Abstract
The phosphatidylinositol 3-kinase (PI3K)–protein kinase B (PKB) pathway regulates survival and chemotherapy resistance of neuronal cells, and its deregulation in neuroblastoma (NB) tumors predicts an adverse clinical outcome. Here, we show that inhibition of PI3K-PKB signaling in human NB cells induces nuclear translocation of FOXO3/FKHRL1, represses the prosurvival protein BIRC5/Survivin, and sensitizes to DNA-damaging agents. To specifically address whether FKHRL1 contributes to Survivin regulation, we introduced a 4-hydroxy-tamoxifen-regulated FKHRL1(A3)ERtm allele into NB cells. Conditional FKHRL1 activation repressed Survivin transcription and protein expression. Transgenic Survivin exerted a significant antiapoptotic effect and prevented the accumulation of Bim and Bax at mitochondria, the loss of mitochondrial membrane potential as well as the release of cytochrome c during FKHRL1-induced apoptosis. In concordance, Survivin knockdown by retroviral short hairpin RNA technology accelerated FKHRL1-induced apoptosis. Low-dose activation of FKHRL1 sensitized to the DNA-damaging agents doxorubicin and etoposide, whereas the overexpression of Survivin diminished FKHRL1 sensitization to these drugs. These results suggest that repression of Survivin by FKHRL1 facilitates FKHRL1-induced apoptosis and sensitizes to cell death induced by DNA-damaging agents, which supports the central role of PI3K-PKB-FKHRL1 signaling in drug resistance of human NB.
INTRODUCTION
Deregulated apoptosis and uncontrolled growth play a pivotal role in the development of aggressive NB tumors. This enhanced growth is in part induced by aberrant expression of neurotrophic factors. Among these, brain-derived neurotrophic factor (BDNF) and its cognate receptor NTRK2/TrkB have been shown to correlate with poor prognosis and resistance to chemotherapeutic agents (Li et al., 2005; Schramm et al., 2005). BDNF/TrkB induces survival signaling via the phosphatidylinositol 3-kinase (PI3K)–protein kinase B (PKB) pathway. Recently, a retrospective study suggests that phosphorylation of PKB at serine 473 and/or threonine 308 may be a prognostic indicator of decreased event-free or overall survival in NB (Opel et al., 2007). Substrates of AKT1/PKB, in particular the members of the FoxO transcription factor family, FOXO1/FKHR, FOXO3/FKHRL1, and FOXO4/AFX, are downstream components of neurotrophin receptor signaling (Zheng et al., 2002; Gilley et al., 2003). We and others have shown that FKHRL1 is phosphorylated by hyperactive PKB in malignant NB (Li et al., 2005; Obexer et al., 2007). The phosphorylation by PKB causes association of FKHRL1 with 14-3-3 proteins, export from the nucleus and repression of FKHRL1 target gene transcription (van der Horst and Burgering, 2007). In the absence of active PKB, FKHRL1 regulates the expression of proteins that are involved in cell cycle arrest, cell division, protection against oxidative stress, and apoptosis (van der Horst and Burgering, 2007; Huang and Tindall, 2007).
Apoptosis is a cellular process regulated either via membrane death receptors or by the balance of pro- and antiapoptotic proteins of the BCL2 family. This protein family is divided into two subgroups: one subgroup is the so-called “multidomain” family that consists of proapoptotic members such as BAX and BAK1/Bak and prosurvival proteins, e.g., BCL2, BCL2L1/Bcl-xl, and MCL1. The other subgroup is the BH3-only family, a large group of apoptosis-inducers such as PMAIP1/Noxa and BCL2L11/Bim that are activated by various stimuli. On cell death decision, cytochrome c is released from mitochondria and induces the formation of the so-called “apoptosome” complex leading to caspase-9 cleavage and activation of effector caspases. The activity of caspases is counteracted by members of the inhibitor of apoptosis protein (IAP) family. In contrast to other IAPs, BIRC5/Survivin contains only a single baculovirus IAP repeat (BIR) and lacks the RING domain. Its apoptosis-protecting function therefore is still under debate: Survivin was shown to inhibit effector caspases via its single BIR domain, but it also was shown to act upstream at the level of mitochondria (Shankar et al., 2001; Liu et al., 2004). Survivin is frequently expressed in various malignancies, among them NB (Adida et al., 1998; Islam et al., 2000). The Survivin gene is located at 17q25 and in NB, gain of chromosome 17q or the distal translocation of 17q is of prognostic relevance and correlates with aggressive tumors (Islam et al., 2000).
Because activated PKB protects NB cells from chemotherapy-induced apoptosis and both active PKB and Survivin are predictive for an adverse clinical outcome, we analyzed a possible connection between PKB and Survivin.
MATERIALS AND METHODS
Cell Lines, Culture Conditions, and Reagents
The NB cell lines SH-EP (kindly provided by Dr. N. Gross, Pediatric Oncology Research, Pediatric Department, University Hospital CHUV, Lausanne, Switzerland) and STA-NB15 (kindly provided by Dr. P. Ambros, St. Anna Children's Hospital, Vienna, Austria) (Narath et al., 2005) as well as Phoenix packaging cells for helper-free production of amphotropic retroviruses (Grignani et al., 1998) were cultured in RPMI 1640 medium (Lonza Verviers SPRL, Verviers, Belgium) containing 10% fetal calf serum (Invitrogen, Paisley, United Kingdom), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine at 5% CO2. All cultures were routinely tested for mycoplasma contamination with the VenorR GeM-mycoplasma detection kit (Minerva Biolabs, Berlin, Germany).
