Abstract
Objectives
To assay if plasma antibody levels in children with autism or developmental delays (DD) differ from those with typical development as an indicator of immune function and to correlate antibody levels with severity of behavioral symptoms.
Methods
Plasma was collected from children with autistic disorder (AU; n=116), DD but not autism (n=32), autism spectrum disorder but not full autism (n=27), and age-matched typically developing (TD) controls (n=96). Samples were assayed for systemic levels of immunoglobulin (IgG, IgM, IgA, and IgE) by enzyme-linked immunosorbent assay. Subjects with autism were evaluated using the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview—Revised, and all subjects were scored on the Aberrant Behavior Checklist (ABC) by the parents. Numerical scores for each of the ABC subscales as well as the total scores were then correlated with Ig levels.
Results
Children with AU have a significantly reduced level of plasma IgG (5.39±0.29 mg/mL) compared to the TD (7.72±0.28 mg/mL; P<0.001) and DD children (8.23±0.49 mg/mL; P<0.001). Children with autism also had a reduced level of plasma IgM (0.670.06mg/mL) compared to TD (0.79±0.05 mg/mL; P<0.05). Ig levels were negatively correlated with ABC scores for all children (IgG: r=−0.334, P<0.0001; IgM: r=−0.167, P=0.0285).
Conclusion
Children with AU have significantly reduced levels of plasma IgG and IgM compared to both DD and TD controls, suggesting an underlying defect in immune function. This reduction in specific Ig levels correlates with behavioral severity, where those patients with the highest scores in the behavioral battery have the most reduced levels of IgG and IgM.
Keywords: Autism, immunoglobulin, behavior, IgG
Introduction
Autism spectrum disorders (ASD) are a group of heterogeneous, behaviorally defined disorders characterized by disturbances in both verbal and non-verbal communication, language, imagination, and social interaction, often with repetitive and stereotyped behavior [APA, 1994]. The prevalence has recently been estimated to be 1:150 in the total population and affects approximately four times as many males as females [MMWR, 2007]. ASD is diagnosed between 2 and 4 years of age, when children begin to take part in structured social interactions. While no specific autism genes have been identified to date, autism is believed to have a complex heritability involving several susceptibility genes that may impact both neurodevelopment and immune function. How genes associated with autism interact among themselves and how their phenotypic penetrance is influenced by epigenetic and environmental factors are poorly understood.
The potential role of the immune system in the etiology of autism arises from emerging evidence of a dysregulated or abnormal immune response in children with ASD [reviewed in Ashwood, Wills, & Van de Water, 2006]. Hypotheses that implicate the immune system in the etiology of a behavioral disorder are somewhat controversial. However, the molecular and cellular mechanisms that interconnect the immune and nervous systems are becoming more clearly understood. For example, cytokines and other products of immune activation have widespread effects on neuronal pathways and can alter behaviors such as mood and sleep throughout life [Hogan, Morrow, Smith, & Opp, 2003; Larson, 2002]. Further, the immune and nervous systems are interdependent such that disruption of either system, especially during early development, has the potential to alter the function of the other [Biber, Zuurman, Dijkstra, & Boddeke, 2002; Huh et al., 2000; Marques-Deak, Cizza, & Sternberg, 2005; Mehler & Kessler, 1998; Mignini, Streccioni, & Amenta, 2003; Rothwell, Luheshi, & Toulmond, 1996]. Elucidation of the mechanisms responsible for the observed abnormalities in immune function noted in autism may therefore provide valuable insights into the etiology of this disorder.
Various immune system components and mediators including cytokine and immunoglobulin (Ig) levels, cell numbers and function including monocytes/macrophages, and natural killer cells have all been investigated in autism [Engstrom et al., 2003; Jyonouchi, Sun, & Le, 2001; Sweeten, Bowyer, Posey, Halberstadt, & McDougle, 2003; Warren, Foster, & Margaretten, 1987; Warren et al., 1990].
However, such findings have often been inconsistent due to numerous methodologic factors, the most notable of which are the use of inappropriate and non-age-matched control groups, as well as heterogeneous, ill-defined subject populations. Despite the current lack of consensus, it is becoming more widely accepted that one or more subsets of children with autism may present with abnormal or dysregulated immunity.
Ig are part of the humoral immune response, the net result of a specific response orchestrated by the complex interaction between dendritic cells, T cells, and Ig-producing B cells. Ig levels are therefore a means to measure not only immune development but successful humoral immune function as well. Ig are of particular interest in childhood disorders because levels are very low at birth and it may take up to 10 years for certain isotypes to reach adult levels [Aksu, Genel, Koturoglu, Kurugol, & Kutukculer, 2006]. Herein, we describe decreased levels of IgG and IgM in children with autism. In addition, we analyzed the relationship between plasma levels of IgG and IgM and behavior.
