic firing rate during REM sleep, with a loss of eye-position codification. By contrast, ABD motoneurons continue to codify eye velocity during rapid eye movements in REM sleep.16
Thus, the precise study of the oculomotor system has been revealed as a useful model for studying tonic and phasic phenomena occurring during REM sleep. The aim of the present work was to characterize the behavior of the oculomotor system during cholinergic i-REM sleep and to compare it with the behavior during spontaneous REM sleep. In this work we have recorded the movement of both eyes—using the search-coil technique—and the activity of identified ABD motoneurons after unilateral microinjection of carbachol into the NRPO of the cat. Present results enable us to demonstrate the similarity between tonic and phasic phenomena underlying eye movements and the behavior of extraocular motoneurons during natural REM sleep and cholinergic i-REM sleep.
MATERIAL AND METHODS
Subjects
Six female adult cats (2.4-3.7 kg) of European strains, obtained from an authorized supplier (University of Cordoba, Spain), were used as experimental subjects. Experiments were performed in accordance with the European Union directive 609/86/EU and current Spanish legislation (RD 1201/2005) for the use of laboratory animals. Every effort was made to minimize the number of animals used and their suffering.
Chronic Preparation
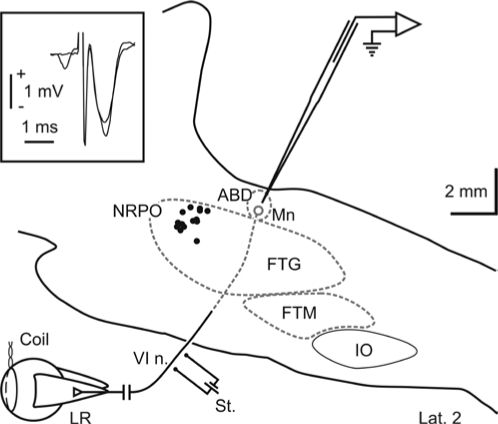
Animals were prepared for chronic recording of eye movements, EEG, electromyography, PGO waves at LGN level, and simultaneous recording of identified ABD motoneurons as previously described.16,21 Briefly, animals were anesthetized with sodium pentobarbitone (35 mg/kg, I.P.) following a protective injection of atropine (0.5 mg/kg, I.M.) aimed at preventing vagal reflexes. Under aseptic conditions, the cats were implanted bilaterally with Teflon-coated stainless-steel coils sutured to the scleral margin of the eye (Figure 1). In the same surgical act, a 6 × 6 mm hole was drilled through the occipital bone to allow access to the posterior brainstem via the cerebellum. Bipolar silver stimulating electrodes were implanted bilaterally on the VIth nerve (Figure 1) at its exit from the brainstem (stereotaxic coordinates, lateral 3.5 and posterior 1, according to Berman22). The final location of the stimulating electrodes was adjusted to evoke the maximum abducting eye movement with the minimum electrical stimulation (50 μs, cathodic square pulses of < 0.1 mA of current intensity). Screw electrodes were implanted bilaterally over the frontal cortex for EEG, bipolar stainless wires in the trapezius neck muscles for electromyography, and bipolar silver electrodes in the LGN (lateral 8.6 and anterior 5.5, according to Berman22) for the recording of PGO waves during i-REM sleep. The final location of each LGN bipolar electrode was adjusted by recording the neuronal activity in response to flashes of light. A head-holding system, consisting of 3 bolts cemented to the skull perpendicular to the stereotaxic plane, was also implanted. Eye coils and polysomnographic recording electrodes were connected to a socket attached to the holding system. The animals received postoperative systemic treatment with antibiotics and antiinflammatory and analgesic drugs. Throughout the experimental period, antibiotics, local anesthetics, and corticoids were topically applied to the cranial window and the eyes.
Figure 1.
Experimental design and injection sites. Teflon-coated stainless-steel coils were fitted to the scleral margin of both eyes. A stimulating electrode (St.) was implanted on the VIth nerve (VI n.) to identify abducens (ABD) motoneurons (Mn) by means of antidromic field potential and collision test (inset). Extracellular activity was recorded with a glass micropipette directed toward the ABD nucleus through the cerebellum. Injection sites are represented with dots in a reconstruction of the sagittal plane of the brainstem 2 mm from the midline. Dotted gray lines represent the edges of neuronal structures not included in the sagittal plane shown. FTG: gigantocellular tegmental field; FTM: magnocellular tegmental field; IO: inferior olive; LR: lateral rectus; NRPO: nucleus reticularis pontis oralis.
Drug Administration
At the beginning of the experiments, the ABD nucleus was localized by the recording of the antidromic field potential produced by the electrical stimulation of their ipsilateral VIth nerve and the NRPO pharmacologically mapped (P-2 to −4, L 2 and H −3.5 to −5.5, according to Berman22) by pressure microinjection of carbachol with a glass micropipette (7-8 μm of tip diameter). At the coordinates in which microinjection of carbachol induced a REM sleep episode with shortest latency, a guide tube (24G) was chronically implanted, keeping its tip at 4 mm from the NRPO. During experimental sessions, microinjections of carbachol were performed by means of an injection tube (30G)—coupled to a 2 μL Hamilton syringe—which was advanced through the guide tube up to the NRPO. Fifty to 300 nanoliters of carbachol (250 μM; Sigma Chemicals, St Louis, MO) prepared in phosphate-buffered saline solution (0.1 M, pH 7.4) were delivered over a period of 4-6 s in each experiment. Control experiments were performed by injecting the vehicle solution alone.
Recording Sessions
One to 2 weeks later, when there was total recovery from surgery, animals were habituated to the experimental set-up. Every 2–4 days, for 2–3 h per day, animals were lightly restrained by elastic bandages, with their head fixed to the recording table by means of the head-holding system. After 1 to 5 sessions, animals remained calm. Their heart and respiratory rates were not different from those of free animals, showing no signs of discomfort or stress. Recording sessions were carried out for a maximum of 8-12 weeks. At the beginning of each session, a glass micropipette filled with 2 M NaCl was advanced through the cerebellum towards either the left or the right ABD nucleus, which was identified by the recording of the antidromic field potential induced by electric stimulation of the ipsilateral VIth nerve (inset in Figure 1). Recorded neurons were identified as motoneurons by antidromic activation and collision test from the electrical stimulation of the ipsilateral VIth nerve. Eye movements, polysomnographic activity, and unitary activity of ABD motoneurons were recorded after carbachol administration. Eye movements were recorded with the scleral search-coil technique,23 and were calibrated at the beginning of each experimental session by rotating ( ± 10 deg) the magnetic field frame about both the horizontal and vertical planes. Eye position, polysomnographic, field, and unitary electrical activities were stored digitally on a computer by means of an analog-digital converter (CED 1401 Plus, Cambridge Instruments, Cambridge, UK). Eye-position signals and unitary electrical activity were sampled at 500 Hz and 17 kHz, respectively. Polysomnographic activities were sampled at 5 kHz. Neural activity signals were filtered at 1 Hz high-pass and 10 kHz low-pass, allowing the recording of PGO waves at ABD level. At the end of the experimental period, animals were anesthetized with sodium pentobarbitone (50 mg/kg I.P.), and transcardially perfused with saline and 4% paraformaldehyde, to anatomically confirm the location of the implanted guide tube and electrodes, and the recording zone.
