Abstract
C1q and members of the defense collagen family are pattern recognition molecules that bind to pathogens and apoptotic cells and trigger a rapid enhancement of phagocytic activity. Candidate phagocytic cell receptors responsible for the enhancement of phagocytosis by defense collagens have been proposed but not yet discerned. Engagement of phagocyte surface-associated calreticulin in complex with the large endocytic receptor, low-density lipoprotein receptor-related protein (LRP/CD91), by defense collagens has been suggested as one mechanism governing enhanced ingestion of C1q-coated apoptotic cells. To investigate this possibility, macrophages were derived from transgenic mice genetically-deficient in LRP resulting from tissue-specific loxP/Cre recombination. LRP-deficient macrophages were impaired in their ability to ingest beads coated with an LRP ligand when compared to LRP-expressing macrophages, confirming for the first time that LRP participates in phagocytosis. When LRP-deficient and -expressing macrophages were plated on C1q-coated slides, they demonstrated equivalently enhanced phagocytosis of sheep red blood cells suboptimally-opsonized with IgG or complement, compared to cells plated on control protein. In addition, LRP-deficient and -expressing macrophages ingested equivalent numbers of apoptotic Jurkat cells in the presence and absence of serum. Both LRP-deficient and -expressing macrophages ingested fewer apoptotic cells when incubated in the presence of C1q-deficient serum compared to normal mouse serum, and addition of purified C1q reconstituted uptake to control serum levels. These studies demonstrate a direct contribution of LRP to phagocytosis and indicate that LRP is not required for the C1q-triggered enhancement of phagocytosis, suggesting that other, still undefined, receptor(s) exist to mediate this important innate immune function.
Keywords: Macrophage, phagocytosis, apoptosis, cell surface molecules, complement
Introduction
Rapid phagocytosis of apoptotic cells is crucial for normal tissue homeostasis such as in the resolution of an inflammatory state and/or immune response. The mechanism by which apoptotic cells are recognized and phagocytosed is complex and involves a multitude of distinct receptor:ligand interactions [1]. Members of the defense collagen family have been well characterized in their ability to enhance phagocytic activity of monocytes, macrophages and dendritic cells [2]. C1q, a member of the defense collagen family, binds to apoptotic cells via its globular head region, while the collagen-like tails can link the apoptotic cell to the phagocyte and signal enhanced ingestion [3–7]. However, the molecular partners involved in both the mechanism governing C1q- (and other defense collagen)-mediated recognition of apoptotic cell-associated molecular patterns by phagocyte surface receptors and the subsequent intracellular signaling that leads to ingestion have yet to be completely defined.
C1q deficiency in humans leads to enhanced susceptibility to infection and development of lupus-like autoimmune disease with essentially complete penetrance [8]. In mice, genetic deficiencies of C1q result in impaired handling of immune complexes and heightened susceptibility to bacterial infection and autoimmunity [9–11]. Recent reports demonstrate the importance of microenvironment and tissue specificity for bridging molecules, such as defense collagens, that link apoptotic cells to phagocytes. For example, C1q deficiency in mice leads to an accumulation of apoptotic bodies in the kidney and resultant glomerulonephritis in 25% of animals [12]. These data are consistent with the hypothesis that C1q deficiency, resulting in inefficient clearance of apoptotic cells, leads to the release of intracellular components, exposure of the immune system to self antigens, and subsequent autoimmunity. In addition, murine deficiency of the defense collagen mannose binding lectin leads to inefficient clearance of apoptotic cells when apoptotic cells are injected intraperitoneally; however, the mutation is not associated with spontaneous autoimmunity [13]. Efforts to elucidate mechanisms involving defense collagen-triggered clearance of apoptotic cells have thus far focused largely on identification of the receptor complex involved in regulating phagocytosis.
CD93, a transmembrane glycoprotein expressed on myeloid cells, endothelial cells and platelets, was originally isolated and cloned based on its ability to modulate the C1q-triggered enhancement of ingestion of target particles suboptimally-opsonized with IgG or complement [14–16]. However, further studies by Norsworthy et al. demonstrated that, while the CD93-deficient mouse was deficient in the in vivo clearance of apoptotic cells, the mechanism governing the clearance defect remained elusive; in vitro analysis of CD93-deficient macrophages demonstrated that these macrophages respond to immobilized C1q with an enhancement of phagocytosis of IgG-opsonized particles and also preferentially engulf apoptotic cells in the presence of C1q-containing serum over apoptotic cells incubated in C1q-deficient serum [17]. These studies prompted further investigation to identify the receptor or receptor complex responsible for the C1q-triggered enhancement of phagocytosis.
It has been suggested that another receptor, the LDL receptor-related protein 1 (LRP or CD91), mediates ingestion of apoptotic cells by forming a phagocyte receptor complex with calreticulin (CRT) and binding to C1q on apoptotic cells or, alternatively, by binding to CRT alone present on apoptotic cells and mediating their phagocytosis [6,7,18,19]. LRP is a large cell surface receptor that mediates endocytosis of numerous ligands, including apoE-enriched lipoproteins, protease inhibitor complexes and matrix proteins (reviewed in [20]). While direct genetic experiments have yet to confirm that LRP is capable of participating in phagocytosis, the LRP cytoplasmic domain associates with GULP [21], an adaptor protein known to participate in phagocytosis, and experiments employing hybrid receptors revealed that the LRP cytoplasmic domain has sufficient information to trigger phagocytosis [22]. Together, these data reveal the potential of LRP to mediate phagocytosis.
Collectively, these studies prompted us to test the hypotheses that LRP can mediate phagocytosis and that LRP is the receptor responsible for C1q-mediated enhancement of phagocytosis. To test these hypotheses, thioglycollate-elicited peritoneal macrophages or bone marrow-derived macrophages (BMDM) from mice generated using a tissue-specific loxP/Cre recombination strategy, which were either genetically-deficient in or normally-expressing LRP, were used to explore the role of LRP in the phagocytosis of ligand-coated microspheres and in the C1q-triggered enhancement of ingestion of a variety of physiologically important targets, including complement- and antibody-opsonized targets and apoptotic cells. The data demonstrate that, while LRP contributes to the phagocytosis of ligand-coated particles, it is not required for the C1q-triggered enhancement of phagocytosis, directly implying that other, still unidentified, receptor(s) exist to mediate this important innate immune function.
Materials and Methods
Reagents
The human Jurkat T cell clone A3 was obtained from ATCC (Manassas, VA). Alexa Fluor 633 and 546 labeling kits, RPMI, penicillin/streptomycin, DMEM, Trypsin-EDTA, and CFSE were purchased from Gibco/Molecular Probes/Invitrogen (Carlsbad, CA). CFSE was reconstituted to 5mM in DMSO and stored at −20°C. FBS was purchased from Hyclone (Logan, Utah). Brewer modified thioglycollate broth was obtained from Becton Dickinson (Franklin Lakes, NJ). Complete protease inhibitor tablets were purchased from Roche Diagnostics (Indianapolis, IN). Enhanced chemiluminescence reagents were from Pierce (Rockford, IL). Versene was obtained from Lonza (Walkersville, MD). Recombinant GST and GST-RAP were purified as described [23]. Anti-tubulin antibody, anti-GST antibody and etoposide were from Sigma (St. Louis, MO). Etoposide was reconstituted at 10 mM in DMSO and stored at −20°C. Fluoresbrite YG carboxylate microspheres (1 µm) were purchased from Polysciences, Inc (Warrington, PA). Annexin V antibody was from BioVision (Mountain View, CA). Anti-CD11b antibody was obtained from eBioscience (San Diego, CA). Human C1q was purified from human plasma by the method of Tenner et al. [24]. Mouse C1q was isolated from mouse sera by ion exchange chromatography. Mouse sera was adjusted to 25 mM EDTA and passed over a column of Biorex70 resin (Biorad, Hercules, CA) of equal volume, which had been pre-equilibrated into 50 mM Tris pH 7.4, 82 mM NaCl, 25 mM EDTA. C1q was eluted with a salt gradient to 300 mM NaCl. Fractions containing functional C1q, as assessed by hemolytic titer, were pooled and concentrated by ultrafiltration (Amicon-Millipore, Billerica, MA). Serum completely lacking C1q was obtained from C1q-deficient mice [12].
Mice
Mice deficient in macrophage LRP were generated using loxP/Cre-mediated recombination essentially as described by Hu et al. [25] except that mice were maintained on an low-density lipoprotein receptor-deficient background for use in atherosclerosis studies, to be reported elsewhere (Lillis et al., in preparation). Briefly, loxP-flanked LRP transgenic mice and mice expressing Cre recombinase driven by the lysozyme M promoter were employed in a breeding scheme which ultimately generated experimental litters with all mice expressing loxP sites on both alleles of LRP. Litters were evenly divided with half of siblings carrying one copy of Cre recombinase under the lysozyme M promoter, which led to recombination and deletion of LRP in macrophages (macLRP−/− mice). The other half of siblings carried no copies of Cre and, therefore, expressed LRP normally in all tissues (LRP+/+ mice). All studies were reviewed and approved by the Institutional Animal Care and Use Committee.