Expression Constructs
The vectors pLIB-FKHRL1(A3)-ERtm-iresNeo and pQ-tetH1-SV40Puro have been described previously (Obexer et al., 2007). The pLIB-MCS2-iresYFP plasmid was constructed by inserting an iresYFP cassette into the BamHI-XhoI sites of the pLIB-MCS2 plasmid. Survivin cDNA was amplified from a liver cDNA library (Clontech, Mountain View, CA) by using the primers 5′-TATAGAATTCATGGGTGCCCCGACGTTG-3′ (forward) and 5′-TATAGTCGACTCAATCCATGGCAGCCAGC-3′ (reverse) and cloned into the EcoRI–SalI sites of the pLIB-MCS2-iresYFP plasmid. For construction of the pQ-tetH1-shSurvivin-SV40Puro vector, oligonucleotides coding for short hairpin RNA (shRNA) double strands containing the Survivin-specific sequence GCATTCGTCCGGTTGCGCT were annealed and inserted into the MunI–BamHI sites of the pQ-tetH1-SV40Puro vector. To obtain the pGL3-survivin-promoter-Luc, a 1900 bp fragment (−1828 to +81) of the human survivin promoter was amplified and inserted into the BglII–HindIII sites of the pGL3-basic plasmid. pLIB-ECFP-FKHRL1-iresPuro was constructed by inserting the FKHRL1 wild-type cDNA from the pECE-HA-FKHRL1 vector (Dijkers et al., 2002) into the MunI-SalI sites of the pLIB-ECFP-MCS2-iresPuro.
Following a recommendation by the HUGO Gene Nomenclature Committee, all official human gene symbols are put in uppercase letters to distinguish them from their often more widely used aliases, e.g. BIRC5 = Survivin.
Production of Retroviruses and Retroviral Infection
Phoenix cells (6.5 × 105) were transfected with 2 μg of pLIB-Survivin-iresYFP, pLIB-MCS2-iresYFP, pQ-tetH1-shSurvivin-SV40Puro, pQ-tetH1-SV40Puro, pLIB-FKHRL1(A3)-ERtm-iresNeo, pLIB-ECFP-FKHRL1-iresPuro, or pLIB-MCS2-iresNeo by using Lipofectamine2000 (Invitrogen). After 48 h, the retrovirus-containing supernatants were filtered through 0.22 μm syringe filters (Sartorius, Göttingen, Germany). pLIB-Survivin-iresYFP and control pLIB-MCS2-iresYFP supernatants were used to infect SH-EP/FKHRL1 (SH-EP/FKHRL1-Surv and SH-EP/FKHRL1-Ctr) and SH-EP (SH-EP-Surv, SH-EP-Ctr) cells. To knock down Survivin, SH-EP/FKHRL1 cells were infected with the retrovirus vector pQ-tetH1-shSurvivin-SV40Puro or the control plasmid pQ-tetH1-SV40Puro (SH-EP/FKHRL1-shSurv, SH-EP/FKHRL1-shCtr). To generate SH-EP/ECFP-FKHRL1 cells for live cell fluorescence analysis of FKHRL1 subcellular localization, SH-EP cells were infected with pLIB-ECFP-FKHRL1-iresPuro retroviral supernatants. Cells infected with the pLIB-Survivin-iresYFP vector (SH-EP and SH-EP/FKHRL1) were enriched to >95% by fluorescence-activated cell sorting (FACS) using an FACS Vantage cell sorter (BD Biosciences, San Jose, CA).
Determination of Apoptosis
Apoptosis was determined by propidium iodide (PI) staining of nuclei and forward/sideward scatter analysis by using a Cytomics FC-500 Series Flow Cytometry System (Beckman Coulter, Fullerton, CA). The experiments were performed as described previously (Ausserlechner et al., 2004). Mitochondrial activity was assessed by the fluorescence dye MitoTracker Red/CMX-Ros (Invitrogen) according to the manufacturer's instructions.
Luciferase Reporter Assay
SH-EP/Ctr and SH-EP/FKHRL1 cells (6 × 105) were transfected with 3 μg of pGL3-survivin-promoter-Luc DNA by using Lipofectamine2000 (Invitrogen). Sixteen hours after transfection, the cells were washed and incubated in presence or absence of 75 nM 4-hydroxy tamoxifen (4OHT) for 24 h. Luciferase activity was assessed using the Luciferase Assay System (Promega, Madison, WI).
Live Cell Fluorescence Microscopy
Imaging of living cells was done using an Axiovert200M microscope (Carl Zeiss, Jena, Germany) equipped with a CO2 chamber and Axiovision 4.6 software (Carl Zeiss). The cells were seeded on glass slides coated with 0.1 mg/ml collagen 1 d before performing the experiments.
Subcellular Fractionation and Immunoblotting
Cytoplasmic and mitochondrial fractions were prepared as follows: 5 × 107 cells were incubated in MSH buffer (210 mM mannitol, 70 mM sucrose, 20 mM HEPES, and 1 mM EDTA, pH 7.4) with protease and phosphatase inhibitors on ice for 1 h. Nuclei and nonlysed cells were removed by centrifugation at 500 × g for 5 min. To obtain the mitochondrial fraction, the supernatant was centrifuged at 25,000 × g for 30 min. The membrane pellet was resuspended in MSH buffer containing protease inhibitor and 1% CHAPS, lysed on ice for 30 min, and centrifuged at 25,000 × g for 30 min. Protein concentration was measured using Bradford reagent.
