Abstract
Before social cognition there is joint processing of information about the attention of self and others. This joint attention requires the integrated activation of a distributed cortical network involving the anterior and posterior attention systems. In infancy, practice with the integrated activation of this distributed attention network is a major contributor to the development of social cognition. Thus, the functional neuroanatomies of social cognition and the anterior–posterior attention systems have much in common. These propositions have implications for understanding joint attention, social cognition, and autism.
Keywords: attention, joint attention, cognitive neuroscience, autism, interconnectivity
Infants follow the direction of other people’s gaze in the first year of life (Scaife & Bruner, 1975). This seminal observation led to a reconsideration of the Piagetian notion of infant egocentrism, and it indicated that the ability to share a common point of reference develops before language. Ultimately, the term joint attention was adopted to refer to this domain. It is an expression of the exquisitely honed human capacity to coordinate attention with a social partner, which is fundamental to our aptitude for learning, language, and sophisticated social competencies throughout life. For example, every teacher’s admonition to students to “pay attention!” is really a request to “pay attention to what I [the teacher] am attending to.” Without the capacity for joint attention, success in many pedagogical contexts would be difficult to achieve. Similarly, joint attention is vital to social competence at all ages. Adolescents and adults who cannot follow, initiate, or join with the rapid-fire changes of shared attention in social interactions may be impaired in their capacity for relatedness and relationships.
Even though it is a vital skill, we know surprisingly little about the development of joint attention. Is joint attention a distinct form of human attention or simply a social manifestation of general aspects of attention? If it is the former, does the development of this distinct form of human attention play a central role in the emergence of human social cognition? In particular, does joint attention develop after social cognition—as current thinking would have it—or does human social cognition follow from the development of joint attention? These questions form the outline of this article, which begins with a more detailed definition of joint attention.
WHAT IS JOINT ATTENTION, AND WHY IS IT IMPORTANT?
Joint-attention behaviors in infancy fall into two categories: responses to the bids of others or spontaneous initiations (Mundy et al., 2007). Responding to joint attention (RJA) refers to infants’ ability to follow the direction of the gaze and gestures of others in order to share a common point of reference. Alternatively, initiating joint attention (IJA) involves infants’ use of gestures and eye contact to direct others’ attention to objects, to events, and to themselves. The function of IJA is to show or spontaneously seek to share interests or pleasurable experience with others (Fig. 1).
Fig. 1.
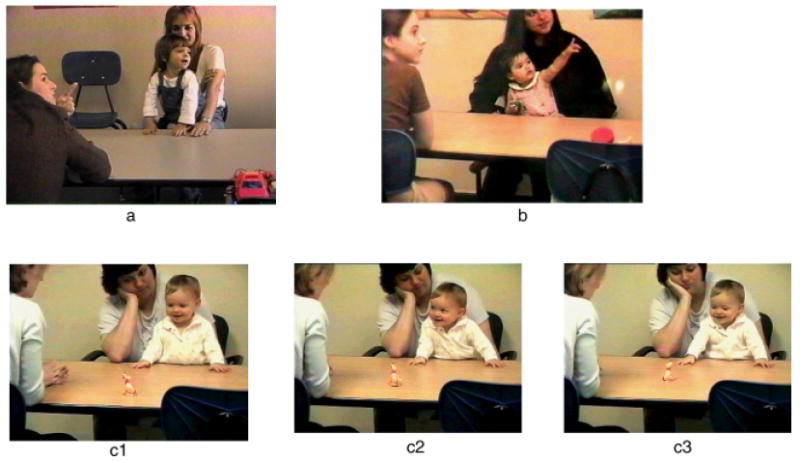
Illustrations of two expressions of joint attention development: responding to joint attention (a) and initiating joint attention (IJA; b, c1,2,3). Two types of IJA behavior are depicted: IJA involving a conventional gesture of pointing to share attention regarding a room poster (b), and IJA involving alternating looking at a toy (c1, c3) with making eye contact with another individual (c2), to share attention with respect to the object.
The development of joint attention heralds a new stage of infant–caregiver interactions that facilitates social learning. For example, early language learning often takes place in unstructured, incidental situations in which a parent refers to a new object. How does the infant know which one of myriad potential referents the parent means? To identify the referent, an infant uses RJA and the direction of gaze of its parent to increase the likelihood of attending to the correct object in incidental novel-word-learning opportunities (Baldwin, 1995). Thus, joint attention serves a self-organizing role in social information processing in early, unstructured social-learning situations.