Cell culture
The human Jurkat T cell clone A3 was maintained in RPMI supplemented with 10% FBS, 100 units/ml penicillin G sodium/100 µg/ml streptomycin sulfate, and 10 mM HEPES, pH 7.4 at 5% CO2. Peritoneal macrophages were harvested from macLRP−/− and sibling control LRP+/+ mice four days after intraperitoneal injection of 1 ml of 5% (wt/vol) Brewer-modified thioglycollate broth by flushing the peritoneum with 3 × 5 ml ice cold PBS. Macrophages were washed with ice cold PBS, and incubated in DMEM supplemented with 10% FBS and penicillin/streptomycin, in 6-well cell culture plates at 37°C, 10% CO2 for 3 days. BMDM were generated as described [26]. Briefly, femurs, tibias and humeri were isolated and shipped insulated overnight in BMDM media (DMEM with 10% FBS, 15% L929 conditioned media, penicillin/streptomycin and 10mM HEPES). Bones were flushed with DMEM supplemented with 2% FBS; red blood cells were lysed, and resident fibroblasts and macrophages were depleted by a preadhesion step for 2–4 hours at 37°C, 5% CO2. Following maturation for four days, 6 ml fresh media was added, and cells were considered fully mature at 7 days. Media was replenished every two to three days to maintain cell viability beyond day 7.
Western blotting
Thioglycollate-elicited peritoneal macrophages were lysed in a 50 mM Tris HCl buffer, pH 7.4, containing 1% NP-40, 150 mM NaCl, 1 mM EDTA, and supplemented with complete protease inhibitor tablets to give final concentrations of 1 mM PMSF and 1 µg/ml each of aprotinin, leupeptin and pepstatin. 30 µg of protein was resolved under non-reducing conditions by 4–12% tris-glycine SDS-PAGE. Proteins were transferred to nitrocellulose at 30 V overnight at 4°C and membrane was blocked with 3% milk in Tris-buffered saline (150 mM NaCl, 20mM Tris, pH 7.4) for 1 hour. LRP was detected by incubation for 1 hour at room temperature with 1 µg/ml rabbit polyclonal anti-LRP antibody 2629 [27]. Membranes were washed, incubated with HRP-conjugated goat-anti-rabbit secondary antibody (1:3,000) and then washed and developed with enhanced chemiluminescence reagents. The LRP blot was stripped and re-probed with 1 µg/ml rabbit polyclonal anti-Mac-1 antibody, ARC-22 (a generous gift from L. Zhang, University of Maryland) and with 1 µg/ml anti-tubulin antibody to confirm equivalent protein loading.
Soluble ligand and microbead uptake assays
Soluble GST-RAP was labeled with Alexa Fluor 633 according to the manufacturer’s instructions. Peritoneal macrophages, isolated as above, were incubated with 5 nM Alexa Fluor 633-labeled GST-RAP in DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C for 30 min, washed twice in ice cold PBS, detached from the plate with Trypsin-EDTA and analyzed by flow cytometry. The number of cells staining positive for Alexa Fluor 633 was expressed as a percentage of total PI-negative (live) cells in each group. For protein-coated microbead uptake assays, green fluorescent microbeads (1 µm) were incubated with 3 mg/ml of GST or GST-RAP for 1 hour with periodic shaking. Beads were washed 3 times in PBS. Plated peritoneal macrophages were incubated with 25 beads/cell in DMEM supplemented with 10% FBS for 30 minutes, washed, removed from the plate with Trypsin-EDTA and analyzed by FACS. This procedure assumes that the fluorescence associated with cells represents internalized beads. The percentage of cells with fluorescence greater than that of cells incubated without beads is expressed as percent phagocytosis. The phagocytic index was determined by multiplying the percent phagocytosis by the mean fluorescence. For fluorescent microscopy, cells were plated on 0.1% gelatin-coated glass coverslips, incubated with beads, which had been coated with GST, GST-RAP or GST-HBD as described above, fixed, immunostained and visualized using a confocal laser-scanning microscope Radiance2000 (BioRad/Zeiss). Cell-associated beads were counted by optical sectioning through cell bodies of several random fields of cells.
To differentiate between beads that were completely engulfed and beads that were cell-associated but not engulfed, four channel fluorescent microscopy was utilized. LRP expressing cells were labeled by pre-loading the cells with Alexa 647 conjugated RAP. Following a wash step to remove non-internalized RAP, Fluoresbrite YG beads conjugated to the specific protein (GST, GST-RAP or GST-HBD) were incubated with cells for 60 minutes, and then cells were washed and fixed. Cells were then stained with anti-GST followed by goat anti-mouse Alexa 568. GST on the surface of the beads is recognized only if the beads are not fully engulfed. Phase images were used to identify LRP-null cells.
Opsonized erythrocyte and apoptotic cell phagocytosis assays
Phagocytosis assays using EAIgG and EAIgMC4b were performed as described previously [28] with the following modification. BMDM were switched to DMEM, supplemented with 10% FBS, penicillin/streptomycin and 10mM HEPES without L929 conditioned culture media for 24 hours prior to performing assays. Cells were harvested from non-tissue-culture-treated plastic Petri dishes with Versene (0.2mg/mL EDTA), washed three times in phagocytosis buffer (RPMI containing 2 mM L-glutamine, penicillin/streptomycin, 5 mM MgCl2) and resuspended in phagocytosis buffer at 1.25 × 105/ml. Macrophages were added to wells previously coated with 4 or 8 µg/ml of either C1q or human serum albumin (used as a control protein), allowed to adhere for 30 minutes at 37°C, and then incubated with targets for an additional 30 minutes. For phagocytosis of apoptotic cells, Jurkat cells were labeled with 5 µM CFSE for 30 minutes at 37°C and, following two washes, resuspended at 5 × 105/ml in complete media with 40 µM etoposide and cultured for 12 to 18 hours. Apoptosis was verified by Annexin V/Propidium Iodide staining. BMDM were harvested and washed as described above, and 5 × 105 macrophages were incubated with 1.5 × 106 apoptotic cells for 15 to 60 minutes at 37°C. Non-adherent cells were removed by washing with PBS, and macrophages were harvested with Trypsin-EDTA. Macrophages were stained with anti-CD11b and detected by flow cytometry. Macrophages that had engulfed apoptotic cells were calculated as (CD11b and CFSE double-positive cells divided by the total number of CD11b-positive cells) × 100. The 30-minute time point has been previously reported in the literature and was also found to be an optimum point to observe the C1q-triggered enhancement of phagocytosis of antibody- and complement-tagged particles in the studies performed here [28–30]. In addition, the 30-minute time point consistently provided reproducible differences between the uptake of live and apoptotic cells.
Statistical analysis
Where indicated, comparison of means was performed using Student’s t-test.
Results
macLRP−/− macrophages are deficient in LRP and defective in the uptake of soluble LRP ligands
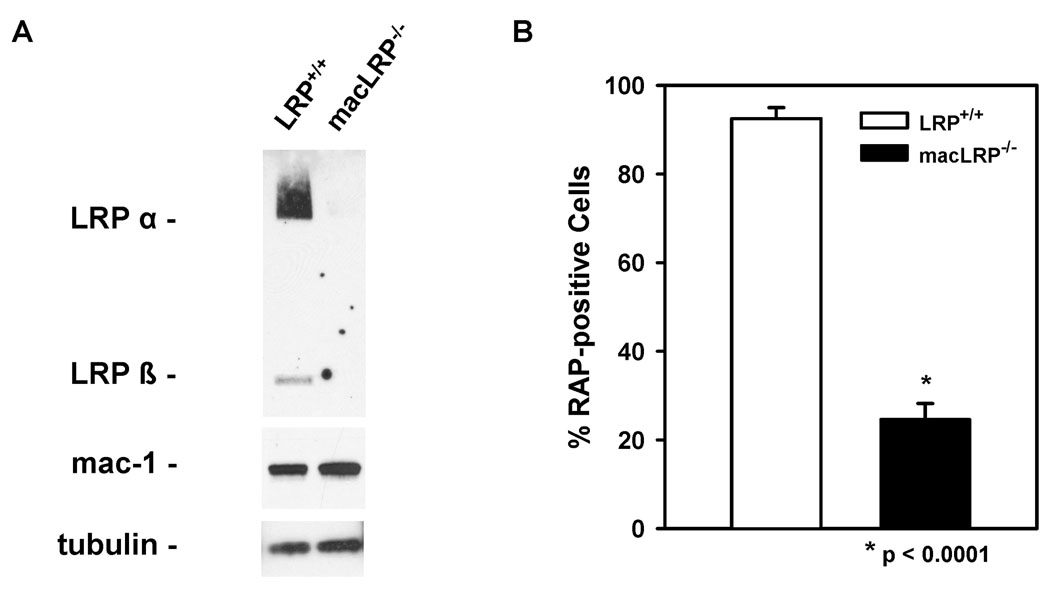
Macrophage-specific LRP-deficient mice were generated using loxP/Cre-mediated recombination as described in Materials and Methods. To evaluate the level of macrophage LRP expression, we examined both thioglycollate-elicited peritoneal macrophages and BMDM from macLRP−/− mice and LRP+/+ siblings. LRP deletion in peritoneal macrophages from LRP-deficient mice was confirmed by immunoblotting for LRP from macrophage extracts (Fig. 1A). Equivalent protein loading between macLRP−/− and LRP+/+ macrophage extracts was confirmed by blotting tubulin and the macrophage marker Mac-1 (CD11b/CD18).
