SYNOPSIS
Objectives
We designed a population-based study of the epidemiology of tuberculosis among foreign-born people in the U.S. and Canada. Challenges included standardizing recruitment and data entry at 22 sites, enrolling individuals who did not speak English and may be undocumented, and obtaining clearance from 36 institutional review boards (IRBs).
Methods
We used stratified sampling to recruit patients through the Tuberculosis Epidemiologic Studies Consortium, a research consortium funded by the Centers for Disease Control and Prevention. Because recruitment sites were overseen by more than 30 local IRBs, we developed a simple process to designate a central IRB. We translated instruments into 10 main languages, arranged for fast translation of consent “short forms” into other languages, used one telephone interpretation service at all sites, and provided extensive interviewer training including mock interviews with simulated patients.
Results
We interviewed 1,696 participants in 19 states and provinces. Participants from 99 countries were interviewed in 40 languages. Twenty-three percent did not speak English at all; 64% needed an interpreter. More than 20% of participants reported they were undocumented. Participants' age, gender, and birthplaces were broadly similar to the target populations. One-third of local IRBs used the central IRB.
Conclusions
Special confidentiality protections, substantial resources for translation and interpretation, and a centralized IRB made possible the recruitment of a representative sample of foreign-born people. The approaches may be applicable to studies of other diseases in multinational populations in the U.S. and Canada.
Research in the United States and Canada increasingly involves multicenter studies among populations with different cultural, linguistic, and national backgrounds.1,2 As the U.S. and Canada become more diverse, studies that do not include foreign-born people will become less generalizable.3 This is true of infectious diseases such as tuberculosis (TB)4 as well as chronic diseases such as cancer, diabetes, and other conditions5–7 that often differentially affect the foreign-born.
TB among foreign-born residents is one of the greatest challenges to TB elimination in the U.S. and Canada. Since 2002, foreign-born people have accounted for an increasing majority of TB cases in the U.S., and comprised 56.7% of the 13,767 cases reported in 2006. As TB rates have fallen faster among native-born residents, the gap between the two groups has widened. Since 1993, the rate ratio of foreign-born to U.S.-born people with TB has more than doubled, from 4.6 (34.0/100,000 and 7.4/100,000, respectively) to 9.5 (21.9/100,000 and 2.3/100,000, respectively).4,8 The U.S. experience mirrors trends in Canada and other Western nations.9,10
To close this gap, national guidelines have called for better data to guide TB control efforts.11,12 No national population-based study has ever gone beyond routinely collected surveillance data to provide an in-depth description of the epidemiology of TB in foreign-born people in the U.S. Critical topics in TB control among the foreign-born include the efficiency of pre-immigration screening, case finding in different immigrant populations, linguistic and cultural barriers that delay care-seeking, and physician and institutional delays in suspecting and diagnosing TB.12
Including foreign-born people in multicenter research requires flexibility and additional resources. In this article, we describe the design, organization, and implementation of a population-based cross-sectional study conducted in the U.S. and Canada to identify missed opportunities for prevention of TB in foreign-born people.
Designing and conducting such a study presented a number of significant challenges. The sampling plan needed to account for 22 recruitment sites with widely varying population sizes (Table 1). Consent forms and questionnaires had to be translated and interpreted for individuals who spoke a total of 40 different languages. In addition, it was expected that many of these individuals would be undocumented and would require additional protection against disclosure.2,13,14 Data from multiple sources would have to be linked together. Thirty-six institutional review boards (IRBs) would have to review and approve the protocol.
