Abstract
p120-catenin is a cytoplasmic binding partner of cadherins and functions as a set point for cadherin expression by preventing cadherin endocytosis, and degradation. p120 is known to regulate cell motility and invasiveness by inhibiting RhoA activity. However, the relationship between these functions of p120 is not understood. Here, we provide evidence that p120 functions as part of a plasma membrane retention mechanism for VE-cadherin by preventing the recruitment of VE-cadherin into membrane domains enriched in components of the endocytic machinery, including clathrin and the adaptor complex AP-2. The mechanism by which p120 regulates VE-cadherin entry into endocytic compartments is dependent on p120's interaction with the cadherin juxtamembrane domain, but occurs independently of p120's prevention of Rho GTPase activity. These findings clarify the mechanism for p120's function in stabilizing VE-cadherin at the plasma membrane and demonstrate a novel role for p120 in modulating the availability of cadherins for entry into a clathrin-dependent endocytic pathway.
INTRODUCTION
VE-cadherin is a central component of endothelial adherens junctions and mediates homophilic interactions between neighboring endothelial cells. Adhesion and signaling events mediated by VE-cadherin play key roles in vascular barrier function and angiogenesis (Vestweber, 2008; Wallez and Huber, 2008). Likewise, several endothelial signaling pathways are known to regulate endothelial cell–cell interactions through regulation of VE-cadherin or other adherens junction molecules (Gavard and Gutkind, 2006; Lampugnani et al., 2006). The cytoplasmic domain of VE-cadherin interacts with members of the armadillo family of proteins, including β-catenin and p120-catenin (p120). These interactions regulate association of cadherins with the actin cytoskeleton and are important for strong cell–cell adhesion (Pokutta and Weis, 2007; Hartsock and Nelson, 2008). Furthermore, gene ablation studies have revealed key roles for VE-cadherin, β-catenin, and p120 in vascular development, underscoring the importance of adherens junction components in vascular biology and vertebrate development (Carmeliet et al., 1999; Gory-Faure et al., 1999; Cattelino et al., 2003; Xiao, Oas, and Kowalczyk, unpublished observations).
An important aspect of the ability of cells to dynamically modulate their adhesive state is the regulation of cadherin availability at the cell surface. One cellular mechanism that is commonly used to rapidly alter the expression of cell surface receptors is membrane trafficking. Cells utilize several endocytic pathways to retrieve receptors from the cell surface. The most common of these is clathrin-dependent, in which transmembrane cargos are sorted into clathrin-coated pits at the plasma membrane. Endocytic adaptor proteins, including the tetrameric adaptor complex AP-2, play a fundamental role in the recruitment and formation of clathrin-coated vesicles at the plasma membrane through the ability to bind to lipids, cargo, clathrin, and clathrin accessory proteins (Bonifacino and Traub, 2003; Maldonado-Báez and Wendland, 2006). Clathrin-independent pathways for receptor endocytosis are increasingly recognized as important portals of internalization (Mayor and Pagano, 2007). Cadherins can be internalized through both clathrin-dependent and -independent pathways, depending on the cadherin and the cellular context (Chiasson and Kowalczyk, 2008; Delva and Kowalczyk, 2008). Evidence suggests that E-cadherin and VE-cadherin are primarily internalized through clathrin-dependent pathways. However, a recent study demonstrated that the desmosomal cadherin desmoglein 3 is internalized through a clathrin- and dynamin-independent pathway (Delva et al., 2008). It remains unclear how the decision for cadherins to enter different endocytic compartments is regulated according to the needs of the cell.
Several years ago, a series of studies revealed a core function for p120 in regulating cadherin-mediated adhesion. p120 acts as a set point for cadherin expression by controlling the amount of cadherin available at cell junctions for adhesion (Davis et al., 2003; Xiao et al., 2003, 2007; Chiasson and Kowalczyk, 2008). In the absence of p120, cadherin-based junctions are destabilized, and the cadherin is targeted for degradation through an endosomal–lysosomal pathway. In addition to regulating cadherin endocytosis, p120 also functions in a signaling capacity as a potent regulator of Rho-GTPase activity within the cell (Anastasiadis, 2007). Rho-GTPases make up a large family of proteins that regulate cytoskeletal organization. The first evidence for the involvement of p120 in regulating Rho-GTPase signaling was the observation of a dramatic branching phenotype in cells expressing high levels of exogenous p120 characterized by the formation of long dendritic processes extending from the cell body (Reynolds et al., 1996; Anastasiadis et al., 2000; Noren et al., 2000). These and other studies have demonstrated that p120 acts as an inhibitor of RhoA, and through this activity p120 appears to regulate cell migration, proliferation, and inflammatory responses.
Although p120's functions in stabilizing cadherins and regulating RhoA signaling are well established, the relationship between these roles is unclear. Early studies suggested that p120-mediated inhibition of RhoA activity is spatially restricted to the cytoplasm and therefore mutually exclusive with the role of p120 at adherens junctions (Anastasiadis et al., 2000; Fox and Peifer, 2007; Yanagisawa et al., 2008). However, a recent study has proposed a potential mechanism linking these functions of p120, at least in certain cellular contexts. After PDGF signaling in fibroblasts, p120 was found to be required for the localized inhibition of RhoA activity through a mechanism that requires the recruitment of p190RhoGAP to cadherin-based junctions. Interestingly, p190RhoGAP not only inhibits RhoA, but also promotes adhesion (Wildenberg et al., 2006). In the absence of p190RhoGAP, N-cadherin is mislocalized from cell junctions. These data provide the first evidence that localized inhibition of Rho activity by p120 at cell junctions may regulate cadherin localization and stability. These data raise the possibility that p120-mediated inhibition of RhoA may in turn regulate entry of cadherins into an endocytic pathway.
In the present article, we directly tested whether p120 inhibition of endocytosis and regulation of RhoA are mechanistically interdependent. Using a series of approaches, we demonstrate that p120 potently inhibits entry of the cadherin into clathrin- and AP-2–enriched membrane domains. VE-cadherin is internalized through a pathway that is dependent on clathrin, dynamin, and the clathrin adaptor complex AP-2. Lastly, we found that the ability of p120 to inhibit cadherin endocytosis can be experimentally uncoupled from p120 inhibition of RhoA, indicating that binding to the cadherin tail, but not the ability of p120 to inhibit Rho, is the crucial function of p120 in preventing endocytosis. These findings clarify the mechanism by which p120 regulates cadherin endocytosis and distinguish this activity from p120 regulation of RhoA.
