Abstract
Cholesterol, a major component of plasma membrane lipid rafts, is important for assembly and budding of enveloped viruses, including influenza and HIV-1. Cholesterol depletion impairs virus assembly and infectivity. This study examined the effects of exogenous cholesterol addition (delivered as a complex with methyl-beta-cyclodextrin (MbCD)) on the production of Molony murine leukemia virus (MoMuLV) retroviral vector and HIV-1-based lentiviral vector pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G). Cholesterol supplementation before and during vector production enhanced the infectivity of retroviral and lentiviral vectors up to 4-fold and 6-fold, respectively. In contrast, the amount of retroviral vector produced was unchanged, and that of lentiviral vector was increased less than 2-fold. Both free cholesterol and cholesterol ester content in 293-gag-pol producer cells increased with cholesterol addition. In contrast, the phospholipids headgroup composition was essentially unchanged by cholesterol supplementation in 293-gag-pol packaging cells. Based on these results, it is proposed that cholesterol supplementation increases the infectivity of VSV-G-pseudotyped retroviral and lentiviral vectors, possibly by altering the composition of the producer cell membrane where the viral vectors are assembled and bud, and/or by changing the lipid composition of the viral vectors.
Keywords: Lipid rafts, Cholesterol, Infectivity, Retroviral vector, Lentiviral vector, Viral vector production
1. Introduction
Cell membrane microdomains called lipid rafts are essential in the assembly of several enveloped viruses such as influenza, Ebola, Rotaviruses, Newcastle disease virus, and HIV-1 [1], [2], [3], [4], [5]. An important property of lipid rafts is that they preferentially include or exclude particular proteins to a variable extent. Two major transmembrane glycoproteins in influenza viruses, hemagglutinin and neuraminidase, are raft-associated proteins [4]. Also, the lipid bilayer of HIV-1 virus contains GPI-linked proteins and ganglioside GM1, both of which are known to partition preferentially into lipid rafts [6]. Conversely, the non-raft protein CD45 is excluded from the lipid bilayer of HIV-1 viruses [6]. Other studies have shown that the HIV-1 Gag and Env proteins are raft-associated proteins [6], [7], [8], [9].
Cholesterol and sphingolipids are essential for the formation and stability of lipid rafts, and their levels are elevated in lipid rafts isolated from mammalian cells [10], [11]. Cholesterol supplementation was shown to increase the percentage of hemagglutinin and neuraminidase associated with lipid rafts in MDCK cells infected with influenza A virus [12]. Cholesterol depletion can disrupt lipid rafts and abrogates the association of raft-associated proteins [13], [14]. Disruption of lipid rafts by cholesterol depletion hinders HIV-1 particle production from virus-producing cells, and cholesterol depletion from virus particles significantly impairs virus infectivity [8]. Depletion of cholesterol from target cells impairs HIV-1 virus infection by decreasing the mobility of HIV-1 coreceptor CCR5 on the cell surface [15]. Other investigators have found that disruption of lipid rafts also decreases production of vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped, pantropic Molony murine leukemia virus (MoMuLV) vector [16].
Cholesterol supplementation has been shown to enhance viral infection and non-viral transfection. For example, cholesterol supplementation can increase the susceptibility of target cells to infection by the enveloped coronavirus murine hepatitis virus [17] and by human adenovirus [18]. Similarly, addition of cholesterol during liposome transfection increases the overall efficiency of non-viral gene delivery [19]. The benefit might be due to the impact of cholesterol on cell membrane fluidity. Cholesterol addition can result in a compensatory increase in the fraction of polyunsaturated fatty acids in phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine to control the membrane fluidity [20].
Beer et al. observed higher cholesterol levels in the membrane of both producer cells and MoMuLV viral vectors when producer cells were cultured at 32 °C compared to those at 37 °C [21], [22]. They suggested that cells may counteract the decrease in membrane fluidity at the lower temperature by increasing cholesterol content in the cell membrane. Curiously, it was also reported that decreased vector stability correlated with a higher level of cholesterol in the viral vector membrane [22].
Because of multiple reported negative correlations between cholesterol depletion, and the processes of virus production and infection, we decided to test whether cholesterol supplementation would affect viral vector production or infection. For this we made use of retroviral and lentiviral vector systems previously described by us [23].
2. Materials and methods
Unless otherwise noted, reagents were obtained from Sigma–Aldrich (St. Louis, MO).
2.1. Cell lines, cell culture and vector plasmids
293-gag-pol [24] and NIH3T3 cells (ATCC, Manassas, VA) were maintained at 37 °C in Dulbecco’s modified Eagle’s medium with high glucose (DMEM) (Mediatech Inc., Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (Omega Scientific Inc., Tarzana, CA) or FetalPlex serum (FP) (Gemini Bio-Products, Woodland, CA), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Mediatech Inc., Cellgro). 120C-S cells were maintained at 37 °C in CD293 medium (Invitrogen, Carlsbad, CA) supplemented with 2% FBS (Hyclone, Logan, UT). This cell line has been developed by adapting 120C cells to suspension culture [25]. HeLa cells (ATCC CCL-2) were maintained at 37 °C in Iscove’s Modified Dulbecco’s Medium (IMDM) (Irvine Scientific, Santa Ana, CA) supplemented with 10% FBS (Hyclone). pVSV-G encodes the pantropic VSV-G envelope protein. The open reading frame for EGFP was introduced into a derivative of pLXSN with a large multiple cloning site [26], and this plasmid is referred to as pLTR-EGFP in this report.
