Abstract
Study Objectives:
Polysomnography (PSG) is the gold standard in diagnosing sleep disordered breathing (SDB). Diagnostic analysis of SDB is time-consuming and labor-intensive and entails long waiting lists for patients. The aim of this study was to assess the validity of a flow-based screening classifier as an automated diagnostic test for Cheyne-Stokes respiration (CSR).
Setting:
Sleep laboratory
Participants:
70 study subjects (28 with obstructive sleep apnea [OSA], 20 with CSR, 11 with CSR+OSA and 11 without SDB)
Measurements:
The nasal cannula flow signal was analyzed by ApneaLink (ResMed, Sydney, Australia), based on a classifier algorithm using pattern recognition. In a simultaneous PSG, results were compared with manual scoring of respiratory events by certified sleep experts.
Results:
For detecting CSR we obtained a sensitivity of 87.1% (95% confidence interval 75.3% to 98.9%), a specificity of 94.9% (95% confidence interval 87.9% to 100%), a positive likelihood ratio of 17.0, and a negative likelihood ratio of 0.14. The area under the curve (AUC) of the according receiver operating characteristic (ROC) curve was 93.4%. This resulted in an accuracy of 91.4% for identifying CSR.
Conclusion:
In this study we demonstrated that the screening classifier was able to detect CSR with high diagnostic accuracy. Hence, ApneaLink equipped with CSR classifier is an appropriate screening tool which may help to prioritize patients with CSR for PSG.
Citation:
Weinreich G; Armitstead J; Töpfer V; Wang YM; Teschler H. Validation of apnealink as screening device for cheyne-stokes respiration. SLEEP 2009;32(4):553–557.
Keywords: ApneaLink, Cheyne-Stokes respiration, obstructive sleep apnea, pattern recognition, sleep disordered breathing, screening
CHEYNE-STOKES RESPIRATION (CSR) IS AN ABNORMAL PERIODIC PATTERN OF BREATHING PREDOMINANTLY IN NREM SLEEP. CSR IS CHARACTERIZED by waxing and waning flow amplitude followed by apnea or hypopnea (Figure 1). This pattern is caused by instability in the feedback control involved in the chemical regulation of breathing. CSR affects 30% to 40% of patients with congestive heart failure1–4 and can also be seen in patients with stroke.5 CSR is associated with sleep disruption and decreased survival rates compared to subjects with a similar ejection fraction but without CSR.6–8
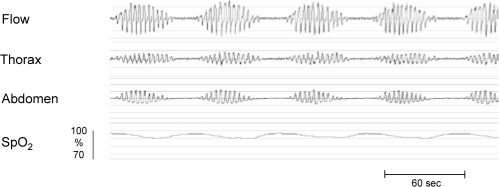
Figure 1.
Typical recording of CSR displaying waxing and waning of flow followed by a central apnea. Lack of thorax and abdomen effort causes cessation of airflow followed by moderate desaturation.
Diagnostic assessment of SDB is based on polysomnography (PSG) recorded during the night. Because diagnostic analysis is labor-intensive and time-consuming, patients must wait 2-10 months for a PSG.9 To prioritize patients for PSG, it is desirable to have a screening device that can distinguish between the various manifestations of SDB. ApneaLink is a single-signal screening device that measures flow via a nasal cannula connected to a pressure transducer. Recent studies showed an excellent correlation of ApneaLink versus PSG regarding the apnea-hypopnea index (AHI).10,11
The newest version of ApneaLink provides a classifier for the auto-detection of CSR. The classifier of ApneaLink is based on pattern recognition, a concept which is also used for voice recognition and radar detection.12–15 The nasal cannula flow signal of ApneaLink is used for pattern recognition and hence classification of CSR.
In recent years attempts have been made to automatically detect SDB. El-Solh et al16 evaluated a neural network based on spectral analysis of arterial oxygen saturation SpO2 in order to determine CSR. Our group analyzed a neural network relying on spectral entropy S of the airflow for identifying OSA and CSR.17 The aim of the present study was to evaluate the diagnostic accuracy of the ApneaLink classifier for CSR against simultaneous PSG.