Several studies support the idea that joint attention reflects mental and behavioral processes that facilitate human learning and development. The frequency with which infants engage in joint attention is related to their language acquisition, even when variance associated with general cognition is controlled (e.g., Morales et al., 2000; Mundy et al., 2007). Joint attention is also associated with the depth of information processing in infants (Striano, Chen, Cleveland, & Bradshaw, 2006), as well as with individual differences in childhood measures of IQ, self-regulation, and social competence (Mundy et al., 2007).
Clinical research indicates autism is characterized by chronic, pronounced impairments in IJA rather than RJA. This IJA impairment is described as “the lack of spontaneous sharing experiences with others such as showing” (e.g., Fig. 1c) in the current American and European psychiatric diagnostic systems (Mundy, 2003). Moreover, individual differences in joint attention are related to the intensity of social symptoms, responsiveness to intervention, and long-term social outcomes in children with autism.
Comparative research also suggests that important distinctions between humans and other primates involve joint attention. Chimpanzees show the capacity for RJA but little evidence of IJA or spontaneous attempts to share experiences with members of their own species (Tomasello & Carpenter, 2005). This suggests that a basic understanding of goal-directed perception and action, manifested in RJA, might be common to advanced primates but that a deeper facility and understanding of sharing attention and intentions, as manifest in IJA, may be unique to human beings (Tomasello & Carpenter, 2005).
Thus, experimental, clinical, and comparative research suggests that joint attention reflects vital aspects of human psychological development. Current models of joint attention provide some insight into the nature of this development.
SOCIAL COGNITION AND JOINT ATTENTION
The social-cognitive model of joint attention proposes that, as infants monitor and represent their own goal-related intentional activity, they also monitor and represent the goal-related behavior of others (Tomasello, Carpenter, Call, Behne, & Moll, 2005). The early development of social cognition, beginning at about 9 to 12 months, allows infants to integrate these two sources of information; infants thereby become able to impute the conditional proposition that if self-intentions lead to goal-related behavior, then goal-related behavior in others must follow from their intentions. Thus, this model suggests that social cognition is necessary for the development of functional joint attention in infancy. Furthermore, social cognition is thought to be equally common to all types of joint attention. Therefore, initiating and responding to joint attention should be highly correlated in development.
Research supports elements of the social-cognitive model. In one revealing study, testers presented gaze-following trials to 9-, 10-, and 11-month-olds. The testers turned their heads either with their eyes open or with their eyes closed (Brooks & Meltzoff, 2005); the task for the infants was to respond to joint attention and turn their attention in the direction of the gaze or head turn of the tester. All the children performed equally well in the “eyes open” condition. However, in the “eyes closed” condition the 10- and 11-month-old infants rarely followed the testers’ head turns, but the 9-month-olds followed at a rate comparable to their rate in the eyes-open condition (see Fig. 2). Thus, the 10-month-olds were able to inhibit following in this condition, presumably based on a social-cognitive awareness of the meaning of the referential intent of visual gaze. However, the 9-month-olds were not able to inhibit their responding behavior because they lacked this aspect of social cognition.
Fig. 2.
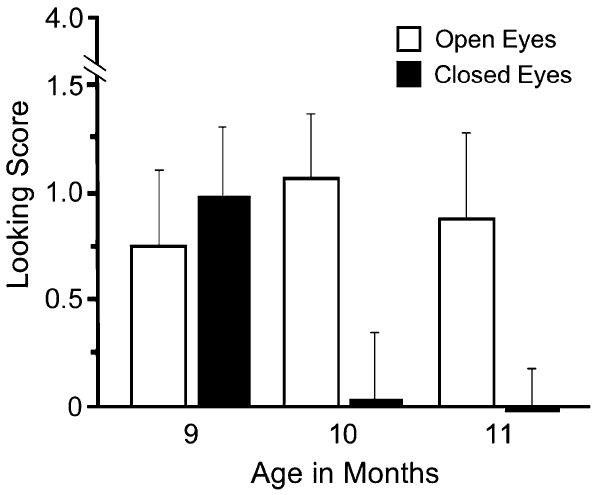
Responsiveness (looking score) of infants to an experimenter’s head turn with eyes closed versus eyes open. While 9-month-olds appear to follow head orientation to look in the correct direction on responding-to-joint-attention trials, 10- and 11-month-olds appear to understand that eye orientation (eyes-open condition) rather than simply head orientation (eyes-closed condition) is critical to sharing attention with others. From Brooks & Meltzoff (2005).