Cytoplasmic and nuclear extracts were prepared using the ProteoJet cytoplasmic and nuclear protein extraction kit (MBI Fermentas, St. Leon-Rot, Germany). Cell extracts for immunoblot analysis were prepared as described previously (Ausserlechner et al., 2004). Samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes by a Hoefer semidry blotting apparatus. The membranes were incubated with primary antibodies specific for Survivin (R&D Systems, Minneapolis, MN), BAX (Cell Signaling Technology, Danvers, MA), Bim (BD Biosciences Pharmingen, San Diego, CA), cytochrome c, Cox4 (Clontech), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Novus Biologicals, Littleton, CO), and α-tubulin (Oncogene Science, Cambridge, MA); washed; and incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom). The blots were developed using enhanced chemiluminescence (GE Healthcare) and analyzed in an AutoChemi system (UVP, Cambridge, United Kingdom). Densitometry of 16-bit digital images was performed using LabWorks 4.6 software (UVP).
Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
SH-EP/FKHRL1 and NB15/FKHRL1 cells were cultured in the presence of 75 nM 4OHT for 0, 3, 6, and 8 h, respectively. Total RNA was isolated from 5 × 106 cells by using TRIzol reagent (Invitrogen), and cDNA was synthesized from 1 μg of total RNA by using the RevertAid kit (MBI Fermentas). RT-PCR was performed on the iCycler instrument (Bio-Rad, Munich, Germany) by using the Survivin (forward 5′-CCACTGAGAACGAGCCAGACTTG-3′ and reverse 5′-AGAAAGGAAAGCGCAACCGG-3′), and GAPDH oligonucleotides (forward 5′-TGTTCGTCATGGGTGTGAACC-3′ and reverse 5′-GCAGTGATGGCATGGACTGTG-3′).
RESULTS
Inhibition of the PI3K-PKB Axis Activates FKHRL1 and Represses Survivin in Human NB Cells
We demonstrated before that in the NB cell lines SH-EP and STA-NB15 FKHRL1 is hyperphosphorylated at S253 and T32, suggesting its inactivation by PKB (Obexer et al., 2007). To investigate, whether this phosphorylation status coincides with cytoplasmic localization of FKHRL1, we constructed an ECFP-FKHRL1 fusion protein and studied FKHRL1 subcellular distribution as a marker of activation in living cells. In untreated SH-EP/ECFP-FKHRL1 cells FKHRL1 localized almost exclusively to the cytoplasm (Figure 1A). On treatment with the PI3K-inhibitor Ly294002, ECFP-FKHRL1 almost completely translocated to the nucleus within 40 min, suggesting rapid activation of this transcription factor upon loss of PI3K survival signaling. In concordance with the ECFP-FKHRL1 fusion protein, also endogenous FKHRL1 accumulated in the nuclear fraction of Ly294002-treated SH-EP cells (Figure 1B). At a concentration of 40 μM Ly294002 the phosphorylation of PKB at serine 473 was completely lost, suggesting that at this concentration PKB is mainly present in its inactive form (Figure 1B, right).
Figure 1.
Inhibition of PI3K activates FKHRL1 and represses Survivin in human NB cells. (A) SH-EP cells expressing an ECFP-FKHRL1 fusion protein were treated with 40 μM Ly294002 for up to 40 min and analyzed by live cell fluorescence microscopy in an Axiovert200M fluorescence microscope. (B) Cytosolic (cyto) and nuclear (nucl) extracts were prepared from SH-EP cells treated with 40 μM Ly294002 for 1 h. Endogenous FKHRL1 expression was determined by immunoblot. Lamin A/C (nuclear) and α-tubulin (cytosolic) served as controls for the purity of subcellular fractions. SH-EP cells were treated with 0, 10, 20, and 40 μM Ly294002 for 45 min, and immunoblot analyses of phospho-PKB-Ser473 and GAPDH as loading control were performed. (C) SH-EP and STA-NB15 cells were cultured in presence of 40 μM Ly294002 for 2 h. Survivin expression was determined by immunoblot. GAPDH was used as loading control.
Because Survivin was reported to be a downstream target of PI3K-PKB signaling, we analyzed whether blockage of PI3K activity affects Survivin steady-state protein expression in NB cells. As shown in Figure 1C, Ly294002 treatment markedly repressed Survivin steady-state levels within 2 h, both in SH-EP and STA-NB15 cells. Because FKHRL1 activation preceded Survivin repression, we next studied whether FKHRL1 may regulate Survivin expression in NB cells.
Conditional FKHRL1(A3)ERtm Induces Apoptotic Cell Death and Decreases Survivin mRNA and Protein in SH-EP and STA-NB15 Cells
To study, whether FKHRL1 contributes to Survivin repression, we infected SH-EP and STA-NB15 cells with a retrovirus coding for a 4OHT-regulated, phosphorylation-independent FKHRL1(A3)ERtm allele (Obexer et al., 2007). Activation of transgenic FKHRL1(A3)ERtm by 75 nM 4OHT induces its accumulation in the nucleus within 2 h (Figure 2A) and programmed cell death in SH-EP/FKHRL1 and NB15/FKHRL1 cells within 48–72 h (Figure 2B). Similar to endogenous FKHRL1 in Ly294002-treated cells (Figure 1B, left), only a fraction of 4OHT-treated FKHRL1(A3)ERtm accumulated in the nucleus, suggesting that partial activation of FKHRL1 suffices to exert a pronounced physiological effect.
Figure 2.