2.2. Transfection
293-gag-pol cells were seeded at 2 × 106 cells in a 60-mm dish with 5 ml DMEM supplemented with 10% FBS or FP, penicillin, and streptomycin 46–48 h before transfection. A total of 6 μg of plasmids pVSV-G (1 μg) and pLTR-EGFP (5 μg) was transfected into cells using lipofectamine-2000 (Invitrogen); this plasmid ratio has previously been shown by us to give a high transduction efficiency [23]. Fresh medium was exchanged 8–9 h after transfection. Afterwards, 5 ml fresh medium was exchanged into culture every 24 h. The supernatants collected at 24 and 48 h were centrifuged at 1900 RCF for 8 min to remove cell debris, and then stored at −80 °C. These were called the 1st and 2nd collections, respectively.
2.3. Induction
To produce lentiviral vector pseudotyped with VSV-G in suspension culture, 120C-S cells were seeded in 20 ml fresh CD293 medium supplemented with 2% FBS in 125-ml shake flasks and induced by addition of 10 mM sodium butyrate and 1 μg/ml doxycylcine. The cell density at induction was 0.5–1.5 × 106 viable cells/ml. Fresh medium supplemented with sodium butyrate and doxycylcine was exchanged every 24 h after induction. The supernatant was filtered through a 0.45-μm filter and stored at −80 °C. The supernatants collected at 24 and 48 h after induction were called the 1st and 2nd collections, respectively.
2.4. Cholesterol supplementation scheme
Cholesterol was added to cultures at different time points before and/or during vector production, and it was delivered as a complex with methyl-beta-cyclodextrin (MbCD) so that it can directly dissolve in medium. The mole percentage of cholesterol in the complex is ∼13%. The cholesterol supplementation scheme used in this study is shown in Fig. 1 .
Fig. 1.
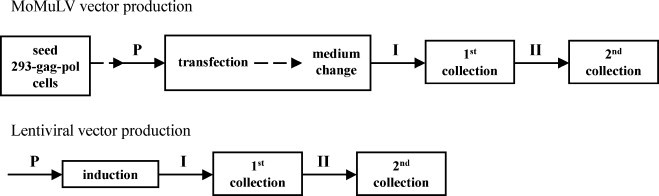
Cholesterol supplementation schemes for both retroviral MoMuLV vector (top) and lentiviral vector (bottom) production. Cholesterol was added to culture at periods P (24 h prior to transfection or induction), I (0–24 h post-transfection or induction), and/or II (24–48 h post-transfection or induction).
2.5. Transduction
NIH3T3 or HeLa cells were seeded at 2 × 104 cells per well in DMEM or IMDM supplemented with 10% FBS or FP in 12-well Plates 24 h before transduction. The viral vectors stored at −80 °C were thawed and used for transduction. Different proportions of viral vector used for transduction were balanced with fresh medium such that the overall amount of transduction medium was 0.8–1 ml. Polybrene was used in the transduction at 8 μg/ml. Transduction medium was replaced with fresh medium 14–16 h after transduction. 62–64 h after the transduction, the target cells were removed with 1X trypsin-EDTA, suspended in DMEM supplemented with 10% FBS or FP, and then analyzed by FACScan (Becton Dickinson, Franklin Lakes, NJ) for EGFP-expressing cells. The EGFP-positive cells were judged to be those cells with fluorescence intensity above that seen in the non-transduced cells in the FL1 channel. For MoMuLV vector, transduction efficiency ranging from 0% to 80% was determined using diluted or partially diluted vector. For lentiviral vector, we reported results in terms of titer, rather than transduction efficiency, because only diluted vector was used in the study.
2.6. Immunoblot for p30gag expression
Briefly, supernatant containing retroviral vectors was pelleted by ultracentrifuge at 39,000 rpm (SW50.1 rotor, Beckman ultracentrifuge, Fullerton, CA) through a 20% sucrose cushion for 1 h at 4 °C and resuspended in 1X phosphate-buffered saline (PBS). Vectors were then lysed using lysis buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1% Triton X-100, 0.02% sodium azide) [27], mixed with sample buffer (2X sample buffer: 4% (w/v) sodium dodecyl sulphate (SDS), 50 mM Tris–HCl, pH 7.0, 24% (v/v) glycerol, 0.01% (w/v) bromphenol blue, 5 μg/ml beta-mercaptoethanol), boiled and then loaded into a SDS-polyacrylamide gel electrophoresis (PAGE) gel. The separated protein bands were transferred onto an Immuno-Blot PVDF membrane (Bio-Rad, Hercules, CA). The membrane was blocked with milk solution (2 g/dL dry milk, 0.05% Tween-20 in 1X PBS) and then hybridized with primary rabbit anti-p30gag antibody (kindly provided by Dr. Rein, National Cancer Institute) and then with peroxidase-conjugated secondary antibody (goat anti-rabbit; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and briefly washed once with 0.5% Tween-20 in PBS. The membrane was then washed with deionized water and analyzed using conventional chemiluminescence methods.
2.7. ELISA analysis of capsid protein P24
The capsid protein P24 in lentiviral vectors was measured following the manufacturer’s instructions using a commercial P24 ELISA Kit (NEN Life Science Products, Boston, MA).
2.8. Equilibrium floatation centrifugation
Lipid raft and non-raft membrane fractions were separated using equilibrium floatation centrifugation as described by Ono [8]. Briefly, 293-gag-pol cells were seeded in 10-cm dishes 72 h before the assay. The cells were washed with cold PBS and scraped in cold FBS. The cells were centrifuged and resuspended in TE (10 mM Tris–HCl, pH 8.0, 1 mM EDTA) plus protease inhibitors. The cells were then homogenized and sonicated. The homogenate was centrifuged to remove the nuclei. The post-nuclear supernatant was treated with or without 0.5% (v/v) Triton X-100 in TNE solution (25 mM Tris–HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA) and incubated for 20 min on ice. 200 μl supernatant was then mixed with 1 ml of 85.5% (w/v) sucrose solution in TNE and placed in an ultracentrifuge tube. Then, 2.8 ml of 65% (w/v) and 1.2 ml of 10% (w/v) sucrose solution in TNE were loaded on the top. The sucrose gradient was then centrifuged at 35,000 rpm at 4 °C for 16 h (SW50.1 rotor, Beckman ultracentrifuge). Twenty fractions of supernatant were collected after centrifugation and mixed with RIPA buffer (2% Igepal, 1% (w/v) sodium deoxycholate, 0.2% sodium dodecyl sulfate in PBS). The samples were stored at −20 °C.
