Abstract
The proper execution of premeiotic S phase is essential to both the maintenance of genomic integrity and accurate chromosome segregation during the meiotic divisions. However, the regulation of premeiotic S phase remains poorly defined in metazoa. Here, we identify the p21Cip1/p27Kip1/p57Kip2-like cyclin-dependent kinase inhibitor (CKI) Dacapo (Dap) as a key regulator of premeiotic S phase and genomic stability during Drosophila oogenesis. In dap−/− females, ovarian cysts enter the meiotic cycle with high levels of Cyclin E/cyclin-dependent kinase (Cdk)2 activity and accumulate DNA damage during the premeiotic S phase. High Cyclin E/Cdk2 activity inhibits the accumulation of the replication-licensing factor Doubleparked/Cdt1 (Dup/Cdt1). Accordingly, we find that dap−/− ovarian cysts have low levels of Dup/Cdt1. Moreover, mutations in dup/cdt1 dominantly enhance the dap−/− DNA damage phenotype. Importantly, the DNA damage observed in dap−/− ovarian cysts is independent of the DNA double-strands breaks that initiate meiotic recombination. Together, our data suggest that the CKI Dap promotes the licensing of DNA replication origins for the premeiotic S phase by restricting Cdk activity in the early meiotic cycle. Finally, we report that dap−/− ovarian cysts frequently undergo an extramitotic division before meiotic entry, indicating that Dap influences the timing of the mitotic/meiotic transition.
INTRODUCTION
During the meiotic cycle, germ cells complete two divisions to produce haploid gametes. Before the two meiotic divisions, the germ cells duplicate their genomes during the premeiotic S phase. Events unique to the premeiotic S phase, such as the expression of REC8, a member of the kleisin family of structural maintenance of chromosome proteins, are required for the full execution of the downstream meiotic program (Watanabe and Nurse, 1999; Watanabe et al., 2001; Strich, 2004). How this specialized meiotic S phase is regulated, as well as how similar it is to the mitotic S phase, has long been a question of interest. Studies from yeast indicate that the mitotic cycle and the meiotic cycle use much of the same basic machinery to replicate their genomes (reviewed in Strich, 2004). For example, the minichromosome maintenance complex (MCM2-7), which functions as a DNA replication helicase, is essential for the duplication of the genome during both the mitotic and premeiotic S phase (Murakami and Nurse, 2001; Lindner et al., 2002). Additionally, both the mitotic and premeiotic S phase require the activity of cyclin-dependent kinases (Cdks) (Stuart and Wittenberg, 1998; Bell and Dutta, 2002a; Benjamin et al., 2003). Yet, despite its fundamental importance to both the maintenance of genomic integrity and the downstream events of meiosis, little is known about the regulation of premeiotic S phase metazoa.
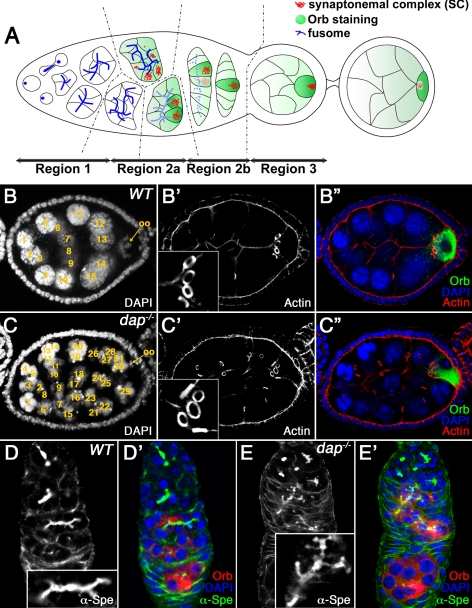
Drosophila provides an excellent model to examine the early events of the meiotic cycle, because the entire process of oogenesis takes place continuously within the adult female. In Drosophila, each ovary is composed of 12–16 ovarioles containing linear strings of maturing follicles also called egg chambers. New egg chambers are generated at the anterior of the ovariole in a region called the germarium that contains both germline and somatic stem cells. The germarium is divided into four regions according to the developmental stage of the cyst (Figure 1A). Oogenesis starts in region 1 when a cystoblast, the asymmetric daughter of the germline stem cell, undergoes precisely four round of mitosis with incomplete cytokinesis to produce a cyst of 16 interconnected germline cells with an invariant pattern of interconnections (individual cells in the cyst are referred to as cystocytes). Stable actin-rich intercellular bridges called ring canals connect individual cystocytes within the cyst (Robinson and Cooley, 1996). Germline cyst formation is accompanied by the growth of the fusome, a vesicular and membrane skeletal protein-rich organelle that forms a branched structure extending throughout all the cells of the cyst (Figure 1A; de Cuevas et al., 1997; McKearin, 1997). After the completion of the mitotic cyst divisions, all 16 cystocytes complete a long premeiotic S phase in region 2a of the germarium (Carpenter, 1981). Subsequently, the two cystocytes with four ring canals form long synaptonemal complexes (SCs) and begin to condense their chromatin, suggesting that they are in pachytene of meiotic prophase I (Figure 1A; Carpenter, 1975). Several of the cells with three ring canals also assemble short SCs, and even cells with only one or two ring canals are occasionally seen to contain traces of SCs. However, as oogenesis proceeds, the SC is restricted to the two pro-oocytes and finally to the single oocyte in region 2b (Carpenter, 1975; Huynh and St Johnston, 2000). The other 15 cystocytes lose their meiotic characteristics, enter the endocycle and develop as polyploid nurse cells.
Figure 1.
dap mutants undergo extraovarian cyst divisions. (A) Schematic representation of the germarium. See text for details. The regions of the germarium (1, 2a, 2b, and 3) are indicated at the bottom. Germ cell cyst formation is accompanied by growth of the fusome (blue dots and lines), which forms a branched structure extending through all the ring canals. In midregion 2a, the SC (red) is assembled in the two pro-oocytes, which progress to pachytene. In region 2a, cytoplasmic proteins, such as Orb (green), accumulate in the germline cyst and are progressively restricted to the oocyte. (B) Wild-type and (C) dap−/− mutant egg chambers, stained with DAPI (B, C; B″, C″, blue), rhodamine-conjugated phalloidin (B′, C′; B″, C″, red), and α-Orb antibody (B″, C″, green). The oocyte is indicated by an arrow. Note that the dap−/− mutant oocyte in C″, marked by the α-Orb staining, is surrounded by five ring canals. (D) Wild-type and (E) dap−/− mutant germaria, stained with α-α-Spe (D, E; D′, E′, green) and α-Orb (D′, E′, red) antibodies and DAPI (D′, E′, blue). The inset in D corresponds to a fusome from a 16-cell cyst. Note that the dap−/− mutant fusome in E, inset, is larger and more branched and corresponds to a 32-cell cyst. Insets in D and E are shown at the same magnification.
During both the mitotic cycle and the meiotic cycle, it is essential that the entire genome is duplicated precisely once during the S phase. In the mitotic cycle, the licensing of the DNA occurs when Cdc6 and Cdt1/Double Parked (Dup) load the MCM2-7 complex onto the origin recognition complex (ORC) to form the prereplication complex (preRC) (reviewed in Bell and Dutta, 2002b; DePamphilis et al., 2006). PreRC formation occurs in late mitosis and G1 when Cdk activity is low. At the onset of S phase, Cdk activity increases, and the preRC initiates bidirectional DNA replication. PreRC formation must be suppressed after the initiation of S phase to prevent rereplication and thus ensure that each segment of the DNA is replicated exactly once per cell cycle (Bell and Dutta, 2002b; DePamphilis et al., 2006). Cdks play a critical role in this process by preventing reestablishment of the preRC through multiple redundant mechanisms (Drury et al., 1997; Fujita et al., 2002; Mendez et al., 2002). Thus, during the mitotic cycle the precise regulation of Cdk activity ensures that each segment of DNA is replicated once, and only once, per cell cycle.