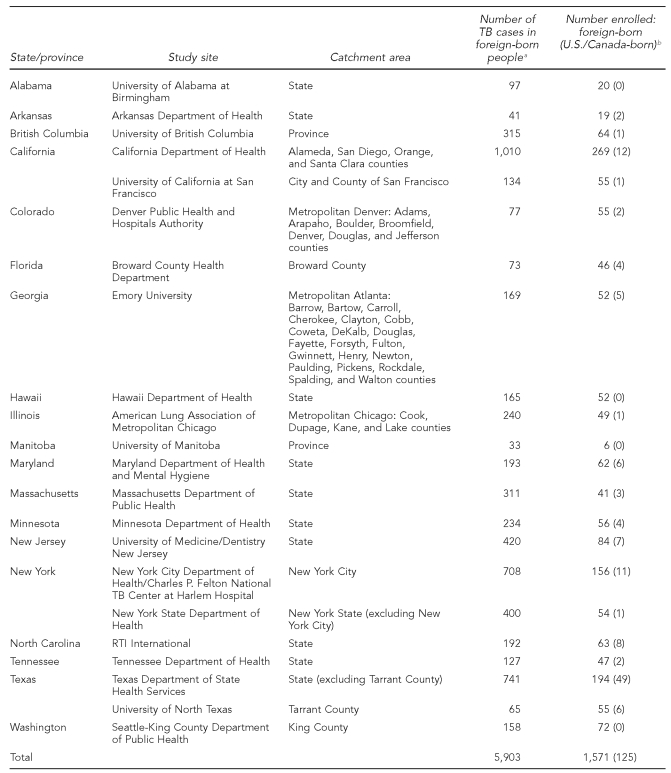
Table 1.
Study site catchment areas and number of TB cases in foreign-born people identified and enrolled during the study recruitment period
aNumber of foreign-born people with TB identified at the study sites between April 2005 and January 2007.
bIncludes four U.S.-born adult source cases and 121 U.S.- or Canadian-born children <5 years of age who had at least one foreign-born parent.
TB = tuberculosis
METHODS
Protocol development
Study team.
The study was conducted by the Tuberculosis Epidemiologic Studies Consortium (TBESC), which was established in 2001 to conduct programmatically relevant epidemiologic, behavioral, economic, laboratory, and operational research with the goal of eliminating TB in the U.S. The TBESC is funded by the Division of Tuberculosis Elimination, Centers for Disease Control and Prevention (CDC).15 The 20 U.S. and two Canadian study sites included academic institutions, public health departments, hospitals, a voluntary health organization, and a not-for-profit research organization (Table 1). In the U.S., the sites' catchment areas accounted for 69% of reported TB cases among the foreign-born in 2005; in Canada, the proportion was 23%.9,16
Data sources.
The study had three data sources: (1) an hour-long structured interview for demographic and epidemiologic information, (2) routine TB surveillance reports for clinical variables, and (3) federal immigration databases consisting of medical screening records of immigrants and refugees.
Study population.
The study population consisted of foreign-born people (i.e., born in a country outside the U.S. and its territories or Canada) with newly reported and verified cases of active TB diagnosed in TBESC catchment areas. In addition, U.S.- or Canadian-born children <5 years of age at diagnosis were eligible if they had a foreign-born parent or legal guardian. Such children were enrolled because it was suspected that they may have risk factors that are more similar to foreign-born children than to U.S.-born children of U.S.-born parents.17 Exclusion criteria included death, mental incapacitation, incarceration at the time of diagnosis or interview, or no longer living in the site's jurisdiction.
Data collection
Participant interviews
Eligible individuals had to be interviewed within 180 days of diagnosis, defined as the day the person was placed on TB medications. Proxy interviews of children <15 years of age were conducted with the child's parent or legal guardian. Children aged 15 to 17 years were interviewed directly or via proxy, according to parental preference. No other proxies were permitted.
Two structured questionnaires were developed: one to directly interview participants and the other to interview a parent or guardian about a child. Both questionnaires addressed:
Demographics (age, gender, nationality, and race/ethnicity) and socioeconomic status, including education, occupation, and income;
Circumstances of the TB diagnosis, including when and where the person was diagnosed and how the person came to medical attention;
Health insurance status before and at the time of diagnosis, and usual source of medical care;
Presentation and duration of TB symptoms;
Care-seeking history: number and types of resources (physicians, traditional healers, friends, self-medication) consulted before diagnosis;
Immigration history: visa status at first entry to the U.S./Canada and at the time of interview;
Missed opportunities for detection and diagnosis: history of previous TB exposure, TB diagnosis, tuberculin skin testing, and treatment for latent TB infection or active TB; and
Knowledge, attitudes, and beliefs about TB.