Analysis of Eye Movements
The ocular disparity between the eyes before and after drug administration was calculated as the difference in their relative position. Upward and leftward rotations of the eyes were considered positive, and downward and rightward ones as negative. Subtraction of the right from the left eye position in the horizontal plane denoted positive values for divergence and negative values for convergence. Similarly, interocular disparity was calculated for the vertical plane, with positive values indicating that the left eye was more upwardly rotated than the right eye, and vice versa. To obtain a representative value for interocular disparity during a time period, interocular disparity values were averaged along this time.
Possible differences in the codification of rapid eye movements before and after carbachol microinjection were examined by analyzing the relationship between eye velocity and amplitude. Eye velocity was calculated as an average difference with an interval of 6 ms (Spike2, Cambridge Instrument, UK). The peak velocity of the eye during the movement was plotted as a function of the amplitude, and a linear regression analysis was performed. The slope of the fitted line was used to find differences in the codification of rapid eye movements during i-REM sleep. Student's t tests and a one-way ANOVA for repeated measures were performed in SPSS (v 14.0, SPSS Inc.). Statistical significance was set at P < 0.05. All results are shown as mean ± SD.
Analysis of ABD Motoneuronal Sensitivity to Eye Position and Velocity
To study the relationship between the firing rate of ABD motoneurons and the position and velocity of the ipsilateral eye after cholinergic activation of the NRPO, each identified ABD motoneuron was recorded just after unilateral microinjection of carbachol. To calculate the sensitivity of each motoneuron to eye position, the mean firing rate in spikes per second (sp/s) during fixations was plotted as a function of the mean eye position in the horizontal plane during the same intervals. In order to avoid interference with rapid eye movements, the first 300 ms after each rapid eye movement were not considered in the analysis. The slope of the regression line for this relationship— k (sp/s/deg)—represents the sensitivity of the neuron to eye position. To characterize the sensitivity of ABD motoneurons to eye velocity during rapid eye movements observed in the course of i-REM sleep, the maximum firing frequency was plotted as a function of the peak velocity of the eye during each rapid eye movement. The slope of the regression line for this relationship— r (sp/s/deg/s)—defined the sensitivity of the neuron to eye velocity. In order to describe the behavior of recorded motoneurons, collected data were included in a first-order linear model24: FR = F0 + k P + rP', where FR is the firing rate, F0 the firing rate at eye position zero, k the sensitivity to eye position (P), and r the sensitivity to eye velocity (P'). The position threshold of motoneurons (Th) was calculated as −F0/ k. Data for each analyzed motoneuron in this study are shown in Table I.
RESULTS
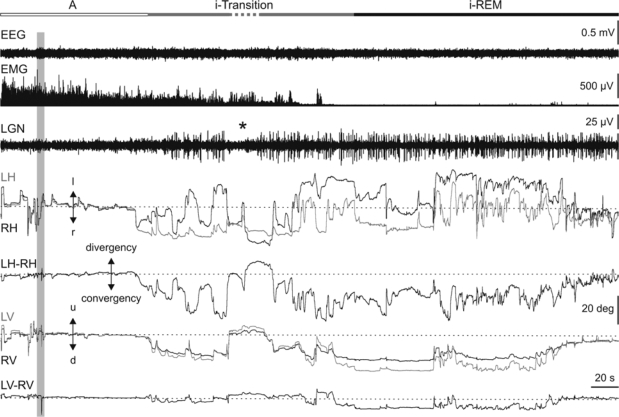
The behavior of the oculomotor system was studied in 6 adult female cats before and after 13 unilateral microinjections of the cholinergic agonist carbachol (50-300 nL; 250 μM) into the NRPO (P-2 to −4, L 2 and H −3.5 to −5.5, according to Berman22; Figure 1). Figure 2 displays an example of EEG, neck electromyography, and PGO wave activities recorded at the LGN, and horizontal and vertical eye movements before and after carbachol microinjection (gray bar). Unilateral microinjections of carbachol into the NRPO induced at short latency (1.0 ± 0.4 min; n = 13) a tonic convergence and downward rotation of the eyes, monocular rapid eye movements of small amplitude, and isolated PGO waves at the LGN. During this period, the neck muscles remained active, although their activity decreased progressively, and EEG activity did not change in either amplitude or frequency. These phasic and tonic components call to mind the different signs that occur during the natural transition between non-REM and REM sleep, and so we designated the period induced transition (i-transition). The duration of the i-transition period was variable, with a mean duration of 13.3 ± 11.4 min (n = 13). Unlike what occurred before a spontaneous transition period, the transition and REM sleep induced by carbachol microinjection were not preceded by a non-REM sleep phase, as can be seen for the homogeneous amplitude of the EEG trace and the absence of divergence and elevation of the eyes. Although each i-transition period was generally followed by a REM sleep, on occasions i-transition was broken by brief periods of alertness in which PGO waves and rapid eye movements disappeared (asterisk in Figure 2).
Figure 2.
Eye movements and interocular disparities during cholinergically induced transition and REM sleep. Representative polygraphic recording and eye movements induced by carbachol (vertical gray bar) in the NRPO. From top to bottom: state qualifying bar for alertness (A, white bar), induced transition (i-Transition, gray), and induced REM (i-REM, black), electroencephalography (EEG), rectified electromyography (EMG) of the neck muscle, lateral geniculate nucleus (LGN) activity, left (gray traces) and right (black traces) horizontal (LH, RH) eye position, ocular disparity in the horizontal plane (LH-RH), left (gray traces) and right (black traces) vertical (LV, RV) eye position, and ocular disparity in the vertical plane (LV-RV). Carbachol microinjection during alertness (A) induced at short latency a transition period (i-Transition) followed by an induced REM (i-REM) sleep period. The i-transition period was characterized by the beginning of convergence and downward rotation of both eyes and miniature monocular isolated rapid eye movements in coincidence with isolated ponto-geniculo-occipital (PGO) waves at the LGN. In this example, the discontinuous state bar indicates an interruption of the i-transition period in which PGO waves, rapid eye movements, convergence, and downward rotation disappeared. The beginning of the i-REM period was identified by the occurrence of a burst of complex binocular rapid eye movements in coincidence with a burst of PGO waves at the LGN. Direction left (l), right (r), up (u), and down (d) of eye movements and calibrations for each trace are indicated.