MATERIALS AND METHODS
Cell Culture
Primary cultures of dermal microvascular endothelial cells (MECs) from human neonatal foreskin were isolated and cultured in MCDB 131 medium as described previously (Xiao et al., 2005). The medium was supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO), l-anyl-glutamine (Mediatech, Herndon, VA), cAMP (Sigma-Aldrich), hydrocortisone (Sigma-Aldrich), epidermal growth factor (Intergen, Purchase, NY), and antibiotic/antimyotic (Invitrogen, Carlsbad, CA). HMEC-1 cells were cultured as previously described (Venkiteswaran et al., 2002). Cells were generally cultured overnight on 0.1% gelatin-coated plates and grown to ∼80% confluency for experiments. For adenovirus production human embryonic kidney cell line QBI-293A (Qbiogene, Carlsbad, CA) were routinely cultured in DMEM supplemented with 10% FBS and antibiotic/antimycotic. Chloroquine was purchased from Sigma-Aldrich and used at 100 μM. Cell-permeable C3 Transferase was purchased from Cytoskeleton (Denver, CO) and used at 1.0 μg/ml. Y27632 was purchased from Calbiochem (San Diego, CA) and used at 10 μM.
Adenovirus Production
Adenoviral reagents for the interleukin (IL)-2R-VE-cadcyto chimera, IL-2R-Dsg3cyto chimera, and p120ctn 1A were generated as described previously (Xiao et al., 2005). To generate the p120-4A K622,628A adenovirus, PCR was performed using a p120-1A K622,628A construct (provided by A. Reynolds, Vanderbilt University, Nashville, TN) as a template and the forward primers consisting of the start site of p120-4A isoform with a NotI restriction site and Kozak (5′-GCGGCCGCGCCACCATGATTGGTGAGGAGGTGCCA-3′) along with the reverse primer containing an SwaI site and the C-terminus of p120 (5′-ATTTAAATGAATCTTCTGCATCAAGGGTGC-3′). The recombinant fragment was placed in pShuttle in front of enhanced green fluorescent protein (EGFP). Adenovirus was then produced using the AdEasy packaging system described previously (Xiao et al., 2005). The dominant negative DynII (K44A) adenovirus (Altschuler et al., 1998) was provided by S. Schmid (The Scripps Institute, La Jolla, CA), and the dominant negative RhoA (N19) adenovirus (Kalman et al., 1999) was provided by D. Kalman (Emory University, Atlanta, GA).
Immunofluorescence
Immunofluorescence was carried out as described previously (Xiao et al., 2005). Antibodies used were as follows: rabbit anti VE-cadherin (Alexis Biochemicals, San Diego, CA), anti-IL-2R IgG from 7G7B6 mouse hybridoma (American Type Culture Collection, Manassas, VA), chicken anti-myc epitope tag (Bethyl Laboratories, Montgomery, TX), a mouse mAb (BD Biosciences Pharmigen, San Diego, CA) or a polyclonal rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) against p120, mouse mAb against dynamin (BD Biosciences Pharmigen), mouse mAb against RhoA (Santa Cruz Biotechnology), mouse mAbs against clathrin (BD Transduction Laboratories, Lexington, KY) or AP-2 (Santa Cruz Biochemicals), a rabbit antibody against caveolin-1 (BD Biosciences Pharmigen). The localization of actin was determined with an Alexa Fluor 488–conjugated phalloidin (Molecular Probes, Eugene, OR). Secondary antibodies conjugated to Alexa Fluors (Molecular Probes) were used for double- and triple-label experiments. Microscopy was performed using either a wide-field fluorescence microscope (model DMR-E; Leica, Wetzlar, Germany) equipped with narrow bandpass filters and a digital camera (model Orca; Hamamatsu, Bridgewater, NJ) or an inverted Leica DMI-6000B microscope equipped with an Infinity II confocal scanning module, 561- and 491-nm lasers, and a Hamamatsu CCD camera (C9100-12). Images were captured, pseudocolored, and processed using Simple PCI software (Compix, Cranberry Township, PA) or Metamorph Software (Universal Imaging, West Chester, PA). For colocalization experiments, a nearest neighbors deconvolution algorithm was performed on successive 0.20-μm focal planes.
Internalization Assay
Assays to follow internalization of transferrin and the IL-2R-VE-cadherin polypeptides were carried out as previously described (Xiao et al., 2005). For colocalization experiments with clathrin and AP-2, cells were labeled at 4°C with IL-2R antibodies and then incubated for 5 min at 37°C to allow for internalization. Cells were then fixed and processed for immunofluorescence without being acid-washed. Potassium depletion was performed as described previously (Xiao et al., 2005), and cells were treated with 5 μg/ml chlorpromazine for 30 min at 37°C. To measure internalization of endogenous VE-cadherin, cells were treated with 100 μM chloroquine for 3 h. Cells were then rinsed, fixed, and processed for immunofluorescence as described above. Internalization was quantified using Simple PCI software to measure either total intracellular fluorescence or object number. Statistical analysis was performed using two-tailed t test. Error bars represent the SEM.
Cross-linking and Immunoprecipitation
To analyze low-affinity interactions between VE-cadherin and AP-2, we performed cross-linking in MECs with DSP [dithiobis(succinimidylpropionate)] as described (Craige et al., 2008; Salazar et al., 2008). Briefly, MECs were grown to confluency in 10-cm dishes and infected with Il-2R-VE-cadherin constructs. On the day of the experiment, cells were placed on ice, rinsed twice with PBS, and incubated with 1 mM DSP (Pierce, Rockford, IL) or vehicle for 2 h on ice. The reaction was quenched by adding 25 mM Tris. The cells were then lysed in buffer A (150 mM NaCl, 10 mM HEPES, 1 mM EGTA, and 0.1 mM MgCl2, pH 7.4) + 0.5% TX-100, scraped from the dish, and incubated on ice for 30 min. Cell homogenates were centrifuged at 16,100 × g for 10 min, and supernatants were diluted to 1 mg/ml in 0.5 ml of buffer A + 0.5% TX-100. The supernatants were incubated overnight at 4°C with Dynal magnetic beads (Invitrogen) conjugated to mAbs against AP-2 (α subunit, BD Transduction Laboratories) or clathrin (Calbiochem, San Diego, CA). The beads were then washed with buffer A + 0.1% TX-100 and eluted with SDS-PAGE sample buffer at 75°C for 5 min. Immunoblotting under reducing conditions reverses the DSP cross-linking and allows for the detection of immunoprecipitated material.