Medical examinations at entry to U.S./Canada.
To identify missed opportunities to prevent TB among refugees and immigrants, study participants who entered legally into the U.S. or Canada were linked by personal identifiers (name and date of birth) to databases at CDC's Division of Global Migration and Quarantine (DGMQ) or Citizenship and Immigration Canada (CIC). These databases provided information for immigrants and refugees who had arrived in the U.S. or Canada in the past 10 years with a radiological diagnosis of active or inactive TB detected during overseas medical screening. Applicants with acid fast bacilli (AFB) detected on sputum smear (and, in Canada, on sputum culture) were excluded from entry to the U.S. and Canada until treated. Matching was based on a probabilistic algorithm developed in collaboration with DGMQ. To ensure confidentiality, all data transmissions between DGMQ/CIC and the sites were via U.S./Canadian mail or private express mail.
Surveillance reports.
As part of nationally required reporting on TB, U.S. health departments enter information on individuals with confirmed cases of TB into a nationally standardized computer reporting system, the TB Information Management System (TIMS).16 Clinical data in the U.S. were obtained by each site from its city or state TIMS database, and by Canadian sites from their provincial TB databases. Data collected included demographic information; country of origin; the history of prior TB diagnosis; anatomical site of TB; results of tuberculin skin testing, microscopic examination for AFB, culture of sputum and other specimens, and drug susceptibility testing; treatment initiation date; and initial drug regimen.
Data management
CDC developed an automated, Web-based data entry system for use in this and other TBESC studies. Data entry personnel at each site logged into a secure, password-protected website; data entry screens incorporated skip patterns and error checks. The data entry was subject to two interactive reviews: one at the site and one centrally at CDC.
Sampling
The goal of the sampling plan was a representative sample of all foreign-born people ≥5 years of age and a complete capture of all eligible children <5 years of age reported with TB in the sites' TB control jurisdictions. Sample size calculations indicated that 1,500 cases would be sufficient to estimate a variety of prevalence estimates with acceptably narrow 95% confidence intervals (CIs).
Sample allocation.
The number of people with active TB to be enrolled at each site was based on prior CDC and Health Canada annual surveillance reports. Sites expecting <50 TB cases in foreign-born people (after exclusion of cases in children <5 years of age) attempted to enroll all eligible people. Sites with 50 to 250 reported TB cases in foreign-born people were to enroll 50 participants selected at random. Sites with more than 250 cases in foreign-born people were to enroll a 20% random sample. All eligible children <5 years of age were to be enrolled.
Sampling methods.
Within each recruitment site, subjects were to be selected using stratified sampling, with strata defined as the constituent counties, local or regional health department jurisdictions, or other geographic subdivisions. Early in the recruitment period, it was noted that response rates among people born in Vietnam and India (two countries accounting for an important proportion of foreign-born TB morbidity) were consistently lower than among other national groups; the recruitment strategy was altered to approach all people born in these two countries. Later, because of higher-than-expected refusal rates and lower-than-expected incidence, all but six sites ultimately ended up approaching all potential participants.
Quality control and assurance
Training.
Training is the first step in assuring quality control. The principal investigators and the protocol team developed a comprehensive training program for interviewers and project coordinators that included protocol review, principles of confidentiality and informed consent, questionnaire administration, and working with interpreters. Two films demonstrated proper interpretation techniques18 and the dangers of using untrained or family interpreters.19
A key aspect of training was practice interviews with simulated patients and interpreters who spoke multiple languages, including English, Spanish, Haitian Creole, and Hindi. All simulated patients were given roles that included immigration history, personality characteristics, and TB-specific clinical and epidemiologic factors. All study personnel were trained to administer the questionnaire in English and in tandem with in-person and telephone interpreters; bilingual staff were also trained in their second language. Trainers observed the interviews via closed-circuit television and provided guidance as needed. Interviews were recorded for further training. After each training, interviewers, simulated patients, and trainers met to get feedback on the training and to refine the pilot questionnaire and procedures.