The p21cip1/p27kip1/p57kip2-like cyclin-dependent kinase inhibitor (CKI) Dacapo (Dap) specifically inhibits Cyclin E/Cdk2 complexes (de Nooij et al., 1996; Lane et al., 1996). In Drosophila, Cyclin E/Cdk2 activity is required for DNA replication during both mitotic cycles and endocycles (Knoblich et al., 1994; Lilly and Spradling, 1996). Similar to what is observed with CKIs in other animals, Dap functions to coordinate exit from the cell cycle with terminal differentiation (de Nooij et al., 1996; Debec et al., 1996). Indeed, high levels of Dap are observed upon exit from the cell cycle in multiple tissues during both embryonic and larval development (de Nooij et al., 1996; Lane et al., 1996; de Nooij et al., 2000; Liu et al., 2002b). Additionally, in the adult ovary, high levels of Dap prevent oocytes from entering the endocycle with the nurse cells as ovarian cysts exit the germarium in stage 1 of oogenesis (Hong et al., 2003). However, in addition to its well-established developmental function, recent work indicates that during developmentally programmed endocycles Dap facilitates the licensing of DNA replication origins by reinforcing low Cyclin E/Cdk2 kinase activity during the Gap phase (Hong et al., 2007). In dap−/− mutants, cells undergoing endocycles have reduced chromatin bound MCM2-7 complex, indicating a reduction in the density of preRCs along the chromatin. Additionally, dap−/− cells accumulate high levels of DNA damage due to the inability to complete genomic replication (Hong et al., 2007). Thus, during developmentally programmed endocycles Dap functions to reinforce low Cdk activity during the Gap phase.
Here, we demonstrate that the CKI Dap promotes genomic stability during the premeiotic S phase of the Drosophila oocyte. Our data indicate that Dap facilitates the licensing of DNA replication origins for the premeiotic S phase by restricting Cyclin E/Cdk2 activity during the early meiotic cycle. These studies represent the first example of a CKI regulating premeiotic S phase and genomic stability in a multicellular animal. Additionally, we find that Dap influences the timing of the mitotic/meiotic switch in ovarian cysts.
MATERIALS AND METHODS
Fly Stocks
The FRT42B, dap4 stock (lane et al., 1996) was a gift of Iswar Hariharan (University of California, Berkeley, CA). hs-FLP1, w1118; Adv1/CyO, y1 w1/Dp(1;Y) y+; mei-P22P22; svspa-pol and w1118; FRT42B ubi-GFP were obtained from the Bloomington Stock Center (University of Indiana, Bloomington, IN). dupa1 (Whittaker et al., 2000b) was a gift of Terry Orr-Weaver (Massachusetts Institute of Technology, Cambridge, MA). The dap4, dupa1 chromosomes were generated by meiotic recombination (Hong et al., 2007). Homozygous dap4 mutant germline cell clones were generated by FLP/FLP recombinase target (FRT)-mediated site-specific recombination (Xu and Rubin, 1993).
Immunocytochemistry
Immunocytochemistry of adult ovary staining was performed as described in McKearin and Ohlstein (1995). For 5-bromo-2′-deoxyuridine labeling, ovaries were dissected and stained according to Calvi and Lilly (2004). 5-Ethynyl-2′-deoxyuridine (EdU) incorporation and labeling were done using the Click-it EdU Imaging kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The following antibodies were used in this study: mouse monoclonal α-MPM2 (1:50; Dako North America, Carpinteria, CA), rabbit polyclonal α-C(3)G (1:3000; Hong et al., 2003), mouse monoclonal α-green fluorescent protein (GFP) (1:200; Roche Diagnostics, Indianapolis, IN), rabbit polyclonal α-GFP (1:500; Invitrogen), mouse monoclonal α-Orb 6H4 (1:50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), mouse monoclonal α-α-Spectrin 3A9 (1:20; Developmental Studies Hybridoma Bank), mouse monoclonal α-proliferating cell nuclear antigen (PCNA) PC10 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA). Guinea pig α-Dup/Cdt1 (1:1000; Whittaker et al., 2000a) was provided by Terry Orr-Weaver. Rabbit α-Dup/Cdt1 (1:200) was kindly provided by Michael Botchan (University of California, Berkeley, CA). Rabbit polyclonal α-α-Spectrin (1:1000; Byers et al., 1987) was a gift of Ron Dubreuil (University of Illinois, Chicago, IL). Mouse monoclonal α-C(3)G (1:500; Anderson et al., 2005) was provided by R. Scott Hawley (Stowers Institute, Kansas City, MO). Mouse monoclonal α-CycE 8B10 and rat polyclonal α-CycE (1:10 and 1:100, respectively; Richardson et al., 1995) were a gift of H. E. Richardson (Peter McCallum Cancer Centre, Melbourne, Australia). Rabbit polyclonal α-γ-H2Av antibodies (1:3000 and 1:500) were provided by Bob Glaser (Wadsworth Center, Albany, NY) and Kim McKim (Waksman Institute, Piscataway, NJ), respectively (Leach et al., 2000; Mehrotra and McKim, 2006). Numbers shown in Tables 1 and 2 were obtained using the rabbit α-γ-H2Av antibody from Bob Glaser. Fluorescence-conjugated secondary antibodies were purchased from Invitrogen and were used at a 1:800 dilution. Actin was labeled with rhodamine-conjugated phalloidin (Invitrogen) at 0.1 μg/ml, and DNA was labeled with 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO) at 1 μg/ml. All samples were mounted in cytofluor (University of Kent, Canterbury, Canterbury, United Kingdom). Samples were examined with a FluoWiew FV1000 microscope (Olympus, Tokyo, Japan).
Table 1.
Average number of γ-H2Av foci in the two pro-oocytes (region 2a) and oocyte (region 2b)
| Region 2a | Early region 2b | Late region 2b | Region 3 | |
|---|---|---|---|---|
| CantonS | 1.5 ± 1.5 | 13.5 ± 2.0 | 5.5 ± 4.7 | 0.2 ± 0.4 |
| dap4/dap4 | 22.1 ± 5.1† | 30.1 ± 8.2† | 28.7 ± 5.2† | 35.0 ± 4.2† |
| dap4, dupa1/dap4/+ | 26.1 ± 6.2‡ | 40.9 ± 10.6‡ | 47.3 ± 9.1‡ | 41.5 ± 6.1 |
For each sample, n is >25.
† p < 0.05 in comparison with wild type,
‡ p < 0.05 in comparison with dap4/dap4.
Table 2.
Average number of γ-H2Av foci per cystocyte (excluding the pro-oocytes and oocytes)
| Region 2a | Early region 2b | Late region 2b | Region 3 | |
|---|---|---|---|---|
| CantonS | 0.6 ± 0.7 | 0.5 ± 0.6 | 0.9 ± 0.8 | 0.2 ± 0.5 |
| dap4/dap4 | 5.8 ± 1.2† | 11.3 ± 1.2† | 11.7 ± 2.8† | 14.3 ± 1.5† |
| dap4, dupa1/dap4/+ | 8.7 ± 1.7‡ | 13.2 ± 3.6‡ | 18.1 ± 3.4‡ | 22.5 ± 6.7‡ |
For each sample, n is >8.
† p < 0.05 in comparison with wild type,
‡ p < 0.05 in comparison with dap4/dap4.
Statistical Analysis
Data were analyzed using a Student's t test. Data were considered statistically significant when p was <0.05.
RESULTS
dap−/− Ovarian Cysts Delay Meiotic Entry
Relative to yeast, little is known about how Cdks influence early meiotic progression in multicellular organisms. In Drosophila, the levels of the G1 Cyclin, cyclin E, influence the number of mitotic divisions that occur before meiotic entry (Lilly and Spradling, 1996; Doronkin et al., 2003; Ohlmeyer and Schupbach, 2003). In wild-type ovaries, a cystoblast undergoes four synchronous mitotic divisions to produce an ovarian cyst with 16 interconnected cells. However, mutations that decrease Cyclin E levels in the female germline result in a reduced number of mitotic cysts divisions and the production of four- or eight-cell cysts (Lilly and Spradling, 1996). Conversely, mutations that raise the levels of Cyclin E protein increase the number of mitotic divisions resulting in 32-cell cysts (Doronkin et al., 2003; Ohlmeyer and Schupbach, 2003). Thus, in the female germ line, the Cyclin E/Cdk2 activity is precisely regulated as cells exit the mitotic cycle and enter the meiotic cycle.