2.9. Lipid extraction
The lipid extraction procedure was a modification of the Bligh & Dyer procedure [28]. Briefly, 293-gag-pol cells were trypsinized and washed with PBS. Then the cells were resuspended in 0.4 ml H2O containing 0.15 mM NaCl and 1 mM EDTA in disposable glass tubes. 1 ml methanol and 0.5 ml chloroform were added to each tube and vortexed briefly after each addition. After adding the chloroform, the mixture was allowed to stand for 15 s after vortexing. Then, 0.5 ml chloroform and 0.5 ml H2O were sequentially added to each tube and vortexed briefly after each addition. The tubes were centrifuged at 1000 RCF at room temperature to separate the chloroform and aqueous layers. The chloroform layer containing the lipid was transferred into a glass tube using a Pasteur pipette. Another 0.5 ml of chloroform was added to the original tube, after which the tube was vortexed and centrifuged. The chloroform layer from this extract was combined with the first exact and stored at −80 °C under nitrogen.
2.10. Phospholipid determination
The chloroform layer was dried under nitrogen at room temperature, and the extract was dissolved in 100 μl chloroform. Then the extract was loaded onto a preheated thin layer chromatography plate (Whatman, Florham Park, NJ) and the plate was developed in a solvent system of chloroform:methanol:acetic acid:0.15 M NaCl (60:30:10:3 v/v). After the 45–55 min run, the phospholipids were visualized by exposure to iodine vapors, identified with the help of authentic standards run on the plates, and scraped off the TLC plate using a razor blade into different glass tubes. Each of the phospholipid fractions was then digested with 400 μl of 70% perchloric acid at 180–190 °C for 20 min. The mixture was allowed to cool to room temperature in a fume hood, and the color developed essentially according to the modified Bartlett procedure [29]. Briefly, 3 ml of dH2O, 200 μl ammonium molybdate, and 80 μl 8-anilino-1-napthalenesulphonic acid (ANSA) were added sequentially, the tubes were vortexed, and placed in boiling water for 10 min. After cooling, the tubes were centrifuged at 900 RCF for 5 min. 300 μl of each mixture was added into a well of a 96-well plate and the absorbance was read at 830 nm using a BioTek Synergy HT microplate reader (Bio-Tek Instruments, Winooski, VT). The phosphorus content was determined by converting the absorbance to concentration using a standard curve, employing KH2PO4 as standard.
2.11. Cholesterol content measurement
The chloroform in the lipid extract was dried under nitrogen at room temperature, and the extract was dissolved in 100 μl isopropanol. The total and free cholesterol contents were determined using Cholesterol E and Free Cholesterol C kits (Wako, Richmond, VA) according to the manufacturer’s instructions with a BioTek Synergy HT microplate reader.
2.12. Statistical analysis
The p values in the study were obtained using a two-tailed, unpaired t-test with equal variances.
3. Results
3.1. Colocalization of MoMuLV Gag protein and lipid rafts in 293-gag-pol packaging cells
Lipid rafts have been shown to be involved in the assembly of several enveloped viruses including influenza, Ebola, and HIV-1 [1], [2], [3], [4]. We investigated the association of MoMuLV Gag protein with lipid rafts using equilibrium floatation centrifugation. Untransfected 293-gag-pol cells supplemented with or without 0.03 mM cholesterol were homogenized and sonicated. The supernatant was treated with or without 0.5% Triton X-100 at 4 °C and the cell lysates were loaded on a discontinuous sucrose gradient solution. After centrifugation, lipid rafts and associated Gag protein floated to the top fractions of the gradient (Fig. 2 ), which also contained the majority of the well-known lipid raft-associated protein calveolin (data not shown). Lipid rafts float to the top of the gradient because they are detergent-resistant microdomains (DRM) [8]. Combined with the fact that rafts typically constitute less than 40% of the cell membrane [30], these results suggest colocalization of rafts and MoMuLV Gag in 293-gag-pol cells. As expected, the association of Gag with DRM was disrupted by treatment with Triton X-100 at 37 °C and most of the Gag protein was found in the bottom fractions (7–9) of the gradient (Fig. 2A).
Fig. 2.
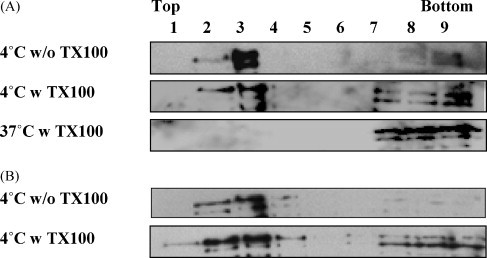
MoMuLV Gag protein is associated with detergent-resistant membrane of untransfected 293-gag-pol packaging cells. Cells treated without (A) or with (B) 0.03 mM cholesterol for 48 h were homogenized and sonicated. Postnuclear supernatants were treated with (w) or without (w/o) 0.5% Triton X-100 (TX100) on ice or at 37 °C before equilibrium flotation centrifugation. Fractions containing MoMuLV Gag protein were detected by immunoblotting. Fractions (1–9) represent the sucrose gradient (10–85.5%). Fraction 1 corresponds to the top and fraction 9 corresponds to the bottom.