Activation of transgenic FKHRL1(A3)ERtm fusion protein by 4OHT leads to its nuclear accumulation and induces apoptotic cell death in human NB cells. (A) SH-EP/FKHRL1 cells expressing the conditional, phosphorylation-independent FKHRL1(A3) ERtm transgene were treated for 2 h with 75 nM 4OHT and subjected to subcellular fractionation. The protein levels of FKHRL1(A3) ERtm in the cytosolic and the nuclear fraction were determined by immunoblot. α-Tubulin (cytosolic) and lamin A/C (nuclear) were used to control the purity of the fractions. (B) The cell lines SH-EP/FKHRL1 and NB15/FKHRL1 were incubated with 75 nM 4OHT for the times indicated and subjected to FACS analysis of PI-stained nuclei.
As shown in Figure 3A, activation of transgenic FKHRL1(A3)ERtm reduced endogenous Survivin expression within 8 h and strongly repressed it after 24 and 36 h (38 and 9% of controls, respectively), which correlated with an increase of apoptosis at these time points in SH-EP/FKHRL1 cells. In NB15/FKHRL1 cells, Survivin decreased after 36 h of 4OHT-treatment to 34% of controls, which correlated with the later onset of nuclear fragmentation in these cells.
Figure 3.
Activation of the conditional FKHRL1(A3)ERtm fusion protein by 4OHT causes repression of Survivin on mRNA and protein level. (A) For analysis of Survivin expression FKHRL1(A3)ERtm-transgenic and mock-transfected cells were treated with 75 nM 4OHT for the times indicated, and protein extracts were analyzed by immunoblotting by using antibodies directed against Survivin and α-Tubulin. Densitometry of 16-bit digital images was done using LabWorks 4.6 software. (B) Activation of FKHRL1(A3)ERtm by 4OHT (75 nM) represses Survivin on mRNA level as analyzed by means of quantitative RT-PCR in SH-EP/FKHRL1 and NB15/FKHRL1 cells. Shown is the mean of three independent experiments each performed in triplicate. (C) SH-EP/Ctr and SH-EP/FKHRL1 cells were transfected with a survivin promoter firefly luciferase vector, and cultured in the presence (black bars) or absence of 75 nM 4OHT (white bars) for 24 h to analyze changes of survivin promoter steady-state activity during FKHRL1 activation. Relative luciferase activity was calculated as percentage of untreated controls. Shown is the mean of three independent experiments each performed in triplicate. (D) SH-EP/FKHRL1 cells were treated with 75 nM 4OHT and 10 μg/ml cycloheximide (CHX) for 6 h alone or in combination, respectively. Survivin mRNA expression was quantified by quantitative RT-PCR.
Next, we addressed by quantitative RT-PCR, whether the reduction of Survivin protein was due to transcriptional repression: Survivin mRNA was repressed within 6 h in SH-EP/FKHRL1 and within 3 h in NB15/FKHRL1 cells (Figure 3B). To assess whether Survivin repression was due to reduced survivin promoter activity, a luciferase reporter plasmid harboring 1900 bp of the human survivin promoter was transfected into SH-EP/FKHRL1 and SH-EP/Ctr cells. On FKHRL1 activation, basal survivin promoter activity was reduced to 12% of untreated controls (Figure 3C). Because the repression of Survivin by FKHRL1 can be blocked by cycloheximide treatment (Figure 3D), a newly synthesized cofactor might be essential for FKHRL1-mediated transcriptional repression, suggesting that FKHRL1 indirectly reduces Survivin promoter activity.
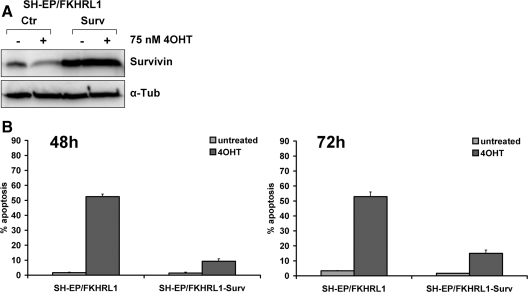
Transgenic Expression of Survivin Inhibits FKHRL1-induced Apoptosis
Survivin was introduced into SH-EP/FKHRL1 cells by using a retroviral bicistronic vector that expresses Survivin and the yellow fluorescent protein (YFP). In these cells, the repression of Survivin during FKHRL1 activation was efficiently prevented for 36 h (Figure 4A). Ectopic Survivin protected SH-EP/FKHRL1 cells against FKHRL1-induced apoptosis, reducing the number of apoptotic cells from 50 to <10% after 48 h and to 15% after 72 h of 4OHT treatment (Figure 4B). In contrast to its antiapoptotic effect in SH-EP/FKHRL1 cells, transgenic Survivin did not significantly affect the antiproliferative effect of FKHRL1 as measured by [3H]thymidine incorporation (see Supplemental Figure), suggesting that loss of Survivin does not contribute to FKHRL1-induced cell cycle arrest. The strong induction of the cell cycle inhibitor p27Kip1 (Figure 5D) might also explain why overexpression of Survivin has no significant impact on the antiproliferative effect of FKHRL1. To further investigate dose-dependent effects of Survivin, we lowered endogenous Survivin expression by retroviral shRNA technology.
Figure 4.
Transgenic expression of Survivin inhibits FKHRL1-induced apoptosis in SH-EP/FKHRL1 cells. (A) SH-EP/FKHRL1 cells were infected with the retroviral vector pLIB-Survivin-iresYFP and sorted by FACS. The transgenic expression of Survivin in SH-EP/FKHRL1-Surv cells was analyzed by immunoblot. α-Tubulin was used as loading control. (B) SH-EP/FKHRL1-Ctr and SH-EP/FKHRL1-Surv cells were subjected to apoptosis detection by PI-FACS analysis after addition of 75 nM 4OHT for 48 and 72 h. Each panel represents the mean ± SEM of three independent experiments.