Thus, cholinergic activation of the NRPO induced 2 types of phenomena affecting the oculomotor system differentially during i-transition and i-REM sleep: tonic phenomena, denoted by modulation of the alignment of the visual axes, and phasic phenomena consisting of rapid eye movements associated with the occurrence of PGO waves.
Tonic Phenomena Affecting the Oculomotor System After Cholinergic Activation of the NRPO
Before carbachol microinjections, alertness periods were characterized by a low amplitude EEG, phasic and tonic neck muscular activity, absence of PGO waves at the LGN, and spontaneous eye movements consisting of saccades—which rotated both eyes from a visual target to another—and visual fixations in between (Figure 2 left). Interocular disparity during alertness—calculated as the differences in the horizontal (LH-RH in Figure 2) and vertical (LV-RV in Figure 2) position between the eyes—showed mean values of 0.18 ± 0.22 deg (n = 5) and 0.02 ± 0.11 deg (n = 5) for the horizontal and vertical planes, respectively. After carbachol microinjection, the beginning of the i-transition period was characterized by a slow, progressive convergence of the eyes in the horizontal plane. Although its intensity was variable, this convergence was present through the whole i-REM sleep, showing a mean interocular disparity in the horizontal plane of 11.50 ± 3.38 deg (n = 5). In the vertical plane, the beginning of the i-transition period was characterized by a downward rotation of both eyes, which remained downwardly rotated during the whole i-REM sleep period. The mean value of the maximum stabilized downward eye position to which the eyes tended was –19.05 ± 6.33 deg (n = 5). The mean vertical interocular disparity during i-REM sleep was 0.73 ± 1.25 deg (n = 5). When interocular disparity values during i-REM sleep were compared with those during alertness before microinjection, differences were found for the horizontal plane (P < 0.01; n = 5) but not for the vertical one.
Phasic Phenomena Affecting the Oculomotor System After Cholinergic Activation of the NRPO
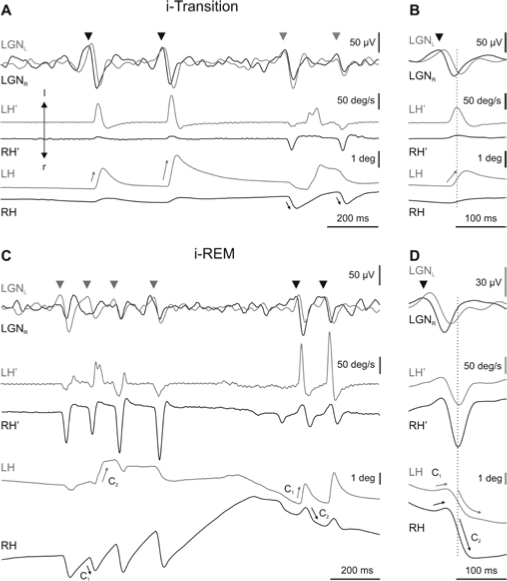
Tonic convergence and downward rotation during i-REM sleep were broken by the occurrence of rapid eye movements associated to PGO waves. During rapid eye movements, the degree of convergence of the eyes decreased and the eyes quickly rotated upward in the vertical plane (Figure 2). Both rapid eye movements and PGO waves developed progressively after microinjection of carbachol into the NRPO. During the i-transition period, rapid eye movements were isolated and mainly monocular, with low amplitude and velocity (Figure 3A, B). As the i-transition period progressed, eye movements became bigger, faster, and binocular. At the beginning of the i-REM sleep period, eye movements become complex and grouped in clusters (Figure 3C, D). Each of these simple or complex rapid eye movements was associated to a PGO wave at LGN level (Figure 3). During the i-transition periods, isolated eye movements were restricted to the horizontal plane. These movements consisted of very small abducting rotations ( < 5 deg) that mainly affected the eye ipsilateral to the direction of rotation (Figure 3A, straight arrows on eye position traces). Along the i-transition periods, pairs of monocular (oppositely directed) eye movements were coupled, in such a way that the time latency between these 2 consecutive eye movements decreased, becoming zero during i-REM sleep. Thus, during i-REM sleep, each rapid eye movement comprised 2 consecutive components with opposite directions (Figure 3C, D). The first component (C1 in Figure 3C, D)—a very brief eye movement—was followed by a larger second component (C2 in Figure 3C, D) directed to the contralateral side. With respect to these components, earlier and bigger (primary) PGO waves (arrowheads in Figure 3) were recorded at the LGN contralateral to the direction of the first component, and delayed and smaller (secondary) PGO waves at the LGN contralateral to the direction of the second component. Figures 3B and D show an average (n = 40) of PGO waves and velocity and position of the eyes in the horizontal planes triggered by the peak eye velocity during i-transition and i-REM sleep, respectively. Moreover, rapid eye movements occurred in clusters of up to 9 consecutive high-velocity movements during i-REM sleep. The mean intracluster frequency was 7.4 ± 1.4 Hz (n = 200, 4 animals).
Figure 3.
Each part of the figure shows, from top to bottom, the activity in left and right lateral geniculate nucleus (LGNL and LGNR) (superimposed for easy comparison) and the velocity (LH', RH') and position (LH, RH) of the left and right eyes, respectively. Recording of the left and right LGN and eyes are in gray and black, respectively. A. During induced transition (i-Transition), primary ponto-geniculo-occipital waves (PGO) (head arrows) coincided with monocular rapid eye movements of the contralateral eye. C. During induced REM (i-REM) sleep, each cluster of complex-two-component (C1, C2) rapid eye movements coincided with a cluster of PGO waves. During i-REM, primary PGO waves occurred contralateral to the direction of the first component of the movement of the eye. B and D show an average (n = 40) of LGN activities and eye velocity and position triggered by the peak velocity of the isolated rapid eye movement during i-transition and by the second component of complex eye movement during i-REM sleep, respectively.