3.2. Cholesterol increased MoMuLV transduction efficiency in a dose- and addition-time-dependent manner
To test the effects of cholesterol supplementation on transduction efficiency of NIH3T3 cells using MoMuLV vectors produced from transfected 293-gag-pol cells, we first examined different doses of cholesterol provided to 293-gag-pol cells 24 h before transfection and maintained in culture during vector production. Supplementation with 0.03 mM cholesterol increased the transduction efficiency of NIH3T3 cells for vector collected in both the 1st and 2nd collections (Table 1A ). The increase was about 1.7-fold for the 1st collection and 2-fold for the 2nd collection. Supplementation with 0.005 mM cholesterol had little effect. In contrast, supplementation with 0.1 mM cholesterol greatly inhibited producer cell growth (data not shown), and thus resulted in very low transduction efficiencies.
Table 1A.
Effects of cholesterol dose and addition time on transduction efficiency of NIH3T3 cells. All values were normalized to those under control condition at each collection, in which the same volume of viral vector produced without cholesterol supplementation was used to transduce the cells. All data represent the mean ± S.D. Cholesterol was supplemented at periods P + I + II (Fig. 1) and the transduction efficiencies under the control condition were between 5% and 20% (n = 3).
| Dose | 1st collection | 2nd collection |
|---|---|---|
| 0.005 mM | 1.00 ± 0.17 | 1.25 ± 0.32 |
| 0.03 mM | 1.74 ± 0.07 | 2.05 ± 0.63 |
| 0.1 mM | 0.47 ± 0.48 | 0.32 ± 0.11 |
To explore the optimal addition time, 0.03 mM cholesterol was supplemented to producer cell cultures using different schemes (Table 1B ). The effects of cholesterol addition using various time schemes were better demonstrated at the 1st collection than at the 2nd collection. Supplementation of cholesterol before transfection led to higher transduction efficiency compared to schemes without addition of cholesterol before transfection, especially for the 1st collection. In general, addition of 0.03 mM cholesterol both before and after transfection gave the highest transduction efficiency and therefore was used for the rest of the study. It is likely that the transduction efficiency could be further increased by more extensive optimization of the cholesterol dose (e.g., between 0.03 and 0.1 mM) and/or incubation time.
Table 1B.
0.03 mM cholesterol was added at periods P, I, and/or II in various combinations and the transduction efficiency under control condition was (26 ± 1)% (n = 2).
| Time | 1st collection | 2nd collection |
|---|---|---|
| P | 1.14 ± 0.01 | 1.28 ± 0.01 |
| P + I | 1.40 ± 0.20 | 1.45 ± 0.17 |
| P + II | 1.08 ± 0.04 | 1.48 ± 0.11 |
| P + I + II | 1.41 ± 0.25 | 1.52 ± 0.01 |
| I | 1.13 ± 0.01 | 1.62 ± 0.20 |
| II | 0.95 ± 0.07 | 1.38 ± 0.13 |
| I + II | 1.17 ± 0.10 | 1.50 ± 0.15 |
3.3. Cholesterol effects on MoMuLV transduction efficiency as a function of serum type and concentration
The beneficial effects of cholesterol supplementation were further evaluated using two different sera – fetal bovine serum (FBS) and FetalPlex serum (FP) – each at concentrations of 10% and 5%. In general, there were benefits with cholesterol supplementation for all of the conditions, although the fold-increase in transduction efficiency was different under different conditions (Fig. 3 ). For both 10% and 5% FBS, the increase in transduction efficiency with cholesterol addition was greater at the 2nd collection than at the 1st collection (Fig. 3A, Table 2 ). There was less differential benefit at the 2nd collection for FP, especially at a concentration of 10% (Fig. 3B, Table 2). The relative increase in transduction efficiency using vectors collected from culture with cholesterol supplementation compared to that without cholesterol addition was greater for lower absolute transduction efficiency in the control condition. This is expected because at the higher values the control transduction efficiency is already approaching the maximum possible for the system.
Fig. 3.
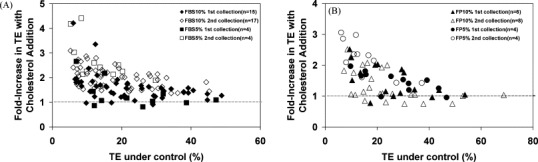
Effects of cholesterol supplementation on transduction efficiency (TE) of NIH3T3 cells using either FBS (A) or FP (B), each at a serum concentration of 5% or 10%. 0.03 mM cholesterol was added at periods P + I + II (Fig. 1). A range of TE values was obtained by using different volumes of viral vector to transduce the target cells. All values were normalized to those under control condition at each collection, in which the same volume of viral vector produced without cholesterol supplementation was used to transduce the cells.
Table 2.
Statistical analysis of the benefits of cholesterol supplementation on transduction efficiency of NIH3T3 cells under different conditions (FBS or FP, the 1st or 2nd collection, 5% or 10% serum). Control condition in the table indicates that the same volume of viral vector produced without cholesterol supplementation was used to transduce the cells at each collection. The numbers in the table represent p values obtained using a two-tailed, unpaired t-test with equal variances.
| Condition | Transduction efficiency under control conditions |
|||
|---|---|---|---|---|
| 0–10% | 10–20% | 20–40% | >40% | |
| FBS 10% 1st collection vs. control | <0.05 | <0.05 | <0.05 | <0.05 |
| FBS 10% 2nd collection vs. control | <0.05 | <0.05 | <0.05 | 0.07 |
| FBS 10% 2nd collection vs. FBS 10% 1st collection | 0.39 | <0.05 | <0.05 | 0.12 |
| FBS 5% 2nd collection vs. FBS 5% 1st collection | n/a | <0.05 | <0.05 | n/a |
| FP 10% 1st collection vs. control | n/a | 0.10 | <0.05 | 0.65 |
| FP 10% 2nd collection vs. control | <0.05 | <0.05 | 0.39 | 0.80 |
| FP 10% 2nd collection vs. FP 10% 1st collection | 0.39 | 0.87 | 0.20 | 0.30 |
| FP 5% 2nd collection vs. FP 5% 1st collection | n/a | 0.07 | 0.80 | n/a |
The 293-gag-pol cell number was maintained or increased slightly after transfection with or without cholesterol supplementation. Compared to that in control culture, the cell number in culture with cholesterol was slightly lower (data not shown). Thus, the observed increase in transduction efficiency with cholesterol supplementation cannot be attributed to increased producer cell numbers.