The study team also developed a three-day multi-media training module for staff hired after the in-person trainings were completed. The module included an online component, conference calls, and telephone-based interviews with simulated patients.
Piloting.
As part of piloting, each site tested the questionnaires as well as its implementation plan in collaboration with health department personnel. The pilot confirmed that the questionnaire could be administered in a median of one hour, even with the use of an interpreter. The pilot questionnaires were also reviewed by a group of ethnically diverse community leaders who suggested ways to simplify questions and elicit sensitive information. One significant recommendation was to drop Likert scales from items about knowledge, attitudes, and behaviors, because many cultures are unfamiliar with such scales. The items were rewritten as questions with yes/no answers.
Recruitment plans
Identification of participants.
Each site maintained a list of all foreign-born people and U.S.-born children <5 years of age reported with TB within its jurisdiction; this list was updated periodically throughout the study. This information, along with variables for determining eligibility, was downloaded from each site's local TIMS database or a comparable local database that is the basis for TIMS reporting. Each Canadian site had access to a similar system in its own province. This information was used to identify and select eligible people for interviews and to track recruitment.
Language assessment.
Once a site identified a potential participant, the site research coordinator contacted the person's local case manager, physician, or other health-care provider, who would briefly introduce the study and obtain verbal agreement for the interviewer to contact the potential participant directly. In some cases, efficiencies were achieved when the research coordinator or interviewer worked or volunteered in the responsible health department and could make initial contact.
The interviewer's first task was to assess the participant's English-language ability by asking a standardized question that has been repeatedly included in the long-form questionnaire of the U.S. decennial census:20 “I would like to know how well you speak English. Would you say you speak English very well, well, not well, or not at all?” Those individuals who said they spoke English “very well” were consented and interviewed in English; all others were consented and interviewed with the assistance of an interpreter or bilingual interviewer in their chosen language.
Translation and interpretation.
Questionnaires and consent forms were translated from English into 10 languages. Eight languages reflected the most common origins of foreign-born people with TB at the national level (Chinese, French, Haitian Creole, Hindi, Korean, Spanish, Tagalog, and Vietnamese), and two languages accommodated locally important populations (Ilocano in Hawaii and Somali in Minnesota). All interpreters used these documents, including the professional translation service that conducted phone-based interpretation for all TBESC sites, and the in-person interpreters working at individual sites. For participants who spoke languages other than these 10, professional interpretation of study documents occurred at the time of the interview.
Facilitating interviews.
To accommodate participants' work schedules, concerns about confidentiality, and other lifestyle issues, interviewers were allowed to interview participants at whatever times and places were convenient for the participants, consistent with safety and confidentiality: after hours and on weekends, in private homes, restaurants, libraries, and other places in addition to clinics, hospitals, and offices. To pay for transportation and interview time, each participant was offered $30.
Human subjects protection
A study conducted at 22 sites that includes participants who do not speak English and who may be in the country illegally presents special problems related to (1) participant confidentiality and (2) the complexities associated with obtaining dozens of IRB approvals. The study team worked with CDC to obtain additional protection for participants and with local IRBs to streamline the IRB approval process.
Confidentiality.
In addition to the usual steps taken to ensure confidentiality (e.g., removal of patient identifiers before data entry), each site was also provided with a Certificate of Confidentiality. Created by federal legislation in 1986 and issued by the Department of Health and Human Services (DHHS), a Certificate of Confidentiality states that research study participants' information is protected against outside requests for access, such as subpoenas, Freedom of Information Act requests, and inquiries from federal agencies. Certificates of Confidentiality are authorized in situations in which participants are asked for extremely sensitive information (e.g., current visa status) or about possibly illegal activities (e.g., drug use). DHHS, through CDC, approved Certificates of Confidentiality for all U.S. sites.