Methods
Sample Collection
All children (n=271) including children with autism (AU; n=116), children with the broader definition of ASD (ASD; n=27), children who are developmentally delayed (DD) but do not have autism (n=32), and typically developing (TD) control children (n=96) participated in the Childhood Autism Risks From Genetics and the Environment (CHARGE) study [Hertz-Picciotto et al., 2006]. Those children enrolled in the study met the following criteria: (a) they were between the ages of 24 and 60 months at the time, (b) lived with at least one biologic parent, (c) had a parent who spoke English or Spanish, (d) were born in California, and (e) resided in the catchment areas of a specified list of regional centers in Northern California. The CHARGE study is an ongoing population-based case–control study with subjects sampled from three strata: children with AU or ASD, children with DD but not autism or ASD, and children selected from the general population (i.e., TD). The demographics of the study population are illustrated in Table I. The age distribution of all subjects is further illustrated in Figure 1. This study protocol followed the ethical guidelines of the most recent Declaration of Helsinki [Edinburgh, 2000], and was approved by the institutional review boards at the University of California, Davis, and The State of California Department of Developmental Services. Informed consent was obtained prior to participation.
Table I.
Age and Behavior Distribution of Children With Autism, ASD, Delay, and Typical Development, n =271
| n | Age (mo) | Range | Male | Female | ABC | Range | |
|---|---|---|---|---|---|---|---|
| Autism | 116 | 42.8±9.8 | 25–64 | 106 | 10 | 56.3±23.6 | 15–126 |
| Early onset | 60 | 41.8±9.9 | 25–64 | 56 | 4 | 53.8±23.1 | 16–126 |
| Regressive | 56 | 43.7±9.6 | 27–62 | 50 | 6 | 59.0±24.2 | 15–121 |
| ASD | 27 | 39.8±9.4 | 27–62 | 22 | 5 | 45.0±31.3 | 9–120 |
| Delayed | 32 | 44.1±10.6 | 24–60 | 19 | 13 | 30.7±30.8 | 1–106 |
| Typical | 96 | 39.8±8.2 | 26–59 | 72 | 24 | 7.3±9.8 | 0–45 |
ASD, autism spectrum disorder; ABC, Aberrant Behavior Checklist.
Figure 1.
Distribution of age within behavioral diagnosis. There was no significant difference in age between groups.
Measures and Procedures
To confirm and further detail the initial diagnosis, all children were assessed at the University of California, Davis, M.I.N.D. Institute. The diagnosis of autism was confirmed in all subjects using the Autism Diagnostic Interview—Revised (ADI-R) [Lord et al., 1997] and the Autism Diagnostic Observation Schedule (ADOS) module 1 or 2 [DiLavore, Lord, & Rutter, 1995; Joseph, Tager-Flusberg, & Lord, 2002; Lord, Leventhal, & Cook, 2001; Owley et al., 2001]. The ADI-R provides a standardized, semi-structured interview and a diagnostic algorithm for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [APA, 1994], and International Classification of Diseases, Tenth Revision, definitions of autism [Steinhausen & Erdin, 1992]. All CHARGE study clinical assessment personnel have attained research reliability on the ADI-R and the ADOS and include bilingual and bicultural staff that administered assessment measures in Spanish for Spanish-speaking families unable to participate in English. Bilingual, bicultural assessors experienced in standardized assessments in English were sensitive to translating the assessment items in culturally relevant terms. Although the assessment measures used have not yet been translated and validated in Spanish, these measures are used clinically in assessing non- English-speaking children, and the use of bilingual and bicultural assessors eliminated the use of non-trained interpreters for administration and facilitated the inclusion of a large segment of the sample population. Children classified with ASD (ASD sample) did not meet the full criteria for autism on either or both the ADI-R and ADOS instruments, but did meet the criteria on either the communication or the social interaction domain of the ADI-R prior to 36 months, were within 2 points of the cut-off on the other domain, and were above the social+communication cut-off for ASD on the ADOS module 1 or 2. The sample designation AU-ASD refers to the combined AU and ASD subgroups. The Social Communication Questionnaire was used to screen for behavioral and developmental characteristics of ASD among the DD and TD controls; children who scored above the screening cut-off score of 15 were fully assessed using the ADI-R and the ADOS. Those who met the criteria for autistic disorder were classified as AU; similarly any general population children who met the criteria for DD based on the Vineland Scales of Adaptive Behavior [Sparrow, Balla, & Cicchetti, 1984] and the Mullen Scales of Early Learning [Mullen, 1995] were classified as DD (scores for cognitive and adaptive function below 70). Controls who did not meet the criteria for ASD or for DD were classified as TD.