3.4. Effects of cholesterol addition method and the addition of methyl-beta-cyclodextrin alone on MoMuLV transduction efficiency
Since cholesterol was also present in the supernatant of viral vector after vector production and during transduction, it is possible that the beneficial effects of cholesterol supplementation on transduction efficiency were due in part to either interaction between viral vectors and cholesterol after vector assembly or other effects during the transduction process. To examine these possibilities, viral vectors produced under control conditions were either incubated with 0.03 mM cholesterol for 1 h at 37 °C before transduction or supplemented with the same concentration of cholesterol during transduction. There was no benefit or a slight decrease for either treatment, while viral vectors produced with cholesterol supplementation yielded about a 2-fold increase in transduction efficiency (Fig. 4 ). This indicates that cholesterol must be added during vector production to obtain the benefits.
Fig. 4.
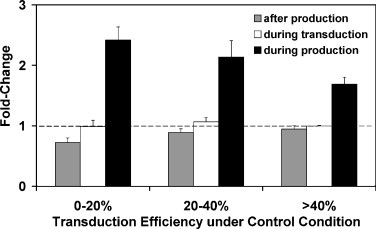
Cholesterol supplementation after vector production had no effect on transduction efficiency using vectors from the 2nd collection. For supplementation after vector production, 0.03 mM cholesterol was added at 37 °C 1 h before transduction to viral vector produced under control conditions. For supplementation during transduction, cholesterol was added only during transduction. For supplementation during production, cholesterol was added to producer cells before and after transfection. All values were normalized to those under control condition at the 2nd collection, in which the same volume of viral vector produced without cholesterol supplementation was used to transduce the cells. Data points represent the mean ± S.D. (n = 2).
Cholesterol was delivered to cell culture as a complex with methyl-beta-cyclodextrin. To evaluate the effects of MbCD alone on vector production, 0.25 mM of MbCD (the same concentration as that in the complex with cholesterol) was added to producer cells both before and after transfection. MbCD alone had little effect on transduction efficiency (Fig. 5 ). As expected, a higher concentration of empty MbCD (5 mM) greatly decreased the transduction efficiency even when exposed to producer cells for only 9 h (Fig. 5 insert).
Fig. 5.
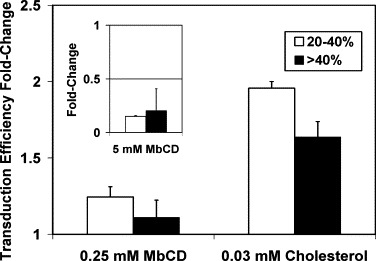
Effects of adding MbCD alone during vector production on transduction efficiency using vectors from the 2nd collection. MbCD with the same concentration (0.25 mM) as that used for cholesterol supplementation was added either alone (0.25 mM MbCD) or complexed with cholesterol (0.03 mM cholesterol) to 293-gag-pol cells during vector production. The insert figure represents the results when 5 mM unloaded MbCD was added to 293-gag-pol cells 9 h before the 2nd collection. All values were normalized to those under control condition at the 2nd collection, in which the same volume of viral vector produced without cholesterol supplementation was used to transduce the cells. Data points represent the mean ± S.D. (n = 2). Results are shown separately for cultures with low (20–40%) and high (>40%) transductions efficiency under control conditions.
3.5. Effects of cholesterol supplementation on MoMuLV vector production and stability
Next, we examined the total amount of vector produced, as determined from the amount of p30gag, with or without cholesterol addition. We found that for all collections the amount of p30gag was very similar with or without cholesterol (Fig. 6 ). Together with the increase in transduction efficiency, this suggests that the viral vector infectivity (defined as transduction efficiency per unit p30gag), rather than the amount of vector produced, increased with cholesterol supplementation.
Fig. 6.
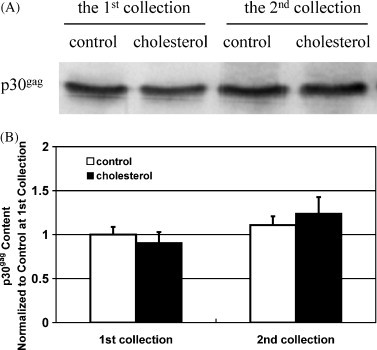
Gag protein amounts in viral vectors determined by immunoblot for both control conditions and cholesterol supplementation. A typical immunoblot result for p30gag in viral vectors from the 1st and 2nd collections with or without 0.03 mM cholesterol supplementation is shown in (A). For culture with cholesterol, cholesterol was added at periods P + I + II (Fig. 1). In (B) the amount of p30gag (determined from densitometry analysis of immunoblots) in viral vectors with or without cholesterol supplementation for each collection was normalized to that of the 1st collection under control conditions. Data points represent the mean ± S.D. (n = 3). p = 0.26 for the 1st collection and p = 0.35 for the 2nd collection with or without cholesterol supplementation.