IRB review.
Thirty-six IRBs had to review and approve the study protocol and its consent documents prior to study enrollment; the number of IRBs exceeds the number of recruitment sites because several sites were collaborations among health departments and universities or other institutions. Preserving the integrity of consent forms during the review process was essential because of the 11 study languages (10 foreign languages plus English) and the three consent forms (parental permission, adolescent assent, and adult consent). After CDC review and approval of the consent forms, study researchers developed a standard letter that explained the difficulty of making site-specific changes, pointed out that the consent met all federal requirements, and asked that the IRB approve the consents with no changes. Ultimately, all 36 IRBS approved the consents as written.
Federal regulations allow local IRBs to cede oversight of protocols to a single IRB—called a central IRB—to improve efficiency.21 The study team developed a simple process that allowed a local IRB to designate the CDC IRB as the central IRB for the study. While local IRBs would still review and approve the initial protocol, the central IRB could review and approve all amendments and continuations. In the course of the study, one-third of the IRBs agreed to use CDC or another central IRB, thereby saving local resources.
Consent forms.
All adults provided written informed consent; parents provided written permission for children <18 years of age, and adolescents aged 15 to 17 years provided written assent. All consent forms were written at a sixth-grade reading level. For people who spoke one of the 11 primary languages of the study, fully translated consent forms were available. For people who were illiterate or did not read any of the 11 languages for which we had full consent forms, interviewers were instructed to read the consent word for word.
In situations in which a potential participant speaks a relatively uncommon language, federal regulations permit the use of a short form in the person's language that explains, in general terms, what a person should know about a study before participating (i.e., what the study is about, how much time it will require, whether compensation is provided, and the other required elements of consent).22 The study team created a one-page short form that explained the required aspects of an epidemiologic study, modeled on a short form for clinical studies.23 This form, which the participant reads and signs, is used in combination with a verbal translation of the full consent form by a trained interpreter. Our ability to produce a short form within one week meant that we could easily interview patients speaking rare additional languages that we could not have easily predicted. By the conclusion of the study, we had produced short forms in 28 languages.
RESULTS
From April 1, 2005, through January 31, 2007, we recruited 1,696 study participants (113% of the recruitment goal of 1,500), including 1,511 foreign-born people ≥15 years of age. Of the 22 sites, 12 reached at least 100%, four reached at least 90%, three reached at least 80%, and three reached <50% of their recruitment goals.
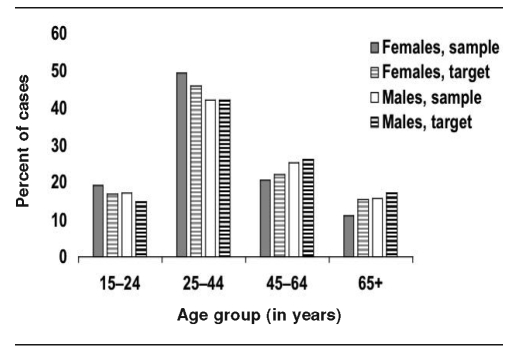
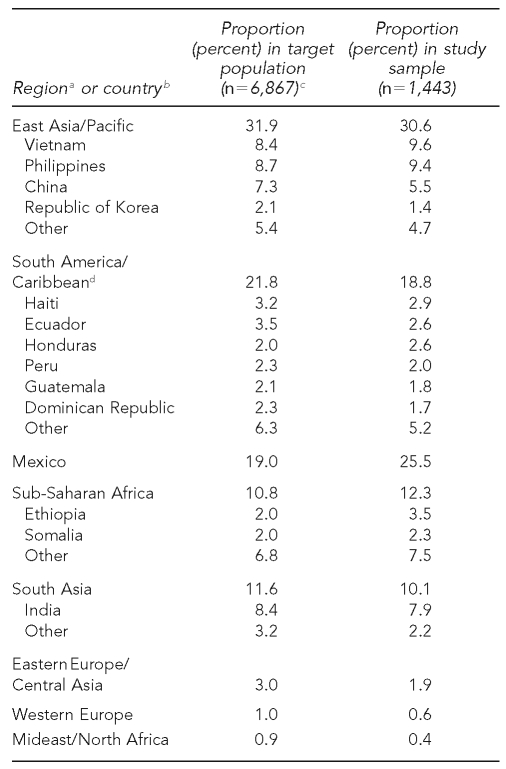
The Figure and Table 2 compare age, gender, and birth countries of adolescents and adults recruited by U.S. study sites (study sample) with all foreign-born people reported to CDC's TB surveillance system from those sites during the recruitment period who were alive and not incarcerated at diagnosis (i.e., the target population). The study sample was broadly representative of the target population with regard to these key variables.