Note that Brooks and Meltzoff’s (2005) data also indicate that 9-month-olds engage in joint attention behavior before they fully develop aspects of social-cognitive awareness. Indeed, RJA may be measured at least as early as 6 months and predicts language ability at 24 months, attesting to the functional validity of measuring RJA at that age (Morales et al., 2000). These observations challenge the social-cognitive model. So do observations that IJA and RJA are not highly correlated in development; that they display different patterns of age-related growth in infancy; and that they have unique associations with subsequent development (Mundy et al., 2007). An alternative to the social-cognitive perspective appears to be needed to account for these observations.
ATTENTION AND JOINT ATTENTION
Mundy, Card, and Fox (2000) suggested that joint attention is an outcome of two interacting attention-regulation systems described by Michael Posner and his colleagues (e.g., Posner & Rothbart, 2007). One is the posterior orienting and perceptual attention system, which plays a primary role in RJA development in infancy. This relatively involuntary system begins to develop in the first months of life and prioritizes orientation toward biologically meaningful stimuli. It is supported by the parietal and superior temporal cortices (Fig. 3), which serve aspects of representational development, imitation, and the perception of the eye and head orientations of others, as well as the perception of spatial relations between self, other, and the environment. The neural substrates and functions of this system are common to many primates. A shorthand way of expressing one goal-related cognitive output from this system is the individual’s understanding that “where others’ eyes go, their behavior follows” (Jellema, Baker, Wicker, & Perrett, D., 2000).
Fig. 3.
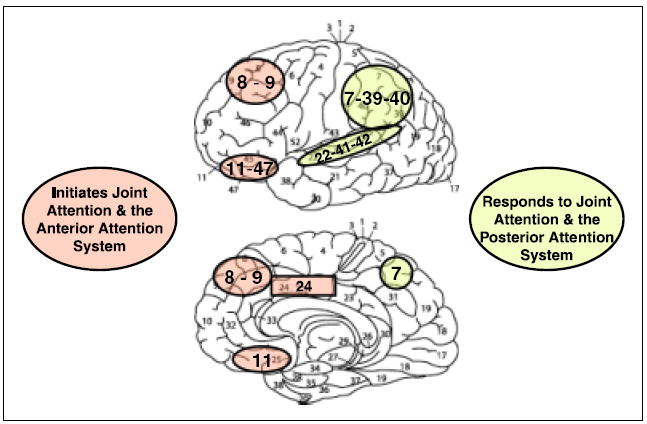
Lateral (side) and medial (interior) areas of the cerebral cortex associated with initiating joint attention and the anterior attention system. These include Brodmann areas 8 (frontal eye fields), 9 (prefrontal association cortex), 24 (dorsal anterior cingulate), and 11 and 47 (orbital prefrontal association cortex). Those regions associated with the Responding to Joint Attention and the posterior attention system include areas 7 (posterior parietal association area); 22, 41, and 42 (superior temporal cortex); and 39 and 40 (parietal, temporal, and occipital association cortices).
Alternatively, IJA is supported by the later-developing anterior attention system. This system controls volitional, goal-directed attention allocation that is constrained and corrected by reward-related self-appraisal of behavior. It involves a neural network that includes the frontal eye fields (dorsal frontal cortical areas associated with intentional eye control), the pre-frontal association cortex, the orbital frontal cortex, and the anterior cingulate (Fig. 3). If part of the cognitive output from the posterior system can be characterized as “where others’ eyes go, their behavior follows,” part of the cognitive output of the anterior system may be characterized as “where my eye’s go, my behaviors follows.”
The system also regulates integrated activity across the anterior and posterior attention systems. This integration yields the distinct form of human attention we call joint attention (Mundy, 2003). Electroencephalography and imaging data attest to the integrated activation of the anterior and posterior systems in human joint attention (for examples, see Mundy et al., 2000; Williams, Waiter, Perra, Perrett, & Whiten, 2005). Moreover, this attention network has much in common with the neural substrates of social cognition (Mundy, 2003). Functionally, starting between 4 and 6 months of age, the anterior attention system integrates the internal monitoring of one’s own control of gaze direction, and its relations to goal-directed behavior, with external monitoring of the relations between others’ gaze direction and their behavior. The interaction and juxtaposition of information from internal and external monitoring about overt attention (active looking) serves as an important engine of cognitive development. For example, it enhances the developmental differentiation of awareness of self-agency with respect to the control of attention versus others’ agency and attention control. Moreover, the same integration of the anterior and posterior attention systems that enables the comparative monitoring of overt attention (looking behavior) ultimately plays the same role when individuals begin to be able to attend to internal representations. That is, when individuals begin to be able to direct covert attention to representations, the integrated anterior–posterior attention system enables them to monitor representations about the behavior of self and others. This contributes to their ability to begin to impute the conditional proposition that if self-control of attention relates to one’s own goal-directed behavior, then goal-related behavior in others must follow from their self-control of attention. As this integrative anterior–posterior capacity to attend to overt or covert information about self and others matures with advances in representational abilities, a fully functional, adaptive, human social-cognitive system emerges (Fig. 4).