Figure 1. LRP is effectively deleted in peritoneal macrophages.
(A) 30 µg of cell lysate proteins from thioglycollate-elicited peritoneal macrophages were separated by SDS-PAGE, and LRP was detected by immunoblot analysis with a rabbit polyclonal antibody. (B) Thioglycollate-elicited peritoneal macrophages from LRP+/+ mice (open bars) and macLRP−/− mice (solid bars) were incubated with 5 nM soluble GST-RAP labeled with Alexa Fluor 633 for 30 minutes and analyzed by FACS. Macrophages from 5 LRP+/+ and 4 macLRP−/− mice were analyzed in triplicate. Means were compared using the Student’s t test and differ at the p < 0.001 level. Error bars denote SEM.
Receptor associated protein (RAP) binds with high-affinity to LRP, functioning in vivo in the endoplasmic reticulum as a molecular chaperone. To confirm the loss of LRP function in LRP-deficient macrophages, internalization assays were performed using thioglycollate-elicited peritoneal macrophages from LRP-deficient and sibling control mice using Alexa Fluor 633-labeled recombinant GST-RAP (5nM). FACS analysis indicated that cells from macLRP−/− mice were defective in their ability to internalize Alexa Fluor labeled-RAP when compared to macrophages from LRP+/+ mice (p < 0.0001) (Fig. 1B). These studies indicate that peritoneal macrophages from macLRP−/− mice are deficient in LRP protein expression as well as in their ability to internalize LRP ligands.
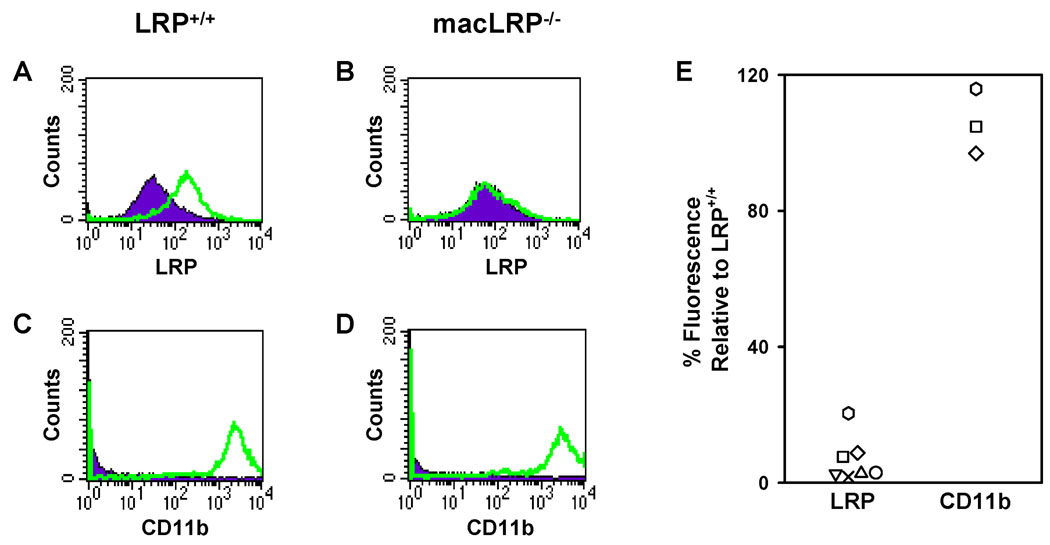
Bone marrow-derived macrophages were also examined for LRP expression by flow cytometry. Surface staining for LRP showed deletion of LRP in BMDM from macLRP−/− mice (Fig. 2A and B), while staining for CD11b (a well-characterized macrophage marker) was unchanged between groups (Fig. 2C and D). Multiple preparations of BMDM yielded consistently diminished levels of LRP on macLRP−/− macrophages compared to sibling controls (Fig. 2E). The highest number of LRP-positive macrophages detected in a macLRP−/− preparation was 64.6%; however, these positive cells only expressed 28.6% of the mean fluorescence level of LRP+/+ control cells and, therefore, had only 20% of the level of LRP expression compared to cells from their sibling controls (% positive cells × mean fluorescence relative to sibling controls). The lowest number of LRP-positive macrophages detected in a macLRP−/− preparation was 7.5% with a mean fluorescence relative to control of 34.2%, or 2.6% of the LRP expression of the control cells. Therefore, the range in LRP expression on macLRP−/− BMDM relative to LRP+/+ controls was between 2.6% and 20.4%. Taken together, these results demonstrate an efficient deletion or diminution of LRP from both peritoneally-derived and bone marrow-derived murine macrophages, and hence these macrophages were used to assess the role of LRP in regulating C1q-dependent and independent phagocytosis of a variety of target particles.
Figure 2. Profile of LRP and CD11b expression by FACS on LRP-expressing and LRP-deficient bone marrow-derived macrophages (BMDM).
(A and B) LRP expression (open histogram) and isotype control staining (closed histogram) are shown. (A) 72.16% of LRP+/+ BMDM express LRP with a mean fluorescence of 143.16. (B) 6% of macLRP−/− BMDM express LRP and have a decreased mean fluorescence of 21.84. (C and D) Expression of CD11b (open histogram), a well-characterized macrophage receptor, and isotype control staining (closed histogram) are equivalent on LRP+/+ (C) and macLRP−/− (D) BMDM. (E) Summary figure demonstrating relative mean fluorescence intensity of LRP and CD11b on macLRP−/− macrophages from the BMDM preparations relative to LRP+/+ control sibling expression used in the studies presented here.
LRP-deficient macrophages are defective in the phagocytosis of 1 µm ligand-coated microbeads
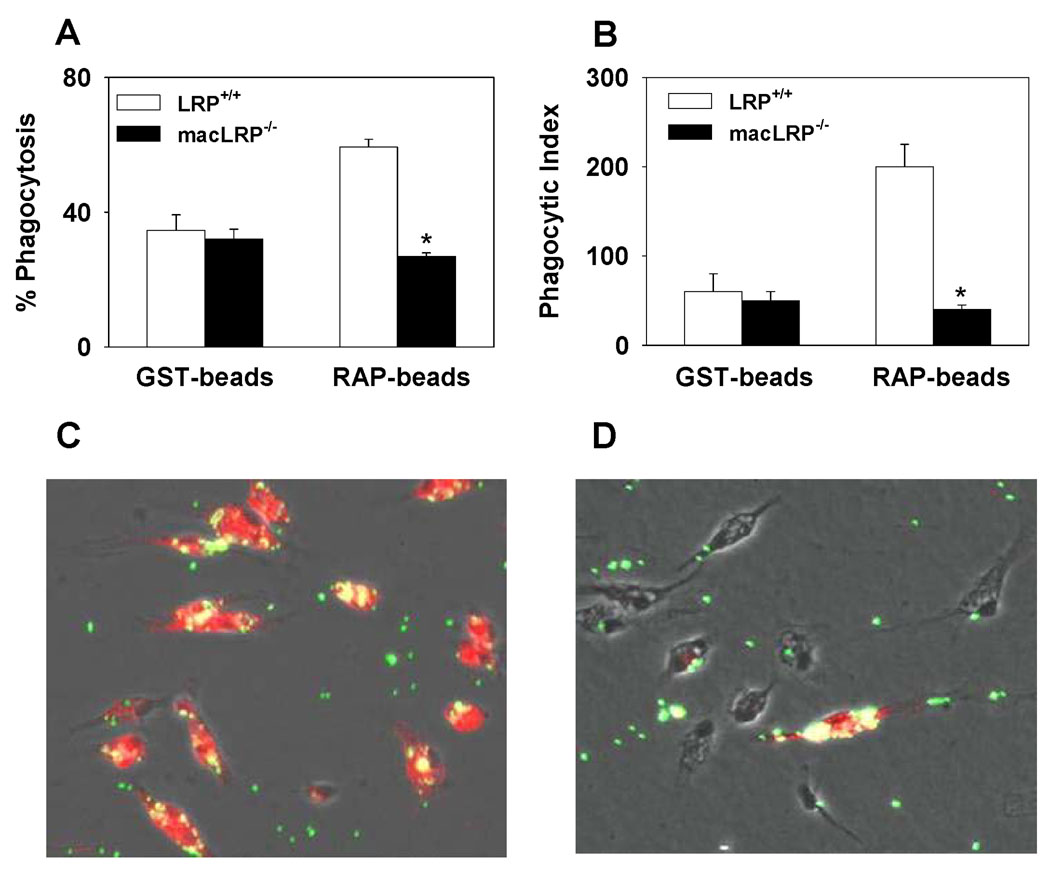
To determine whether LRP has the potential to mediate phagocytosis, thioglycollate-elicited peritoneal macrophages from LRP+/+ or macLRP−/− mice were incubated with 1 µm fluorescent beads coated with GST-RAP or GST alone. Cells were washed, and phagocytosis of the microparticles was measured by flow cytometry. Similar percentages of LRP+/+ and macLRP−/− macrophages internalized GST-coated beads, with 34.1 ± 4.6% and 32.1 ± 3.8% of cells taking up GST-coated beads, respectively (Fig. 3A). The phagocytic indices for the GST-coated beads were also similar between LRP+/+ and macLRP−/− cells (59.9 ± 20 and 48 ± 10.9, respectively) (Fig. 3B). When cells were incubated with GST-RAP-coated beads, LRP-expressing macrophages exhibited an increase in both the percent phagocytosis, from 34.1 ± 4.6% with GST-coated beads to 58.6 ± 2.4% with GST-RAP-coated beads (p = 0.002) (Fig. 3A), and a greater than 3-fold increase in the phagocytic index from 59.9 ± 20.3 to 199.4 ± 24.9, respectively (p = 0.002) (Fig. 3B). In contrast, LRP-deficient cells failed to demonstrate enhanced ingestion of GST-RAP-coated beads compared to GST-coated beads. The percentage of macLRP−/− macrophages that ingested GST-RAP-coated beads was equivalent to those ingesting GST-coated beads (27.3 ± 2.1% and 32.1 ± 3.8%, respectively) (Fig. 3A). Likewise, the phagocytic indices for LRP-deficient macrophages ingesting GST-RAP and GST-coated beads were also similar (36.2 ± 5.6 and 48.0 ± 10.9, respectively) (Fig. 3B), demonstrating no response to the presence of RAP on the beads in the absence of LRP.