Figure.
Age and gender of adolescents and adults recruited by U.S. study sites
Table 2.
Comparison of region/country of birth of reported cases (target population) and enrolled participants (study sample) ≥15 years of age at all U.S. sites
aBased on groupings developed by the World Bank. Available from: URL: http://web.worldbank.org/WBSITE/EXTERNAL/COUNTRIES/0,,pagePK:180619~theSitePK:136917,00.html [cited 2008 Aug 13].
bSpecific birth countries listed include those accounting for at least 2% of the target or study population.
cSource: Division of TB Elimination, Centers for Disease Control and Prevention, Atlanta
dIncludes all countries south of Mexico.
Almost 90% of study participants were willing and able to report their visa status. Of the 1,511 foreign-born adolescents and adults, 22 (1.5%) refused and 19 (1.3%) didn't know their visa status. An additional 149 people (9.9%) from New York City were not asked their visa status because of a city ordinance prohibiting such questions. Of the 1,321 subjects who provided their visa status at the time of interview, 308 (23.3%) reported that they were undocumented.
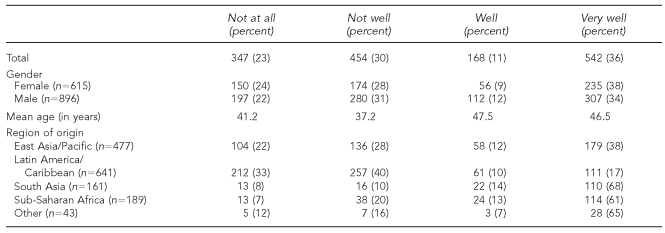
Participants were interviewed in 40 languages using bilingual interviewers, in-person interpreters, and telephone interpreters; prominent languages included English (38.1%), Spanish (33.2%), Vietnamese (7.3%), and Chinese (5.5%). Only nine people could not be recruited because they spoke or signed a language for which no interpreter could be found. As expected, English-language facility varied by birthplace (Table 3).
Table 3.
Responses of 1,511 foreign-born adults and adolescents (aged 15 to 17 years) to the question, “How well do you speak English?”, by gender, age, and place of origin
Based on the survey design and total recruitment, the approximate design effect is 1.2. The design effect is a measure of how closely the study design approximates a simple random sample. The closer the design effect is to the value of 1, the more the design is similar to one in which all subjects are recruited as a simple random sample. Calculation of the design effect is based on the size of the population of interest at each recruitment site and the expected within-site correlation of the primary variables of interest. The equivalent number of cases sampled via simple random sampling is given by the actual number recruited divided by the design effect.24 Therefore, our sample is equivalent to a randomly selected sample size of 1,422 people. Prevalence estimates of 50.0% will be accurate to within 62.6% (with 95% CI), while estimates of 25.0%, 10.0%, and 5.0% will be accurate to within 62.3%, 61.6%, and 61.1%, respectively. For any subsample of size ≥226, prevalence estimates ranging from 5.0% to 50.0% will be accurate to within 3.0%.