To define the pathways that control Cyclin E/Cdk2 activity during oogenesis, we examined whether the CKI Dap influences the cell cycle program of premeiotic and/or early meiotic ovarian cysts. Consistent with Dap functioning to inhibit Cyclin E/Cdk activity before meiotic entry, 25% of dap−/− ovarian cysts undergo a fifth mitotic cyst division to produce egg chambers with 32 cells (n = 102, Figure 1C and Supplemental Figure 1). Importantly, dap−/− egg chambers with 32 cells contain only a single oocyte, as indicated by the preferential accumulation of the oocyte marker Orb in a single cell (Figure 1C″). Additionally, in egg chambers with 32 cells, the oocyte always contains five ring canals, demonstrating that the cyst has undergone five divisions (Figure 1C′, inset, and C″). Finally, in dap−/− mutants the fusome, a germline specific organelle that connects all the cells within an individual cyst, often contains extra branches (Figure 1E, inset). Together, these data confirm that the dap−/− egg chambers containing 32 germline cells are the result of an extramitotic division, rather than the fusion of two 16-cell cysts. Thus, mutations in the CKI dap phenocopy mutations that increase Cyclin E levels in the female germ line. These data demonstrate that dap is a component of the pathway that regulates the transition from the mitotic to the meiotic cycle and further support the role of Cyclin E/Cdk2 activity in determining the number of mitotic cyst divisions that occur before meiotic entry.
Dap Inhibits Cyclin E/Cdk2 Activity in Early Ovarian Cysts
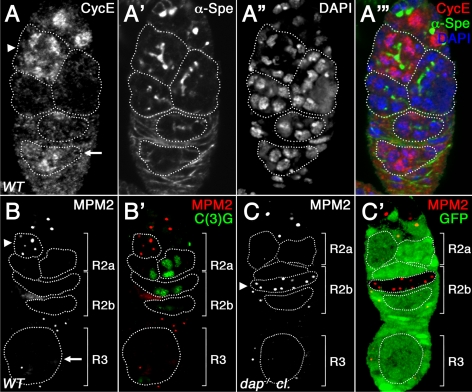
The increased number of ovarian cyst divisions observed in dap−/− mutants is consistent with increased Cyclin E/Cdk2 activity before meiotic entry (Lilly and Spradling, 1996; Lilly et al., 2000; Ohlmeyer and Schupbach, 2003). Therefore, to assess Cyclin E/Cdk2 activity in wild-type versus dap−/− ovarian cysts, we used the α-mitotic protein monoclonal-2 (MPM2) antibody (Davis et al., 1983). The α-MPM2 antibody, raised against a phospho-epitope from human mitotic cells, detects Cyclin E/Cdk dependent staining of the histone locus body in Drosophila (Calvi et al., 1998; White et al., 2007). During the mitotic cyst divisions, α-MPM2–positive histone loci bodies are observed as small foci in a fraction of dividing cysts, consistent with the oscillation of Cyclin E/Cdk2 activity during the ovarian cyst divisions (Figure 2B, arrowhead). After the completion of the mitotic cyst divisions, all 16 cystocytes enter into premeiotic S phase in early region 2a of the germarium (Carpenter, 1975). We find that ovarian cysts in early region 2a are often Cyclin E positive (Figure 2A, A′′′, arrowhead) and contain MPM2-positive foci (Supplemental Figure 2, arrowhead). However, when cysts enter prophase of meiosis I and construct an SC in late region 2a and into early region 2b, we observe no MPM2-positive histone loci bodies, suggesting that the level of Cyclin E/Cdk2 activity has fallen (Figure 2B′). Finally, in region 3 of the germarium, coincident with a burst of Cyclin E expression that accompanies the asynchronous entry of the nurse cells into the first endocycle (Figure 2, A and A′′′, arrow), a fraction of nurse cells again have α-MPM2–positive foci (Figure 2B, arrow). In contrast to the developmentally dynamic α-MPM2 expression observed in wild-type ovarian cysts, in dap−/− mutant germaria all ovarian cysts, at all stages of development, contain α-MPM2–positive foci (Figure 2C, arrowhead; data not shown). These results strongly suggest that in dap−/− mutants, the baseline level of Cyclin E/Cdk activity is increased both before and after ovarian cysts enter the meiotic cycle. Additionally, these data suggest that the dynamics of Cyclin E oscillations are altered in the dap−/− background or that the low levels of Cyclin E protein present in region 2b and region 3 of the germarium are sufficient to activate Cdk2 in the absence of the inhibitor Dap.
Figure 2.
dap mutants have increased Cyclin E/Cdk2 activity. (A) Wild-type germarium, labeled with α-Cyclin E (A; A′′′, red) and α-α-Spe antibodies (A′; A′′′, green) and DAPI (A″; A′′′, blue). Cyclin E is expressed in the mitotically active cysts in region 1 and in 16-cell cysts in early region 2a (number of cells in the cyst is determined by the fusome branching pattern, arrowhead), is absent from late region 2a and region 2b, and reappears in region 3 as the nurse cells enter the endocycle (arrow). (B) Wild-type and (C) dap−/− mutant clones stained with α-MPM2 (B, C; B′, C′, red), α-C(3)G (B′, green), and α-GFP (C′, green) antibodies. dap−/− clones are identified by the absence of α-GFP staining. Note that MPM2 and C(3)G are never expressed in the same cysts. (C) In region 2b of dap−/− ovarian cysts, all cells have α-MPM2 positive histone loci bodies (arrowhead).
dap−/− Ovarian Cysts Have Increased γ-H2Av Staining
The extramitotic cyst division, as well as the dramatically increased number of ovarian cysts with α-MPM2–positive histone loci bodies, suggest that dap−/− ovarian cysts have inappropriately high cyclin E/Cdk2 activity as they enter meiosis. We wanted to determine whether the increased Cdk activity observed in dap−/− ovarian cysts, influences progression through the premeiotic S phase. In mammals and yeast, deregulated Cdk activity during the mitotic cycle inhibits the formation of preRCs, often resulting in genomic instability (Spruck et al., 1999; Lengronne and Schwob, 2002; Tanaka and Diffley, 2002; Ekholm-Reed et al., 2004). This genomic instability stems from inappropriately high Cdk activity during G1, reducing the number of licensed origins assembled along the chromatin before S phase. Ultimately, the low density of DNA replication origins leads to intraorigin distances that are too large to be transversed by DNA polymerase during a single S phase. This causes DNA replication forks to stall and ultimately collapse, resulting in DNA damage and the production of double-stranded breaks (DSBs) (Spruck et al., 1999; Tanaka and Diffley, 2002; Ekholm-Reed et al., 2004).
To determine whether the increased Cyclin E/Cdk2 activity observed in dap−/− ovarian cysts results in DNA damage during the premeiotic S phase, we used an anti-phosphoprotein antibody specific for phosphorylated H2Av (γ-H2Av) (Madigan et al., 2002). One of the earliest responses to DNA damage is the phosphorylation of H2A histone variants near the sites of DSBs (Modesti and Kanaar, 2001; Madigan et al., 2002). In the Drosophila ovary, meiotic DSBs are generated after the initiation of SC formation in late region 2a of the germarium (Carpenter, 1975; Jang et al., 2003; Mehrotra and McKim, 2006). Accordingly, γ-H2Av nuclear foci are first observed in the two pro-oocytes, which are in early pachytene, in late region 2a (Jang et al., 2003; Mehrotra and McKim, 2006 and Figure 3A, arrowhead). Additionally, a small number of DSB are also observed in the pronurse cells cysts in late region 2a (Table 2). As meiosis proceeds and the DSBs are repaired, γ-H2Av–positive foci are reduced and ultimately disappear in late region 2b (Jang et al., 2003; Figure 3A, arrow). By germarial region 3, there is no detectable γ-H2Av signal in the oocyte (Figure 3A), whereas γ-H2Av staining is again observed in nurse cells as they begin to endoreplicate their DNA and become polyploid (Mehrotra and McKim, 2006; Hong et al., 2007; Figure 3A, asterisk).