Figure 3. Defective internalization of GST-RAP-coated 1 µm fluorescent microspheres in LRP-deficient macrophages.
(A and B) Peritoneal macrophages from LRP+/+ and macLRP−/− mice were incubated with either GST-coated or GST-RAP-coated 1 µm microspheres (25 beads/cell) and analyzed by FACS. LRP-deficient cells are defective in the phagocytosis of GST-RAP-coated beads compared to LRP-expressing cells as indicated by both a lower percent phagocytosis (A) and phagocytic index (B). Percent phagocytosis is defined as the number of cells taking up one or more beads divided by the total number of live cells. Phagocytic index is calculated by multiplying the percent phagocytosis by the mean fluorescence of each sample. (C and D) Fluorescent microscopy of peritoneal macrophages from LRP+/+ (C) and macLRP−/− (D) mice incubated with GST-RAP-coated microspheres demonstrates fewer beads associated with LRP-deficient cells. Cells plated on glass coverslips were incubated with microbeads at 37°C for 30 minutes and washed. Cells were fixed, permeabilized and probed for LRP expression with an Alexa 546-conjugated antibody. Merged images of the cell bodies (phase contrast), beads (green) and LRP (red) are shown, with yellow indicating overlapping fluorescence. A representative field from one of four sibling pairs examined is shown.
Individual macrophages incubated with fluorescent beads, as well as with an anti-LRP antibody to reveal LRP expression, were also examined by fluorescence microscopy to verify the phagocytic defect in macLRP−/− cells (Fig. 3C and D). The results reveal significant uptake of GST-RAP-coated beads in cells expressing LRP (Fig. 3C). Yellow fluorescence, indicating the merge of green, GST-RAP-coated microbeads and red, Alexa-546-conjugated anti-LRP, is not observed in cells deficient in LRP (Fig. 3D). The presence of an LRP-expressing cell in the macLRP−/− samples is consistent with the reported LysMCre-mediated recombination efficiency of 90% [31]. This single LRP-expressing cell serves as an internal control within the macLRP−/− sample and confirms that LRP-expressing cells ingest GST-RAP-coated beads more efficiently than cells deficient in LRP.
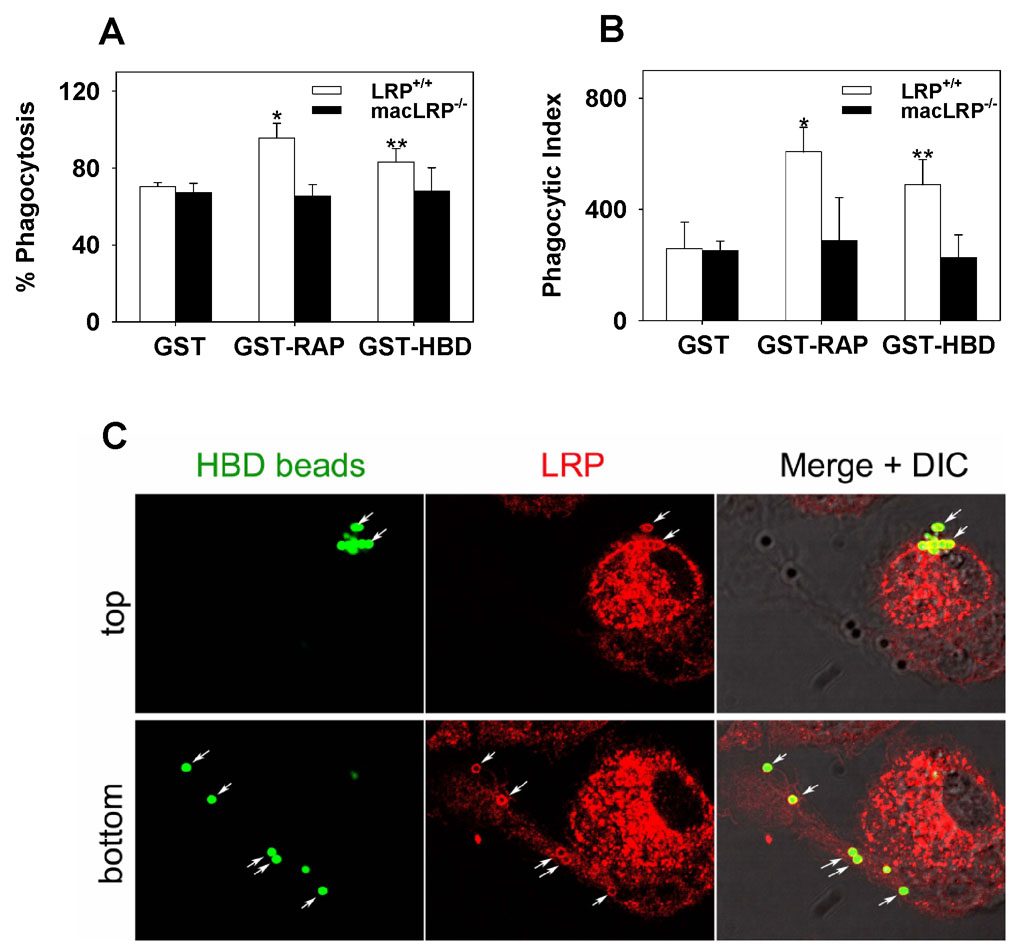
To verify the ability of LRP to mediate phagocytosis of a physiologically relevant ligand, peritoneal macrophages were also incubated with microbeads coated with the heparin binding domain (HBD) derived from thrombospondin fused to GST or, as a control, GST alone. Thrombospondin-1 is an LRP ligand, and the LRP binding site is located within its HBD [32,33]. After incubation, the cells were washed, and phagocytosis of the microparticles was measured by microscopy. Anti-GST IgG was used to detect beads that were surface-bound but not internalized, and only internalized beads were scored. LRP+/+ and macLRP−/− macrophages internalized similar levels of GST-coated beads, with 70.4 ± 2.0 and 67.2 ± 4.8% of cells taking up GST-coated beads, respectively in this experiment (Fig. 4A). The phagocytic indices for the GST-coated beads were also similar between LRP+/+ and macLRP−/− cells (259.2 ± 94.6 and 252.5 ± 33.6, respectively) (Fig. 4B). When the cells were incubated with GST-HBD-coated beads, LRP-expressing macrophages exhibited an increase in both the percent phagocytosis, from 70.3% with GST-coated beads to 83.2% with GST-HBD-coated beads (p = 0.014) (Fig. 4A) and a 1.9-fold increase in the phagocytic index from 259.2 ± 94.6 to 488.6 ± 90.5, respectively (p = 0.0001) (Fig. 4B). In contrast, LRP-deficient cells failed to demonstrate enhanced ingestion of GST-HBD-coated beads compared to GST-coated beads. The percentage of macLRP−/− macrophages that ingested GST-HBD-coated beads was equivalent to those ingesting GST-coated beads (67.2 ± 4.8% and 67.9 ± 12%, respectively) (Fig. 4A). Likewise, the phagocytic indices for LRP-deficient macrophages ingesting GST-HBD and GST-coated beads were also similar (226.9 ± 81 and 252.5 ± 33.6, respectively) (Fig. 4B), demonstrating no response to the presence of HBD on the beads in the absence of LRP. In the same experiment, phagocytosis of GST-RAP coated beads was also measured, and, similar to the results shown in Fig 3, this data confirmed that the percentage of cells ingesting beads was greater in LRP-expressing cells than in LRP-deficient cells (95.5 ± 7.6 versus 65.5 ± 5.9%, respectively, p=0.005). Likewise, the phagocytic index of GST-RAP coated beads was considerably higher in LRP-expressing cells when compared to LRP-deficient cells (607.8 ± 88 versus 288.4 ± 154, respectively, p=0.03).