Definitions of Onset
Using clinical characteristics reported in the Early Development Questionnaire [Ozonoff, Williams, & Landa, 2005] and answers to questions regarding loss of language (Q11) and social skills (Q25) from the ADI-R, the autism population was further divided into two groups based on the clinical onset of autistic symptoms: firstly, children with regression who initially developed but subsequently lost previously acquired language and/or social skills (n=56; 50 males, 6 females) and, secondly, children with early onset autism characterized by early deficits in the requisite behavioral domains (n=60; 56 males, 4 females) [Hansen et al., 2008].
All instruments and forms were administered in either English or Spanish, depending on the language in which the parent or child felt most comfortable.
Aberrant Behavior Checklist (ABC)
Children were administered the ABC behavioral assessment prior to blood draw. The ABC is a series of 58 questions that are answered by parents as a means to assess the severity of behavioral symptoms associated with autism with a total score range of 0–174. The 58 questions are further broken down into five subscales: subscale I (irritability) range 0–45, subscale II (lethargy) range 0–48, subscale III (stereotypy) range 0–21, subscale IV (hyperactivity) range 0–48, and subscale V (inappropriate speech) range 0–12.
Plasma Collection
Ten milliliters of blood from each child was collected in yellow top citrate tubes (BD Biosciences, Franklin Lakes, NJ) according to the study protocol and centrifuged at 900g for 10 min to pellet cells. Plasma was collected and immediately frozen in 0.5mL aliquots at −80°C until assayed for Ig levels.
ELISA
Levels of total IgG, IgM, IgA, and IgE were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits purchased from ALerCHEK Inc. (Portland, ME). Kits were run according to the manufacturer’s instructions. Briefly, samples were diluted 1:100,000 (IgG), 1:10,000 (IgM and IgA), or 1:10 (IgE) and added to 96 well plates pre-coated with capture antibody. After 1-hr incubation and subsequent washing, horseradish peroxidase- conjugated detection antibodies were added and TMB (3,3′, 5,5′ tetramethyl benzidine)/peroxide substrate used for development. Data are reported as median mg/mL (IgG, IgM, IgA) or in international units (IU/mL) (IgE). Intra- and inter-assay variability was controlled for using control standards on each plate. The coefficient of variance was less than 10% on any given plate.
Statistical Analysis
All analyses were carried out using SAS version 9.1 (SAS Institute Inc., Cary, NC). The Kruskal–Wallis test was used to compare median levels of total Ig levels with diagnostic groups. The Spearman correlation coefficient (r) was calculated to assess the association between Ig levels and behavior scores: 0.2< (r)>0.29 was considered as a weak correlation, 0.30<(r)>0.39 was considered as moderate, 0.40<(r)>0.69 was considered as strong, and 0.70<(r)>1.00 was considered as very strong. P-values<0.05 were considered as statistically significant.
Results
Decreased Levels of IgG and IgM in Children With Autism
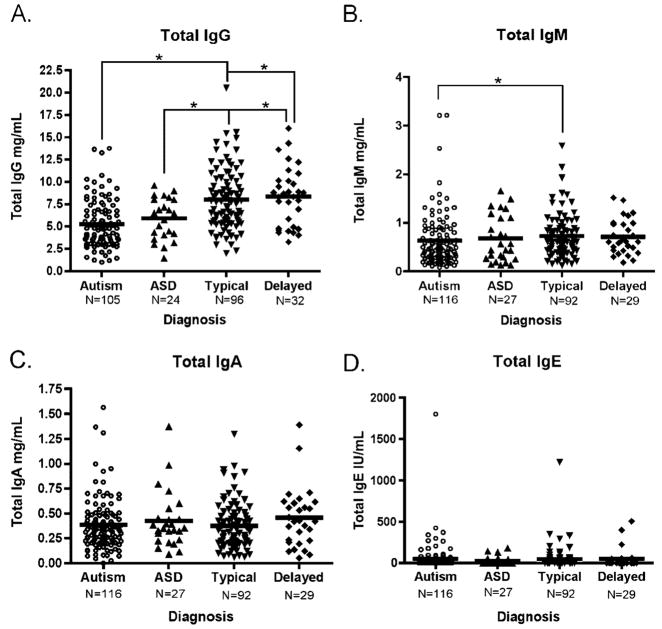
Plasma Ig levels were analyzed in this study as one measure of humoral immune function. A pronounced difference in plasma Ig levels between children with autism and TD controls was noted in the concentration of plasma IgG. Children with AU demonstrated a significantly lower median level of total IgG (4.77 mg/mL, interquartile range (IQR) 3.27–6.77) compared with both TD controls (7.76 mg/mL, IQR 5.52–10.20, P<0.0001) and children with DD (8.54 mg/mL, IQR 4.85–10.14, P<0.0001) (Fig. 2). Correction for age, sex, and allergy season did not alter significance. Allergy season was defined as “high” from February to June and “low” from July to January. Therefore, blood samples collected during months of February through June were ranked as high allergy season. An indicator variable for allergy season (high=1, low=0) was then fitted into a linear regression model to adjust for seasonality.