Figure 3.
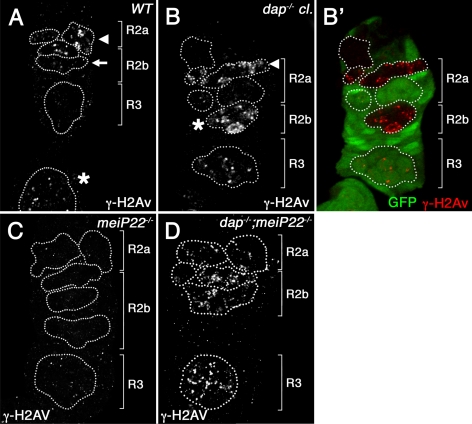
γ-H2Av foci accumulate in dap−/− ovarian cysts and are independent of meiotic DSBs. Wild-type (A), dap−/− mutant clones (B), mei-P22P22/P22 (C), and dap−/−; mei-P22−/− double mutant germaria (D), stained with α-γ-H2Av (A, B, C, D; B′, red) and α-GFP (B′, green) antibodies. dap−/− clones are identified by the absence of α-GFP staining. (A) In wild-type, γ-H2Av foci are restricted to region 2a of the germarium, where meiotic DSBs form, whereas in B, dap−/− mutant cysts they increase in number (B, arrowhead) and persist into region 2b (B, asterisk). (D) The DNA damage induced in dap−/− cysts is not suppressed in a mei-P22−/− mutant background.
Beginning in region 2a of the germarium, which marks the onset of meiosis, we find that in dap−/− ovarian cysts, the two pro-oocytes have at least 3 times as many γ-H2Av foci as observed in similarly staged wild-type cysts (Table 1). Additionally, in dap−/− mutants the γ-H2Av foci persist into late region 2b and region 3 (Figure 3, B and B′). In wild-type cysts, the majority of DSBs are repaired by region 3 of the germarium, with only an occasional focus observed in wild-type oocytes (0.2 γ-H2Av foci observed, Table 1; Mehrotra and McKim, 2006). In contrast, in dap−/− mutants, oocytes retain ∼30 γ-H2Av foci in region 2b and region 3 (Figure 3B, asterisk, and Table 1). The number of DSBs, as measured by γ-H2Av staining, is also increased in the pronurse cells in dap−/− mutants. In wild-type ovaries, there is less than one γ-H2Av foci per pronurse cell in cysts from region 2a and region 2b of the germarium (Table 2; Mehrotra and McKim, 2006). However, in dap−/− cysts this number increases greater than 10-fold, with ∼12 and 14 γ-H2Av foci found in pronurse cells from region 2b and region 3, respectively (Table 2 and Figure 3B, asterisk). Thus, in dap−/− germaria, ovarian cysts have an increased number of γ-H2Av foci and therefore increased levels of DNA damage in both the pro-oocyte and pronurse cells.
Are the increased levels of DNA damage found in dap−/− ovarian cysts simply due to the inability of the mutants to repair the DSBs that initiate meiotic recombination? To address this question, we examined γ-H2Av staining in dap4, mei-P22P22 double-mutant females. mei-P22 is required for DSB formation during meiosis (Liu et al., 2002a). As demonstrated previously, we find that γ-H2Av–positive foci are not observed in mei-P22 ovarian cysts (Liu et al., 2002a; Klattenhoff et al., 2007; Figure 3C). In contrast, dap4, mei-P22P22 double-mutant ovarian cysts retain large numbers of γ-H2Av foci (Figure 3D). These results demonstrate that the DNA damage observed in dap−/− ovarian cysts is independent of the production of the DSBs that initiate meiotic recombination.
dap−/− Ovarian Cysts Accumulate DNA Damage during Premeiotic S Phase
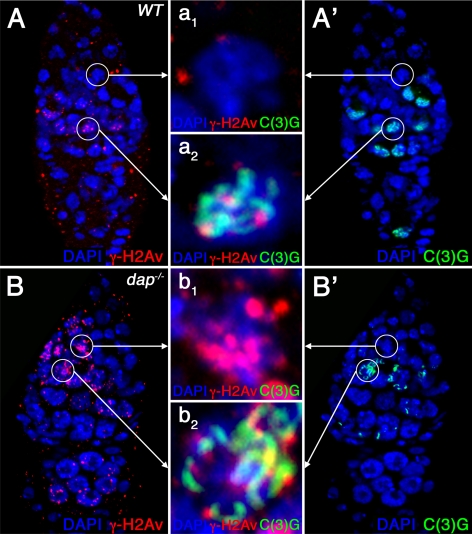
The observation that dap−/− cysts accumulate DNA damage independently of meiotic DSB formation, as well the more uniform accumulation of γ-H2Av staining throughout the cyst, suggest that in dap−/− ovaries DNA damage may be induced during the premeiotic S phase. In Drosophila, the production of meiotic DSBs occurs after the assembly of the mature SC in prophase of meiosis I (Carpenter, 1975; Jang et al., 2003; Mehrotra and McKim, 2006). Therefore, to establish the timing of DSB formation in dap−/− ovarian cysts, we double stained wild-type and dap−/− ovaries with antibodies against the SC component C(3)G and γ-H2Av (Page and Hawley, 2001). In wild-type ovaries, γ-H2Av foci are absent in the earliest region 2a cysts that contain at most small patches of SC staining (zygotene stage, Figure 4, A–A1). Indeed, γ-H2Av foci are not observed until SC formation seems complete in the two pro-oocytes (pachytene stage, Figure 4, A–A2) as visualized by C(3)G staining (McKim et al., 1998; Jang et al., 2003; Mehrotra and McKim, 2006). In contrast, in dap−/− germaria, α-γ-H2Av staining is often observed in the earliest cysts in region 2a, well before the appearance of α-C(3)G staining (Figure 4, B–B1). Importantly, in both wild-type and mutant ovaries, γ-H2Av foci are not observed during the mitotic cyst divisions in region 1 of the germarium (Figure 3, A and B). Thus, in dap−/− ovarian cysts α-γ-H2Av staining is first observed after the completion of the mitotic cyst divisions but before the construction of the mature SC. These data are consistent with dap−/− mutants incurring DNA damage during the premeiotic S phase.
Figure 4.
DNA damage occurs before SC formation in dap−/− ovarian cysts. Wild-type (A) and dap−/− mutant germaria (B) labeled with α-γ-H2Av (A, B, red) and α-C(3)G (A′, B′, green) antibodies and DAPI (A–B′, blue). The magnified images in the middle (a1–b2) show C(3)G protein (green) and γ-H2Av foci (red) (a1 and b1) in one cell of a 16-cell cyst in early region 2a before the appearance of α-C(3)G staining and in one of the two pro-oocytes in region 2a after the appearance of α-C(3)G staining (a2 and b2). Note that whereas no α-γ-H2Av staining is seen in a1, wild-type 16-cell cysts of region 2a before the appearance of C(3)G, (b1) dap−/− mutant cells of the same region show several γ-H2Av foci. In meiotic region 2a, when C(3)G protein is expressed, an increased number of γ-H2Av foci are observed in dap−/− mutant pro-oocytes (b2) compared with wild type (a2).