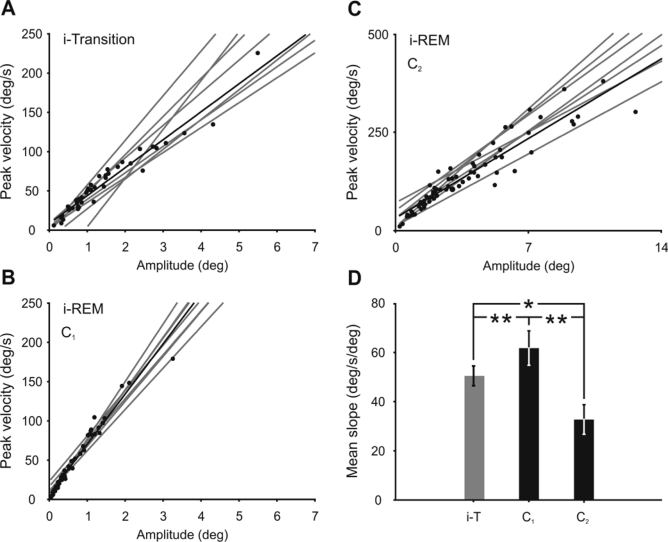
To test the differences in the codification of isolated rapid eye movements during i-transition and the 2 components of complex eye movements during i-REM sleep, the relationship between peak eye velocity and amplitude was analyzed. In the horizontal plane, the mean slope of the relationship between eye velocity and amplitude during the i-transition period was 41.8 ± 9.1 deg/s/deg (n = 8) (Figure 4A), while during i-REM sleep the mean slope was 61.8 ± 7.0 deg/s/deg (n = 8) (Figure 4B) and 32.8 ± 6.0 deg/s/deg (n = 8) (Figure 4C) for the first and second component, respectively. All comparisons were statistically significant (**P < 0.01 and *P < 0.05) (Figure 4D).
Figure 4.
Comparison of dynamic properties of rapid eye movements induced by cholinergic activation of the NRPO. Relationship between peak eye velocity and amplitude in the horizontal plane of monocular eye movements during induced transition (i-Transition) (A) and of the first (C1, B) and second (C2, C) components of complex rapid eye movements during induced REM (i-REM) sleep. Gray lines represent regression lines for each analyzed period, and dots and black lines the data and the regression line for a representative example. The scales in plot C have been proportionally modified to facilitate slope comparison. D. Mean slope comparison for each type of rapid eye movement during induced transition (i-T), and the first and second components of complex eye movements during i-REM sleep. Differences between all types of rapid eye movement were significant (**P < 0.01; *P < 0.05).
Qualitative Behavior of ABD Motoneurons During i-REM Sleep.
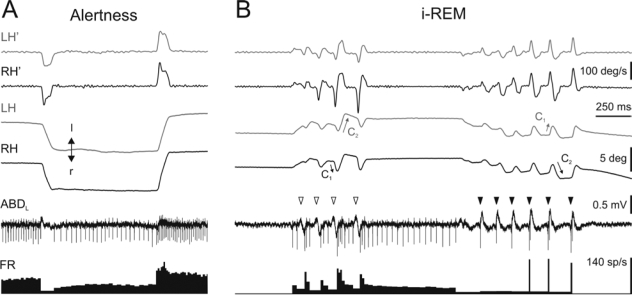
With the aim of characterizing motoneuronal activity underlying rapid eye movements during i-REM sleep, the activity of 22 identified ABD motoneurons was recorded after cholinergic activation of the NRPO. During alertness, the activity of ABD motoneurons showed an increase in their firing rate as the eyes rotated ipsilateral to the recorded side (Figure 5A). During eye fixations, ABD motoneurons showed a tonic discharge that was proportional to the abducting rotation of the ipsilateral eye. During saccades directed toward the recorded side, ABD motoneurons produced a burst of action potentials whose maximum firing rate was proportional to the peak eye velocity. During saccades directed contralateral to the recorded side, ABD motoneurons showed a pause.
Figure 5.
Activity of abducens motoneurons during alertness and induced REM sleep. Example of the activity of an identified motoneuron recorded in the left abducens (ABDL) nucleus during alertness and induced REM (i-REM) sleep. During alertness (A), ABD motoneurons showed a burst and tonic firing rate (FR) that was proportional to the velocity and position of the ipsilateral eye during rotations in the abducting direction. During i-REM sleep, the activity of ABD motoneurons was dependent on the occurrence of ponto-geniculo-occipital (PGO) waves. During biphasic PGO waves recorded at the ABD level (empty arrowheads), motoneurons produced a pause followed by a burst and tonic discharge in their firing, whereas during triphasic PGO waves (filled arrowheads), motoneurons produced a very short burst followed by a pause. This activity of ABD motoneurons induced a complex rapid eye movement with two consecutive opposed components (C1 and C2). Positive and negative values denoted left- (l) and right- (r) directed movements in the horizontal plane.
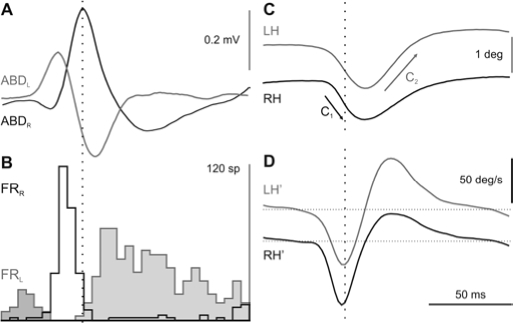
During i-REM sleep, ABD field activities were characterized by the presence of biphasic or triphasic waves that corresponded to respectively primary or secondary PGO waves at the ipsilateral LGN. ABD-PGO waves were associated to a complex rapid eye movement whose first component was contralateral or ipsilateral, depending on whether the recorded wave was biphasic (white arrowheads in Figure 5B) or triphasic (black arrowheads in Figure 5B), respectively. The presence of each type of ABD-PGO wave was associated to the behavior of ABD motoneurons. Triphasic ABD-PGO waves coincided with a very short burst—usually 1 to 3 action potentials—followed by a pause in ABD motoneuron activity. This burst induced a small ipsilateral rotation of the eyes, faster in the ipsilateral eye (C1 in Figure 5B). During the occurrence of biphasic ABD-PGO waves, ABD motoneurons displayed a pause in their firing rate followed by a burst and tonic discharge. This burst and tonic discharge induced an ipsilateral rotation that was bigger in the ipsilateral eye (C2 in Figure 5B). In order to establish the temporal and spatial relationship between ABD-PGO waves, the activity of ABD motoneurons, and eye movements, simultaneous recording was performed at both ABD nuclei. Figure 6 shows the average (n = 80) of ABD-PGO wave activities, the firing rate of 2 identified ABD motoneurons—1 in each ABD nucleus—and the position and velocity of the eyes. The 2 types of ABD-PGO wave occurred simultaneously, in such a way that when one type of PGO wave was recorded in one ABD nucleus, the other type was recorded in the contralateral one (Figure 6A). Accordingly, motoneurons displayed opposed firing discharge profiles in each ABD nucleus (Figure 6B). Thus, whereas in one ABD nucleus, motoneurons showed a burst followed by a pause, in the other ABD nucleus they showed a pause followed by a burst. This push-pull activation of ABD nuclei produced the 2 consecutive components that constituted the rapid eye movements (Figure 6C, D) during i-REM sleep.