Figure 2.
Median plasma immunoglobulin levels as determined by ELISA. Plasma from children diagnosed with AU, ASD, DD, or TD controls was assayed for levels of total immunoglobulin by ELISA. Children with AU displayed a significantly reduced level of total IgG when compared to both TD controls and children with developmental delay (A). Total IgM levels were also significantly reduced in children with AU compared to TD controls (B). In contrast, both total IgA (C) and IgE (D) levels showed no difference between any of the groups. Analysis performed using Kruskal–Wallis test, *P=0.01, **P<0.001. Correction for age and allergy season did not alter significance. ELISA, enzyme-linked immunosorbent assay; ASD, autism spectrum disorder; DD, developmental delay; TD, typically developing; Ig, immunoglobulin.
The ASD group, in which children exhibit autism-like behaviors but do not fully meet the strict criteria in our study for an AU diagnosis, had plasma IgG levels that while higher than those of the AU group (6.47 mg/mL, IQR 4.12–8.04, P=0.01) remained significantly lower than the TD control group. Moreover, children with AU had significantly lower levels of plasma IgM (0.48 mg/mL, IQR 0.29–0.85) than TD controls (0.65 mg/mL, IQR 0.45–0.89, P=0.01).
The diminished IgM levels within the AU group were more pronounced in the regressive AU phenotype (0.41 mg/mL, IQR 0.27–0.75) compared with the early onset AU phenotype (0.56 mg/mL, IQR 0.30–0.89). However, neither the ASD population (0.57 mg/mL, IQR 0.27–1.11) nor those subjects with DD (0.62 mg/mL, IQR 0.43–0.97) demonstrate a significant difference when compared to any of the control groups for plasma IgM.
Of interest, values for total IgA show no significant difference when comparing the subjects with AU (0.33 mg/mL, IQR 0.22–0.48) or ASD (0.35 mg/mL, IQR 0.23–0.45) to either the TD (0.34 mg/mL, IQR 0.21–0.48) or DD (0.42 mg/mL, IQR 0.23–0.60) subject groups. Likewise, total median levels of IgE do not significantly differ among any of the diagnostic groups. The IgE levels for subjects with AU and ASD are typically very low with a large range of values within each subject group (AU: 0.40 IU, IQR 0.00–42.39; ASD: below detection IU, IQR 0.00–33.84). IgE levels were also low in the DD group (4.00 IU, IQR 0.00–43.84), while TD subjects had the highest plasma IgE median values (10.76 IU, IQR 0.00–30.04). However, levels of IgE did not significantly differ among any of the diagnostic groups.