METHODS
Subjects
The study population consisted of 70 subjects referred to our sleep laboratory for PSG testing (Table 1). Firstly, we included 41 cardiology patients with congestive heart failure and high pre-test probability of CSR. Secondly, to test the device in a mixed population (CSR, OSA, CSR+OSA, no SDB), we included 29 subjects with high pre-test probability of OSA referred from general practitioners. An institutional review board approved the study, and subjects provided informed consent prior to participation in this study.
Table 1.
Demographic Characteristics, SDB Classification, and Comorbidities for All 70 Subjects
| Demographic Characteristic | Results |
|---|---|
| Age | 60.3 ± 11.5 years |
| Sex, f/m | 15/55 |
| BMI | 27.5 ± 4.4 kg/m2 |
| OSA | 28 |
| CSR | 20 |
| OSA + CSR | 11 |
| no SDB | 11 |
| Congestive heart failure | 41 |
| Hypertension | 36 |
| Diabetes | 13 |
| Asthma | 9 |
| Chronic obstructive bronchitis | 14 |
| Allergies | 5 |
Polysomnography
Polysomnography was performed using a digital polygraph (Embla, Broomfield, USA) including 2 electroencephalographic (EEG) channels, 2 electrooculograms (EOG), submental electromyogram (EMG), tibialis EMG, rib cage and abdominal inductance pneumograms, pulse oximeter (Nonin 8500, Minnesota, USA), body position, and nasal cannula connected via a T-piece allowing simultaneous recording of flow signal by ApneaLink and the PSG system. Sleep staging and arousals were scored using 30-s epochs according to Rechtschaffen and Kales19 and American Sleep Disorders Association.20 An apnea was defined as cessation of flow for ≥ 10 s, and a hypopnea as reduction of ≥ 50% in flow amplitude lasting ≥ 10 s. The respiratory event was scored as obstructive apnea if it met apnea criteria and was associated with continued or increased respiratory effort throughout the entire period of absent airflow.21 The respiratory event was scored as central apnea if it met apnea criteria and was associated with absent respiratory effort throughout the entire period of absent airflow. According to the definitions of the American Academy of Sleep Medicine21 CSR was defined if i) ≥ 5 central apneas or hypopneas/h occurred, or ii) the cyclic crescendo and decrescendo change in breathing amplitude had a duration of ≥ 10 consecutive minutes. In PSG an apnea-hypopnea index (AHI) was calculated by dividing the total number of apneas and hypopneas by the total hours of sleep time recorded over the night.
ApneaLink
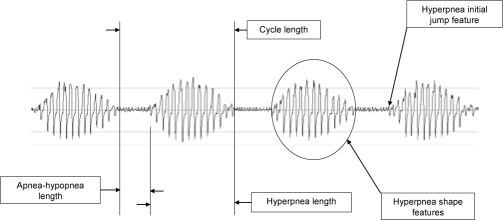
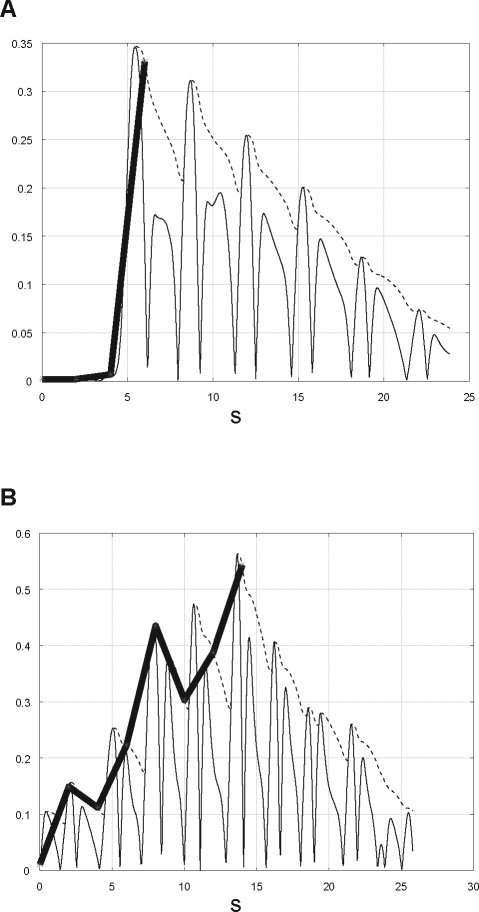
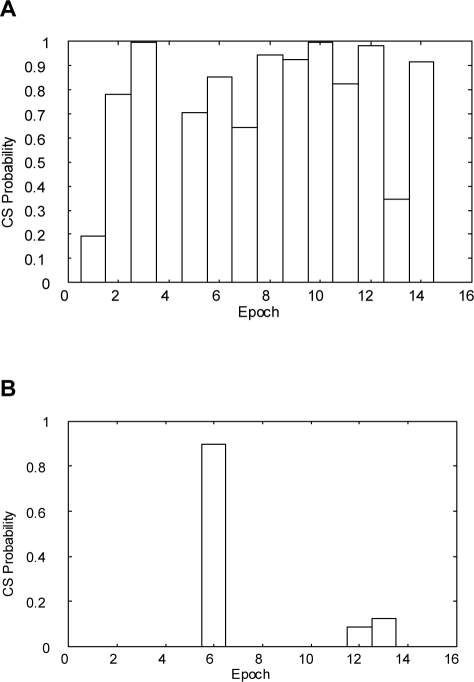