To test if there was any difference in the stability of vector produced in culture with or without cholesterol, the viral vectors were incubated for different periods of time at 37 °C and then used for transduction. The results in Fig. 7 show that vectors produced with or without cholesterol supplementation decayed at a similar rate if the amount of vector prior to incubation at 37 °C yielded the same transduction efficiency. For both conditions, a higher transduction efficiency at time zero resulted in a smaller decay rate. This effect is to be expected because the transduction efficiency does not increase linearly with the amount of viral vector. In conclusion, the retroviral vector stability did not change with cholesterol supplementation during vector production.
Fig. 7.
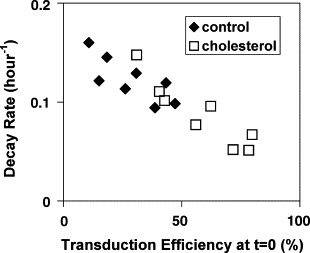
Comparison of the stability of retroviral vector produced with or without cholesterol supplementation. MoMuLV vectors were incubated for different periods of time at 37 °C and then used for transduction. The x-axis represents the transduction efficiency for vectors without incubation at 37 °C and the y-axis represents the vector decay rate obtained from the experiment.
3.6. Phospholipid distribution and cholesterol content in 293-gag-pol producer cells
To determine the effects of cholesterol supplementation on the plasma membrane composition of 293-gag-pol cells, different classes of phospholipids extracted from untransfected 293-gag-pol cell membranes were separated by thin layer chromatography and measured by analysis of phosphorous content. The phospholipid head group composition did not change significantly with cholesterol supplementation (Fig. 8 ).
Fig. 8.
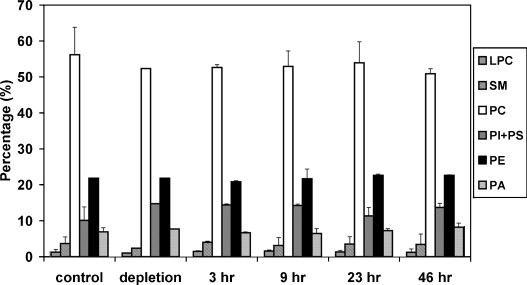
Phospholipid composition in untransfected 293-gag-pol cells with or without cholesterol supplementation. Packaging cell line 293-gag-pol (without transfection) was incubated with 0.03 mM cholesterol for 3, 9, 23, or 46 h, respectively. Alternatively, cholesterol-free methyl-beta-cyclodextrin (10 mM) was added to 293-gag-pol cells and incubated for 30 min at 37 °C to deplete cholesterol (depletion). LPC: lysophosphatidylcholine; SM: sphingomyelin; PC: phosphatidylcholine; PI: phosphatidylinositol; PS: phsphatidylserine; PE: phosphatidylethanolamine; PA: phosphatidic acid. Data points represent the mean ± S.D. (n = 2).
Next, we measured the cholesterol content in untransfected 293-gag-pol cells with or without cholesterol addition. Preliminary results showed that both free and total cholesterol contents increased as the time of incubation with cholesterol increased in untransfected 293-gag-pol cells (data not shown). Based on this result, we measured the cholesterol content in transfected producer cells. Cholesterol supplementation increased both free cholesterol and total cholesterol in the producer cells (Fig. 9 ). The longer producer cells were cultured with cholesterol, the higher was the cholesterol content. The free and total cholesterol contents were greater than for control cells at the time of transfection (t = 0) because of the 24-h incubation with cholesterol before transfection.
Fig. 9.
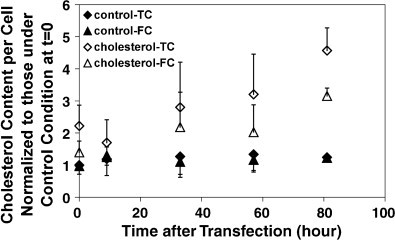
Total cholesterol (TC) and free cholesterol (FC) content in transfected 293-gag-pol cells with or without 0.03 mM cholesterol supplementation during vector production. Cholesterol was added at periods P + I + II (Fig. 1). All values were normalized to the total cholesterol amount under control conditions prior to transfection. Data points represent the mean ± S.D. (n = 3–7).
Interestingly, the cholesterol content in producer cells without cholesterol supplementation did not vary much after transfection.
3.7. Cholesterol supplementation during production increases lentiviral vector infectivity
The effects of cholesterol on the production of a lentiviral vector by an inducible packaging cell line in suspension culture were also evaluated. Similar to the case for retroviral vector production, addition of cholesterol before and after induction gave the highest titer of lentiviral vector (data not shown). A concentration of 0.01 mM cholesterol was supplemented because higher concentrations of cholesterol caused cell death (data not shown). The fold-change of P24 Gag protein, titer, and infectivity for lentiviral vector produced with cholesterol supplementation relative to that without cholesterol are listed in Table 3 . Similar to that for retroviral vector production, cholesterol supplementation increased lentiviral vector infectivity (defined as titer/P24), especially at the 2nd collection. The increase in P24 Gag protein levels with cholesterol addition, if any, was much less than the increase in infectivity. Lentiviral vector stability was not evaluated.
Table 3.