Ig Levels Negatively Correlate With ABC Scores
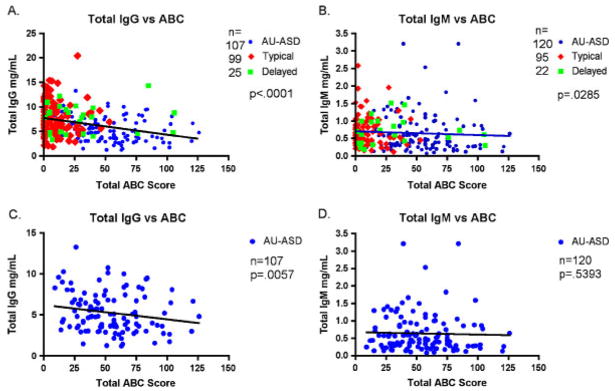
In addition to the evaluation of plasma Ig levels by subject diagnosis, we also examined the possible association between IgG and IgM levels and the ADOS and ABC behavior scores. When all subject groups were combined, IgG levels were significantly negatively correlated with total ABC score (r=−0.334, P<0.0001) (Fig. 3A and B). Thus, the lower the plasma IgG level, the higher the ABC score, indicating increased severity of behavioral symptoms that are associated with autism. The same relationship was noted for IgM, although to a lesser degree of significance (r=−0.167, P=0.03). When the AU/ASD subject population is analyzed without the control subjects for IgG and IgM vs. ABC outcome, there remains a significant negative correlation between total plasma IgG and ABC score (r=−0.242, P=0.0057) (Fig. 3C), whereas the correlation between IgM and ABC scores is lost (r=−0.051, P=0.5393) (Fig. 3D). The ABC scores can be further stratified into five different subscales that measure individual behavior categories. Table II summarizes the correlations between Ig levels and the individual ABC subscale scores in the ASD group. While all but subscale V (inappropriate speech) were significant or approaching significance (P range 0.063–0.011), subscale II (lethargy) was the most highly correlated with a low plasma IgG level (r=−0.236, P=0.0071). Low IgA levels did show a highly significant correlation with subscale III (stereotypy); however, there was no correlation between IgA levels and total ABC score. Imunoglobulin levels were also compared to both ADOS and ADI-R scores. However, neither the ADOS nor the ADI-R scores showed a statistical correlation with either IgG or IgM levels in children with autism.
Figure 3.
Immunoglobulin levels correlate with behavioral symptoms. Total IgG and IgM levels were plotted against total ABC scores. Total IgG (A) and IgM (B) levels show a negative correlation to total ABC scores when all groups are included. Figures (C) and (D) show IgG and IgM plotted against total ABC scores for affected children with AU/ASD only. Analysis was performed using Spearman correlation. Ig, immunoglobulin; ABC, Aberrant Behavior Checklist; ASD, autism spectrum disorder.
Table II.
Spearman Correlation Between ABC Subscale Scores and Immunoglobulin Levels of Children With AU/ASD, n =143
| IgM |
IgA |
IgG |
IgE |
|||||
|---|---|---|---|---|---|---|---|---|
| Measurement | r | P= | r | P= | R | P= | r | P= |
| Subscale I: irritability | −0.064 | 0.4386 | −0.008 | 0.9252 | −0.177 | 0.0447 | −0.107 | 0.1966 |
| Subscale II: lethargy | −0.064 | 0.4406 | −0.149 | 0.0721 | −0.236 | 0.0071 | −0.041 | 0.6228 |
| Subscale III: stereotypy | −0.115 | 0.168 | −0.22 | 0.0075 | −0.164 | 0.0635 | 0.004 | 0.9617 |
| Subscale IV: hyperactivity | −0.029 | 0.726 | −0.093 | 0.2615 | −0.221 | 0.0116 | −0.13 | 0.1184 |
| Subscale V: inappropriate speech | 0.022 | 0.7895 | 0.059 | 0.4808 | −0.002 | 0.9839 | −0.026 | 0.753 |
| Total score | −0.051 | 0.5393 | −0.1 | 0.2308 | −0.242 | 0.0057 | −0.104 | 0.2096 |
ABC, Aberrant Behavior Checklist; ASD, autism spectrum disorder; Ig, immunoglobulin.
Discussion
Our current data suggest that children with autism demonstrate decreased levels of IgG and IgM. Despite the strict diagnostic criteria we used to classify individuals with autism, there remains phenotypic heterogeneity of behavioral outcome in the study population. This phenotypic heterogeneity, as represented by variations in ABC scores, is negatively correlated with IgG and IgM levels strengthening the association between immune dysfunction and at least a subset of behaviors associated with autism.
Previous studies have reported changes in several aspects of the immune system in children with autism including alterations in immune cell numbers, immune cell phenotype, the presence of autoantibodies, altered cytokine profiles, immune pathology in the gut, altered levels of complement proteins, and altered levels of Ig as reviewed by Ashwood et al. [2006]. Of these immune abnormalities, altered levels of Ig are one of the most commonly reported. However, these reports are extremely varied and the results appear to be contradictory in several cases. For example, IgG has been reported to be both increased [Croonenberghs et al., 2002; Trajkovski, Ajdinski, & Spiroski, 2004] and decreased [Ashwood et al., 2003; Gupta, Rimland, & Shilling, 1996] in children with autism compared to controls. IgM has also been reported to be both increased [Gupta, Rimland et al., 1996; Trajkovski et al., 2004] and decreased [Ashwood et al., 2003], while IgA has been reported to be either decreased [Ashwood et al., 2003; Gupta, Aggarwal, & Heads, 1996; Warren et al., 1997] or unchanged [Stern et al., 2005; Trajkovski et al., 2004]. However, IgE has only been reported to be elevated in children with autism [Ashwood et al., 2003; Gupta, Aggarwal et al., 1996].