Figure 4. Defective phagocytosis of 1 µm fluorescent microspheres coated with the heparin binding domain (HBD) from thrombospondin in LRP-deficient macrophages and confocal microscopy analysis of LRP distribution during phagocytosis.
(A and B) Peritoneal macrophages from LRP+/+ and macLRP−/− mice were incubated with either GST-coated, GST-RAP-coated or GST-HBD-coated 1 µm microspheres (50 beads/cell). Following incubation for 1 h, cells were analyzed by microscopy. Beads located on the surface were identified with anti-GST IgG and were not counted. (A) LRP-deficient cells are defective in the phagocytosis of GST-RAP- and GST-HBD-coated beads compared to LRP-expressing cells as indicated by a lower percent phagocytosis. Percent phagocytosis is defined as the number of cells taking up one or more beads divided by the total number of cells, and the results are shown as the mean ± SD. (*p = 0.005, ** p = 0.014). (B) LRP-deficient cells are defective in the phagocytosis of GST-RAP- and GST-HBD- coated beads as indicted by a lower phagocytic index, which is defined as the number of beads ingested per 100 cells. The results are shown as the mean ± SD. (*p = 0.035, **p = 0.0001). (C) Cells plated on glass coverslips were incubated with 1 µm GST-HBD-coated microspheres (green) for 30 minutes and washed. Cells were fixed, permeabilized and probed for LRP with an Alexa Fluor 546-conjugated antibody (red). Merged images demonstrate colocalization of LRP and GST-HBD-coated microspheres (yellow). A stack of optical sections through the cell body was collected (26 sections, 0.2 µm apart). The top panels show the top of the cell, while the bottom panels show the bottom of the cells.
In order to examine the distribution of LRP during the phagocytosis of GST-HBD coated beads, confocal microscopy was performed. Optical sectioning through the cell body showing the top of the cell or the bottom of the cell (Fig. 4C) confirmed that the fluorescent GST-HBD-coated beads were surrounded by LRP immunostaining. Collectively, these studies demonstrate that particles displaying an LRP ligand on their surface exhibit enhanced ingestion by macrophages and that this increased phagocytosis occurs in an LRP-dependent fashion, as LRP-deficient cells show no enhanced uptake of the GST-RAP- or GST-HBD-coated beads compared to control GST-coated beads. These data provide direct evidence that macrophage LRP contributes to phagocytosis.
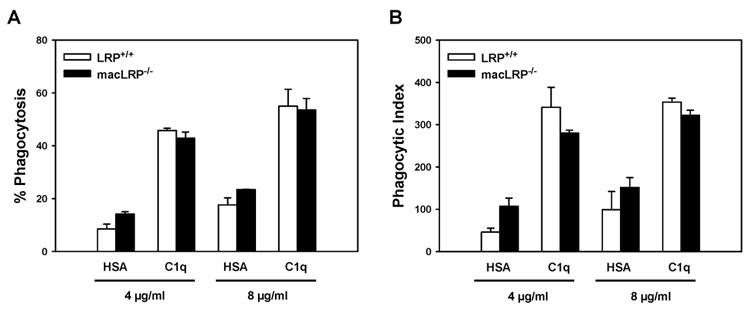
LRP-deficient macrophages demonstrate C1q-mediated enhanced phagocytosis of EAIgG or EAIgMC4b
Having demonstrated that macrophage LRP participates in phagocytosis of particles bearing LRP ligands on their surfaces, we sought to determine if LRP is required to mediate C1q-enhancement of phagocytosis, as previously suggested [34]. To test this hypothesis, we isolated BMDM from LRP+/+ and macLRP−/− mice and evaluated their ability to engulf sheep red blood cells suboptimally-opsonized with IgG (EAIgG) following adhesion to 4 or 8 µg/ml C1q-coated surfaces or, as a control, human serum albumin (HSA)-coated surfaces. As expected, there was an increase in both the percent phagocytosis and phagocytic index when LRP-expressing cells were adherent to 4 or 8 µg/ml C1q (Fig. 5). Additionally, LRP-deficient macrophages responded to C1q with an enhancement of percent phagocytosis and phagocytic index comparable to that of LRP+/+ macrophages under all conditions tested (that is, 4 and 8 µg/ml C1q as the coating concentration, and using two additional different concentrations of IgG to opsonize the targets, data not shown). For example, at 4 µg/ml HSA, LRP+/+ and macLRP−/− macrophages demonstrated 8.5 ± 1.8 % and 14.1 ± 0.9 % phagocytosis respectively and this increased to 45.7 ± 0.9 % and 42.9 ± 2.3 %, respectively, when cells were plated on C1q (Fig. 5A). Only 13% of the macLRP−/− macrophages used in the representative experiment shown in Figure 5 expressed LRP, and these LRP-expressing macrophages expressed only 15.1% of the mean fluorescence level expressed by control LRP+/+ cells. Since 42.9% of the macLRP−/− macrophages ingested targets in this experiment, these data demonstrate that LRP is not required for the C1q-triggered enhancement of phagocytosis of antibody-opsonized targets.
Figure 5. LRP-deficient BMDM respond to C1q with an enhancement of phagocytosis of antibody-coated sheep red blood cells (EAIgG).
BMDM were adhered to surfaces coated with 4 or 8 µg/ml HSA or C1q for 30 minutes and incubated with sheep erythrocytes suboptimally-opsonized with anti-sheep IgG (EAIgG) for an additional 30 minutes. Non-ingested EAIgG were lysed. Cells were fixed and stained, and slides were scored by phase microscopy. Percent phagocytosis is the number of BMDM that phagocytosed at least one target per 100 cells, and phagocytic index is the number of targets ingested per 100 cells. Data points represent 200 cells/well from duplicate wells ± SD. Shown is one experiment representative of two.
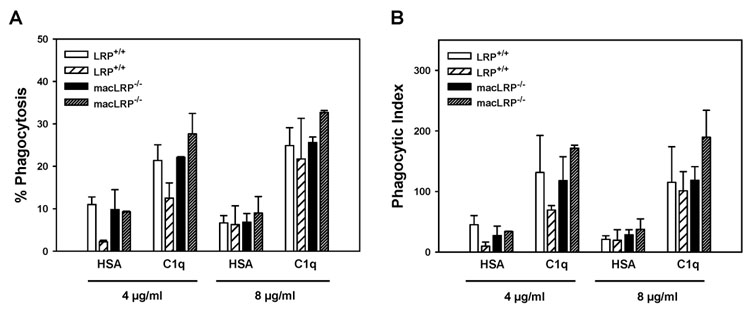
Phagocytosis of EAIgG is mediated by Fcγ receptors. To determine if multiple forms of C1q-triggered enhanced phagocytosis were active in the macLRP−/− cells, we compared the ability of macLRP−/− macrophages to engulf sheep red blood cells opsonized with complement component C4b (EAIgMC4b). Both LRP+/+ and macLRP−/− macrophages responded to C1q with an enhancement of percent phagocytosis and phagocytic index of EAC4b, and there was no significant difference between the genotypes (Fig. 6). For example, in the experiment shown in Figure 6, both LRP+/+ and macLRP−/− macrophages responded to 8 µg/ml C1q with a 3.7-fold enhancement of phagocytosis. In the experiment shown, 43.6 and 44% of macLRP−/− macrophages expressed LRP; however, of the macLRP−/− cells expressing LRP, LRP expression levels were only 16.2 to 18.3% of LRP+/+ macrophage controls. Therefore, there was a greater than five-fold reduction in LRP expression on macLRP−/− cells; however, there was no effect on the C1q-triggered enhancement of phagocytosis. These data demonstrate that LRP is not required for the C1q-triggered enhancement of phagocytosis of antibody- or complement-coated particles.
Figure 6. LRP-deficient BMDM respond to C1q with an enhancement of phagocytosis of complement-opsonized sheep red blood cells (EAIgMC4b).
Two individual LRP+/+ BMDM preparations and two macLRP−/− BMDM preparations were processed as described in Figure 5, except that cells were treated with 10 ng/ml phorbol 12, 13-dibutyrate (PDBu). Bars represent average ± SD from duplicate wells. Shown is one experiment representative of two.