Figure 5.
Knockdown of Survivin by shRNA increases FKHRL1-induced apoptosis in SH-EP/FKHRL1 cells. (A) The pQ-tetH1-shSurvivin-SV40Puro retrovirus vector was infected into SH-EP/FKHRL1 cells. Knockdown of endogenous Survivin was analyzed by immunoblot. (B) SH-EP/FKHRL1-shCtr (infected with the empty plasmid pQ-tetH1-SV40Puro) and SH-EP/FKHRL1-shSurv cells were treated with 20 nM 4OHT for 24 and 48 h. Apoptosis induction was determined by PI-FACS analysis. (C) Mitochondrial activity was assessed by CMX-Ros staining in untreated and 4OHT-treated (20 nM) SH-EP/FKHRL1-shCtr and SH-EP/FKHRL1-shSurv cells after 48 h. Shown is the mean of three independent experiments. (D) SH-EP/FKHRL1 cells were treated either with 20 nM or with 75 nM 4OHT for 0, 8, or 24 h, and the expression of Survivin and p27Kip1 was determined by immunoblot. GAPDH served as loading control. Densitometry was performed using LabWorks 4.6 software.
Knockdown of Endogenous Survivin Sensitizes SH-EP Cells to FKHRL1-induced Apoptosis
To study whether Survivin steady-state levels determine sensitivity of NB cells to FKHRL1-induced apoptosis, we infected SH-EP/FKHRL1 cells either with a Survivin–shRNA-expressing or an empty pQ-tetH1-SV40Puro retrovirus. Bulk-selected SH-EP/FKHRL1-shSurv cells showed markedly reduced endogenous Survivin expression compared with mock-infected controls (Figure 5A). We next assessed whether lowering the 4OHT dose to activate transgenic FKHRL1(A3)ERtm affects cell death kinetics. 4OHT (75 nM) induced 49% apoptosis after 48 h (Figure 2B). As shown in Figure 5B, addition of only 20 nM 4OHT reduced programmed cell death to 11% after 48 h. This indicates that the ability of FKHRL1 to induce apoptosis is dose dependent. Importantly, in Survivin–shRNA-expressing SH-EP/FKHRL1- shSurv cells 20 nM 4OHT markedly increased apoptosis compared with controls with 54% apoptotic cells after 48 h. This is comparable with the death rate induced by a threefold higher 4OHT concentration (Figure 2B). The knockdown of Survivin not only increased nuclear fragmentation as a hallmark of apoptosis but also accelerated the loss of mitochondrial membrane potential during FKHRL1-activation as shown in Figure 5C. To assess, whether reduced cell death at 20 nM 4OHT corresponds with reduced activity of ectopic FKHRL1(A3)ERtm, we assessed steady-state protein levels of Survivin and p27Kip1 in cells treated with 20 or 75 nM 4OHT, respectively (Figure 5D). After 8 h in presence of 75 nM 4OHT, Survivin was decreased to 52% of control, whereas with 20 nM 4OHT Survivin steady-state levels remained unchanged. However, after 24 h, Survivin expression was lowered to 35 and to 15% of untreated controls with 20 and 75 nM 4OHT, respectively. p27Kip1 was strongly induced at both concentrations after 24 h. However, with 75 nM 4OHT approximately twofold higher protein levels were achieved compared with 20 nM 4OHT, suggesting a dose-dependent gene regulation by FKHRL1(A3)ERtm. The combined data demonstrate that cellular Survivin steady-state levels are rate limiting for FKHRL1-induced apoptosis in NB cells.
Survivin Purifies with the Mitochondrial Compartment and Affects Mitochondrial Accumulation of Proapoptotic BCL2 Proteins
In SH-EP/FKHRL1 cells, FKHRL1-induced apoptosis is associated with loss of mitochondrial membrane potential, cytochrome c release, and cleavage of caspase-9 (Obexer et al., 2007). We therefore studied whether transgenic Survivin may prevent apoptosis upstream of caspases by preserving mitochondrial activity during FKHRL1-induced apoptosis. On FKHRL1 activation, the number of CMX–Ros-negative cells was markedly reduced in the population of Survivin-overexpressing cells after 48 h (42.2%) compared with controls (13.4%), suggesting that transgenic Survivin expression affects apoptosis signaling at the level of mitochondria (Figure 6A). In concordance with the CMX-Ros staining, Survivin prevented the appearance of cytoplasmic cytochrome c after 8 and 24 h (Figure 6B). We described before that the BH3-only protein Bim is strongly induced 8 h after FKHRL1 activation in SH-EP and STA-NB15 cells (Obexer et al., 2007). Interestingly, in SH-EP/FKHRL1-Surv cells Bim was present in the mitochondrial fraction at lower levels than in SH-EP/FKHRL1-Ctr cells. BAX was found equally distributed between cytoplasm and mitochondria in SH-EP/FKHRL1-Surv cells, and no accumulation at the mitochondria upon FKHRL1 activation was observed at the investigated time points (Figure 6B, top). As described for other cell types (Dohi et al., 2007), Survivin purified with cytoplasmic and mitochondrial preparations (Figure 6B, bottom). After 24 h of 4OHT-treatment in SH-EP/FKHRL1-Ctr cells Survivin was reduced both in the cytoplasmic and the mitochondrial fraction, whereas in SH-EP/FKHRL1-Surv cells Survivin repression was only visible in the cytoplasmic fraction. The combined data demonstrate that a pool of Survivin localizes to the mitochondria, affects the expression of the proapoptotic BCL2 proteins Bim and Bax, and delays the release of cytochrome c into the cytoplasm in NB cells.