While these reports would appear to be contradictory, differences in data might be due to lack of consistency in the subject populations and controls that were used. In previous studies, small sample sizes (range 18–40 autism subjects) and variations in the types of controls (siblings vs. age-matched general population) could affect these results. For example, in two of the studies [Ashwood et al., 2003; Stern et al., 2005], the comparison of Ig levels in subjects with autism was made against a population standard rather than a matched case–control study as described herein. Geographic location of the subjects and controls would also influence the levels of IgE in plasma, as would the season during which the sample was obtained due to individual response to regional and seasonal allergens. In addition, as a highly heterogeneous disorder, the behavioral phenotype of the subjects studied may also affect the outcome. However, we believe that one of the most critical factors to consider with respect to Ig concentration is age. Ig levels do not reach adult levels until 1 year of age for IgM, age 6–8 years for IgG, and age 10 years for IgA [Aksu et al., 2006]. In previously reported studies regarding differences in Ig levels, the ages of the study populations varied widely, with a range of 3–12 [Gupta, Aggarwal et al., 1996] in one study and up to 5–31 [Warren et al., 1997] in another. Comparing Ig levels within a broad age range, especially during the ages spanning immune development, may introduce artifacts. In the current study, we examined IgG, IgM, IgA, and IgE levels in the plasma from subjects during a very narrow age window (2–5 years of age) corresponding to the earliest time an official diagnosis could be obtained. This allowed us to better compare our index and control subjects. Differences in immunodetection technique would also have the potential to introduce variability between studies. However, all previous studies [except for IgA detection by Warren et al., 1997] used nephelometry to detect Ig levels, minimizing the likelihood that technique is entirely responsible for the previous conflicting results. In the current study, we employed the use of ELISA technology for plasma Ig assessment, a technique that appears to yield similar results when compared to nephelometry for total Ig determination [Ginel, Margarito, Lucena, & Molleda, 1997; Raux et al., 1999].
While the Ig levels reported in the current study as well as those reported in the Ashwood et al. [2003] and Stern et al. [2005] studies are not considered clinically low, they do indicate suboptimal humoral function in children with autism. B cells are responsible for the production of Ig; however, the amount and type of antibody secreted is dependent upon the interaction of multiple aspects of the immune system. Physical interaction between antigen presenting cells, T cells, and B cells as well as the effects of soluble factors such as cytokines and chemokines all play a role in this process [Janeway, Travers, Walport, & Shlomchik, 2005]. The alterations of Ig levels observed in the current study are indicative of an underlying dysfunction in the immune system. While this may affect the way children manage a pathogenic insult, it is not our contention that host/pathogen or host/vaccine interactions are a causative factor in the etiology of autism.
The immune system and the nervous system are highly interconnected. Beginning early in development, the relationship between the immune and nervous systems is exceedingly complex and continues throughout life [Haddad, Saade, & Safieh-Garabedian, 2002; Steinman, 2004; Wrona, 2006]. Immune system factors, such as major histocompatibility complex I, cytokines, and chemokines are important during many stages of neuro-development and central nervous system plasticity, functioning, and maintenance. Likewise, several proteins associated with the nervous system, such as neuropeptides, have a broad range of effects on the development of the immune system and its function (suppression as well as activation), including the innervation of immune system-associated organs, such as the lymph nodes and spleen [Biber et al., 2002; Huh et al., 2000; Marques-Deak et al., 2005; Mehler & Kessler, 1998; Mignini et al., 2003; Rothwell et al., 1996]. A carefully established equilibrium and timing of the previously mentioned parameters is vital for normal immune and central nervous system functioning. Changes in either system during this critical stage have the potential to cause lifelong alterations, such as changes in receptor distribution and/or number, as well as modifications in neuropeptide, cytokine, hormone, or neurotransmitter release. Finally, several genes associated with autism are relevant to both the nervous system and the immune system. These include PTEN (a negative regulator of the PI3K/AKT pathway) [Boccone et al., 2006; Butler et al., 2005; Goffin, Hoefsloot, Bosgoed, Swillen, & Fryns, 2001; Kwon et al., 2006], MET (a receptor tyrosine kinase) [Campbell et al., 2006, 2007], and Ca(V)1.2 (an L-type calcium channel) [Splawski et al., 2004]. Alterations in these genes can affect function in both systems.