LRP-deficient and LRP-expressing BMDM exhibit equivalent C1q-independent and C1q-enhanced phagocytosis of apoptotic cells
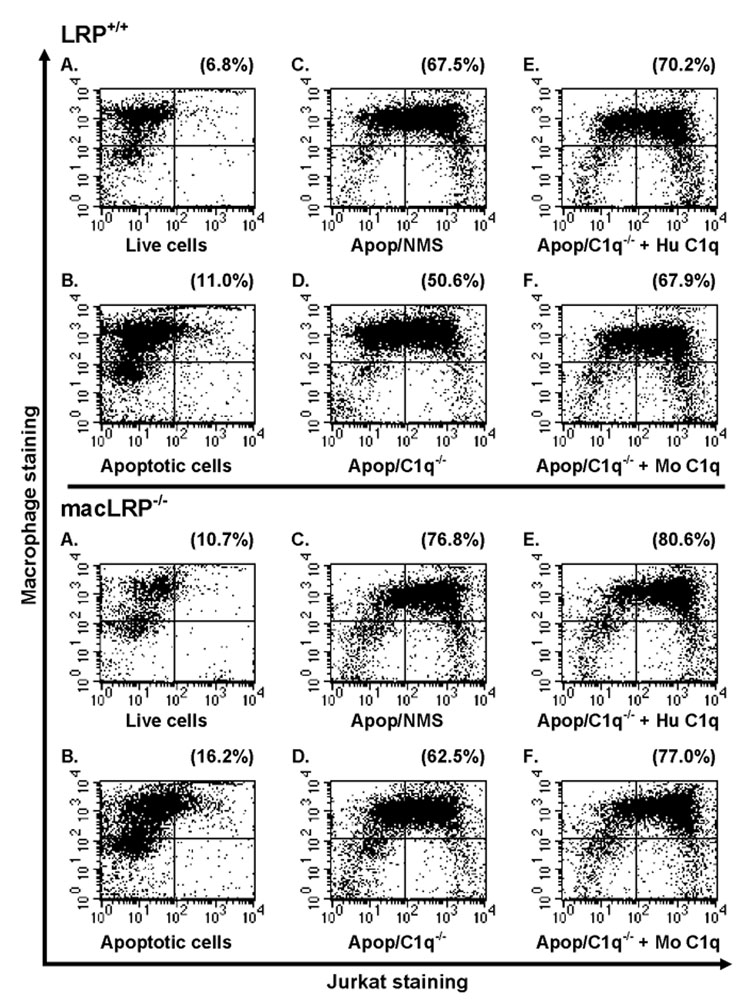
In order to determine if LRP plays a role in the phagocytosis of apoptotic cells, we examined phagocytosis of apoptotic Jurkat cells by LRP-expressing and LRP-deficient BMDM under a variety of serum-free or serum-containing conditions. BMDM from LRP+/+ and macLRP−/− mice were incubated with live or apoptotic Jurkat cells at a ratio of 3 Jurkat cells for every 1 BMDM for 30 minutes at 37°C. Cells were stained for CD11b, a common macrophage marker, and analyzed by flow cytometry. Representative dot plots from one of four independent experiments are shown comparing uptake of Jurkat cells by LRP+/+ and macLRP−/− BMDM (Fig. 7). Only 13% of the macLRP−/− macrophages used in the representative experiment shown expressed LRP, and these LRP-expressing macrophages expressed only 15.1% of the mean fluorescence level expressed by control LRP+/+ cells. In a replicate experiment (not shown), only 7.5% of macLRP−/− cells expressed LRP while 15.5% of cells engulfed apoptotic cells in a C1q-dependent manner (42% phagocytosis for C1q deficient serum and 57.5% phagocytosis when reconstituted with C1q). These data demonstrate that LRP is not required for the C1q-dependent or -independent phagocytosis of apoptotic cells under the specified conditions. Results from each of the four experiments performed were averaged and are shown in Figure 8. No averages are plotted for apoptotic cells phagocytosed in the presence of C1q-deficient serum supplemented with mouse C1q because this condition was used in only one experiment.
Figure 7. Phagocytosis of apoptotic Jurkat T cells by BMDM is enhanced by C1q-containing serum in both LRP-expressing and LRP-deficient phagocytes.
BMDM from LRP+/+ (top) and macLRP−/− (bottom) mice were incubated with: (A) live or (B) apoptotic CFSE-labeled Jurkat T cells for 30 minutes at 37°C in serum free conditions, or with apoptotic cells incubated in the presence of (C) 10% normal mouse serum (NMS), (D) 10% C1q-deficient NMS (C1q−/−), or 10% C1q−/− serum supplemented with either (E) human C1q (Hu C1q) or (F) mouse C1q (Mo C1q) at a final concentration of 75 µg/ml. Cells were washed, harvested and stained with a PE-conjugated anti-CD11b antibody for FACS analysis. Percent phagocytosis was calculated by dividing CFSE/CD11b double-positive cells by the total number of CD11b-positive cells and multiplying by 100 (Upper right quadrant / (Upper right + upper left quadrants)*100) and is shown in the upper right corner. With the exception of the serum condition C1q−/− + Mo C1q, which was only performed once, results for all conditions shown are from one of four independent experiments performed which yielded similar results.
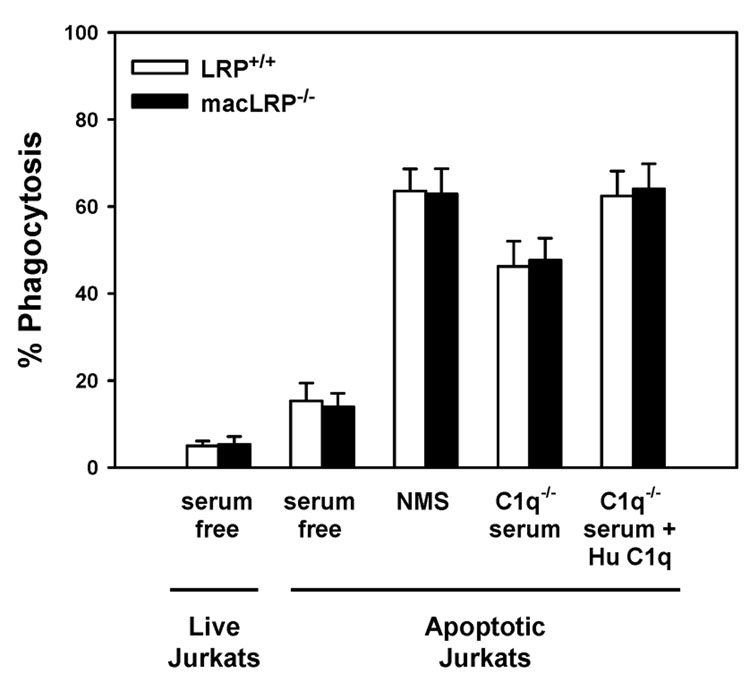
Figure 8. LRP-deficient BMDM exhibit C1q-mediated enhanced phagocytosis of apoptotic Jurkat T cells.
BMDM from LRP+/+ and macLRP−/− mice were incubated with live or apoptotic Jurkat T cells for 30 minutes at 37°C in serum free conditions or in the presence of 10% normal mouse serum (NMS), C1q-deficient serum (C1q−/−), or C1q-deficient serum supplemented with human C1q (C1q−/− + Hu C1q) (final concentration of 75 µg/ml). Cells were processed as described in Figure 7. For both LRP+/+ and macLRP−/− BMDM, means were significantly different at the p < 0.01 level or lower when comparing the following conditions within genotypes: serum free with NMS, NMS with C1q−/− serum, and C1q−/− serum with C1q−/− serum + Hu C1q by Student’s t test. There were no statistically significant differences between LRP-expressing and LRP-deficient cells in any of the conditions tested. Results shown are averaged from 4 independent experiments. Error bars represent SEM.
In serum-free conditions, apoptotic Jurkat cells were phagocytosed more efficiently than live Jurkat cells by both LRP-expressing and LRP-deficient macrophages, with 5.0 ± 1.1% and 5.3 ± 1.8% phagocytosing live cells, respectively, and 15.3 ± 4.1 and 14.0 ± 3.0% ingesting apoptotic Jurkat cells, respectively (n = 4). Previous studies suggested that LRP was required for phagocytosis of apoptotic cells, even in the absence of defense collagens, by a direct interaction between phagocyte LRP and CRT on the apoptotic cell [7]. However, we observed no difference between the LRP+/+ and macLRP−/− macrophages in their ability to phagocytose apoptotic cells in serum-free conditions, suggesting that phagocytosis occurs independently of LRP in this C1q-free system.
Multiple factors in serum, in addition to C1q and other complement factors, contribute to the efficiency of phagocytosis of apoptotic cells [35]. Indeed, we observed a similar increase in the phagocytosis of apoptotic Jurkat cells in the presence of normal mouse serum (NMS) by BMDM from both LRP+/+ and macLRP−/− mice. LRP-expressing and LRP-deficient macrophages exhibited a 4.2 and 4.5-fold increase in ingestion of apoptotic targets in the presence of serum, respectively, when compared to the serum-free samples (Fig. 7 and Fig. 8). These data demonstrate that phagocytosis of apoptotic Jurkat cells in the presence or absence of serum occurs with equal efficiency in LRP+/+ and macLRP−/− macrophages, suggesting that LRP is not required for ingestion of apoptotic Jurkat cells.