Figure 6.
Temporal relationship between PGO waves, abducens motoneuron activity, and horizontal eye movements. Average (n = 80) of simultaneously recorded (left side in gray; right side in black) ponto-geniculo-occipital (PGO) waves in both abducens (ABD) nuclei, the firing rate (FR) of 2 identified ABD motoneurons—each recorded in a different ABD nucleus—and the left and right eye position (LH, RH) and velocity (LH', RH') in the horizontal plane. The average was triggered by the triphasic PGO waves. The peri-stimulus histogram for the firing rate was constructed with a bin size of 5 ms. When a biphasic PGO wave was recorded in one ABD, a triphasic PGO wave was recorded in the other ABD nucleus, and vice versa. During the recording of biphasic PGO waves, ABD motoneurons were firstly inhibited and thereafter activated, whereas during the triphasic waves, motoneurons were briefly activated and then inhibited. This push-pull response of the two ABD nuclei shaped a conjugated eye movement directed first toward the side on which the triphasic PGO wave (C1, black arrows) occurred, followed by another toward the contralateral side (C2, gray arrows).
Eye Signal Codification of ABD Motoneurons During i-REM Sleep
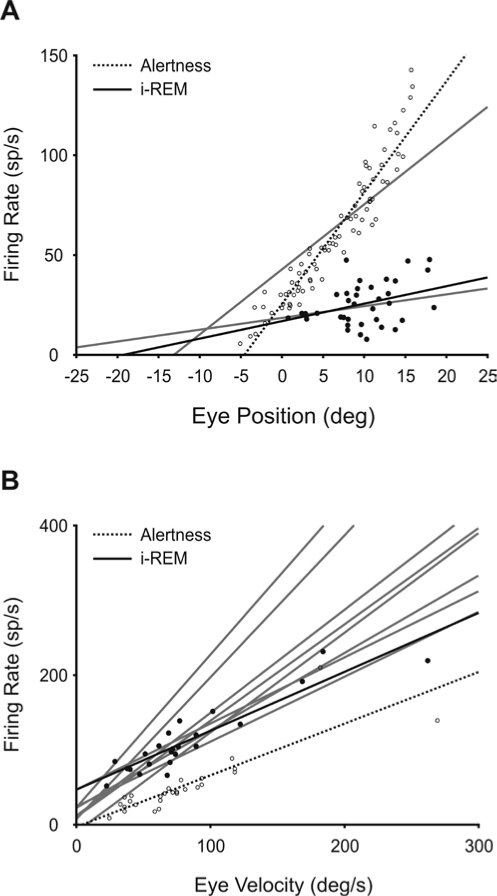
In order to know the eye signal codification of ABD motoneurons during i-REM sleep, the sensitivity to the position ( k) and velocity ( r) of 9 identified ABD motoneurons was analyzed (Table 1). During i-REM sleep, only 3 of 9 analyzed ABD motoneurons maintained some tonic activity, but displayed a high variability in their discharge and a negligible relationship with eye position (closed circles in Figure 7A). In these few motoneurons, the value of R2 was less than 0.2 (P < 0.05). The mean slope of the relationship between firing rate and eye position ( k) during i-REM sleep was 1.6 ± 1.5 sp/s/deg (n = 3) ( k in Table 1). By contrast, the maximum firing discharge during the burst was proportional to the velocity of rotation of the ipsilateral eye in the abducting direction (closed circles in Figure 7B; Table 1). The mean slope of the relationship between firing rate and peak eye velocity during i-REM sleep was 1.3 ± 0.5 sp/s/deg/s (n = 9) ( r in Table 1). Open and closed circles and dotted and continuous lines in Figure 7 represent the data and linear regression lines for the same motoneuron recorded during alertness and i-REM respectively.
Table 1.
Eye Position and Velocity Sensitivity for 9 Identified Abducens Motoneurons Recorded During Induced Rapid Eye Movement (i-REM) Sleep
| Th | k | R2 | r | R2 | |
|---|---|---|---|---|---|
| 1 | - | - | - | 2.0 | 0.95 |
| 2 | −31.4 | 0.6 | 0.15 | 0.9 | 0.65 |
| 3 | - | - | - | 1.3 | 0.83 |
| 4 | - | - | - | 1.0 | 0.84 |
| 5 | −19.4 | 0.9 | 0.13 | 0.8 | 0.84 |
| 6 | −13.1 | 3.3 | 0.20 | 0.9 | 0.71 |
| 7 | - | - | - | 1.9 | 0.69 |
| 8 | - | - | - | 1.4 | 0.56 |
| 9 | - | - | - | 1.3 | 0.79 |
| Mean ± SD | −21.3 ± 9.3 | 1.6 ± 1.5 | 1.3 ± 0.5 |
To describe the behavior of identified abducens motoneurons, collected data were included in a first-order linear model24: FR = fo + k P + r P', where FR represents the firing rate of the motoneuron, fo the rate at eye position zero, k the sensitivity to the position (P), and r the sensitivity to the eye velocity (P'). The position of the eye at which abducens motoneurons started to discharge (Th) was calculated as −fo/k. During i-REM sleep, some motoneurons did not show tonic discharge (−).
Figure 7.
Codification of eye position and velocity by abducens motoneurons during induced REM sleep. Identified abducens (ABD) motoneurons (n = 9) were recorded during induced REM (i-REM) sleep, and their firing rate was analyzed with respect to the position (A) and velocity (B) of the ipsilateral eye in the horizontal plane. During i-REM sleep, the majority of ABD motoneurons lost their tonic activity, and in those cases in which some tonic activity remained, the correlation of activity with eye position (black dots and regression line in A) was poor with respect to the case of alertness (open circles and dashed line). B. By contrast, the maximum firing discharge of ABD motoneurons correlated with the peak velocity of the eye during rapid eye movements (black dots and regression line), as during alertness (open circles and dashed line). Gray lines correspond to the linear regression lines for each analyzed motoneuron. Parametric and statistical values for each motoneuron are given in Table 1.