Small Interfering RNA Knockdown of AP-2
Small Interfering RNA (siRNA) siRNA oligonucleotides to the AP-2 μ subunit and control, nontargeting siRNA were purchased from Dharmacon (Lafayette, CO) and have been previously described (Urs et al., 2008; Motley et al., 2003). HMEC-1 cells were seeded in 60-mm dishes at 50% confluency. Cells were transfected on consecutive days with 200 pM siRNA using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Twenty-four hours after the second transfection, cells were trypsinized and plated onto coverslips or 35-mm dishes to assay internalization or efficiency of knockdown. Cells were infected with adenovirus expressing IL-2R-VE-cadcyto at the time of plating. Internalization assays were performed as described above.
RESULTS
VE-Cadherin Is Internalized via a Clathrin-, AP-2–, and Dynamin-dependent Pathway
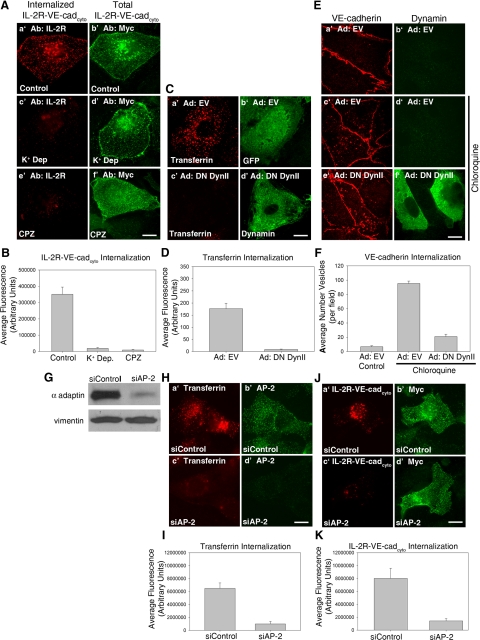
The cytoplasmic domain of VE-cadherin mediates internalization of the cadherin from the cell surface, and p120 is a potent inhibitor of this process (Xiao et al., 2005). However, the molecular machinery utilized for VE-cadherin internalization and the mechanism of p120 inhibition are not fully understood. In previous studies, we demonstrated that a chimeric molecule consisting of the cytoplasmic domain of VE-cadherin fused to the extracellular domain of the IL-2 receptor (IL-2R-VE-cadcyto) is rapidly internalized from the cell surface (Xiao et al., 2005). To further elucidate the endocytic machinery used by VE-cadherin, the IL-2R-VE-cadcyto chimera was expressed in primary cultures of human MECs, and a series of pharmacological and genetic approaches were utilized to inhibit endocytosis. Cells were subjected to K+ depletion (Salazar and Gonzalez, 2002; Xiao et al., 2005) or treatment with chlorpromazine (Wang et al., 1993) to inhibit clathrin-dependent endocytosis. To measure internalization of IL-2R-VE-cadcyto chimera, MECs were incubated at 4°C with antibodies directed against IL-2R to label the cell surface. After incubation at 37°C for 5 min to allow for endocytosis, cells were washed with a low pH acid wash buffer to remove antibody remaining at the cell surface, making it possible to distinguish internalized IL-2R-VE-cadcyto from that at the cell surface. An myc epitope at the carboxy-terminal tail of the VE-cadherin cytoplasmic domain was used to identify cells expressing the IL-2R-VE-cadcyto. As shown in Figure 1, untreated cells expressing the IL-2R-VE-cadcyto chimera were found to display high levels of internalized IL-2R-VE-cadcyto after 5 min of internalization at 37°C (Figure 1, A, a′ and b′, and B). As expected from previous data (Xiao et al., 2005) K+ depletion dramatically reduced the extent of internalization of the IL-2R-VE-cadcyto chimera (Figure 1, A, c′ and d′, and B). Treatment with chlorpromazine, which disrupts localization of the clathrin adaptor complex AP-2 to membranes and prevents clathrin assembly at the plasma membrane (Wang et al., 1993), also strongly inhibited internalization of the IL-2R-VE-cadcyto chimera (Figure 1, A, e′ and f′, and B).
Figure 1.
VE-cadherin endocytosis is mediated through a clathrin-, AP-2–, and dynamin-dependent pathway. (A and B) Cell surface IL-2R-VE-cadcyto was labeled with IL-2R antibodies at 4°C. Cells were incubated at 37°C for 5 min, washed in a low pH buffer at 4°C to remove surface bound antibody, and then processed for immunofluorescence to visualize intracellular IL-2R-VE-cadcyto (A, a′, c′, and e′). Antibodies against the myc epitope tag at the carboxyl terminal domain of the IL-2R-VE-cadcyto were used to verify expression of the polypeptide (A, b′, d′, and f′). Note that untreated cells exhibited high levels of internalized IL-2R-VE-cadcyto, whereas treatment with K+ depletion or chlorpromazine completely inhibited internalization. (C–F) MECs were infected with adenoviruses expressing either GFP (C, a′ and b′) or dominant negative DynII (C, c′ and d′). The DynII completely blocked internalization of Alexa Fluor 555–conjugated transferrin (C and D). To monitor internalization of VE-cadherin, MECs were either untreated (E, a′ and b′) or treated with 100 μM chloroquine for 3 h (E, c′–f′). Note that intracellular vesicular accumulation of VE-cadherin is reduced in cells expressing DynII (E, e′ and f′, and F). (G–K) HMEC-1 cells were transfected with an siRNA oligo targeted to the AP-2 μ subunit or a nontargeting control siRNA. Lysates prepared from cells treated with control siRNA or AP-2 siRNA were separated by SDS-PAGE and immunoblotted for AP-2 to confirm AP-2 knockdown (G). Western blot and immunofluorescence reveals ∼75% reduction in AP-2 levels in siRNA-treated cells compared with control, as well as an 85% decrease in transferrin internalization (G–I). IL-2R-VE-cadcyto internalization assays were performed and AP-2 siRNA-treated cells (J, c′ and d′) exhibited an 80% decrease in internalization of IL-2R-VE-cadcyto compared with control (J, a′ and b′), indicating a requirement for AP-2 in VE-cadherin internalization (K). Error bars, SEM; n > 20 cells. Scale bar, 20 μm.
To determine if dynamin, which is required for scission of clathrin-coated vesicles from the plasma membrane (Ungewickell and Hinrichsen, 2007), is involved in VE-cadherin endocytosis, a dominant negative dynamin II (DN DynII) mutant was utilized (Altschuler et al., 1998). Transferrin internalization was entirely blocked by expression of DN DynII (Figure 1, C and D). Intracellular accumulation of endogenous VE-cadherin after treatment with chloroquine to inhibit lysosomal degradation was used as a measure of VE-cadherin endocytosis (Xiao et al., 2003). Chloroquine-treated cells expressing DN DynII (Figure 1, E, e′ and f′, and F) had limited vesicular staining of VE-cadherin compared with cells expressing empty vector (Figure 1, E, c′ and ′, and F), suggesting a role for dynamin in VE-cadherin endocytosis.