Figure 6.
Ectopic Survivin purifies with the mitochondrial fraction and prevents FKHRL1-induced cell death at the level of mitochondria. (A) Mitochondrial activity was assessed by CMX-Ros staining in untreated and 4OHT-treated (75 nM, 48 h) SH-EP/FKHRL1-Ctr and SH-EP/FKHRL1-Surv cells. (B) Cytosolic and mitochondrial extracts of either untreated or 75 nM 4OHT-treated SH-EP/FKHRL1-Ctr and SH-EP/FKHRL1-Surv cells were prepared after 8 h (top) and 24 h (bottom) and analyzed for the expression of Survivin, cytochrome c, Cox4, BAX, and Bim. α-Tubulin (cytosolic) and Cox4 (mitochondrial) served as markers for the purity of subcellular fractions.
Survivin Repression Is Critical for FKHRL1-enhanced Sensitivity to Doxorubicin and Etoposide
Because hyperactivation of the PI3K-PKB pathway has been shown to protect NB cells from chemotherapy-induced cell death (Li et al., 2007), we studied whether FKHRL1 may influence the sensitivity to chemotherapy. SH-EP cells were treated with the drugs doxorubicin, etoposide, and vinblastine and transgenic FKHRL1(A3)ERtm was activated by 50 nM 4OHT. FKHRL1 per se induced ∼15% apoptosis within 24 h compared with 2% in the untreated controls (Figure 7A). Treatment with 2.5 μg/ml etoposide or 0.25 μg/ml doxorubicin induced 8 and 12% apoptosis, respectively, whereas 50 ng/ml vinblastine treatment increased cell death to ∼31% within 24 h. However, when FKHRL1 was activated by 4OHT, sensitization to doxorubicin- and etoposide-treated cells was observed, raising apoptosis levels to >50% within 24 h. In contrast, active FKHRL1 did not markedly elevate vinblastine-induced apoptosis. This suggests that FKHRL1 sensitizes neuroblastoma cells specifically to DNA-damaging agents such as doxorubicin and etoposide. We next asked whether Survivin may protect NB cells against doxorubicin- and etoposide-induced apoptosis. For that purpose, we stably overexpressed Survivin in SH-EP cells (Figure 7B) and subjected these cells to doxorubicin, etoposide, and vinblastine treatment for 48 h. As shown in Figure 7C, Survivin exerted a protective effect on doxorubicin- and etoposide-treated cells but only slightly lowered vinblastine-induced cell death after 48 h, suggesting a critical role of Survivin in the death pathways activated by these drugs. The fact that FKHRL1 strongly represses Survivin and sensitizes NB cells to doxorubicin- and etoposide-induced apoptosis together with the observation that Survivin overexpression specifically inhibits cell death by these DNA-damaging drugs suggests a role of Survivin in FKHRL1-mediated drug sensitization. To address this question in detail, SH-EP/FKHRL1-Ctr and SH-EP/FKHRL1-Surv cells were cultured for 48 and 72 h in presence or absence of 50 nM 4OHT and/or doxorubicin and/or etoposide (Figure 7, D and E). In controls, the activation of FKHRL1 induced 52% apoptosis within 48 h, which was further increased to 73 and 69% by the combination with doxorubicin or etoposide, respectively. Transgenic Survivin markedly reduced FKHRL1- induced apoptosis after 48 h from 52 to 9% and reduced apoptosis rates from 73 to 34% in doxorubicin/4OHT and from 69 to 26% in etoposide/4OHT-treated cells. This protection by transgenic Survivin was apparent up to 72 h of 4OHT treatment (Figure 7E). The combined data suggest that Survivin repression by FKHRL1 contributes to enhanced drug sensitivity of NB cells.
Figure 7.
Survivin expression counteracts FKHRL1-induced sensitization to doxorubicin and etoposide induced cell death. (A) SH-EP/FKHRL1 cells were treated either with the chemotherapeutics doxorubicin (0.25 μg/ml), etoposide (2.5 μg/ml), and vinblastine (50 ng/ml) alone or in combination with 50 nM 4OHT. Apoptosis was determined by PI-FACS analysis after 24 h. (B) Transgenic Survivin was analyzed by immunoblot analysis. (C) SH-EP and transgenic Survivin-expressing SH-EP-Surv cells were treated with the chemotherapeutics doxorubicin (0.25 μg/ml), etoposide (2.5 μg/ml), and vinblastine (50 ng/ml). Apoptosis was determined by PI-FACS analysis after 48 h. (D and E) SH-EP/FKHRL1-Ctr and SH-EP/FKHRL1-Surv cells were treated either with the chemotherapeutics doxorubicin (0.25 μg/ml) and etoposide (2.5 μg/ml) alone or in combination with 4OHT (50 nM) for 48 and 72 h and analyzed by FACS analysis of PI-stained nuclei. Each panel represents the mean ± SEM of three independent experiments.