Fig. 4.
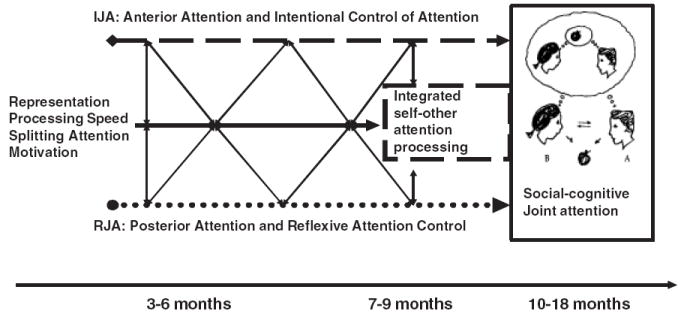
The attention-systems model of joint attention and social cognition. The posterior attention system path associated with the development of responding to joint attention (RJA) is illustrated with a dotted line and the anterior attention system path associated with the development of initiating joint attention (IJA) is illustrated with a dashed line. The central solid line in the figure denotes the development of other processes, such as representational ability, speed of processing, motivation, and the executive control of attention, that influence joint-attention development, as well as each other, during infancy. The diagonal arrows reflect the dynamic and coactive nature of joint-attention development, whereby the maturation of attention, cognitive, and affective systems interact in reciprocal cause-and-effect relations with experience, including the experiences children create for themselves through their own actions. Finally, the development of integrated self-and-other attention processing is considered to be a social-attention executive function of the anterior system that emerges in the 7-to-9-months period, represented by the dashed box. The capacity to integrate and share overt aspects of attention provides a foundation for the ability to share covert aspects of attention, such as representations, and social cognition.
Elements of this perspective are consistent with current social-cognitive models. However, this model emphasizes that learning about and from the early self-control of attention may be a primary step toward the later understanding of intentional behavior in others. Another explicit tenet of this model is that the rapid, real-time functional interactions and maturation of a distributed cortical attention network make a fundamental contribution to the human development of social cognition (Fig. 4). This notion has much in common with current connectionist theories that integrated patterns of activity across distal cortical neural networks are fundamental to human cognitive abilities including developments in representational thinking and self-consciousness. A corollary here is that processes affecting the organization, accuracy, and speed of integrated processing, within or across the anterior and posterior attention systems, may contribute to phylogenetic or human developmental differences in joint attention and social cognition. Finally, to be fully realized, integrated processing of the anterior–posterior systems requires practice with sharing overt attention early in life. Thus, in this model, practice with joint attention in the first 9 months of life is thought of as a major contributor to, rather than a product of, the development of social cognition (Fig. 4).
FUTURE DIRECTIONS
The attention-systems model begins to unify the study of joint attention and social cognition with the rich theoretical and empirical literature on the neurodevelopment of attention. Such unification may raise useful hypotheses. For example, activity in the frontal eye fields (Fig. 3) has been one of the most consistent cortical correlates of social cognition in imaging studies (Mundy, 2003). The self-control of direction of gaze by the frontal eye fields, and the effects of that eye gaze on the behaviors of other people, may be among the first sources of information to generate an awareness of self-intentioned action for many infants. If self-awareness of intentional action is vital to understanding intentional action in others, then systems controlling intentional visual attention may be expected to make a primary contribution to social cognition. If this is true, then it leads to the testable hypothesis that the frontal topography of social cognition may be expected to be different for children who are blind from birth.
The attention-systems model also recognizes that IJA and RJA reflect distinct but interacting processes (Fig. 4). This recognition may facilitate a deeper understanding of human individual differences as well as phylogenetic differences in joint attention and social cognition. For example, the reflexive nature of the posterior attention system may help us understand the capacity for RJA in infants prior to the 9- to 10-month onset of social cognition (cf., Brooks & Meltzoff, 2005).
In autism, IJA impairments are more profound than RJA impairments. IJA also makes more demands on interconnected activity across the anterior and posterior attention systems in infancy than does RJA (Mundy et al., 2000). Interestingly, recent connectivity theory suggests that problems in communication between brain regions, especially anterior and posterior cortical connections, may be primary to the cognitive impairments of autism (e.g., Cherkassky, Kana, Keller, & Just, 2006). In the context of these observations, the attention-systems model leads to the hypothesis that IJA deficits may be one of the earliest behavioral manifestations of abnormal neural connectivity in autism.