Inhibition of VE-cadherin endocytosis by inhibitors of clathrin-mediated endocytosis suggests a role for adaptor molecules involved in the formation and transport of clathrin-coated vesicles. To determine if the adaptor complex AP-2 is required for VE-cadherin endocytosis, we used siRNA to deplete expression of AP-2. For these experiments, HMEC-1 cells, an immortalized line of dermal microvascular endothelial cells, were used to overcome transfection inefficiencies in primary cells. Treatment of cells with siAP-2, but not siControl, reduced expression of AP-2 by 75% as measured by Western blot (Figure 1G). Treatment with siAP-2 also inhibited internalization of transferrin, a well-characterized AP-2 cargo, by 80% (Figure 1, H and I). Internalization of IL-2R-VE-cadcyto was also reduced by 80% in siAP-2–treated cells compared with siControl-treated cells (Figure 1, J and K). A dominant negative AP-2 mutant also reduced internalization of endogenous VE-cadherin in chloroquine-treated cells (data not shown). Together, these results demonstrate that VE-cadherin endocytosis is dependent on components of the clathrin endocytic machinery, including AP-2 and dynamin.
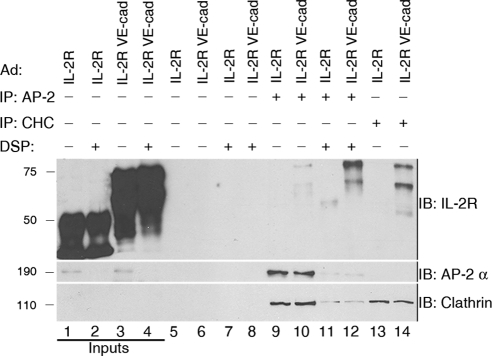
To test the hypothesis that the VE-cadherin cytoplasmic tail forms a complex with clathrin, we immunoprecipitated clathrin from MEC lysates expressing either IL-2R or IL-2R-VE-cadcyto chimera. Reducing SDS-PAGE and immunoblotting with IL-2R antibodies revealed that the IL-2R-VE-cadcyto chimera coprecipitated with clathrin but the IL-2R lacking the VE-cadherin tail did not (Figure 2, cf. lanes 13 and 14), demonstrating that clathrin specifically associates with the VE-cadherin cytoplasmic domain. To analyze interactions between VE-cadherin and adaptor complexes, we used limited whole cell cross-linking followed by immunoprecipitation. DSP, a homobifunctional cell-permeable cross-linker, was used to stabilize cargo-adaptor interactions (Craige et al., 2008). In cells treated with DSP, IL-2R-VE-cadcyto, but not IL-2R, was consistently immunoprecipitated with AP-2 (Figure 2, lanes 11 and 12). IL2-R and IL-2R-VE-cadcyto did not coprecipitate with beads without antibody (Figure 2, lanes 5–8), demonstrating that clathrin and AP-2 specifically interact with the VE-cadherin cytoplasmic tail.
Figure 2.
The VE-cadherin cytoplasmic tail specifically associates with clathrin and AP-2. MECs expressing IL-2R or IL-2R-VE-cadcyto were treated with DSP (lanes 2, 4, 7–8, and 11–12) or DMSO vehicle (lanes 1, 3, 5–6, 9–10, and 13–14), and extracted in detergent, and proteins were immunoprecipitated with beads alone (lanes 5–8), beads coated with AP-2 α antibodies (lanes 8–12), or beads coated with clathrin antibodies (CHC; lanes 13–14). Western blot analysis using antibodies against IL-2R to detect IL-2R or IL-2R-VE-cadcyto demonstrates that the IL2R-VE-cadcyto specifically interacts with AP-2 (lane 12) and clathrin (lane 14). Inputs represent 5% of sample.
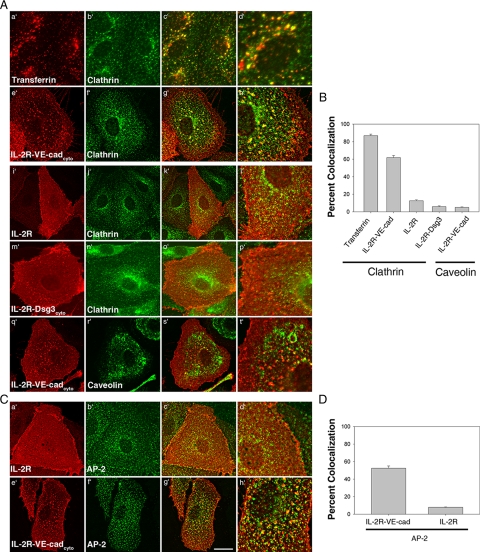
To determine if the association between clathrin, AP-2, and the VE-cadherin tail could be observed in cells, the localization of these proteins was examined by deconvolution immunofluorescence microscopy. Transferrin was used as a positive control for colocalization with clathrin. A chimeric molecule consisting of the extracellular domain of the IL-2 receptor fused to the cytoplasmic tail of the desmosomal cadherin desmoglein 3 (IL-2R-Dsg3cyto), which has been shown to be internalized through a clathrin-independent pathway (Delva et al., 2008), was used as a negative control. To measure colocalization, cells expressing IL-2R, IL-2R-VE-cadcyto, or IL-2R-Dsg3cyto were labeled with IL-2R antibodies at 4°C and transferred to 37°C for 5 min to allow for internalization. For transferrin, uninfected cells were labeled with fluorescently conjugated transferrin at 4°C and transferred to 37°C for 5 min. In contrast to internalization assays, cells were not acid-washed, allowing for the visualization of clusters of the chimeric molecules at early endocytic steps. As expected, extensive colocalization was observed between transferrin and clathrin (Figure 3, A, a′–d′, and B). We also observed significant levels of colocalization between IL-2R-VE-cadcyto and clathrin (Figure 3, A, e′–h′, and B). This colocalization was dependent on the VE-cadherin cytoplasmic tail, because only minimal colocalization was observed between IL-2R or IL-2R-Dsg3cyto and clathrin (Figure 3, A, i′–p′, and B). Furthermore, the IL-2R-VE-cadcyto did not colocalize with caveolin-1 (Figure 3, A, q′–t′, and B), providing additional evidence that VE-cadherin is internalized through a clathrin-dependent pathway. Significant colocalization was also observed between IL-2R-VE-cadcyto and AP-2 (Figure 3, C, e′–h′, and D), but not between IL-2R and AP-2 (Figure 3, C, a′–d′, and D) or IL-2R-Dsg3cyto and AP-2 (data not shown). These data indicate that VE-cadherin associates with clathrin and AP-2 and localizes to membrane domains enriched in both proteins during internalization.