DISCUSSION
In the last few decades, many studies have been performed on pontine cholinergic induction of REM sleep, indicating that the NRPO is a key structure for the generation and maintenance of phasic and tonic phenomena underlying REM sleep. Microinjections of cholinergic agonists in the NRPO induce long- term increases of REM sleep that are blocked by atropine, a selective muscarinic antagonist. However, there are some differences in the response to cholinergic agonist between felines and rodents6. Whereas in rats25,26 and mice27 the cholinergic agonist carbachol seems to play a facilitative role for REM sleep—increasing the amount of REM sleep by hours without changing the latency of the first episode—in cats it induces REM-sleep-like activities a few minutes after injections.28–33 Although the origin of these differences is unknown, cholinergic i-REM sleep in cats has been employed as a useful model for studying neural mechanisms underlying classical signs of REM sleep, such as muscular atonia,9–11 cortical and hippocampal activities,12,13 and PGO waves at LGN level.8,14 Nevertheless, except for some partial descriptions,8,29 eye movement kinematics and neural oculomotor control have remained uncharacterized during cholinergic i-REM sleep. In this paper, eye movements and ABD motoneuron activities during carbachol i-REM sleep were compared with those recently described15,16 as taking place during spontaneous REM sleep. This comparison may help to establish the validity of the carbachol model for the study of the oculomotor system, providing in-depth knowledge of the mechanisms responsible for eye movements during REM sleep.
Tonic and Phasic Phenomena Affecting the Oculomotor System During Cholinergic i-REM Sleep
In all the experiments, carbachol microinjection into the NRPO induced at very short latency a tonic convergence and downward rotation of the eyes and single monocular rapid eye movements paired with PGO waves, in the presence of low-amplitude EEG and muscular tonus. With the exception of the low amplitude and high frequency of the EEG, this state was identical to that observed during the spontaneous transition to REM sleep.15 In contrast to the natural sleep-wake cycle, where transition periods usually last less than one minute,15 the i-transition period persisted for a long time, reaching more than 30 minutes in 2 of the performed microinjections. Similar states have been previously described as dissociated states,7,28,29 in which PGO waves were reported to be independent of rapid eye movements.34 A recent description of eye movements during transition and REM sleep—using the binocular search-coil technique, a much more precise technique than the more popular electro-oculography—strongly suggests that low-amplitude rapid eye movement during the i-transition period has passed unnoticed in previous studies.15 Nevertheless, it is important to emphasize that in the present experiments, no i-REM sleep was observed without a previous i-transition phase. This i-transition period was similar to the spontaneous transition period—which is characterized by isolated PGO waves at the LGN35–37 and cortical slow waves38–41 preceding spontaneous REM sleep—except that no synchronization in the EEG was observed.
Tonic phenomena during i-REM sleep were characterized by a downward rotation and convergence of the eyes in the vertical and horizontal planes, respectively. Misalignment between the visual axes of the eyes during spontaneous REM sleep has been described previously in monkeys,42 and more recently the existence has been demonstrated of a dependence of interocular disparity on each of the different phases of the sleep-wake cycle in the cat.15 The results shown in this paper completely agree with those obtained during spontaneous REM sleep, demonstrating the existence of common mechanisms underlying tonic phenomena during spontaneous and cholinergic i-REM sleep at the NRPO. Cholinergic activation of the NRPO also induced other tonic phenomena during i-REM sleep, such as an EEG desynchronization and loss of muscular tonus.8,9,11,32,43,44
Previous works have also reported that administration of carbachol in the NRPO induces phasic phenomena such as PGO waves at the LGN, muscular twitches, high-amplitude inhibitory postsynaptic potentials in motoneurons, and rapid eye movements, all similar to those occurring during spontaneous REM sleep.11,29,32,43,45–47 As previously reported,8,32 carbachol microinjection induced with a short latency a REM-sleep-like state without previous non-REM sleep.
As during the spontaneous sleep-wake cycle in the cat,15 rapid eye movements during i-transition periods were qualitatively different from those observed during i-REM sleep. During i-transition, rapid eye movements were mainly monocular, isolated, and in the abducting direction. Each eye movement was associated to the occurrence of a primary PGO wave recorded at the LGN contralateral to the direction of rotation, as during the spontaneous transition period.15 These eye movements contrasted with the more complex 2-component and clustered eye movements during i-REM sleep. We found that during i-REM, primary PGO waves occurred at the LGN contralateral to the one at which the first component was directed, and ipsilateral to the second component. These data agree with a previous description regarding spontaneous REM sleep.15 By contrast, most authors agree that rapid eye movements during spontaneous REM sleep are directed toward the side where the primary PGO wave is recorded.17–20 This discrepancy is understandable, taking into account that the second component of eye movement is usually bigger than the first, so that it is not surprising that the first component has hitherto passed unnoticed.
Regarding the eye-velocity/amplitude relationship, present results coincide with those reported by Marquez-Ruiz and Escudero15 in the case of spontaneous transition and during REM sleep, and by Vanni-Mercier et al.48 in the case of the second component during spontaneous REM sleep. The slope of this relationship for all these eye movements is higher than those reported for saccades during alertness, although some discrepancies exist with other authors who reported values for those during REM sleep equal to42,49 or lower than50,51 those during alertness.
Finally, the similarity in the internal burst frequency between spontaneous15 and cholinergic i-REM sleep supports the existence of a common bursting generator mechanism underlying rapid eye movements and the concomitant PGO waves during spontaneous and pharmacological i-REM state.8,14
ABD Motoneuron Behavior Underlying Eye Movements Induced by Cholinergic Activation of the NRPO
During the last few years, the model of carbachol i-REM sleep has been used to study how muscular atonia is induced in different populations of lumbar,9,44,46 masseter,14,48,52 and hypoglossal44,47 motoneurons in cats. The present study is the first characterizing the behavior of the oculomotor motoneurons under the carbachol model of REM sleep. During i-REM sleep, the activity of identified ABD motoneurons was characterized by a low or absent tonic activity and by phasic activation and inhibition activities associated to PGO waves. Biphasic and triphasic PGO waves recorded in the ABD nucleus corresponded with, respectively, primary and secondary PGO waves at the ipsilateral LGN, as previously reported during spontaneous REM sleep.16,18,19 As described for spontaneous REM sleep,16 each type of ABD-PGO wave was present simultaneously in each ABD, inducing an antagonic behavioral pattern in the activities of ABD motoneurons in each ABD nucleus. Thus, when biphasic PGO waves were recorded in one ABD nucleus, the ipsilateral motoneurons showed a pause followed by a burst-tonic activity. At this time, a triphasic PGO wave was recorded in the contralateral ABD nucleus, and motoneurons in this ABD showed a short burst denoted by one or two action potentials followed by a silence.