Effects of cholesterol supplementation on lentiviral vector production. 120C-S cells were induced at 0.5–1.5 × 106 viable cells/ml. Cholesterol (0.01 mM) was added at periods P + I + II (Fig. 1) during vector production. Viral vectors were then diluted and used to transduce HeLa cells to obtain the vector titer. All values were normalized to those under control condition at each collection, in which the same volume of viral vector produced without cholesterol supplementation was used to transduce the cells. Data points represent the mean ± S.D. (n = 2–3).
| Vector quality | The 1st collection | The 2nd collection |
|---|---|---|
| P24 | 0.96 ± 0.17 | 1.89 ± 0.12 |
| Titer | 1.66 ± 0.01 | 11.71 ± 3.54 |
| Infectivity | 1.76 ± 0.32 | 6.15 ± 1.49 |
4. Discussion
Several studies have investigated the association between lipid rafts and the assembly and infection of murine leukemia virus. The envelope protein 4070A of amphotropic murine leukemia virus has been shown to colocalize with detergent resistant microdomains [31]. In addition, Lu et al. have demonstrated that the association of ecotropic MLV receptor with rafts is very important during the early stage of virus infection [32]. Here, we demonstrate that MoMuLV Gag is also preferentially localized in lipid rafts in 293-gag-pol cells, commonly used to produce MoMuLV vectors (Fig. 2). Therefore it was postulated that modulation of lipid raft composition in the producer cell membrane could possibly affect the assembly and infectivity of pantropic retroviral vector.
To alter the composition of producer cell membranes, we supplemented growth media with cholesterol, a major component in lipid rafts, before and during MoMuLV vector production. The cholesterol content in the producer cells increased directly as a consequence of cholesterol supplementation (Fig. 9). Viral vectors produced with cholesterol supplementation had higher infectivity compared to those without cholesterol addition (Fig. 3). Although depletion of cholesterol from virus-producing cells could disrupt lipid rafts, hinder viral particle production and decrease virus infectivity [5], [8], the cholesterol amount in producer cells without cholesterol supplementation did not decrease during vector production (Fig. 9). Thus, the supplemented cholesterol did not act by restoring its deficiency in producer cells. Rather, the increased cholesterol content increased the infectivity of vectors that were assembled and budded from rafts, possibly by altering cellular lipid raft composition. Consequently, it is also possible that the lipid composition of the vial vectors was changed similar to that observed by Beer et al. [21], [22], and that this led to changes in viral vector membrane fluidity. It is known that lipid fatty acid composition tends to change to offset the associated change in membrane fluidity under certain circumstances such as low temperature [33]. In this case, increased cholesterol content might trigger a change in membrane fatty acid composition. It has been reported that the level of polyunsaturated species of the major cell membrane phospholipids increased with the incorporation of cholesterol as an adaptive mechanism to control the membrane fluidity [20]. This is consistent with a decrease in the saturated fatty acid (16:0) content in the major phospholipid (PC) with cholesterol supplementation in our study (data not shown). Another possible mechanism is that, by altering cellular lipid raft composition, a greater fraction of vector particles contained viral RNA during assembly and budding. If this were true, a higher percentage of viral vectors produced with cholesterol supplementation would become infectious compared to those without cholesterol addition.
Various cholesterol addition schemes were compared in this transient vector production system. Among all of the cholesterol addition methods, supplementation with cholesterol both before and after transfection or induction gave vector with the highest transduction efficiency. It is therefore proposed that the producer cells need a certain amount of time to incorporate and, perhaps more importantly, process cholesterol in order to produce vector with higher infectivity.
In this study, two different sera, FBS and FP, were supplemented before and during retroviral vector production. Generally, the beneficial effects of cholesterol supplementation were higher using FBS compared to FP (Fig. 3). Interestingly, it was also observed that FP has a higher concentration of total cholesterol compared to FBS.
The observed benefits of cholesterol supplementation on transduction efficiency were due to effects on producer cells, rather than directly on viral vectors, because incubation of cholesterol with viral vectors after production did not increase the overall transduction efficiency (Fig. 4). Similarly, there was no effect on transduction efficiency when we added cholesterol directly into the transduction medium. These results support the hypothesis that the beneficial effects on vector infectivity with cholesterol supplementation happen during the vector assembly process and/or during budding from the producer cell membrane, which has been shown by others to occur from lipid rafts on the producer cells. Cholesterol supplementation may increase the fraction of Gag protein associated with lipid rafts, as has been reported for influenza virus hemagglutinin and neuraminidase [12].
In our experiments, cholesterol was delivered as a complex with MbCD. Empty MbCD has been shown to disrupt lipid rafts in producer cells. Consistent with other studies on HIV-1 virus [8], addition of 5 mM MbCD to 293-gag-pol producer cells greatly decreased the transduction efficiency of pantropic retroviral vectors (Fig. 5).
Finally, the beneficial effect of cholesterol supplementation during vector production on vector infectivity was observed both for pantropic MoMuLV retroviral vector and pantropic lentiviral vector, although the extent was different. The amount of lentiviral vector produced, as measured by the Gag protein P24 content, increased at the 2nd collection with cholesterol supplementation (Table 3), while the amount of retroviral vector, as measured by the Gag protein p30gag content, was not affected (Fig. 6). There was also a greater increase in vector infectivity with cholesterol supplementation for the lentiviral vector system. These differences may be due in part to the differences between the two production systems. The retroviral vector production system was transient and the producer cells were adherent. In contrast, lentiviral vector production was inducible and the producer cells were in suspension. There may also be an inherent difference for the different vectors.
5. Conclusions
Taken together, the transduction efficiency or titer for two VSV-G pseudotyped viral vectors, MoMuLV and lentiviral vector, was increased by cholesterol supplementation. The benefits are mainly due to an increase in vector infectivity. Combined with other methods that improve viral vector production, the results in this study will contribute to improving the quality of vector production for both research and clinical purposes.
Acknowledgements
This work was supported by research grants NSF-BES-9813479 (WMM) NIH-HL 68585 (PVS), and NIH-CA-82177 (AA). We wish to thank Dr. Eric Barklis, Oregon State University, for kindly providing rabbit anti-p30gag antibody, and Cell Genesys for sponsoring YC as an intern during the lentiviral vector study.