Figure 3.
The VE-cadherin cytoplasmic tail specifically colocalizes with clathrin and AP-2. MECs expressing IL-2R, IL-2R-VE-cadcyto, or IL-2R-Dsg3cyto were labeled for 30 min at 4°C with IL-2R antibodies, transferred to 37°C for 5 min, and then processed for immunofluorescence microscopy. As a positive control, internalization of transferrin receptor was monitored by labeling MECs with fluorescently conjugated transferrin at 4°C, transferring cells to 37°C for 5 min, and then processing for immunofluorescence microscopy. (A and B) Colocalization of transferrin, IL-2R, IL-2R-VE-cadcyto, or IL-2R-Dsg3cyto with clathrin or caveolin was monitored. Colocalization was quantified as the percentage of transferrin, IL-2R, IL-2R-VE-cadcyto, or IL-2R-Dsg3cyto that colocalize with clathrin or caveolin, using Metamorph software. (C and D) Colocalization of IL-2R and IL-2R-VE-cadcyto with AP-2 was also measured. Colocalization was quantified as the percentage of transferrin, IL-2R, or IL-2R-VE-cadcyto that colocalize with AP-2. Error bars, SEM; n = 25 cells. Scale bar, 20 μm.
p120 Prevents VE-Cadherin from Entering a Clathrin- and AP-2–enriched Membrane Domain
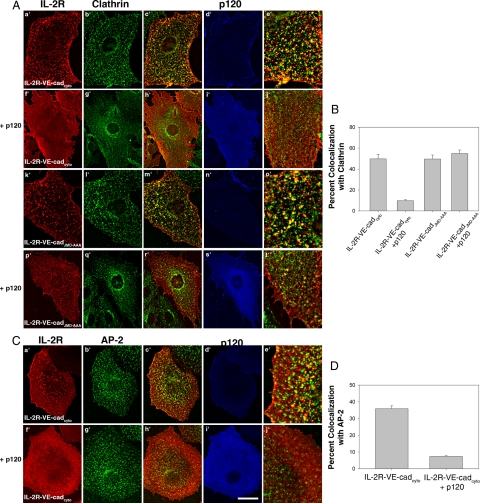
To test the hypothesis that p120 inhibits the entry of VE-cadherin into membrane domains enriched in clathrin and AP-2, we monitored the localization of the IL-2R-VE-cadcyto chimera in cells expressing exogenous p120. Interestingly, in cells expressing exogenous p120 the IL-2R-VE-cadcyto chimera did not form punctate clusters as seen in control cells (Figure 4, A and C, a′–j′). Additionally, colocalization between the IL-2R-VE-cadcyto chimera and clathrin or AP-2 was dramatically reduced in these cells (Figure 4, B and D). To test whether this function of p120 requires its interaction with the juxtamembrane domain (JMD) of VE-cadherin, we expressed a mutant IL-2R-VE-cadcyto construct that contains a triple alanine substitution in the p120-binding site of VE-cadherin (IL-2R-VE-cadJMD-AAA; Xiao et al., 2005). This mutation completely abrogates the binding of p120 to VE-cadherin (Calkins et al., 2003). The IL-2R-VE-cadJMD-AAA displays a punctate localization and colocalizes with clathrin after incubation at 37°C in cells containing endogenous levels of p120 (Figure 4A, k′–o′). However, unlike the wild-type IL-2R-VE-cadcyto chimera, the distribution and colocalization of the IL-2R-VE-cadJMD-AAA chimera was not affected by exogenous expression of p120 (Figure 4, A, p′–t′, and B). These results indicate that p120 prevents VE-cadherin from localizing to clathrin- and AP-2–enriched membrane domains in a manner that requires its binding to the VE-cadherin JMD.
Figure 4.
Exogenous expression of p120 prevents IL-2R-VE-cadcyto from colocalizing with clathrin and AP-2 in a manner dependent on the interaction of p120 with the VE-cadherin JMD. (A) p120 was coexpressed in MECs with either IL-2R-VE-cadcyto or IL-2R-VE-cadJMD-AAA, which is unable to bind to p120, and internalization assays were conducted as described in Figure 3. Cells were fixed and processed for triple-label immunofluorescence to monitor the IL-2R-VE-cadherin polypeptides, p120, and clathrin. (B) Colocalization was quantified as the percentage of IL-2R-VE-cadcyto or IL-2R-VE-cadJMD-AAA that colocalize with clathrin. In the presence of p120, colocalization between IL-2R-VE-cadcyto and clathrin is greatly reduced. High levels of colocalization between IL-2R-VE-cadJMD-AAA and clathrin in cells expressing exogenous p120 demonstrate that p120 must bind to the VE-cadherin JMD to prevent VE-cadherin recruitment into clathrin-enriched membrane domains. (C) Colocalization between IL-2R-VE-cadcyto and AP-2 was also monitored. In the presence of p120, IL-2R-VE-cadcyto colocalization with AP-2 is dramatically reduced (D). Error bars, SEM; n = 25 cells. Scale bar, 20 μm.
p120 Inhibits VE-Cadherin Endocytosis Independently of RhoA
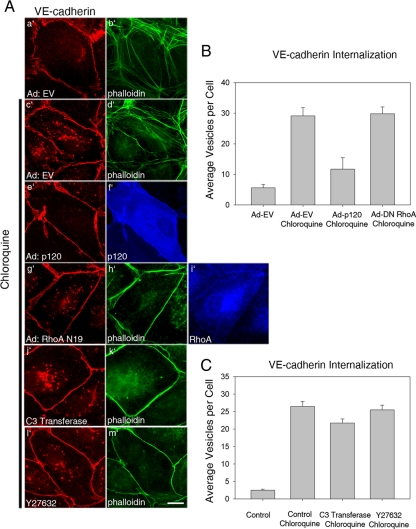
In addition to its central function in regulating cadherin expression at adherens junctions, an important role has also been established for p120 in regulating Rho GTPases, particularly as an inhibitor of RhoA activity. To determine if this function of p120 is integral to its ability to stabilize VE-cadherin at the plasma membrane by preventing VE-cadherin endocytosis, we utilized several methods to inhibit Rho activity in endothelial cells and measured the effect on internalization of endogenous VE-cadherin. As expected, expression of exogenous p120 inhibited the accumulation of intracellular VE-cadherin after chloroquine treatment (Figure 5A, e′ and f′). Expression of a dominant negative RhoA mutant (N19; Figure 5A, g′– i′), treatment with the selective Rho inhibitor C3 toxin (Figure 5A, j′ and k′) or treatment with Y27632 (Figure 5A, l′ and m′), an inhibitor of ROCK, a downstream Rho effector, were effective in reducing RhoA activity within endothelial cells, as indicated by the dramatic loss of actin stress fibers. However, none of these treatments had any discernable impact on internalization of VE-cadherin in chloroquine-treated cells (Figure 5, B and C), suggesting that inhibition of RhoA does not phenocopy the activity of p120.