In order to examine the role of C1q in the serum-mediated increase in phagocytosis, we performed the same phagocytosis assay in the presence of mouse serum genetically devoid of C1q (C1q−/−) [12]. When the assay was performed using C1q−/− mouse serum and the results compared to NMS, we observed a 27.2% and 25.9% decrease in phagocytosis of apoptotic Jurkat cells by LRP+/+ and macLRP−/− BMDM, respectively (Fig. 7 and Fig. 8). These results confirm that C1q is one of the factors in NMS which contributes to the serum-induced enhancement of phagocytosis. Furthermore, supplementing the C1q−/− serum with human C1q returned the percent phagocytosis values to 98.2% and 101.9% of the values obtained in the presence of NMS in LRP+/+ and macLRP−/− macrophages, respectively (n = 4). Importantly, there were no differences in percent phagocytosis between BMDM from LRP+/+ and macLRP−/− macrophages in any of the conditions tested. These studies verify that C1q is one factor in serum responsible for the increased phagocytosis of apoptotic Jurkat cells by BMDM and that the C1q-mediated enhancement of phagocytosis in complete serum occurs independently of LRP.
Discussion
The objectives of this study were to investigate the role of LRP in phagocytosis and to determine if LRP is the receptor or a required component of a receptor complex responsible for C1q-triggered enhancement of phagocytosis. To accomplish these objectives, we employed a targeted deletion of the LRP gene. As expected from crossing LysMCre mice with loxP-flanked LRP, we observed an effective deletion of the LRP gene from both thioglycollate-elicited peritoneal macrophages and BMDM. Effective LRP deletion was confirmed by immunoblotting, FACs analysis, and functional assays. One of these functional assays demonstrated that LRP-deficient macrophages were unable to mediate the endocytosis of fluorescently labeled RAP. While RAP functions in vivo as a molecular chaperone, it binds tightly to LRP, is rapidly internalized by this receptor, and serves as a useful ligand to monitor LRP functional activity. Our results with fluorescently labeled RAP definitively demonstrate that the macLRP−/− macrophages are defective in processes known to be mediated by LRP.
To investigate the potential of LRP to mediate phagocytosis, we examined the ability of LRP-expressing and LRP-deficient macrophages to mediate the phagocytosis of microbeads coated with GST-RAP, GST-HBD or with GST. The finding that GST-RAP and GST-HBD-coated microbeads are more efficiently ingested by LRP-expressing cells provides direct evidence that endogenous LRP contributes to phagocytosis in professional phagocytes. These results are also consistent with hypotheses from past published work. Kinchen et al. reported that a receptor in C. elegans, CED-1, through interaction with the adaptor protein CED-6, initiates reorganization of actin necessary for phagocytosis [36], and they proposed that, in the mammalian system, LRP could play a similar role to CED-1. The human homologue of CED-6, GULP, is an adaptor protein that was shown to interact with phosphorylated forms of the LRP intracellular domain [21,37]. Additionally, the CED-1 homologue in Drosophila, Draper, was shown by others to play a critical role in phagocytosis of apoptotic cells [38,39]. Finally, it was previously demonstrated that the LRP cytoplasmic domain contains sufficient information to trigger phagocytosis [22]. Our studies confirm that LRP has the capacity to mediate phagocytosis of particles bearing LRP ligands on their surfaces.
We next used this genetic model to test the hypothesis that LRP is the receptor responsible for mediating C1q enhancement of phagocytosis in professional phagocytes and/or that LRP is required for the C1q-dependent or independent uptake of apoptotic cells [6,18,34]. MacLRP−/− BMDM displayed enhanced ingestion of antibody- and complement-opsonized red blood cells when adherent to C1q, which was comparable to levels demonstrated by LRP+/+ control macrophages. In addition, apoptotic Jurkat cells were ingested equivalently by LRP+/+ and macLRP−/− macrophages in the presence or absence of serum. Ingestion of Jurkats by both LRP-deficient and LRP-expressing macrophages was reduced in the presence of C1q−/− serum compared to NMS; however, ingestion at the NMS level could be reconstituted for both LRP+/+ and macLRP−/− when purified C1q was added back. These studies demonstrate that C1q-enhanced phagocytosis, via multiple physiologically important pathways, can occur independently of LRP, suggesting that other, as of yet unidentified, C1q receptors or receptor complexes exist.
The use of suboptimally-opsonized erythrocytes coated with either IgG or C4b enabled us to look at C1q enhancement of FcR- and complement-mediated phagocytosis. Previous studies identified a six amino acid stretch in the collagen-like tail of C1q responsible for triggering the enhanced ingestion [29]; however, the phagocyte receptor (or receptor complex) that binds the six amino acid stretch has remained elusive. Our results unequivocally show that C1q enhances both FcR- and complement-mediated phagocytosis equally well in LRP-deficient and LRP-expressing macrophages, demonstrating that LRP is not a required receptor through which C1q mediates this enhancement. Importantly, previous studies suggesting a required role for LRP in C1q-enhanced ingestion have focused on phagocytosis of apoptotic cells [6,7,18,34], and it is possible that enhanced ingestion of antibody- and complement-opsonized particles following adhesion to C1q-coated surfaces (as demonstrated in Fig. 5 and Fig. 6) occurs via an alternative mechanism. In support of this hypothesis, previous studies showed that adhesion to C1q triggers an inhibition rather than an enhancement of uptake of apoptotic cells [34]. However, here we show that under serum-free and a variety of serum-containing conditions apoptotic Jurkat cells were ingested equally well by LRP-expressing and LRP-deficient macrophages, suggesting that LRP is not essential for the phagocytosis of apoptotic cells.
Multiple receptors have been suggested to be involved in ingestion of apoptotic cells including LRP, αvβ3, CD36 and others (reviewed in [1]). Ogden and colleagues reported that anti-LRP and anti-CRT antibodies inhibited human monocyte derived macrophage phagocytosis of apoptotic Jurkat cells and erythrocytes bearing C1q tails on their surfaces [34]. They suggested that C1q, when bound to apoptotic cells, is recognized by CRT on the phagocyte, which signals through LRP to induce phagocytosis. Similarly, Vandivier and colleagues reported that two collectins, lung surfactant proteins SP-A and SP-D, performed a similar function to C1q and enhanced ingestion of apoptotic neutrophils by alveolar macrophages in a CRT/LRP-dependent fashion, again using antibodies against CRT and LRP to block function [18]. Here we demonstrate that the presence of C1q in serum enhances ingestion of apoptotic Jurkat cells by macrophages. As previously shown by other laboratories [40,41] there was a significant reduction in the phagocytosis of apoptotic cells in the presence of C1q-deficient serum compared to NMS, and a full recovery when C1q-deficient serum was supplemented with purified C1q. It should be noted that in addition to preventing direct C1q-mediated enhancement of phagocytosis via a C1q receptor, use of C1q-deficient serum also prevents activation of the classical complement pathway [41]. Thus, the enhancement of phagocytosis seen upon adding back purified C1q to serum, which contains other soluble complement factors, could be due to uptake mediated by C3 activation fragments, C3b or iC3b, via complement receptor types 1 or 3 or other complement receptors [41]. Further studies will be required to determine if purified C1q, in the absence of other complement components, facilitates enhanced ingestion of apoptotic cells with macLRP−/− macrophages.
A second model addressing the role of LRP in the clearance of apoptotic cells was proposed by Gardai and colleagues, suggesting that phagocyte LRP binds CRT on the surface of apoptotic cells and that this interaction is required for phagocytosis [7]. This was referred to as the “trans” model because it described the interaction between LRP and CRT on two separate cells, and it differentiated this model from the “cis” model [34], which states that CRT and LRP are both found on the engulfing cell where they function as a complex to bind C1q on the apoptotic cell and mediate phagocytosis. Gardai et al. reported that blocking antibodies against CRT reduced ingestion of apoptotic fibroblasts, neutrophils and Jurkat cells by fibroblasts (used as engulfing cells) and that apoptotic, CRT-deficient, murine embryonic fibroblasts were less efficiently cleared than apoptotic, wild-type murine embryonic fibroblasts from the peritoneum of normal mice, suggesting a role for CRT as a surface ligand important for the removal of apoptotic cells [7]. In addition, anti-LRP antibodies and treatment with RAP were used to demonstrate a decrease in LRP-mediated phagocytosis of apoptotic neutrophils by the J774 macrophage cell line. The “trans” model would predict that we should observe a decrease in phagocytosis of apoptotic cells by LRP-deficient macrophages in serum free conditions (i.e., in the absence of C1q or other serum factors). However, macLRP−/− macrophages consistently ingested comparable numbers of apoptotic cells compared to LRP+/+ macrophages over four repeat experiments. These data indicate that LRP is not essential for the phagocytosis of apoptotic cells and suggest that either redundant pathways of ingestion are available, which fully compensate for the loss of LRP, or that the “trans” pathway is not the major mechanism for engulfment of non-opsonized Jurkat cells.