References
- 1.Campbell S., Gaus K., Bittman R., Jessup W., Crowe S., Mak J. The raft-promoting property of virion-associated cholesterol, but not the presence of virion-associated Brij 98 rafts, is a determinant of human immunodeficiency virus type I infectivity. J. Virol. 2004;78:10556–10565. doi: 10.1128/JVI.78.19.10556-10565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell S.M., Crowe S.M., Mak J. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 3.Isa P., Realpe M., Romero P., Lopez S., Arias C.F. Rotavirus RRV associates with lipid membrane microdomains during cell entry. Virology. 2004;322:370–381. doi: 10.1016/j.virol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Keller P., Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laliberte J.P., McGinnes L.W., Peeples M.E., Morrison T.G. Integrity of membrane lipid rafts is necessary for the ordered assembly and release of infectious Newcastle disease virus particles. J. Virol. 2006;80:10652–10662. doi: 10.1128/JVI.01183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen D.H., Hildreth J.E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindwasser O.W., Resh M.D. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 2001;75:7913–7924. doi: 10.1128/JVI.75.17.7913-7924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono A., Freed E.O. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono A., Waheed A.A., Freed E.O. Depletion of cellular cholesterol inhibits membrane binding and higher-order multimerization of human immunodeficiency virus type 1 Gag. Virology. 2007;360:27–35. doi: 10.1016/j.virol.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown D.A., London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 11.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 12.Barman S., Nayak D.P. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J. Virol. 2007;81:12169–12178. doi: 10.1128/JVI.00835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel B.U., Mohandas N., Harrison T., McManus H., Rosse W., Reid M., Haldar K. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J. Biol. Chem. 2001;276:29319–29329. doi: 10.1074/jbc.M101268200. [DOI] [PubMed] [Google Scholar]
- 14.Suomalainen M. Lipid rafts and assembly of enveloped viruses. Traffic. 2002;3:705–709. doi: 10.1034/j.1600-0854.2002.31002.x. [DOI] [PubMed] [Google Scholar]
- 15.Steffens C.M., Hope T.J. Mobility of the human immunodeficiency virus (HIV) receptor CD4 and coreceptor CCR5 in living cells: implications for HIV fusion and entry events. J. Virol. 2004;78:9573–9578. doi: 10.1128/JVI.78.17.9573-9578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickl W.F., Pimentel-Muinos F.X., Seed B. Lipid rafts and pseudotyping. J. Virol. 2001;75:7175–7183. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorp E.B., Gallagher T.M. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 2004;78:2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imelli N., Meier O., Boucke K., Hemmi S., Greber U.F. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J. Virol. 2004;78:3089–3098. doi: 10.1128/JVI.78.6.3089-3098.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koster F., Finas D., Schulz C., Hauser C., Diedrich K., Felberbaum R. Additive effect of steroids and cholesterol on the liposomal transfection of the breast cancer cell line T-47D. Int. J. Mol. Med. 2004;14:769–772. [PubMed] [Google Scholar]
- 20.Blom T.S., Koivusalo M., Kuismanen E., Kostiainen R., Somerharju P., Ikonen E. Mass spectrometric analysis reveals an increase in plasma membrane polyunsaturated phospholipid species upon cellular cholesterol loading. Biochemistry. 2001;40:14635–14644. doi: 10.1021/bi0156714. [DOI] [PubMed] [Google Scholar]
- 21.Beer C., Buhr P., Hahn H., Laubner D., Wirth M. Gene expression analysis of murine cells producing amphotropic mouse leukemia virus at a cultivation temperature of 32 and 37 degrees C. J. Gen. Virol. 2003;84:1677–1686. doi: 10.1099/vir.0.18871-0. [DOI] [PubMed] [Google Scholar]
- 22.Beer C., Meyer A., Muller K., Wirth M. The temperature stability of mouse retroviruses depends on the cholesterol levels of viral lipid shell and cellular plasma membrane. Virology. 2003;308:137–146. doi: 10.1016/s0042-6822(02)00087-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Miller W.M., Aiyar A. Transduction efficiency of pantropic retroviral vectors is controlled by the envelope plasmid to vector plasmid ratio. Biotechnol. Prog. 2005;21:274–282. doi: 10.1021/bp049865x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns J.C., Friedmann T., Driever W., Burrascano M., Yee J.K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend K., Chen Y., Dayao J., Farson D., Miller W.M., Lin A. Adaptation of a lentiviral vector producer cell line to reduced-serum, suspension culture for large scale production. 7th Annual Meeting of American Society of Gene Therapy; June 2–6; 2004. [Google Scholar]
- 26.Sears J., Kolman J., Wahl G.M., Aiyar A. Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by Epstein-Barr nuclear antigen 1. J. Virol. 2003;77:11767–11780. doi: 10.1128/JVI.77.21.11767-11780.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones T.A., Blaug G., Hansen M., Barklis E. Assembly of gag-beta-galactosidase proteins into retrovirus particles. J. Virol. 1990;64:2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 29.Marinetti G.V. Chromatographic separation, identification, and analysis of phosphatides. J. Lipid Res. 1962;3:1–20. [Google Scholar]
- 30.Prior I.A., Muncke C., Parton R.G., Hancock J.F. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beer C., Pedersen L., Wirth M. Amphotropic murine leukaemia virus envelope protein is associated with cholesterol-rich microdomains. Virol. J. 2005;2:36. doi: 10.1186/1743-422X-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X., Silver J. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts. Virology. 2000;276:251–258. doi: 10.1006/viro.2000.0555. [DOI] [PubMed] [Google Scholar]
- 33.Cossins A.R. Portland Press; London: 1994. Temperature Adaptation of Biological Membranes. pp. 63–75. [Google Scholar]