DISCUSSION
PKB hyperactivation contributes to chemotherapy resistance of malignant NB and has been implied as a prognostic marker for decreased event-free survival (Opel et al., 2007). We have shown recently that the PKB downstream target FKHRL1 induces apoptosis by up-regulating the BH3-only proteins Bim and Noxa in NB cells (Obexer et al., 2007). FKHRL1 is retained in the cytoplasm in NB cells but rapidly translocates into the nucleus upon inhibition of PI3K (Figure 1, A and B) and represses the antiapoptotic protein Survivin (Figure 3). In primary NB cells, Survivin expression correlates with adverse clinical factors, advanced disease stage (Islam et al., 2000) and is associated with an unfavorable prognosis (Tajiri et al., 2001; Miller et al., 2006). Survivin overexpression was reported to increase proliferation and to protect tumor cells against apoptosis by various stimuli (Ambrosini et al., 1997; Islam et al., 2000). There is also evidence that Survivin is regulated by targets of the PI3K-PKB pathway in neuronal cells: vascular endothelial growth factor-induced expression of Survivin in NB is blocked by the PI3K inhibitor Ly294002, suggesting that Survivin is regulated downstream of PI3K (Beierle et al., 2005). A role of the PI3K-PKB pathway in Survivin regulation is further supported by the observation that dominant-negative PI3K decreases Survivin and c-IAP1 mRNA and protein expression in various NB cell lines (Kim et al., 2004). Our data imply that the transcription factor FKHRL1 might be a missing link between PI3K-PKB and Survivin in NB cells. Importantly, Survivin was not only repressed by FKHRL1 but also protected against FKHRL1-induced apoptosis (Figure 4B) and FKHRL1-induced drug sensitization (Figure 7, D and E). Survivin knockdown by retroviral shRNA technology, in contrast, markedly sensitized SH-EP cells to FKHRL1-induced death (Figure 5, B and C). This supports the notion that Survivin steady-state levels critically modulate FKHRL1- induced apoptosis in NB cells. Whereas its function during mitosis and cytokinesis has been investigated in detail, the role of Survivin in protecting cells from apoptosis is less well understood. Because Survivin contains only a single BIR domain and lacks a RING structure, its activity as a caspase-inhibiting IAP is under debate. Interestingly, we observed that part of Survivin purified with the mitochondrial fraction in SH-EP/FKHRL1 cells. It was shown before that a pool of Survivin localizes to the intramitochondrial membrane space in mammalian cells. Mitochondrial Survivin binds BIRC4/XIAP and thereby prevents XIAP from polyubiquitination and proteasomal degradation (Dohi et al., 2004). In response to mitochondrial permeabilization, the Survivin/XIAP complex is released into the cytosol where it inhibits caspase-9 activation (Dohi et al., 2007). Ceballos-Cancino et al. (2007) recently reported that mitochondrial Survivin prevents etoposide-induced cell death via binding to Smac/Diablo, thereby delaying its release from the mitochondrial intermembrane space into the cytosol. These data are consistent with our observation that repression of endogenous Survivin by FKHRL1 sensitized to the DNA-damaging compounds etoposide and doxorubicin and that transgenic Survivin diminished FKHRL1-induced drug sensitization (Figure 7, D and E). Because FKHRL1-induced cell death in NB cells is controlled at the level of mitochondria (Obexer et al., 2007), the ability of mitochondrial Survivin to bind Smac/Diablo and/or XIAP at the mitochondria might explain, at least in part, why stable Survivin knockdown lowers the threshold for apoptosis induction by FKHRL1 (Figure 5, B and C), whereas Survivin overexpression had the opposite effect (Figure 4B). The loss of endogenous, mitochondrial Survivin correlated with the release of cytochrome c to the cytosol in control cells. Interestingly, in Survivin-transgenic cells, only cytosolic Survivin was down-regulated by FKHRL1, whereas mitochondrial Survivin steady-state levels remained unaltered (Figure 6B, bottom). This differential regulation of mitochondrial and cytoplasmic Survivin might be due to a lower turn over rate of mitochondrial Survivin (Dohi et al., 2007). Thereby, as long as mitochondria are intact (as suggested by mitochondrial retention of cytochrome c) mitochondrial Survivin is protected from proteasomal degradation. The repression of endogenous Survivin by FKHRL1 may accelerate the release of Smac/Diablo during chemotherapy treatment (Ceballos-Cancino et al., 2007), which is otherwise blocked by mitochondrial Survivin. This effect might in particular be important in NB cells, where gain of 17q and elevated expression of Survivin in advanced stage tumors correlates with therapy resistance and adverse outcome (Islam et al., 2000). However, Survivin seems to exert additional effects upstream of mitochondrial outer membrane permeabilization, because ectopic Survivin preserved mitochondrial activity (Figure 6A) and its knockdown accelerated mitochondrial breakdown during FKHRL1-induced cell death (Figure 5C). Furthermore, Survivin expression affected mitochondrial accumulation of proapoptotic Bim and BAX. The molecular basis for this observation is unclear and currently under investigation.
Because both high activity of PKB and deregulated expression of Survivin are prognostic for an adverse outcome in NB, strategies targeting FKHRL1 will have the advantage to activate death control downstream of PKB and to repress Survivin, which protects against DNA-damaging agents such as etoposide and doxorubicin. A decrease in Survivin will considerably lower drug concentrations necessary during chemotherapy and thereby also allow the reduction of therapy-induced side effects and long-term effects.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. G. Nolan (Stanford University School of Medicine, Stanford, CA) for the Phoenix packaging cell line; Dr. P. Coffer (University Medical Center, Utrecht, The Netherlands) for donating plasmids; and C. Kitzbichler, B. Leimgruber, and J. Dörflinger for excellent technical assistance. The work was supported by grants from the Children Cancer Society of Tyrol and Vorarlberg, the Children's Cancer Research Institute (CCRI), the “Südtiroler Krebshilfe”, the OeNB Anniversary Fund (Projects 11436 and 12582), the Tyrolean Science Fund (TWF), and by the “Österreichische Krebshilfe-Oberösterreich.” The Tyrolean Cancer Research Institute and this study are supported by the “Tiroler Landeskrankenanstalten Ges.m.b.H. (TILAK)”, the Tyrolean Cancer Society, and the Department of Health-Care, Autonomy of South Tyrol.