In comparing systems used in previous studies to our results presented here, it should be noted that previous studies utilized Jurkat cells that were rendered apoptotic by UV-irradiation, whereas the studies herein induced apoptosis with etoposide. It is possible that these different methods could lead to a different series of ligands displayed on the surface of apoptotic cells and may account for the discrepancies noted, although no precedence for such a difference was found in a review of the literature. In addition, while Ogden et al. demonstrated that adhesion to C1q-coated surfaces resulted in an inhibition of phagocytosis of apoptotic cells in human monocyte derived macrophages (presumably by sequestering LRP at the base of the cell) [34], we did not detect an inhibition of uptake of apoptotic cells with murine BMDM adherent to C1q (data not shown). Similarly, in our experiments (data not shown) opsonization of apoptotic cells with purified C1q did not reproducibly trigger enhanced ingestion, in contrast to what has been demonstrated in previously published systems [6,34]. Taken together, these data demonstrate that the contribution of LRP to C1q-dependent and -independent enhancement of phagocytosis of apoptotic cells appears to be highly dependent on the specific myeloid cell type and/or apoptotic cell system employed. Nevertheless, our data definitively reveal a system in which engulfment of apoptotic cells is independent of LRP and enhancement of phagocytosis by C1q, in the context of whole serum or purified C1q immobilized on plastic, is also independent of LRP.
Finally, verifying that a given protein mediates a specific function in an in vitro cell-based system is difficult when using antibodies or inhibitors alone. As mentioned in the introduction, identification of the C1q receptor that mediates enhanced ingestion of antibody- and complement-opsonized targets, which may or may not be the same receptor that mediates engulfment of C1q opsonized apoptotic cells, has proven elusive. CD93 was suggested to mediate the C1q-triggered enhancement of phagocytosis based on compelling in vitro antibody studies and other indirect means of testing function (for a full discussion the reader is referred to [2]), but when CD93−/− mice were generated, in vitro studies showed similar C1q-mediated enhancement between CD93−/− and control macrophages [17]. The LRP-deficient macrophages utilized in this study offered a similar opportunity to test proposed roles for LRP, and future studies employing the mice will enable verification of these conclusions in vivo. It is possible that blocking antibodies and/or soluble RAP, used in previous experiments to suggest a non-redundant role for LRP and CRT in engulfment of apoptotic cells, are generating inhibitory signals resulting in diminished phagocytosis. Further experimentation utilizing these reagents in conjunction with macLRP−/− macrophages should help clarify the mechanism by which these receptors modulate phagocytosis. It is also possible that both CD93 and LRP are involved in this function, and that one receptor compensates in the absence of the other. Generation of double-deficient macrophages (LRP−/−/CD93−/−) can be utilized to address this possibility.
In summary, results using murine macrophages genetically deficient in LRP demonstrate that (1) LRP is capable of contributing to the phagocytosis of targets bearing LRP ligands on their surfaces, (2) LRP is not required for C1q-triggered enhancement of phagocytosis of IgG- or C4b-opsonized particles and, as such, is not the C1q receptor utilized in this system and (3) LRP is not required for phagocytosis of apoptotic cells in vitro either directly (in the absence of serum) or by C1q-mediated effects in the presence of complete serum. Despite our conclusions based on the evidence reported here, previously published work suggests that LRP has the potential to mediate phagocytosis of apoptotic cells in other cellular systems (e.g., fibroblasts) [6,7,18,21,34]. Future experiments employing the macLRP−/− mice should enable the identification of specific systems in or conditions under which LRP is involved in the uptake of apoptotic cells and/or other physiologically-relevant, LRP-dependent, phagocytic targets.
Acknowledgements
The authors thank Dr. Deborah Fraser (University of CA, Irvine) for purified mouse C1q, Dr. Li Zhang (University of Maryland) for the anti-Mac-1 antibody and Jessica Morris, (University of Notre Dame) Frances Battey, Mary Migliorini and Susan Robinson (University of Maryland) for invaluable technical assistance.
Footnotes
This work was supported by the Duke University Medical Scientist Training Program, grant T32 GM007171-NIGMS (APL), T32 HL007698 (APL), and grants NIH HL-24066 (SVP), NIH AI-41090 (AJT), HL054710 (DKS), HL050784 (DKS) and AHA 0630068N (SSB).
Abbreviations used in this paper: LRP, low-density lipoprotein receptor-related protein; GST, Glutathione-S-transferase; RAP, receptor associated protein; CRT, calreticulin; BMDM, bone marrow-derived macrophages; EAIgG, sheep erythrocytes opsonized with IgG; HBD, heparin binding domain; HSA, human serum albumin; EAIgMC4b, sheep erythrocytes opsonized with complement component C4b; NMS, normal mouse serum.
Reference List
- 1.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol. Immunol. 2007;44:33. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525. [PubMed] [Google Scholar]
- 4.Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, Gingras AR, Mantovani A, Hack EC, Roos A. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 2002;32:1726. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van KC, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J. Immunol. 2004;173:3044. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 6.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 7.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4 Suppl 3:S279–S293. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidari Y, Bygrave AE, Rigby RJ, Rose KL, Walport MJ, Cook HT, Vyse TJ, Botto M. Identification of chromosome intervals from 129 and C57BL/6 mouse strains linked to the development of systemic lupus erythematosus. Genes Immun. 2006;7:592. doi: 10.1038/sj.gene.6364335. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, Botto M, Walport MJ. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J. Immunol. 2002;168:2538. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- 11.Warren J, Mastroeni P, Dougan G, Noursadeghi M, Cohen J, Walport MJ, Botto M. Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2002;70:551. doi: 10.1128/iai.70.2.551-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 13.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 2005;174:3220. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 14.Guan E, Robinson SL, Goodman EB, Tenner AJ. Cell-surface protein identified on phagocytic cells modulates the C1q-mediated enhancement of phagocytosis. J. Immunol. 1994;152:4005. [PubMed] [Google Scholar]
- 15.Guan EN, Burgess WH, Robinson SL, Goodman EB, McTigue KJ, Tenner AJ. Phagocytic cell molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J. Biol. Chem. 1991;266:20345. [PubMed] [Google Scholar]
- 16.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 17.Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, Taylor PR, Bygrave AE, Thompson RD, Nourshargh S, Walport MJ, Botto M. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J. Immunol. 2004;172:3406. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- 18.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 2002;169:3978. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 19.Stuart GR, Lynch NJ, Day AJ, Schwaeble WJ, Sim RB. The C1q and collectin binding site within C1q receptor (cell surface calreticulin) Immunopharmacology. 1997;38:73. doi: 10.1016/s0162-3109(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 20.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J. Thromb. Haemost. 2005;3:1884. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 21.Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, Ravichandran KS. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP) J. Biol. Chem. 2002;277:11772. doi: 10.1074/jbc.M109336200. [DOI] [PubMed] [Google Scholar]
- 22.Patel M, Morrow J, Maxfield FR, Strickland DK, Greenberg S, Tabas I. The cytoplasmic domain of the low density lipoprotein (LDL) receptor-related protein, but not that of the LDL receptor, triggers phagocytosis. J. Biol. Chem. 2003;278:44799. doi: 10.1074/jbc.M308982200. [DOI] [PubMed] [Google Scholar]
- 23.Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J. Biol. Chem. 1992;267:9035. [PubMed] [Google Scholar]
- 24.Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J. Immunol. 1981;127:648. [PubMed] [Google Scholar]
- 25.Hu L, Boesten LS, May P, Herz J, Bovenschen N, Huisman MV, Berbee JF, Havekes LM, van Vlijmen BJ, Tamsma JT. Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2710. doi: 10.1161/01.ATV.0000249641.96896.e6. [DOI] [PubMed] [Google Scholar]
- 26.Roach T, Slater S, Koval M, White L, Cahir McFarland ED, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr. Biol. 1997;7:408. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 27.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J. Biol. Chem. 1990;265:17401. [PubMed] [Google Scholar]
- 28.Nepomuceno RR, Ruiz S, Park M, Tenner AJ. C1qRP is a heavily O-glycosylated cell surface protein involved in the regulation of phagocytic activity. J. Immunol. 1999;162:3583. [PubMed] [Google Scholar]
- 29.Arora M, Munoz E, Tenner AJ. Identification of a site on mannan-binding lectin critical for enhancement of phagocytosis. J. Biol. Chem. 2001;276:43087. doi: 10.1074/jbc.M105455200. [DOI] [PubMed] [Google Scholar]
- 30.Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, Rochford R, Tenner AJ. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J. Leukoc. Biol. 2006;80:107. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 31.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 32.Mikhailenko I, Krylov D, Argraves KM, Roberts DD, Liau G, Strickland DK. Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J. Biol. Chem. 1997;272:6784. doi: 10.1074/jbc.272.10.6784. [DOI] [PubMed] [Google Scholar]
- 33.Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J. Biol. Chem. 1995;270:9543. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- 34.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2002;2:346. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 36.Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- 37.Ranganathan S, Liu CX, Migliorini MM, von Arnim CA, Peltan ID, Mikhailenko I, Hyman BT, Strickland DK. Serine and threonine phosphorylation of the low density lipoprotein receptor-related protein by protein kinase Calpha regulates endocytosis and association with adaptor molecules. J. Biol. Chem. 2004;279:40536. doi: 10.1074/jbc.M407592200. [DOI] [PubMed] [Google Scholar]
- 38.Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- 39.Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, Henson P, Nakanishi Y. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J. Biol. Chem. 2004;279:48466. doi: 10.1074/jbc.M408597200. [DOI] [PubMed] [Google Scholar]
- 40.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 41.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur. J. Immunol. 2005;35:252. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]