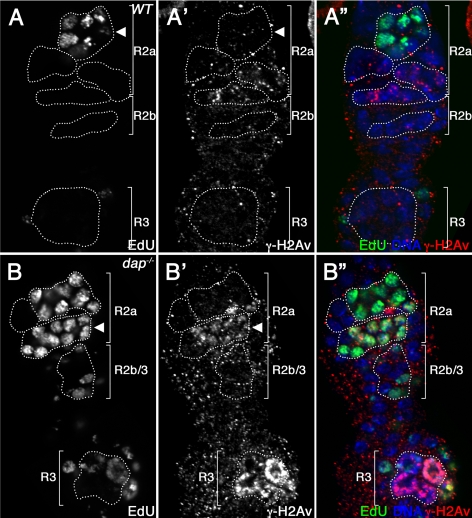
To determine directly whether DNA damage occurs during premeiotic S phase, we colabeled wild-type and mutant ovaries with antibodies against γ-H2Av and the nucleotide analogue EdU. As reported previously, in wild-type females meiotic DSBs are generated after the initiation of SC formation in late region 2a of the germarium (Mehrotra and McKim, 2006). Accordingly, we find that in wild-type germaria, EdU incorporation always occurs in 16-cell cysts before the appearance of γ-H2Av foci. Therefore, in wild-type ovaries, we do not observe ovarian cysts that are positive for both EdU and γ-H2Av (Figure 5, A–A″, arrowhead). In contrast, in dap−/− ovaries γ-H2Av foci accumulate in region 2a before the completion of the premeiotic S phase. Thus, in dap−/− mutants a fraction of ovarian cysts in region 2 of the germarium colabel with EdU and γ-H2Av (Figure 5, B–B″, arrowhead). To confirm that DNA damage accumulates during the premeiotic S phase, we used an antibody against another S phase marker, PCNA. The eukaryotic DNA polymerase processivity factor PCNA is an essential component of the DNA replication and repair machinery and can be used to label cells in the S phase (Celis and Celis, 1985; Eissenberg et al., 1997; Maga and Hubscher, 2003; Kisielewska et al., 2005). As is observed with EdU incorporation, in wild-type ovaries PCNA is always expressed in 16-cell cysts before the appearance of γ-H2Av foci (n = 23; Supplemental Figure 3, A–A″). In contrast, 16% of dap−/− cysts in early region 2a are colabeled with antibodies against both PCNA and γ-H2Av (n = 25; Supplemental Figure 3, B–B″, arrowhead). These observations confirm that dap−/− ovarian cysts accumulate DNA damage during the premeiotic S phase.
Figure 5.
DNA damage accumulates during the premeiotic S phase in dap−/− cysts. Wild-type (A) and dap−/− mutant germaria (B) stained with EdU (A, B; A″, B″, green) and α-γH2Av (A′, B′; A″, B″, red) antibodies and DAPI (A″, B″, blue). Note that in A, wild-type, 16-cell cysts in region 2a of the germarium never colabel with antibodies against both EdU and γ-H2Av. In contrast, a fraction of B dap−/− cysts in region 2a incorporate EdU and contain γ-H2Av foci (B, B′, arrowhead).
Consistent with a lengthening of the premeiotic S phase, in dap−/− mutants an increased proportion of germaria contain at least one EdU-positive 16-cell cyst. Specifically, although 24% (n = 50) of control germaria contain an EdU-positive 16-cell cyst, in dap−/− ovaries this number is increased to 62% of germaria (n = 31). Additionally, in dap−/− germaria, EdU-positive 16-cell cysts are often present in late region 2a and in region 2b (Figure 5B). In contrast, in wild type germaria, EdU-positive 16-cell cysts are restricted to early region 2a. These data are consistent with dap−/− ovarian cysts having a prolonged premeiotic S phase that extends into the later stages of development. Importantly, we recognize that because 25% of dap−/− ovarian cysts undergo a fifth mitotic division to produce 32-cell cysts, one would predict an increase in the number of 16-cell cysts that incorporate EdU. However, the 25% of ovarian cysts that undergo a fifth mitotic division are unlikely to account for the greater than twofold increase in the number of 16-cell cysts that incorporate EdU in dap−/− germaria. Thus, we believe the simplest explanation for this data are that in dap−/− ovaries the premeiotic S phase is extended.
Dap Promotes the Accumulation of Dup/Cdt1 before Premeiotic S Phase
Why might mutations in the CKI dap result in DNA damage during the premeiotic S phase? PreRCs are built by sequential binding at the DNA origins of ORC, Cdt1/Dup and Cdc6, and the MCM2-7 complex (Whittaker et al., 2000b; Bell and Dutta, 2002a). The regulation of preRC assembly is tightly controlled and is an important mechanism by which DNA replication is restricted to a single round per cell cycle (Blow and Dutta, 2005; Machida and Dutta, 2005). Deregulated Cdk activity inhibits adequate preRC formation and resulting in DNA damage and genomic instability (Spruck et al., 1999; Lengronne and Schwob, 2002; Tanaka and Diffley, 2002; Ekholm-Reed et al., 2004). Thus, one model to explain the DNA damage observed in dap−/− ovarian cysts is that inappropriately high Cdk activity inhibits preRC assembly before premeiotic S phase.
In Drosophila, Cyclin E/Cdk2 activity inhibits the accumulation of the preRC component Dup/Cdt1 (Thomer et al., 2004). During the mitotic cycle in somatic cells, Dup/Cdt1 is expressed during the G2, M, and G1 phases and then is rapidly destroyed at the G1/S transition (Whittaker et al., 2000a; Thomer et al., 2004; May et al., 2005). Consistent with these observations, we find that Dup/Cdt1 levels oscillate in mitotically active germline stem cells, cystoblasts, and dividing ovarian cysts. As ovarian cysts enter the meiotic cycle, Dup/Cdt1 accumulates in 16-cell cysts in early region 2a before the initiation of SC formation, shown by C3G expression (Supplemental Figure 4, A and A′, arrowhead). As noted previously, early region 2a of the germarium corresponds to when ovarian cysts enter premeiotic S phase, as indicated by nucleotide incorporation and PCNA expression (Carpenter, 1981; Figure 5A, arrowhead, and Supplemental Figure 3A, arrowhead). Subsequently, in late region 2a and early region 2b, the levels of Dup/Cdt1 protein fall below the level of detection as the cysts enter prophase of meiosis I and progress through the early steps of meiotic recombination (Supplemental Figure 4A, arrows). Thus, Dup/Cdt1 accumulates in 16-cell cysts just before and/or during the premeiotic S phase in early region 2a of the germarium. A possible role for Dup/Cdt1 in licensing DNA replication origins for the premeiotic S phase are consistent with many recent studies indicating that much of the machinery responsible for initiating DNA replication is conserved between the mitotic cycle and the meiotic cycle (Murakami and Nurse, 2001; Lemaitre et al., 2002; Lindner et al., 2002; Ofir et al., 2004).
To examine whether Dap influences the status of Dup/Cdt1 before and during the premeiotic S phase, we compared the expression of Dup/Cdt1 in the first two cysts of region 2a in wild-type versus dap−/− ovaries. From these experiments, we determined that dap−/− ovaries had a fourfold reduction (2.4%; n = 270) in the percentage of early 2a cysts that expressed Dup/Cdt1 relative to wild-type ovaries (10.4%; n = 247; p = 1.8 × 10−4). It is important to note that the relatively low percentage of wild-type ovarian cysts that express Dup/Cdt1 is consistent with the fact that a single germarium rarely contains cysts representing all stages of cyst development. In contrast to meiotic ovarian cysts, the levels of Dup/Cdt1 are not notable altered in the dap−/− background during the mitotic cyst divisions with 20.7% (n = 87) of wild-type ovarian cysts expressing Dup/Cdt1 versus 17.9% (n = 86) of dap−/− mutant cysts. Thus, the levels of Dup/Cdt1 present before the meiotic S phase are specifically reduced in the dap−/− background. Together, our results indicate that Dap promotes the accumulation of the licensing factor Dup/Cdt1 before and/or during the premeiotic S phase in region 2a.