Figure 5.
Inhibition of RhoA activity does not inhibit VE-cadherin endocytosis. (A) MECs were infected with empty adenovirus (a′–d′), virus expressing p120 (1A; e′ and f′), or a RhoA dominant negative mutant (N19; g′-i′). RhoA activity was also inhibited by treating cells with a cell-permeable C3 transferase (j′–k′) or Y27632 (l′ and m′), an inhibitor of Rho kinase. Accumulation of vesicular VE-cadherin was measured in untreated or chloroquine-treated cells as described in Figure 1, and inhibition of Rho activity was verified by loss of stress fibers as detected by Alexa Fluor 488—conjugated phalloidin (b′, d′, h′, k′, and m′). The number of VE-cadherin–containing vesicles was quantified as shown in B and C. Error bar, SEM; n = 50 cells.
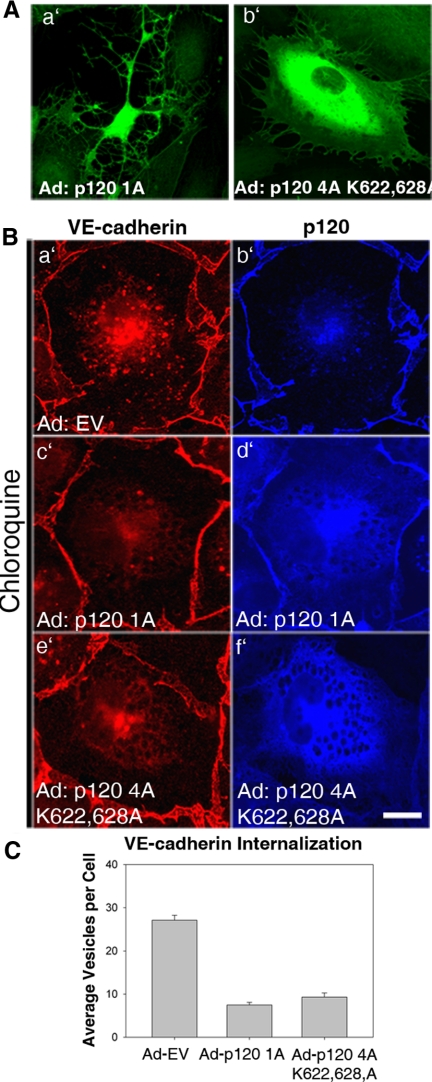
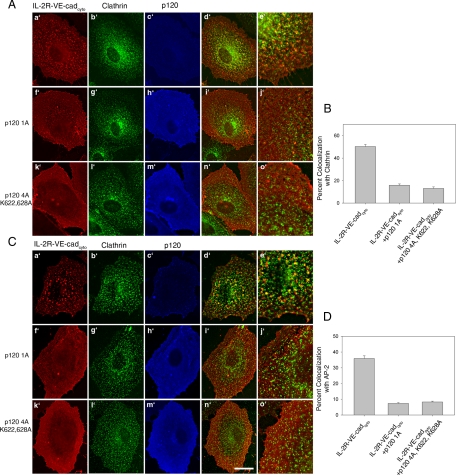
To look specifically at the role of local p120-dependent regulation of RhoA on VE-cadherin stability, we tested the effects of a p120 mutant that is unable to inhibit RhoA activity. The p120 mutant lacks the N-terminal regulatory domain and also contains mutations within the Rho-binding domain (p120 4A K622,628A). Deletion of these domains eliminates p120's ability to inhibit RhoA activity (Castano et al., 2007; Yanagisawa et al., 2008). In MECs expressing high levels of wild-type p120, cells exhibit a dramatic branching phenotype characterized by dendritic outgrowths, which have been shown to result from loss of RhoA activity (Figure 6Aa′). No cells with this branching phenotype could be found in cells expressing the p120 Rho-uncoupled mutant, even at very high levels (Figure 6Ab′). Next, internalization of endogenous VE-cadherin in cells expressing either wild-type p120 (Figure 6B, c′ and d′) or the Rho-uncoupled p120 mutant (Figure 6B, e′ and f′) was assessed. Both wild-type p120 and the Rho-uncoupled p120 mutant significantly reduced the amount of internalized VE-cadherin (Figure 6C). To determine if p120 Rho-uncoupled mutant can block the association of VE-cadherin with clathrin and the AP-2 adaptor, we measured colocalization between the IL-2R-VE-cadcyto and clathrin (Figure 7A) or AP-2 (Figure 7C) in cells expressing either wild-type (Figure 7, A and C, f′–j′) or mutant p120 (Figure 7, A and C, k′–o′). The p120 Rho-uncoupled mutant was able to prevent colocalization between the IL-2R-VE-cadcyto and clathrin (Figure 7B) and AP-2 (Figure 7D) similarly to wild-type p120. Together, these findings suggest that p120 stabilizes VE-cadherin at the plasma membrane by preventing the cadherin from entering a clathrin- and AP-2–dependent endocytic pathway. The mechanism by which p120 functions in this manner requires its interaction with the VE-cadherin cytoplasmic tail, but occurs independently of RhoA activity.
Figure 6.
A p120 Rho-uncoupled mutant is effective at preventing VE-cadherin endocytosis. (A) Wild-type p120 1A or a RhoA-uncoupled mutant (p120 4A K622,628A) were expressed in MECs. Note dendritic appearance of cells expressing wild-type but not mutant p120. (B) Internalization of VE-cadherin was measured in MECs expressing EV (a′ and b′), p120 1A (c′ and d′), or the RhoA-uncoupled p120 4A K622,628A mutant (e′ and f′) by treating cells with chloroquine for 3 h. Total intracellular levels of VE-cadherin were quantified as shown in C. VE-cadherin internalization was dramatically reduced in cells expressing either exogenous wild-type p120 or the RhoA-uncoupled p120 mutant. Error bars, SEM; n = 100 cells. Scale bar, 20 μm.