Of interest, the current data indicate that children with autism have a dysregulated immune system during a critical period in development. The strongest evidence to support an association between immune function and autism behavior is the correlation between low IgG levels and elevated ABC scores. Of particular interest is the fact that IgG levels in children with the broader ASD phenotype are significantly higher than those in children with full-blown autism, but remain statistically lower than those in control children. In the current study, the ASD group represents children who have autism-like behaviors but did not meet the full study criteria set for the autism population. Therefore, these data suggest that the status of the humoral immune system may be reflective of behavioral outcome. Moreover, the separation of total ABC score into its individual subscales stimulates further interest in the association between immune status and behavior. In particular, subscale II (lethargy) was significantly correlated with low IgG levels. While it is too early to make concrete conclusions regarding the relationship between the ABC scores and humoral immune function, one could speculate that diminished IgG levels may result in impaired health and contribute to symptoms of lethargy. Additional studies are needed to explore this potential link further.
One could speculate that there are several possible scenarios involving the immune system and altered neurodevelopment. First, it is possible that the immune dysfunction noted in a substantial portion of children with autism is completely independent from the neuro-developmental changes noted in these children. Second, it also possible that in the context of a multi-system disorder, these data may be reflective of a defect common to both the neural and immune systems, and that a reduction in Ig levels is not directly affecting behavioral outcome. Therefore, by definition, reduced Ig levels may be considered an epiphenomenon in either of these scenarios. However, elucidation of the underlying mechanism for the observed phenomenon may discern the causative factor responsible for dysfunction in the neural system. Finally, evidence of immune dysfunction at an early age may be indicative of altered immune development that could have lasting effects on the function of the nervous system. Recently, it has also been shown that, throughout life, both cognition and neuronal plasticity are dependent to an extent on direct immune –neural interaction [Brynskikh, Warren, Zhu, & Kipnis, 2008; Ziv et al., 2006]. Therefore, due to the intimate connection between the neural and immune systems, elucidation of the pathways responsible for immune dysfunction may bring to light some of the physiological mechanisms responsible for the behavioral changes associated with autism. At this stage, we are clearly unable to discern the etiologic and pathologic factors behind the observed immune dysfunction in children with autism. However, future studies in understanding how the immune system might be implicated in abnormal neurodevelopment, and in the emergence of autism, will be of great interest.
Acknowledgments
Grant sponsor: NIEHS; Grant number: 1P01ES11269-01; Grant sponsor: US EPA; Grant number: R829388.
References
- Aksu G, Genel F, Koturoglu G, Kurugol Z, Kutukculer N. Serum immunoglobulin (IgG, IgM, IgA) and IgG subclass concentrations in healthy children: a study using nephelometric technique. Turkish Journal of Pediatrics. 2006;48:19–24. [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. Journal of Clinical Immunology. 2003;23:504–517. doi: 10.1023/b:joci.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. Journal of Leukocyte Biology. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Biber K, Zuurman MW, Dijkstra IM, Boddeke HWGM. Chemokines in the brain: neuroimmunology and beyond. Current Opinion in Pharmacology. 2002;2:63. doi: 10.1016/s1471-4892(01)00122-9. [DOI] [PubMed] [Google Scholar]
- Boccone L, Dessi V, Zappu A, Piga S, Piludu MB, et al. Bannayan–Riley–Ruvalcaba syndrome with reactive nodular lymphoid hyperplasia and autism and a PTEN mutation. American Journal of Medical Genetics, Part A. 2006;140:1965–1969. doi: 10.1002/ajmg.a.31396. [DOI] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain, Behavior, and Immunity. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. Journal of Medical Genetics. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, D’Oronzio R, Garbett K, Ebert PJ, Mirnics K, et al. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Annals of Neurology. 2007;62:243–250. doi: 10.1002/ana.21180. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, et al. A genetic variant that disrupts MET transcription is associated with autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychological Medicine. 2002;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. Journal of Autism and Developmental Disorders. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Edinburgh. 2000 www.cgmh.org.tw/intr/intr1/c0040/web/C/Declaration%20of%20Helsinki.pdf.