ApneaLink (ResMed, Sydney, Australia) is a single-signal screening device which measures flow via a nasal cannula connected to a pressure transducer. The device is also equipped with finger oximetry, but this feature was not used in the study. The flow signal is recorded with a sampling rate of 100 Hz and is pre-processed by linearizing the signal, filtering the noise, and zeroing the baseline. For analyzing the flow, the signal is split into epochs of 30 min as CSR occurs typically in periods of 20-30 minutes. The classifier of ApneaLink uses various features for identifying CSR. As shown in Figure 2, the main features are the cycle length (for CSR usually 30-90 s), the apnea-hypopnea length, the hyperpnea length, and the shape of the hyperpnea. The typical waxing and waning shape of the flow can be described in mathematical terms as a linear combination of various trigonometric functions. Furthermore, a so-called jump feature is analyzed, as displayed in Figure 3. Usually, the CSR hyperpnea starts with a moderate flow after the end of an apnea, and the hyperpnea of obstructive sleep apnea (OSA) begins typically with the so-called sudden breath. Thus, creating an envelope over a 2-s time base, the jump features of OSA and CSR are significantly different from each other. All features are combined into a pertinent set of parameters and tested by a discriminator providing a probability of CS-like breathing between zero and one. However, if in a definite epoch the discriminator provides a high probability for CSR in patients with both CSR and OSA, it is possible that obstructive events may occur in the same epoch as well. The resulting CSR probability can be marked up on a patient record or displayed as a time-series graph. The classification of each epoch could be displayed in a bar chart (Figure 4). This provides an indication of a patient record at a glance. In this study, the results provided by ApneaLink were based on total recording time, whereas the results derived from PSG were based on total sleep time.
Figure 2.
Classifier features of ApneaLink: apnea-hypopnea length, cycle length, hyperpnea length, hyperpnea shape, and hyperpnea initial jump feature.
Figure 3.
The jump feature is obtained by creating an envelope over a 2-s time base. (A) OSA jump feature: the maximum difference between 2 points is high compared to CSR jump feature. (B) CSR jump feature: the maximum difference between 2 points is low compared to OSA jump feature.
Figure 4.
Classification of each epoch displayed in a bar chart; one epoch = 30 min. (A) A report where many epochs show a high probability of CS-like breathing. (B) A report where a single epoch shows a high probability of CS-like breathing. This epoch could be investigated more closely.