Figure 7.
A p120 Rho-uncoupled mutant prevents entry of VE-cadherin into membrane compartments containing clathrin and AP-2. (A and C) MECs were infected with adenovirus carrying IL-2R-VE-cadcyto and either p120 1A or p120 4A K622,628A. Cells were then processed for immunofluorescence to determine the extent of colocalization between IL-2R-VE-cadcyto and clathrin (A) or AP-2 (C) in cells expressing either wild-type or Rho-uncoupled p120 (p120 4A K622,628A). Colocalization was quantified as the percentage of IL-2R-VE-cadcyto that colocalize with clathrin or AP-2. The RhoA-uncoupled p120 mutant was as effective at preventing colocalization of IL-2R-VE-cadcyto as wild-type p120 (B and D). Error bars, SEM; n = 25 cells. Scale bar, 20 μm.
DISCUSSION
The results presented in this study demonstrate that p120 stabilizes VE-cadherin at the plasma membrane by preventing its entry into a clathrin-, AP-2–, and dynamin-dependent endocytic pathway through a mechanism that does not involve RhoA. These studies identify the endocytic machinery involved in VE-cadherin endocytosis and help to clarify the mechanism by which p120 functions as a set point to regulate cadherin expression levels. Additionally, they establish a novel role for p120 as part of a plasma membrane retention mechanism that modulates the availability of a cargo protein for recruitment into membrane-trafficking pathways.
In previous studies, we demonstrated that the cytoplasmic domain of VE-cadherin mediates rapid endocytosis of the protein and that p120 prevents VE-cadherin from being internalized (Xiao et al., 2005). However, these previous studies did not fully explain how p120 functions to stabilize the cadherin at the plasma membrane. In the present study, we found that internalization of endogenous VE-cadherin or an IL-2R-VE-cadcyto chimera was reduced by inhibition of clathrin-, AP-2–, or dynamin-dependent endocytosis (Figure 1). After a short period of internalization, a significant fraction of the IL-2R-VE-cadcyto chimera colocalizes with clathrin and AP-2 (Figure 3), providing further evidence that VE-cadherin is internalized through a clathrin-mediated pathway. We were also able to specifically immunoprecipitate the IL-2R-VE-cadcyto with antibodies against clathrin and the clathrin adaptor AP-2 (Figure 2). Together, these results provide the first evidence for a role for the AP-2 adaptor complex in the trafficking of VE-cadherin through a clathrin-mediated pathway.
Expression of exogenous p120 dramatically inhibits internalization of VE-cadherin during the earliest stages of endocytosis. The IL-2R-VE-cadcyto colocalizes extensively with clathrin and AP-2. However, in cells expressing exogenous p120, the IL-2R-VE-cadcyto fails to colocalize with clathrin or AP-2. Importantly, the interaction of p120 with the VE-cadherin JMD is crucial for p120's ability to prevent VE-cadherin from clustering with components of the clathrin machinery (Figure 4). These data support previous findings that p120 prevents VE-cadherin endocytosis and indicate that p120 acts to stabilize VE-cadherin at the plasma membrane at an early step in endocytosis, most likely by preventing adaptor binding and entry into a clathrin-enriched membrane domain.
In addition to functioning as a critical regulator of adherens junction stability, p120 also plays a key signaling role in regulating activity of Rho-GTPases (Anastasiadis, 2007). The relationship between the function of p120 in regulating RhoA activity and its role in stabilizing cadherins at cell junctions is not understood. The data presented in this study indicate that p120 regulates cadherin stability at the plasma membrane independently of Rho activity. Internalization of VE-cadherin was not impacted by inhibition of Rho activity by either genetic or pharmacological manipulation (Figure 5). Similarly, a p120 mutant that is unable to inhibit Rho activity retains its ability to prevent VE-cadherin endocytosis (Figure 6) and also prevents the IL-2R-VE-cadcyto from colocalizing with clathrin and AP-2 (Figure 7). Importantly, these data indicate that VE-cadherin endocytosis occurs independently of Rho activity and that p120 stabilizes VE-cadherin at the cell surface through a mechanism that does not require its Rho inhibitory function.
On the basis of our findings that the function of p120 in preventing VE-cadherin endocytosis can be uncoupled from the ability of p120 to inhibit RhoA, we hypothesize that p120 prevents the VE-cadherin cytoplasmic tail from interacting with components of the endocytic machinery that selectively recruit transmembrane receptors into a clathrin-mediated pathway. The identification of a complex containing VE-cadherin, clathrin, and AP-2, together with the inhibitory effect of silencing AP-2 expression on the internalization of VE-cadherin, demonstrates an important role for the AP-2 adaptor complex in the regulation of VE-cadherin endocytosis. In general, clathrin interacts with transmembrane cargo through multiple adaptor and accessory proteins (Bonifacino and Traub, 2003; Robinson, 2004; Sorkin, 2004). Recently, vascular endothelial growth factor (VEGF)-induced internalization of VE-cadherin was found to involve β-arrestin (Gavard and Gutkind, 2006), a clathrin adaptor that often works cooperatively with AP-2 to regulate clathrin-dependent endocytosis. Studies are currently underway to further characterize the interaction between VE-cadherin and AP-2, to identify additional adaptor proteins that may be required for VE-cadherin endocytosis and to determine if p120 prevents them from interacting with the VE-cadherin cytoplasmic tail.
The ability of p120 to prevent VE-cadherin from entering domains enriched in clathrin and AP-2 in a manner that requires p120 binding to the VE-cadherin juxtamembrane domain further supports a model in which p120 functions as a cap on the VE-cadherin cytoplasmic tail to prevent adaptor interactions. An alternative model is that p120 stabilizes cadherins at the plasma membrane by inhibiting the activity of another component of the endocytic machinery. On the basis of this hypothesis, one would predict that p120 may function as a general inhibitor of clathrin-dependent endocytosis. Thus far, we have found no evidence that this is the case. p120 has no effect on internalization of transferrin receptor or epidermal growth factor receptor (EGFR; Xiao et al., 2005, our unpublished observation). Additionally, the requirement for p120 binding to the cadherin tail for the inhibitory effect suggests that p120's function is cadherin-specific. Therefore, we currently favor a model in which the association of p120 with the VE-cadherin JMD prevents the clathrin adaptor AP-2 from recruiting VE-cadherin into clathrin-coated pits.