- Engstrom AH, Ohlson S, Stubbs EG, Maciulus A, Caldwell V, et al. Decreased expression of CD95 (FAS/APO-1) on CD4+ T-lymphocytes from participants with autism. Journal of Developmental and Physical Disabilities. 2003;15:155–163. [Google Scholar]
- Ginel PJ, Margarito JM, Lucena R, Molleda JM. Concentrations of plasma immunoglobulins in the dog as determined by laser nephelometry. Comparison with radial immunodiffusion and enzyme-linked immunosorbent assay. European Journal of Clinical Chemistry and Clinical Biochemistry. 1997;35:223–228. doi: 10.1515/cclm.1997.35.3.223. [DOI] [PubMed] [Google Scholar]
- Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. American Journal of Medical Genetics. 2001;105:521–524. doi: 10.1002/ajmg.1477. [DOI] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, Heads C. Dysregulated immune system in children with autism: beneficial effects of intravenous immune globulin on autistic characteristics. Journal of Autism and Developmental Disorders. 1996;26:439–452. doi: 10.1007/BF02172828. [DOI] [PubMed] [Google Scholar]
- Gupta S, Rimland B, Shilling PD. Pentoxifylline: brief review and rationale for its possible use in the treatment of autism. Journal of Child Neurology. 1996;11:501–504. doi: 10.1177/088307389601100622. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune–endocrine interactions: a role for the hypothalamic–pituitary–adrenal revolving axis. Journal of Neuroimmunology. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, et al. Regression in autism: prevalence and associated factors in the CHARGE study. Ambulatory Pediatrics. 2008;1:25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats. Journal of Neuroimmunology. 2003;137:59–66. doi: 10.1016/s0165-5728(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. New York: Garland Science Publishing; 2005. [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2002;43:807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. Journal of Neuroimmunology. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SJ. Behavioral and motivational effects of immune-system activation. Journal of General Psychology. 2002;129:401–414. doi: 10.1080/00221300209602104. [DOI] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH., Jr Quantifying the phenotype in autism spectrum disorders. American Journal of Medical Genetics. 2001;105:36–38. [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, et al. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. Journal of Autism and Developmental Disorders. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Marques-Deak A, Cizza G, Sternberg E. Brain–immune interactions and disease susceptibility. Molecular Psychiatry. 2005;10:239–250. doi: 10.1038/sj.mp.4001643. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Cytokines in brain development and function. Advances in Protein Chemistry. 1998;52:223–251. doi: 10.1016/s0065-3233(08)60437-4. [DOI] [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Autonomic and Autacoid Pharmacology. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- MMWR. Prevalence of autism spectrum disorders— autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveillance Summary. 2007;56:12–28. [PubMed] [Google Scholar]
- Mullen E. The Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Owley T, McMahon W, Cook EH, Laulhere T, South M, et al. Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1293–1299. doi: 10.1097/00004583-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: the delays-plus-regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, et al. Comparison of the distribution of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Research and Human Retroviruses. 1999;15:1365–1376. doi: 10.1089/088922299310070. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G, Toulmond S. Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacology & Therapeutics. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Erdin A. Abnormal psychosocial situations and ICD-10 diagnoses in children and adolescents attending a psychiatric service. Journal of Child Psychology and Psychiatry. 1992;33:731–740. doi: 10.1111/j.1469-7610.1992.tb00908.x. [DOI] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nature Immunology. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Stern L, Francoeur MJ, Primeau MN, Sommerville W, Fombonne E, Mazer BD. Immune function in autistic children. Annals of Allergy, Asthma & Immunology. 2005;95:558–565. doi: 10.1016/S1081-1206(10)61019-8. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112:e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- Trajkovski V, Ajdinski L, Spiroski M. Plasma concentration of immunoglobulin classes and subclasses in children with autism in the Republic of Macedonia: retrospective study. Croatian Medical Journal. 2004;45:746–749. [PubMed] [Google Scholar]
- Warren RP, Foster A, Margaretten NC. Reduced natural killer cell activity in autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:333–335. doi: 10.1097/00004583-198705000-00008. [DOI] [PubMed] [Google Scholar]
- Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, et al. Brief report: immunoglobulin A deficiency in a subset of autistic subjects. Journal of Autism and Developmental Disorders. 1997;27:187–192. doi: 10.1023/a:1025895925178. [DOI] [PubMed] [Google Scholar]
- Warren RP, Yonk LJ, Burger RA, Cole P, Odell JD, et al. Deficiency of suppressor–inducer (CD4+CD45RA+) T cells in autism. Immunological Investigations. 1990;19:245–251. doi: 10.3109/08820139009041839. [DOI] [PubMed] [Google Scholar]
- Wrona D. Neural–immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. Journal of Neuroimmunology. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neuroscience. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]