Statistical Analysis
Using ROC curves, we obtained the best cut-off point of ApneaLink classifier probability threshold compared to the diagnosis of sleep experts in terms of sensitivity, specificity, and positive and negative likelihood ratios.21,22 The patient was considered to be positive for CSR if the probability value of CSR exceeded a specific threshold. The accuracy was the number of true positive and true negative cases divided by all cases.
RESULTS
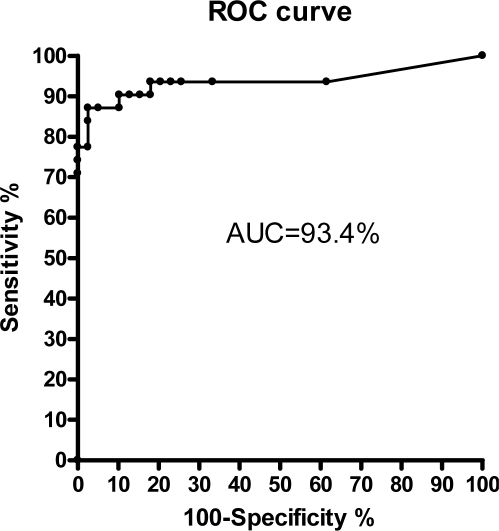
The ROC curve with different probability thresholds is displayed in Figure 5. The AUC was 93.4%. Best values for sensitivity and specificity regarding the CSR probability were yielded at a threshold of 0.5. We obtained a sensitivity of 87.1% and a specificity of 94.9% as shown in Table 2. We found 2 false positive results reflecting a low CSR probability of 0.56 and 0.57 (close to the threshold of 0.5). There were 4 false negative results: one included apneas of short cycle length and the other 3 contained no continuous clear CSR pattern, so that the experts relied heavily on non-flow information such as thorax and abdomen signals.
Figure 5.
Receiver operator characteristic (ROC) curve for the diagnosis of CSR given by sleep experts using PSG compared to the simultaneous ApneaLink using the CSR classifier results. CSR probability was varied between 0 and 1 in equidistant steps of Δp = 0.05.
Table 2.
Diagnostic Accuracy for CSR Regarding Classifier Probability Threshold of 0.5
| Sensitivity % (95% CI) | Specificity % (95% CI) | LR+ | LR− | Accuracy % |
|---|---|---|---|---|
| 87.1 (75.3-98.9) | 94.9 (87.9-100) | 17.0 | 0.14 | 91.4 |
CI denotes confidence interval, LR refers to likelihood ratio.
DISCUSSION
This study shows for the first time the utility of ApneaLink as a screening device for detecting CSR in a group of subjects with OSA, CSR, and no SDB. The ApneaLink classifier based on pattern recognition of nasal canula flow signal provided a high diagnostic accuracy for the detection of CSR when compared to the results of manually scored simultaneous PSG. The classifier of ApneaLink recognized CSR with a sensitivity of 87.1% and a specificity of 94.9%.
A limitation of ApneaLink at the current stage of development is that the classifier discriminates between CSR and non-CSR only. In theory, mixed apneas that look like central apneas of CSR are classified as CSR. Nevertheless, in this study, no false positive cases caused by incorrect classification of mixed apneas were identified.
Our study tested CSR detection by comparing ApneaLink with simultaneous PSG. Erman et al11 showed that the diagnostic accuracy regarding the AHI decreased when ApneaLink in a home setting was compared with ApneaLink from an attended laboratory-based case. The reason for different diagnostic accuracy under those conditions was night-to-night variation of the occurrence of respiratory events.23,24 Also, long-term loss of flow signal when the nasal cannula accidentally falls off during sleep occurs more often in the unattended home setting than in the attended laboratory-based PSG setting. Therefore, for future purposes a study should be undertaken to evaluate the diagnostic accuracy of the CSR classifier based on results obtained from a comparison between home studies and in-laboratory studies.