Also recall that RJA is common to advanced primates but that a facility for IJA and an understanding of shared intentions may be unique to human beings (Tomasello & Carpenter, 2005). Imaging data indicates that the parietal (joint) attention system is well represented in primates but that the anterior system is not (Astafiev et al., 2003; Jellema et al., 2000). Therefore, studying the nature of frontal processes associated with joint attention may be fundamental to understanding the uniquely human nature of social cognition.
Further advances in this area will require neurocognitive methods that provide data on synchronous patterns of activity across the distributed cortical network associated with joint attention and social cognition (e.g., connectivity revealed by functional magnetic resonance imaging or electroencephalograph coherence), rather than unitary regions of interest. It will also be important to understand how the self–other processing involved in joint attention relates to other precursors of social cognition, such as imitation (see Meltzoff, 2007). A deeper appreciation of the interpersonal and motivation factors (e.g., reward value of sharing experiences and attention to self) that are critical to some types of joint attention will also be indispensable. In this regard it may be informative to examine the continuity between individual differences in infant initiations of joint attention and later-emerging forms of social behavior (Mundy et al., 2007). The use of “capitalization,” or the tendency of older individuals to enhance social bonds by sharing good experiences with others, might be one example of such a related form of mature social behavior (Gable, Reis, Impett, & Asher, 2004). Indeed, examining the degree to which joint attention is integral to the development of human learning and social relatedness after infancy may be an essential goal of new research and theory in this arena of psychological science.
Acknowledgments
Preparation of this paper was supported by National Institutes of Health Grant MH071273 and the University of Miami Marino Autism Research Institute and National Institute of Child Health and Human Development Grant T32 HD07473.
References
- Astafiev S, Shulman G, Stanley C, Snyder A, Essen D, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking and pointing. Journal of Neuroscience. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DA. Understanding the link between joint attention and language. In: Moore C, Dunham PJ, editors. Joint Attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 131–158. [Google Scholar]
- Brooks R, Meltzoff A. The development of gaze following and its relations to language. Developmental Science. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassy V, Kana R, Keller T, Just M. Functional connectivity in baseline resting state network in autism. Neuroreport for Rapid Communication of Neuroscience Research. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Gable S, Reis H, Impett E, Asher A. What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. Journal of Personality and Social Psychology. 2004;87:228–245. doi: 10.1037/0022-3514.87.2.228. [DOI] [PubMed] [Google Scholar]
- Jellema T, Baker C, Wicker B, Perrett D. Neural representation for the perception of intentionality of actions. Brain and Cognition. 2000;44:280–302. doi: 10.1006/brcg.2000.1231. [DOI] [PubMed] [Google Scholar]
- Meltzoff A. ‘Like me’: A foundation for social cognition. Developmental Science. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Mundy P, Delgado C, Yale M, Messinger D, Neal R, Schwartz H. Responding to joint attention across the 6- through 24-month age period and early language acquisition. Journal of Applied Developmental Psychology. 2000;21:283–298. [Google Scholar]
- Mundy P. The neural basis of social impairments in autism: The role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology & Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Vaughan Van Hecke A, Delgado C, Parlade M, Pomeras Y. Individual differences in the development of joint attention in infancy. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Card J, Fox N. Fourteen-month cortical activity and different infant joint attention skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Posner M, Rothbart M. Educating the human brain. Washington, DC: American Psychological Association; 2007. [Google Scholar]
- Scaife M, Bruner J. The capacity for joint visual attention in the infant. Nature. 1975;253:265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Striano T, Chen X, Cleveland A, Bradshaw S. Joint attention social cues influence infant learning. European Journal of Developmental Psychology. 2006;3:289–299. [Google Scholar]
- Tomasello M, Carpenter M. The emergence of social cognition in three young chimpanzees. Monographs of the Society for Research in Child Development. 2005;70(1):vii–132. doi: 10.1111/j.1540-5834.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences. 2005;28:675–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Perra O, Perrett DI, Whiten A. An fMRI study of joint attention experience. NeuroImage. 2005;25:133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
Recommended Reading
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63(4):1–174. [PubMed] [Google Scholar]
- Eilan N, Hoerl C, McCormack T, Roessler J, editors. Joint attention: Communication with other minds: Issues in philosophy and psychology. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Mundy P, Sigman M. Joint attention, social competence and developmental Psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology, volume 1: Theory and Methods. 2. Hoboken, NJ: Wiley; 2006. pp. 293–332. [Google Scholar]
- Vaughan A, Mundy P, Acra CF, Block J, Delgado C, Parlade M, et al. Infant joint attention, temperament, and social competence in preschool children. Child Development. 2007;78:53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