Dup/Cdt1 Is Limiting in dap−/− Ovarian Cysts
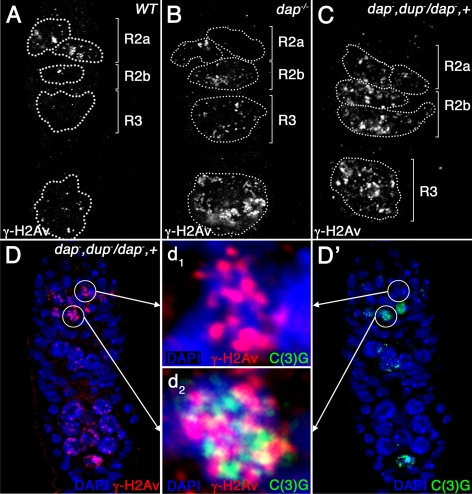
We have shown that dap−/− ovarian cysts have low levels of the replication-licensing factor Dup/Cdt1. However, it was unclear whether the low levels of Dup/Cdt1 contribute to the observed DNA damage phenotype. To determine whether Dup/Cdt1 levels are limiting during the premeiotic S phase, we removed one copy of dup/cdt1 and compared the number of γ-H2Av foci in dap−/− versus dap−, dupa1/dap−, + ovarian cysts. From these studies, we found an increased number of γ-H2Av foci in dap−, dupa1/dap−, + double mutant ovarian cysts compared with dap−/− single mutant ovarian cysts (Figure 6 and Tables 1 and 2). Thus, dup/cdt1 dominantly enhances the DNA damage phenotype observed in early ovarian cysts. These data support the model that the accumulation of DSBs in dap−/− ovarian cysts, during and soon after the premeiotic S phase, is the result of the inadequate preRC formation due to low levels of Dup/Cdt1. Intriguingly, removing one copy of dup/cdt1 in the dap−/− background does not enhance the cyst division phenotype, with dap−/− (31 ± 6.4%) and dap−, dupa1/dap−, + (24 ± 4.2%) ovaries containing similar percentages of 32 cell cysts. These data strongly suggest that the extramitotic cyst division is not a direct result of reducing the number of preRCs assembled before premeiotic S phase.
Figure 6.
dup/cdt1 dominantly enhances the DNA damage phenotype in dap−/− ovarian cysts. Wild-type (A), dap−/− mutant (B), and dap−, dupa1/dap−, + mutant germaria (C and D) stained with α-γ-H2Av (A–C; D, red) and C(3)G (D′, green) antibodies. The magnified images in the middle (d1 and d2) show C(3)G protein (green) and γ-H2Av foci (red) in one cell of a 16-cell cyst in early region 2a before the appearance of α-C(3)G staining (d1) and in one of the two pro-oocytes in region 2a after the appearance of α-C(3)G staining (d2). Note that γ-H2Av foci are increased in dap−, dupa1/dap−, + cysts (C and D) compared with wild-type (A) and dap−/− (B) cysts.
DISCUSSION
The production of a mature gamete requires the precise duplication of the genome during the premeiotic S phase. However, the regulation of premeiotic S phase remains poorly understood in metazoa. Here, we demonstrate that the regulation of Cyclin E/Cdk activity by the CKI Dap is an important factor influencing both meiotic entry and the execution of the premeiotic S phase in Drosophila ovarian cysts.
Cells in the mitotic cycle and the meiotic cycle face a similar challenge. To maintain the integrity of the genome, they must replicate their DNA once, and only once, during the S phase. In mitotic cells, this goal is accomplished, at least in part, through the precise regulation of Cdk activity throughout the cell cycle (Bell and Dutta, 2002b; DePamphilis et al., 2006). During the mitotic cycle, Cdk activity inhibits preRC formation (Bell and Dutta, 2002b; DePamphilis et al., 2006). This inhibitory relationship, restricts the assemble of preRCs to a short window from late mitosis to G1, when Cdk activity is low, and provides an important mechanism by which mitotic cells prevent DNA rereplication. However, the inhibitory effect of Cdk activity on preRC assembly necessitates that cells have a strictly defined period of low Cdk activity before S phase, to assemble preRCs for the next round of DNA replication. In mammals and yeast, compromising this period of low Cdk activity by overexpression G1 cyclins results in decreased replication licensing and genomic instability (Spruck et al., 1999; Lengronne and Schwob, 2002; Tanaka and Diffley, 2002; Ekholm-Reed et al., 2004).
One means by which cells inhibit Cdk activity is the expression of CKIs (Harper, 1997). In the mitotic cycle of budding yeast, the deletion of the CKI Sic1, which contains a Cdk inhibitor domain that is structurally conserved with the inhibitor domain present in the dap homologue p27Kip1, results in inadequate replication licensing and genomic instability due to the precocious activation of Cdks in G1 (Nugroho and Mendenhall, 1994; Schneider et al., 1996; Lengronne and Schwob, 2002). Our data strongly suggest that Dap plays a similar role in defining a critical period of low Cdk activity during the early meiotic cycle in Drosophila females.
Based on our results, we propose that the Dap facilitates the licensing of DNA replication origins in ovarian cysts by restricting the inhibitory effects of Cyclin E/Cdk2 kinase activity on preRCs formation before premeiotic S phase. Our data support the model that in the absence of Dap, ovarian cysts enter premeiotic S phase with a reduced number of licensed origins and thus fail to complete genomic replication. This hypothesis is supported by several observations. First, relative to wild-type, dap−/− ovarian cysts spend an increased proportion of their time in premeiotic S phase, as evidenced by the increased proportion of 16-cell cysts that incorporated EdU. The lengthening of premeiotic S phase is in line with the hypothesis that dap−/− ovarian cysts initiate DNA replication from a reduced number of licensed origins. Second, dap−/− ovarian cysts accumulate DNA damage during the premeiotic S phase. The accumulation of DNA damage during the premeiotic S phase is consistent with decreased preRC assembly resulting in intraorigin distances that are too large to be negotiated by DNA polymerase during a single S phase. Third, dap−/− meiotic cysts have decreased levels of the preRC component Dup/Cdt1. Moreover, our genetic analysis indicates that Dup/Cdt1 levels are indeed limiting for premeiotic S phase in the dap−/− background. Specifically, we find that reducing the dose of dup/cdt1 dramatically increases the levels of DNA damage observed in dap−/− ovarian cysts in region 2a and 2b of the germarium. In Drosophila, the levels of Dup/Cdt1 are negatively regulated by Cyclin E/Cdk2 activity (Harper, 1997).
The use of the CKI Dap to restrict Cdk activity and thus promote the formation of preRCs before S phase is observed in multiple cell types beyond the oocyte. In previous work, we found that in dap−/− mutants, cells in developmentally programmed endocycles also accumulate DNA damage and have dramatically reduced levels of Dup/Cdt1 (Hong et al., 2007). Thus, Dap functions to promote the accumulation of Dup/Cdt1 in multiple developmental and cell cycle contexts in Drosophila. Indeed, in select mitotic cycles removing one copy of dup/cdt1 in a dap−/− background results in DNA damage and cell death. However, in most mitotic cycles the requirement for Dap is redundant with other mechanisms that restrict Cyclin E/Cdk2 activity (Hong et al., 2007).
Why Dap is required for preRC assembly in some cell types but not others remains unclear. However, it is interesting to note that DNA replication that occurs outside the confines of the canonical mitotic cycle, during the meiotic S phase and the S phase of developmentally programmed endocycles, is most dependent on Dap function (Hong et al., 2007). Thus, the increased reliance on the CKI Dap to establish a period of low Cdk activity before the onset of DNA replication may be explained by the absence of cell cycle programs that are specific to the mitotic cycle. For example, the tight transcriptional control of S phase regulators during the mitotic cycle may make the presence of Dap unnecessary for proper S phase execution. Alternatively, there may be differential regulation of the machinery that controls the regulated destruction of cyclins in the archetypical mitotic cycle versus the variant cell cycles of meiosis and the endocycle (Narbonne-Reveau et al., 2008; Zielke et al., 2008). In the future, determining why Dap plays a nonredundant role in the regulation of DNA replication during the meiotic cycle, but not the mitotic cycle, will be an important avenue of study.