To our knowledge, ApneaLink is the first commercially available screening device that is aimed to detect CSR based on pattern recognition using the flow signal. We believe that the diagnostic accuracy could be improved if the ApneaLink classifier for CSR were based on a 2-channel screening device with additional use of pulse oximetry. In a recent study El-Solh16 demonstrated that a neural network based on spectral analysis of arterial oxygen saturation SpO2 could detect CSR with a sensitivity of 100% and a specificity of 99%. The astonishingly high accuracy reported in this study is explained by the use of a strict definition of CSR, which was defined as > 85% of SDB events being classified as central events. Based on these results, however, combining pattern recognition of flow and SpO2 in a single classifier would be a promising approach for detecting CSR. The higher diagnostic accuracy of such a 2-channel device would result from a more precise classification of respiratory events if signals from one or both channels are of low quality.
In summary, this study shows that ApneaLink equipped with CSR classifier is a useful device for detecting CSR. Due to the high prevalence of CSR in patients with congestive heart failure there is a need for suitable screening devices that are easy to use and available at low cost. ApneaLink is an appropriate screening tool that may help to prioritize patients with high pre-test probability of CSR for PSG.
DISCLOSURE STATEMENT
This study was sponsored by a research grant from ResMed Corporation. Dr. Weinreich has participated in paid speaking engagements. Dr. Armitstead is the Manager of Engineering Research for ResMed Ltd. Dr. Töpfer has participated in paid speaking engagements. Dr. Teschler has participated in paid speaking engagements and is on the advisory board of ResMed Corporation. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 2.Cherniack NS, Longobardo G, Evangelista CJ. Causes of Cheyne-Stokes. Neurocrit Care. 2005;3:271–9. doi: 10.1385/NCC:3:3:271. [DOI] [PubMed] [Google Scholar]
- 3.Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171:1048–52. doi: 10.1164/rccm.200411-1591OC. [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 6.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 7.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 8.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 9.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Teschler T, Weinreich G, Hess S, Wessendorf TE, Teschler H. Validation of microMESAM as screening device for sleep disordered breathing. Pneumologie. 2003;57:734–40. doi: 10.1055/s-2003-812423. [DOI] [PubMed] [Google Scholar]
- 11.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop CM. Neural networks for pattern recognition. Oxford University Press; 1995. [Google Scholar]
- 13.Duda RO, Hart PE, Stork DG. Pattern classification. New York: Wiley; 2001. [Google Scholar]
- 14.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning. Springer; 2001. [Google Scholar]
- 15.Theodoridis S, Koutroumbas K. Pattern recognition. Academic Press; 2006. [Google Scholar]
- 16.El-Solh AA, Magalang UJ, Mador MJ, et al. The utility of neural network in the diagnosis of Cheyne-Stokes respiration. J Med Eng Technol. 2003;27:54–8. doi: 10.1080/0309190021000043693. [DOI] [PubMed] [Google Scholar]
- 17.Weinreich G, Armitstead J, Teschler H. Pattern recognition of obstructive sleep apnoea and Cheyne-Stokes respiration. Physiol Meas. 2008;29:869–78. doi: 10.1088/0967-3334/29/8/002. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. Washington, DC: U.S. Government Printing Office; 1968. A Manual of standardized terminology, techniques, and scoring system for the sleep stages of human subjects. [Google Scholar]
- 19.American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:174–83. [PubMed] [Google Scholar]
- 20.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 21.Flemons WW, Littner MR. Measuring agreement between diagnostic devices. Chest. 2003;124:1535–42. doi: 10.1378/chest.124.4.1535. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 23.Fietze I, Dingli K, Diefenbach K, et al. Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir J. 2004;24:987–93. doi: 10.1183/09031936.04.00100203. [DOI] [PubMed] [Google Scholar]
- 24.Sahlin C, Svanborg E, Stenlund H, Franklin KA. Cheyne-Stokes respiration and supine dependency. Eur Respir J. 2005;25:829–33. doi: 10.1183/09031936.05.00107904. [DOI] [PubMed] [Google Scholar]