Regarding eye-signal codification, ABD motoneurons failed to encode eye-position signals after unilateral microinjection of carbachol into the NRPO. Eye-position signal deficit during i-REM sleep could be due to a premotor disfacilitation and/or to an active inhibition acting on ABD nuclei. Previous studies in spontaneously sleeping animals, where fuzzy codification of eye-position signal was also reported, describe an increase in antidromic field potential latency of the ABD nucleus during REM sleep, suggesting that ABD motoneurons are actively inhibited during this state.16 However, a disfacilitation at the prepositus hypoglossi nucleus—responsible for eye-position signals in the horizontal plane21,53–55—should be considered, since neurons recorded in the interstitial nucleus of Cajal (the vertical eye-position generator)56 lost vertical position signals during spontaneous REM sleep.57 By contrast, ABD motoneurons continue codifying eye-velocity signals during i-REM sleep, as during spontaneous REM sleep.16
In conclusion, present results show that eye movements and the underlying ABD motoneuronal activities induced by cholinergic activation of the NRPO are very similar to those present during natural spontaneous REM sleep in cats. Thus, in this species, microinjection of carbachol seems to activate common structures responsible for exclusive oculomotor system behavior observed during spontaneous and cholinergic i-REM sleep. Present results add evidence that the NRPO participates in the generation and maintenance of eye movements during REM sleep, validating—from the standpoint of oculomotor behavior—the carbachol model and enabling continuation of the exploration and systematic study of phasic and tonic phenomena underlying eye movements during REM sleep.
ACKNOWLEDGMENTS
This research was supported by grants MCYT-BFU2005-01579 and the Consejeria de Innovacion, Ciencia y Empresa of the Junta de Andalucia, Spain. The authors wish to acknowledge the editorial help of Mr. Roger Churchill.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Steriade M, McCarley RW. Brain control of wakefulness and sleep. New York: Kluwer Academic-Plenum Publishers; 2005. [Google Scholar]
- 2.Jouvet M. Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- 3.Hernandez-Peon R, Chavez-Ibarra G, Morgane PJ, Timo-Iaria C. Limbic cholinergic pathways involved in sleep and emotional behavior. Exper Neurol. 1963;8:93–111. [Google Scholar]
- 4.George R, Haslett WL, Jenden DJ. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–52. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- 5.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–61. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 6.Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–68. [PubMed] [Google Scholar]
- 7.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–64. [PubMed] [Google Scholar]
- 8.Garzon M, De Andres I, Reinoso-Suarez F. Sleep patterns after carbachol delivery in the ventral oral pontine tegmentum of the cat. Neuroscience. 1998;83:1137–44. doi: 10.1016/s0306-4522(97)00494-6. [DOI] [PubMed] [Google Scholar]
- 9.Fung SJ, Boxer PA, Morales FR, Chase MH. Hyperpolarizing membrane responses induced in lumbar motoneurons by stimulation of the nucleus reticularis pontis oralis during active sleep. Brain Res. 1982;248:267–73. doi: 10.1016/0006-8993(82)90584-4. [DOI] [PubMed] [Google Scholar]
- 10.Pereda AE, Morales FR, Chase MH. Medullary control of lumbar motoneurons during carbachol-induced motor inhibition. Brain Res. 1990;514:175–9. doi: 10.1016/0006-8993(90)90455-k. [DOI] [PubMed] [Google Scholar]
- 11.Takakusaki K, Matsuyama K, Kobayashi Y, Kohyama J, Mori S. Pontine microinjection of carbachol and critical zone for inducing postural atonia in reflexively standing decerebrate cats. Neurosci Lett. 1993;153:185–8. doi: 10.1016/0304-3940(93)90318-f. [DOI] [PubMed] [Google Scholar]
- 12.Garzon M, De Andres I, Reinoso-Suarez F. Sleep patterns after carbachol delivery in the ventral oral pontine tegmentum of the cat. Neuroscience. 1997;83:1137–44. doi: 10.1016/s0306-4522(97)00494-6. [DOI] [PubMed] [Google Scholar]
- 13.Kinney GG, Vogel GW, Feng P. Brainstem carbachol injections in the urethane anesthetized rat produce hippocampal theta rhythm and cortical desynchronization: a comparison of pedunculopontine tegmental versus nucleus pontis oralis injections. Brain Res. 1998;809:307–13. doi: 10.1016/s0006-8993(98)00878-6. [DOI] [PubMed] [Google Scholar]
- 14.Kohlmeier KA, Lopez-Rodriguez F, Morales FR, Chase MH. Relationship between sensory stimuli-elicited IPSPs in motoneurons and PGO waves during cholinergically induced muscle atonia. J Neurophysiol. 1997;78:2145–55. doi: 10.1152/jn.1997.78.4.2145. [DOI] [PubMed] [Google Scholar]
- 15.Marquez-Ruiz J, Escudero M. Tonic and phasic phenomena underlying eye movements during sleep in the cat. J Physiol. 2008;586:3461–77. doi: 10.1113/jphysiol.2008.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escudero M, Marquez-Ruiz J. Tonic inhibition and ponto-geniculo-occipital-related activities shape abducens motoneuron discharge during REM sleep. J Physiol. 2008;586:3479–91. doi: 10.1113/jphysiol.2008.153254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cespuglio R, Laurent JP, Jouvet M. Etude des relations entre l'activite ponto-geniculo-occipitale (PGO) et la motricite oculaire chez le chat sous reserpine. Brain Res. 1975;83:319–35. doi: 10.1016/0006-8993(75)90939-7. [DOI] [PubMed] [Google Scholar]
- 18.Sakai K, Cespuglio R. Evidence for the presence of eye movement potentials during paradoxical sleep in cats. Electroencephalogr Clin Neurophysiol. 1976;41:37–48. doi: 10.1016/0013-4694(76)90213-3. [DOI] [PubMed] [Google Scholar]
- 19.Sakai K, Petitjean F, Jouvet M. Effects of ponto-mesencephalic lesions and electrical stimulation upon PGO waves and EMPs in unanesthetized cats. Electroencephalogr Clin Neurophysiol. 1976;41:49–63. doi: 10.1016/0013-4694(76)90214-5. [DOI] [PubMed] [Google Scholar]
- 20.Nelson JP, McCarley RW, Hobson JA. REM sleep burst neurons, PGO waves, and eye movement information. J Neurophysiol. 1983;50:784–97. doi: 10.1152/jn.1983.50.4.784. [DOI] [PubMed] [Google Scholar]
- 21.Escudero M, de la Cruz RR, Delgado-Garcia JM. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J Physiol. 1992;458:539–60. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman AL. Madison, WI: University of Wisconsin; 1968. The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. [Google Scholar]