Endocytic adaptor proteins play a fundamental role in the recruitment and formation of clathrin-coated vesicles at the plasma membrane. The recruitment of transmembrane cargo receptors into clathrin-coated vesicles by AP-2 and the formation of a stable network of interactions between cargo, adaptors, and clathrin is a key step in vesicle formation (Maldonado-Báez and Wendland, 2006). For these reasons, the regulation of the interaction between adaptor proteins and cargo molecules is crucial to the process of clathrin-coated vesicle formation and trafficking. The ability of p120 to prevent cadherin recruitment into clathrin- and AP-2–enriched membrane domains reveals a previously unappreciated mechanism by which clathrin-mediated endocytosis is regulated. It remains to be determined whether this mechanism for endocytic regulation is unique to the cadherin tail-p120 interaction or if other receptors and clathrin cargo are similarly regulated by cytoplasmic-binding partners.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Dr. Kathleen Green and members of the Kowalczyk lab for their help and advice and Dr. Branch Craige for assistance with the cross-linking and immunoprecipitation assays. We are grateful for reagents provided by Dr. Klaus Fruh (Vaccine and Gene Therapy Institute, Oregon Health and Science University, Beaverton, OR), Dan Kalman, Albert Reynolds, Sandra Schmid, and Dr. Gary Thomas (The Vollum Institute, Portland, OR). This work was supported by National Institute of Health Grants R01AR050501 (A.P.K.) and R01HL077870 (P.A.V.). C.M.C. was supported by a predoctoral fellowship from the American Heart Association.
Abbreviations used:
- AP-2
adaptor complex 2
- DN DynII
dominant negative dynamin II
- IL-2R
interleukin-2 receptor
- JMD
juxtamembrane domain
- MEC
human microvascular endothelial cell
- p120
p120 catenin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0735) on February 11, 2009.
REFERENCES
- Altschuler Y., Barbas S. M., Terlecky L. J., Tang K., Hardy S., Mostov K. E., Schmid S. L. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis P. Z. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Anastasiadis P. Z., Moon S. Y., Thoreson M. A., Mariner D. J., Crawford H. C., Zheng Y., Reynolds A. B. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Calkins C. C., Hoepner B. L., Law C. M., Novak M. R., Setzer S. V., Hatzfeld M., Kowalczyk A. P. The armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J. Biol. Chem. 2003;278:1774–1783. doi: 10.1074/jbc.M205693200. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Castano J., Solanas G., Casagolda D., Raurell I., Villagrasa P., Bustelo X. R., Garcia de Herreros A., Dunach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol. Cell. Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattelino A., et al. The conditional inactivation of the β-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson C. M., Kowalczyk A. P. Cadherin trafficking and junction dynamics. In: LaFlamme S., Kowalczyk A., editors. Cell Junctions: Adhesion, Development, and Disease. Weinheim: Wiley-VCH; 2008. pp. 251–270. [Google Scholar]
- Craige B., Salazar G., Faundez V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol. Biol. Cell. 2008;19:1415–1426. doi: 10.1091/mbc.E07-12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A., Ireton R. C., Reynolds A. B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E., Jennings J. M., Calkins C. C., Kottke M. D., Faundez V., Kowalczyk A. P. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin- and dynamin-independent mechanism. J. Biol. Chem. 2008;283:18303–18313. doi: 10.1074/jbc.M710046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E., Kowalczyk A. P. Regulation of cadherin trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. T., Peifer M. Cell Adhesion: separation of p120's powers? Curr. Biol. 2007;17:R24–R27. doi: 10.1016/j.cub.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Gavard J., Gutkind J. S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Gory-Faure S., Prandini M. H., Pointu H., Roullot V., Pignot-Paintrand I., Vernet M., Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta Biomembranes. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D., Gomperts S. N., Hardy S., Kitamura M., Bishop J. M. Ras family GTPases control growth of astrocyte processes. Mol. Biol. Cell. 1999;10:1665–1683. doi: 10.1091/mbc.10.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani M. G., Orsenigo F., Gagliani M. C., Tacchetti C., Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Báez L., Wendland B. Endocytic adaptors: recruiters, coordinators and regulators. Trends Cell Biol. 2006;16:505–513. doi: 10.1016/j.tcb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mayor S., Pagano R. E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren N. K., Liu B. P., Burridge K., Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Weis W. I. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Reynolds A. B., Daniel J. M., Mo Y.-Y., Wu J., Zhang Z. The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp. Cell Res. 1996;225:328–337. doi: 10.1006/excr.1996.0183. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Salazar G., Gonzalez A. Novel mechanism for regulation of epidermal growth factor receptor endocytosis revealed by protein kinase A inhibition. Mol. Biol. Cell. 2002;13:1677–1693. doi: 10.1091/mbc.01-08-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G., Zlatic S., Craige B., Peden A. A., Pohl J., Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol-4-kinase type II alpha in neuronal and non-neuronal cells. J. Biol. Chem., 2009;284:1790–1802. doi: 10.1074/jbc.M805991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Ungewickell E. J., Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr. Opin. Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Urs N. M., Kowalczyk A. P., Radhakrishna H. Different mechanisms regulate lysophosphatidic acid (LPA)-dependent versus phorbol ester-dependent internalization of the LPA1 receptor. J. Biol. Chem. 2008;283:5249–5257. doi: 10.1074/jbc.M710003200. [DOI] [PubMed] [Google Scholar]
- Venkiteswaran K., Xiao K., Summers S., Calkins C. C., Vincent P. A., Pumiglia K., Kowalczyk A. P. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am. J. Physiol. Cell Physiol. 2002;283:C811–821. doi: 10.1152/ajpcell.00417.2001. [DOI] [PubMed] [Google Scholar]
- Vestweber D. VE-Cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta Biomembranes. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Rothberg K. G., Anderson R. G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg G. A., Dohn M. R., Carnahan R. H., Davis M. A., Lobdell N. A., Settleman J., Reynolds A. B. p120-Catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Xiao K., Allison D. F., Buckley K. M., Kottke M. D., Vincent P. A., Faundez V., Kowalczyk A. P. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Garner J., Buckley K. M., Vincent P. A., Chiasson C. M., Dejana E., Faundez V., Kowalczyk A. P. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Oas R. G., Chiasson C. M., Kowalczyk A. P. Role of p120-catenin in cadherin trafficking. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Huveldt D., Kreinest P., Lohse C. M., Cheville J. C., Parker A. S., Copland J. A., Anastasiadis P. Z. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastatic disease. J. Biol. Chem. 2008;283:18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.