The 22 sites identified 5,903 foreign-born people diagnosed within their jurisdictions during the study period. Of those, 1,400 were ineligible because they were identified more than 180 days after diagnosis (n=651), had moved out of the jurisdiction or were diagnosed in another jurisdiction (n=241), were dead at diagnosis or died before they could be interviewed (n=221), were incarcerated at diagnosis (n=132), were incapable of providing consent (n=58), were subsequently determined not to have TB (n=28), or for another reason (n=69). This resulted in a sampling frame of 4,503 eligible individuals, from which 3,722 were randomly selected (including sites that sampled at 100%). The mean study response rate (proportion of people approached who agreed to participate) was 53% (range 32, 97). Future data analyses will take into account the study sampling design and be statistically weighted for nonresponse.
DISCUSSION
This is the first national epidemiologic study of TB to include interviews of foreign-born people—a minority population that contributes the majority of newly reported TB cases in the U.S. and Canada.
A study in which all participants were born in another country and speak English as a second -language or not at all presents numerous recruitment challenges. Many subjects lacked proper immigration documents, making them potentially more apprehensive about efforts to recruit them. This study demonstrated that it is possible to overcome these challenges on a national level. Factors that aided the successful recruitment of such a large, diverse study population included the availability of a consortium infrastructure, special confidentiality protections for vulnerable subjects, substantial resources devoted to translation and interpretation, and extensive training and oversight. Another key factor was getting all 36 local IRBs to collaborate and permit the use of standard, centrally approved consent documents at all 22 study sites. This eliminated the potential for introducing errors in the local revision process25 and permitted central translation of the adult, pediatric, and adolescent consent documents into 10 languages other than English.
Findings from this study will help public health practitioners and private clinicians close the gap in TB rates between native-born and foreign-born people in the U.S. and Canada. Initial analyses of these data include:
Epidemiology of TB among the foreign-born
Highly infectious (i.e., AFB smear-positive) foreign-born TB cases: epidemiology and implications for TB control needs
Barriers to care and delays in diagnosis of TB among the foreign-born due to patient or health system factors
The effect of federal and state screening for TB among the foreign-born: implications for national policies
Contact investigations and targeted testing for latent TB infection among the foreign-born
Epidemiology of TB in foreign-born youth and children
Knowledge, attitudes, and beliefs about TB among foreign-born TB cases
CONCLUSION
We believe that the approaches we used may be applicable to the design of other multisite epidemiologic studies of diseases that involve foreign-born populations in the U.S. and Canada. Both countries annually receive large numbers of immigrants, refugees, and visitors from many nations. In such diverse societies, scientists need to reach out to many ethnic, linguistic, and national groups to make study findings applicable to all. Our experience offers guidance in meeting the challenges such studies pose.
REFERENCES
- 1.McWilliams R, Hoover-Fong J, Hamosh A, Beck S, Beaty T, Cutting G. Problematic variation in local institutional review of a multi-center genetic epidemiology study. JAMA. 2003;290:360–6. doi: 10.1001/jama.290.3.360. [DOI] [PubMed] [Google Scholar]
- 2.Schmidley AD. Current Population Reports, P23-206. Washington: U.S. Government Printing Office; 2001. Dec, [cited 2008 Aug 29]. Profile of the foreign-born population in the United States: 2000. Also available from: URL: http://www.census.gov/prod/2002pubs/p23-206.pdf. [Google Scholar]
- 3.Laren LJ. Current Population Reports, P20-551. Washington: U.S. Government Printing Office; 2004. Aug, [cited 2008 Aug 29]. The foreign-born population in the United States: 2003. Also available from: URL: http://www.census.gov/prod/2004pubs/p20-551.pdf. [Google Scholar]
- 4.Trends in tuberculosis incidence—United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56(11):245–50. [PubMed] [Google Scholar]
- 5.Murphy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 6.The DPP Research Group. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials. 2002;23:157–71. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 7.Janson SL, Alioto ME, Boushey HA. Asthma Clinical Trials Network. Attrition and retention of ethnically diverse subjects in a multicenter randomized controlled research trial. Control Clin Trials. 2001;22(6 Suppl):S236–43. doi: 10.1016/s0197-2456(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US) Reported tuberculosis in the United States, 2006. Atlanta: Department of Health and Human Services (US); 2007. Sep, [Google Scholar]
- 9.Public Health Agency of Canada. Tuberculosis in Canada, 2005. [cited 2008 Aug 27]. Available from: URL: http://www.phac-aspc.gc.ca/publicat/2008/tbcan05/index-eng.php.