- 23.Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–70. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 24.Robinson DA. Oculomotor unit behavior in the monkey. J Neurophysiol. 1970;33:393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- 25.Marks GA, Birabil CG. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 1998;86:29–37. doi: 10.1016/s0306-4522(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 26.Deurveilher S, Hars B, Hennevin E. Pontine microinjection of carbachol does not reliably enhance paradoxical sleep in rats. Sleep. 1997;20:593–607. [PubMed] [Google Scholar]
- 27.Lydic R, Douglas CL, Baghdoyan HA. Microinjection of neostigmine into the pontine reticular formation of C57BL/6J mouse enhances rapid eye movement sleep and depresses breathing. Sleep. 2002;25:835–41. doi: 10.1093/sleep/25.8.835. [DOI] [PubMed] [Google Scholar]
- 28.Mitler MM, Dement WC. Cataplectic-like behavior in cats after micro-injections of carbachol in pontine reticular formation. Brain Res. 1974;68:335–43. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- 30.Morales F, Boxer P, Chase MH. Behavioral state-specific inhibitory postsynaptic potentials impinge on cat lumbar motoneurons during active sleep. Exp Neurol. 1987;98:418–35. doi: 10.1016/0014-4886(87)90252-4. [DOI] [PubMed] [Google Scholar]
- 31.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–84. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 32.Reinoso-Suarez F, De Andres I, Rodrigo-Angulo ML, Rodriguez-Veiga E. Location and anatomical connections of a paradoxical sleep induction site in the cat ventral pontine tegmentum. Eur J Neurosci. 1994;6:1829–36. doi: 10.1111/j.1460-9568.1994.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 33.Lai YY, Siegel JM. Muscle atonia in REM sleep. In: Mallick BN, Inoue S, editors. Rapid eye movement sleep. New Delhi, India: Narosa Publishing House; 1998. pp. 69–90. [Google Scholar]
- 34.Lopez-Rodriguez F, Kohlmeier K, Morales FR, Chase MH. State dependency of the effects of microinjection of cholinergic drugs into the nucleus pontis oralis. Brain Res. 1994;649:271–81. doi: 10.1016/0006-8993(94)91074-x. [DOI] [PubMed] [Google Scholar]
- 35.Steriade M, Pare D, Bouhassira D, Deschenes M, Oakson G. Phasic activation of lateral geniculate and perigeniculate thalamic neurons during sleep with ponto-geniculo-occipital waves. J Neurosci. 1989;9:2215–29. doi: 10.1523/JNEUROSCI.09-07-02215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- 37.Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–65. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarley RW, Hobson JA. Cortical unit activity in desynchronized sleep. Science. 1970;167:901–3. doi: 10.1126/science.167.3919.901. [DOI] [PubMed] [Google Scholar]
- 39.Trachsel L, Tobler I, Borbely AA. Electroencephalogram analysis of non-rapid eye movement sleep in rats. Am J Physiol. 1988;255:27–37. doi: 10.1152/ajpregu.1988.255.1.R27. [DOI] [PubMed] [Google Scholar]
- 40.Glin L, Arnaud C, Berracochea D, Galey D, Jaffard R, Gottesmann C. The intermediate stage of sleep in mice. Physiol Behav. 1991;50:951–3. doi: 10.1016/0031-9384(91)90420-s. [DOI] [PubMed] [Google Scholar]
- 41.Gottesmann C. Detection of seven sleep-waking stages in the rat. Neurosci Biobehav Rev. 1992;16:31–8. doi: 10.1016/s0149-7634(05)80048-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W, King WM. Binocular eye movements not coordinated during REM sleep. Exp Brain Res. 1997;117:153–60. doi: 10.1007/s002210050209. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Rodriguez F, Kohlmeier KA, Yamuy J, Morales FR, Chase MH. Muscle atonia can be induced by carbachol injections into the nucleus pontis oralis in cats anesthetized with alpha-chloralose. Brain Res. 1995;699:201–7. doi: 10.1016/0006-8993(95)00899-2. [DOI] [PubMed] [Google Scholar]
- 44.Fung SJ, Yamuy J, Xi MC, Engelhardt JK, Morales FR, Chase MH. Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res. 2000;885:262–72. doi: 10.1016/s0006-8993(00)02955-3. [DOI] [PubMed] [Google Scholar]
- 45.Kohlmeier KA, Lopez-Rodriguez F, Liu RH, Morales FR, Chase MH. State-dependent phenomena in cat masseter motoneurons. Brain Res. 1996;722:30–8. doi: 10.1016/0006-8993(96)00173-4. [DOI] [PubMed] [Google Scholar]
- 46.Xi MC, Liu RH, Yamuy J, Morales FR, Chase MH. Electrophysiological properties of lumbar motoneurons in the alpha-chloralose-anesthetized cat during carbachol-induced motor inhibition. J Neurophysiol. 1997;78:129–36. doi: 10.1152/jn.1997.78.1.129. [DOI] [PubMed] [Google Scholar]
- 47.Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–5. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- 48.Vanni-Mercier G, Pelisson D, Goffart L, Sakai K, Jouvet M. Eye saccade dynamics during paradoxical sleep in the cat. Eur J Neurosci. 1994;6:1298–306. doi: 10.1111/j.1460-9568.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 49.Herman JH, Barker DR, Roffwarg HP. Similarity of eye movement characteristics in REM sleep and the awake state. Psychophysiology. 1983;20:537–43. doi: 10.1111/j.1469-8986.1983.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs AF, Ron S. An analysis of rapid eye movements of sleep in the monkey. Electroencephalogr Clin Neurophysiol. 1968;25:244–51. doi: 10.1016/0013-4694(68)90022-9. [DOI] [PubMed] [Google Scholar]
- 51.Aserinsky E, Lynch JA, Mack ME, Tzankoff SP, Hurn E. Comparison of eye motion in wakefulness and REM sleep. Psychophysiology. 1985;22:1–10. doi: 10.1111/j.1469-8986.1985.tb01551.x. [DOI] [PubMed] [Google Scholar]
- 52.Kohlmeier KA, Lopez-Rodriguez F, Morales FR, Chase MH. Effects of excitation of sensory pathways on the membrane potential of cat masseter motoneurons before and during cholinergically induced motor atonia. Neuroscience. 1998;86:557–69. doi: 10.1016/s0306-4522(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 53.Cheron G, Godaux E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus-vestibular complex of the cat. J Physiol. 1987;394:267–90. doi: 10.1113/jphysiol.1987.sp016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol. 1987;57:1383–409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- 55.McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J Neurophysiol. 1992;68:319–32. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- 56.Fukushima K, Kaneko CR, Fuchs AF. The neuronal substrate of integration in the oculomotor system. Prog Neurobiol. 1992;39:609–39. doi: 10.1016/0301-0082(92)90016-8. [DOI] [PubMed] [Google Scholar]
- 57.Fukushima K, Fukushima J. Activity of eye-movement-related neurons in the region of the interstitial nucleus of Cajal during sleep. Neurosci Res. 1990;9:126–39. doi: 10.1016/0168-0102(90)90028-d. [DOI] [PubMed] [Google Scholar]