In addition to its role in the regulation of premeiotic S phase, we find that dap influences the number of mitotic cyst divisions that occur before meiotic entry. In dap−/− mutants, ∼25% of ovarian cysts complete a fifth mitotic division to produce ovarian cysts with 32 cells. Similarly, mutations that compromise the degradation of the Cyclin E protein also result in production of 32-cell cysts (Doronkin et al., 2003; Ohlmeyer and Schupbach, 2003). In line with these observations, females with reduced levels of Cyclin E produce ovarian cysts that undergo only three mitotic divisions and thus contain eight cells (Lilly and Spradling, 1996). Why Cyclin E/Cdk2 activity influences the timing of meiotic entry is not fully understood. However, our data suggest that the cyst division phenotype is not a direct result of reducing the number of preRCs assembled for the premeiotic S phase. Specifically, we find that in dap−/− females reducing the dose of dup/cdt1 does not increase the number of ovarian cysts that undergo an extra division. In contrast, reducing the dose of dup/cdt1 in dap−/− females significantly enhances the meiotic DNA damage phenotype. These data strongly suggest that the extramitotic cyst division observed in dap−/− ovarian cyst is not the direct result of high CyclinE/Cdk2 activity inhibiting preRC formation.
Intriguingly, Cdk2 is not the only Cdk that influences the number of ovarian cyst divisions in Drosophila females. Surprisingly, increasing the activity of the mitotic kinase Cdk1 results in the production of egg chambers with eight-cell cysts (Mata et al., 2000; Sugimura and Lilly, 2006). Moreover, decreased Cdk1 activity results in ovarian cysts undergoing five mitotic divisions to produce egg chambers with 32 cells. Thus, Cdk1 and Cdk2 seem to have opposing roles in the regulation of the ovarian cysts divisions and/or meiotic entry. One of several possible explanations for these data, is that the number of ovarian cyst divisions is influenced by the amount of time cystocytes spend in a particular phase (G1, S, G2, and M) of the cell cycle (Mata et al., 2000). In the mitotic cycle of the Drosophila wing, there is a compensatory mechanism that ensures that changes in the length of one phase of the cell cycle result in alterations in the other phases of the cell cycle to ensure normal division rates (Reis and Edgar, 2004). This compensatory mechanism is likely to be operating in multiple cell types and may account for why Cdk1 and Cdk2 activity have opposite effects on the number of ovarian cyst divisions. Alternatively, Cdk1 and Cdk2 may act on truly independent pathways that have opposing roles in regulating the number of mitotic cyst divisions and/or the timing of meiotic entry. Ultimately, why Cdk1 and Cdk2 activity have opposite effects on the number of ovarian cyst divisions that occur before meiotic entry awaits the identification of essential downstream targets of these kinases.
In summary, we have defined two novel functions for a p21Cip/p27Kip1/p57Kip2-like CKI during the meiotic cycle, the regulation of the mitotic/meiotic transition and the maintenance of genomic stability during the premeiotic S phase.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Botchan, Ron Dubreuil, Bob Glaser, R. Scott Hawley, Kim McKim, Terry Orr-Weaver, Helena Richardson, the Developmental Hybridoma Bank, and the Bloomington Stock Center for Drosophila stocks and antibodies. We also thank Eva Decotto and Michael Lichten for comments on the manuscript. This research was supported by the Intramural Research Program of the Eunice Kennedy-Shriver National Institute of Child Health and Human Development at the National Institutes of Health.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-09-0916) on February 11, 2009.
REFERENCES
- Anderson L. K., Royer S. M., Page S. L., McKim K. S., Lai A., Lilly M. A., Hawley R. S. Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex. Proc. Natl. Acad. Sci USA. 2005;102:4482–4487. doi: 10.1073/pnas.0500172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002a;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002b;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- Benjamin K. R., Zhang C., Shokat K. M., Herskowitz I. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17:1524–1539. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow J. J., Dutta A. Preventing re-replication of chromosomal DNA. Nat. Rev. Mol. Cell Biol. 2005;6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Dubreuil R., Branton D., Kiehart D. P., Goldstein L. S. Drosophila spectrin. II. Conserved features of the alpha-subunit are revealed by analysis of cDNA clones and fusion proteins. J. Cell Biol. 1987;105:2103–2110. doi: 10.1083/jcb.105.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi B. R., Lilly M. A. Fluorescent BrdU labeling and nuclear flow sorting of the Drosophila ovary. Methods Mol. Biol. 2004;247:203–213. doi: 10.1385/1-59259-665-7:203. [DOI] [PubMed] [Google Scholar]
- Calvi B. R., Lilly M. A., Spradling A. C. Cell cycle control of chorion gene amplification. Genes Dev. 1998;12:734–744. doi: 10.1101/gad.12.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T. Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma. 1975;51:157–182. doi: 10.1007/BF00319833. [DOI] [PubMed] [Google Scholar]
- Carpenter A. T. EM autoradiographic evidence that DNA synthesis occurs at recombination nodules during meiosis in Drosophila melanogaster females. Chromosoma. 1981;83:59–80. doi: 10.1007/BF00286016. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc. Natl. Acad. Sci. USA. 1985;82:3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M., Lilly M. A., Spradling A. C. Germline cyst formation in Drosophila. Annu. Rev. Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- de Nooij J. C., Graber K. H., Hariharan I. K. Expression of the cyclin-dependent kinase inhibitor Dacapo is regulated by cyclin E. Mech. Dev. 2000;97:73–83. doi: 10.1016/s0925-4773(00)00435-4. [DOI] [PubMed] [Google Scholar]
- de Nooij J. C., Letendre M. A., Hariharan I. K. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell. 1996;87:1237–1247. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- Debec A., Kalpin R. F., Daily D. R., McCallum P. D., Rothwell W. F., Sullivan W. Live analysis of free centrosomes in normal and aphidicolin-treated Drosophila embryos. J. Cell Biol. 1996;134:103–115. doi: 10.1083/jcb.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Blow J. J., Ghosh S., Saha T., Noguchi K., Vassilev A. Regulating the licensing of DNA replication origins in metazoa. Curr. Opin. Cell Biol. 2006;18:231–239. doi: 10.1016/j.ceb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Doronkin S., Djagaeva I., Beckendorf S. K. The COP9 signalosome promotes degradation of cyclin E during early Drosophila oogenesis. Dev. Cell. 2003;4:699–710. doi: 10.1016/s1534-5807(03)00121-7. [DOI] [PubMed] [Google Scholar]
- Drury L. S., Perkins G., Diffley J. F. The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast. EMBO J. 1997;16:5966–5976. doi: 10.1093/emboj/16.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Ayyagari R., Gomes X. V., Burgers P. M. Mutations in yeast proliferating cell nuclear antigen define distinct sites for interaction with DNA polymerase delta and DNA polymerase epsilon. Mol. Cell. Biol. 1997;17:6367–6378. doi: 10.1128/mcb.17.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm-Reed S., Mendez J., Tedesco D., Zetterberg A., Stillman B., Reed S. I. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J. Cell Biol. 2004;165:789–800. doi: 10.1083/jcb.200404092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Ishimi Y., Nakamura H., Kiyono T., Tsurumi T. Nuclear organization of DNA replication initiation proteins in mammalian cells. J. Biol. Chem. 2002;277:10354–10361. doi: 10.1074/jbc.M111398200. [DOI] [PubMed] [Google Scholar]
- Harper J. W. Cyclin dependent kinase inhibitors. Cancer Surv. 1997;29:91–107. [PubMed] [Google Scholar]
- Hong A., Lee-Kong S., Iida T., Sugimura I., Lilly M. A. The p27cip/kip ortholog dacapo maintains the Drosophila oocyte in prophase of meiosis I. Development. 2003;130:1235–1242. doi: 10.1242/dev.00352. [DOI] [PubMed] [Google Scholar]
- Hong A., Narbonne-Reveau K., Riesgo-Escovar J., Fu H., Aladjem M. I., Lilly M. A. The cyclin-dependent kinase inhibitor Dacapo promotes replication licensing during Drosophila endocycles. EMBO J. 2007;26:2071–2082. doi: 10.1038/sj.emboj.7601648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J. R., St Johnston D. The role of BicD, Egl, Orb and the microtubules in the restriction of meiosis to the Drosophila oocyte. Development. 2000;127:2785–2794. doi: 10.1242/dev.127.13.2785. [DOI] [PubMed] [Google Scholar]