- 10.Institut de Veille Sanitaire. Surveillance of tuberculosis in Europe: report on tuberculosis cases notified in 2004. Saint-Maurice (France): Institut de Veille Sanitaire; 2006. Feb, [cited 2008 Aug 27]. Also available from: URL: http://www.eurotb.org/rapports/2004/eurotb_report_2004.pdf. [Google Scholar]
- 11.Institute of Medicine, Committee on the Elimination of Tuberculosis in the United States. Ending neglect: the elimination of tuberculosis in the United States. Washington: National Academy Press; 2000. [PubMed] [Google Scholar]
- 12.Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 13.Independent Task Force on Immigration and America's Future. Immigration and America's future: a new chapter. Washington: Migration Policy Institute; 2006. Sep, [Google Scholar]
- 14.Weis SE, Moonan PK, Pogoda JM, Turk LE, King B, Freeman-Thompson S, et al. Tuberculosis in the foreign-born population of Tarrant County, Texas, by immigration status. Am J Respir Crit Care Med. 2001;164:953–7. doi: 10.1164/ajrccm.164.6.2102132. [DOI] [PubMed] [Google Scholar]
- 15.Katz D, Albalak R, Wing JS, Combs V. Setting the agenda: a new model for collaborative tuberculosis epidemiologic research. Tuberculosis. 2007;87:1–6. doi: 10.1016/j.tube.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (US) Reported tuberculosis in the United States, 2005. Atlanta: Department of Health and Human Services (US); 2006. Sep, [Google Scholar]
- 17.Lobato MN, Hopewell PC. Mycobacterium tuberculosis infection after travel to or contact with visitors from countries with a high prevalence of tuberculosis. Am J Respir Crit Care Med. 1998;158:1871–5. doi: 10.1164/ajrccm.158.6.9804106. [DOI] [PubMed] [Google Scholar]
- 18.Francis J. Making the connection: an introduction to interpretation skills for TB control. San Francisco: Francis J. Curry National Tuberculosis Center; 2003. Jul, [Google Scholar]
- 19.Grainger-Monsen M, Haslett J. Stanford University Center for Biomedical Ethics. Harriman (NY): Fanlight Productions; 2003. Worlds apart: a four-part series on cross-cultural healthcare. [Google Scholar]
- 20.Kominski R. How good is “how well”? An examination of the Census English-speaking ability question. Presented at the American Statistical Association Annual Meeting; 1989 Aug 6–11; Washington. [Google Scholar]
- 21.U.S. Federal Code. Central Institutional Review Board. Title 45 of the Code of Federal Regulations, sec. 46.114. [Google Scholar]
- 22.U.S. Federal Code. Protection of human subjects. Title 45 of the Code of Federal Regulations, sec. 46.117(b)(2) [PubMed] [Google Scholar]
- 23.Tuberculosis Trials Consortium, Division of TB Elimination, Centers for Disease Control and Prevention (US) The Tuberculosis Trials Consortium: a model for clinical trials collaborations. Public Health Rep. 2001;116(Suppl 1):S41–9. doi: 10.1093/phr/116.s1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohr SL. Sampling: design and analysis. Pacific Grove (CA): Duxbury Press; 1999. [Google Scholar]
- 25.Burman W, Breese P, Weis S, Bock N, Bernardo J, Vernon A, Tuberculosis Trials Consortium The effects of local review on informed consent documents from a multicenter clinical trials consortium. Control Clin Trials. 2003;24:245–55. doi: 10.1016/s0197-2456(03)00003-5. [DOI] [PubMed] [Google Scholar]