Abbreviations used:
- 4OHT
4-hydroxy-tamoxifen
- FKHRL1
forkhead transcription factor-like 1
- NB
neuroblastoma
- Surv
Survivin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0699) on February 18, 2009.
REFERENCES
- Adida C., Berrebi D., Peuchmaur M., Reyes-Mugica M., Altieri D. C. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998;351:882–883. doi: 10.1016/S0140-6736(05)70294-4. [DOI] [PubMed] [Google Scholar]
- Ambrosini G., Adida C., Altieri D. C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Ausserlechner M. J., Obexer P., Bock G., Geley S., Kofler R. Cyclin D3 and c-MYC control glucocorticoid-induced cell cycle arrest but not apoptosis in lymphoblastic leukemia cells. Cell Death. Differ. 2004;11:165–174. doi: 10.1038/sj.cdd.4401328. [DOI] [PubMed] [Google Scholar]
- Beierle E. A., Nagaram A., Dai W., Iyengar M., Chen M. K. VEGF-mediated survivin expression in neuroblastoma cells. J. Surg. Res. 2005;127:21–28. doi: 10.1016/j.jss.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Ceballos-Cancino G., Espinosa M., Maldonado V., Melendez-Zajgla J. Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene. 2007;26:7569–7575. doi: 10.1038/sj.onc.1210560. [DOI] [PubMed] [Google Scholar]
- Dijkers P. F., Birkenkamp K. U., Lam E. W., Thomas N. S., Lammers J. W., Koenderman L., Coffer P. J. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T., et al. An IAP-IAP complex inhibits apoptosis. J. Biol. Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- Dohi T., Xia F., Altieri D. C. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol. Cell. 2007;27:17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J., Coffer P. J., Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F., Kinsella T., Mencarelli A., Valtieri M., Riganelli D., Grignani F., Lanfrancone L., Peschle C., Nolan G. P., Pelicci P. G. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- Huang H., Tindall D. J. Dynamic FoxO transcription factors. J. Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Islam A., et al. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- Kim S., Kang J., Qiao J., Thomas R. P., Evers B. M., Chung D. H. Phosphatidylinositol 3-kinase inhibition down-regulates survivin and facilitates TRAIL-mediated apoptosis in neuroblastomas. J. Pediatr. Surg. 2004;39:516–521. doi: 10.1016/j.jpedsurg.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Li Z., Jaboin J., Dennis P. A., Thiele C. J. Genetic and pharmacologic identification of Akt as a mediator of brain-derived neurotrophic factor/TrkB rescue of neuroblastoma cells from chemotherapy-induced cell death. Cancer Res. 2005;65:2070–2075. doi: 10.1158/0008-5472.CAN-04-3606. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang J., Liu Z., Woo C. W., Thiele C. J. Downregulation of Bim by brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from paclitaxel but not etoposide or cisplatin-induced cell death. Cell Death Differ. 2007;14:318–326. doi: 10.1038/sj.cdd.4401983. [DOI] [PubMed] [Google Scholar]
- Liu T., Brouha B., Grossman D. Rapid induction of mitochondrial events and caspase-independent apoptosis in Survivin-targeted melanoma cells. Oncogene. 2004;23:39–48. doi: 10.1038/sj.onc.1206978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Ohashi K., Zhu X., McGrady P., London W. B., Hogarty M., Sandler A. D. Survivin mRNA levels are associated with biology of disease and patient survival in neuroblastoma: a report from the children's oncology group. J. Pediatr. Hematol. Oncol. 2006;28:412–417. doi: 10.1097/01.mph.0000212937.00287.e5. [DOI] [PubMed] [Google Scholar]
- Narath R., Lorch T., Greulich-Bode K. M., Boukamp P., Ambros P. F. Automatic telomere length measurements in interphase nuclei by IQ-FISH. Cytometry A. 2005;68:113–120. doi: 10.1002/cyto.a.20190. [DOI] [PubMed] [Google Scholar]
- Obexer P., Geiger K., Ambros P. F., Meister B., Ausserlechner M. J. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- Opel D., Poremba C., Simon T., Debatin K. M., Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]
- Schramm A., Schulte J. H., Astrahantseff K., Apostolov O., Limpt V., Sieverts H., Kuhfittig-Kulle S., Pfeiffer P., Versteeg R., Eggert A. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005;228:143–153. doi: 10.1016/j.canlet.2005.02.051. [DOI] [PubMed] [Google Scholar]
- Shankar S. L., Mani S., O'Guin K. N., Kandimalla E. R., Agrawal S., Shafit-Zagardo B. Survivin inhibition induces human neural tumor cell death through caspase-independent and -dependent pathways. J. Neurochem. 2001;79:426–436. doi: 10.1046/j.1471-4159.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- Tajiri T., Tanaka S., Shono K., Kinoshita Y., Fujii Y., Suita S., Ihara K., Hara T. Quick quantitative analysis of gene dosages associated with prognosis in neuroblastoma. Cancer Lett. 2001;166:89–94. doi: 10.1016/s0304-3835(01)00434-7. [DOI] [PubMed] [Google Scholar]
- van der Horst A., Burgering B. M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Zheng W. H., Kar S., Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J. Neurochem. 2002;80:1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.