- Jang J. K., Sherizen D. E., Bhagat R., Manheim E. A., McKim K. S. Relationship of DNA double-strand breaks to synapsis in Drosophila. J. Cell Sci. 2003;116:3069–3077. doi: 10.1242/jcs.00614. [DOI] [PubMed] [Google Scholar]
- Kisielewska J., Lu P., Whitaker M. GFP-PCNA as an S-phase marker in embryos during the first and subsequent cell cycles. Biol. Cell. 2005;97:221–229. doi: 10.1042/BC20040093. [DOI] [PubMed] [Google Scholar]
- Klattenhoff C., Bratu D. P., McGinnis-Schultz N., Koppetsch B. S., Cook H. A., Theurkauf W. E. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev. Cell. 2007;12:45–55. doi: 10.1016/j.devcel.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Knoblich J. A., Sauer K., Jones L., Richardson H., Saint R., Lehner C. F. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Lane M. E., Sauer K., Wallace K., Jan Y. N., Lehner C. F., Vaessin H. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell. 1996;87:1225–1235. doi: 10.1016/s0092-8674(00)81818-8. [DOI] [PubMed] [Google Scholar]
- Leach T. J., Mazzeo M., Chotkowski H. L., Madigan J. P., Wotring M. G., Glaser R. L. Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. J. Biol. Chem. 2000;275:23267–23272. doi: 10.1074/jbc.M910206199. [DOI] [PubMed] [Google Scholar]
- Lemaitre J. M., Bocquet S., Mechali M. Competence to replicate in the unfertilized egg is conferred by Cdc6 during meiotic maturation. Nature. 2002;419:718–722. doi: 10.1038/nature01046. [DOI] [PubMed] [Google Scholar]
- Lengronne A., Schwob E. The yeast CDK inhibitor Sic1 prevents genomic instability by promoting replication origin licensing in late G(1) Mol. Cell. 2002;9:1067–1078. doi: 10.1016/s1097-2765(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Lilly M. A., de Cuevas M., Spradling A. C. Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev. Biol. 2000;218:53–63. doi: 10.1006/dbio.1999.9570. [DOI] [PubMed] [Google Scholar]
- Lilly M. A., Spradling A. C. The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996;10:2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- Lindner K., Gregan J., Montgomery S., Kearsey S. E. Essential role of MCM proteins in premeiotic DNA replication. Mol. Biol. Cell. 2002;13:435–444. doi: 10.1091/mbc.01-11-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Jang J. K., Kato N., McKim K. S. mei-P22 encodes a chromosome-associated protein required for the initiation of meiotic recombination in Drosophila melanogaster. Genetics. 2002a;162:245–258. doi: 10.1093/genetics/162.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. H., Li L., Vaessin H. Transcription of the Drosophila CKI gene dacapo is regulated by a modular array of cis-regulatory sequences. Mech. Dev. 2002b;112:25–36. doi: 10.1016/s0925-4773(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Machida Y. J., Dutta A. Cellular checkpoint mechanisms monitoring proper initiation of DNA replication. J. Biol. Chem. 2005;280:6253–6256. doi: 10.1074/jbc.R400037200. [DOI] [PubMed] [Google Scholar]
- Madigan J. P., Chotkowski H. L., Glaser R. L. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 2002;30:3698–3705. doi: 10.1093/nar/gkf496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga G., Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J. Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- Mata J., Curado S., Ephrussi A., Rørth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell. 2000;101:511–522. doi: 10.1016/s0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]
- May N. R., Thomer, Murenen M., K F., Calvi B. R. Levels of the origin-binding protein Double parked and its inhibitor Geminin increase in response to replication stress. J. Cell Sci. 2005;118:4207–4217. doi: 10.1242/jcs.02534. [DOI] [PubMed] [Google Scholar]
- McKearin D. The Drosophila fusome, organelle biogenesis and germ cell differentiation: if you build it. Bioessays. 1997;19:147–152. doi: 10.1002/bies.950190209. [DOI] [PubMed] [Google Scholar]
- McKearin D., Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- McKim K. S., Green-Marroquin B. L., Sekelsky J. J., Chin G., Steinberg C., Khodosh R., Hawley R. S. Meiotic synapsis in the absence of recombination. Science. 1998;279:876–878. doi: 10.1126/science.279.5352.876. [DOI] [PubMed] [Google Scholar]
- Mehrotra S., McKim K. S. Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. PLoS Genet. 2006;2:e200. doi: 10.1371/journal.pgen.0020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J., Zou-Yang X. H., Kim S. Y., Hidaka M., Tansey W. P., Stillman B. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol. Cell. 2002;9:481–491. doi: 10.1016/s1097-2765(02)00467-7. [DOI] [PubMed] [Google Scholar]
- Modesti M., Kanaar R. DNA repair: spot(light)s on chromatin. Curr. Biol. 2001;11:R229–R232. doi: 10.1016/s0960-9822(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Murakami H., Nurse P. Regulation of premeiotic S phase and recombination-related double-strand DNA breaks during meiosis in fission yeast. Nat. Genet. 2001;28:290–293. doi: 10.1038/90142. [DOI] [PubMed] [Google Scholar]
- Narbonne-Reveau K., Senger S., Pal M., Herr A., Richardson H. E., Asano M., Deak P., Lilly M. A. APC/CFzr/Cdh1 promotes cell cycle progression during the Drosophila endocycle. Development. 2008;135:1451–1461. doi: 10.1242/dev.016295. [DOI] [PubMed] [Google Scholar]
- Nugroho T. T., Mendenhall M. D. An inhibitor of yeast cyclin-dependent protein kinase plays an important role in ensuring the genomic integrity of daughter cells. Mol. Cell. Biol. 1994;14:3320–3328. doi: 10.1128/mcb.14.5.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir Y., Sagee S., Guttmann-Raviv N., Pnueli L., Kassir Y. The role and regulation of the preRC component Cdc6 in the initiation of premeiotic DNA replication. Mol. Biol. Cell. 2004;15:2230–2242. doi: 10.1091/mbc.E03-08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmeyer J. T., Schupbach T. Encore facilitates SCF-Ubiquitin-proteasome-dependent proteolysis during Drosophila oogenesis. Development. 2003;130:6339–6349. doi: 10.1242/dev.00855. [DOI] [PubMed] [Google Scholar]
- Page S. L., Hawley R. S. c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev. 2001;15:3130–3143. doi: 10.1101/gad.935001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H., O'Keefe L. V., Marty T., Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Robinson D. N., Cooley L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 1996;6:474–479. doi: 10.1016/0962-8924(96)84945-2. [DOI] [PubMed] [Google Scholar]
- Schneider B. L., Yang Q. H., Futcher A. B. Linkage of replication to start by the Cdk inhibitor Sic1. Science. 1996;272:560–562. doi: 10.1126/science.272.5261.560. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., Won K. A., Reed S. I. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- Strich R. Meiotic DNA replication. Curr. Top. Dev. Biol. 2004;61:29–60. doi: 10.1016/S0070-2153(04)61002-7. [DOI] [PubMed] [Google Scholar]
- Stuart D., Wittenberg C. CLB5 and CLB6 are required for premeiotic DNA replication and activation of the meiotic S/M checkpoint. Genes Dev. 1998;12:2698–2710. doi: 10.1101/gad.12.17.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis T., Edgar B. A. Negative regulation of dE2F1 by cyclin-dependent kinases controls cell cycle timing. Cell. 2004;16:253–264. doi: 10.1016/s0092-8674(04)00247-8. [DOI] [PubMed] [Google Scholar]
- Sugimura I., Lilly M. A. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell. 2006;10:127–135. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Diffley J. F. Deregulated G1-cyclin expression induces genomic instability by preventing efficient pre-RC formation. Genes Dev. 2002;16:2639–2649. doi: 10.1101/gad.1011002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer M., May N. R., Aggarwal B. D., Kwok G., Calvi B. R. Drosophila double-parked is sufficient to induce re-replication during development and is regulated by cyclin E/CDK2. Development. 2004;131:4807–4818. doi: 10.1242/dev.01348. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature. 1999;400:461–464. doi: 10.1038/22774. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Yokobayashi S., Yamamoto M., Nurse P. Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature. 2001;409:359–363. doi: 10.1038/35053103. [DOI] [PubMed] [Google Scholar]
- White A. E., Leslie M. E., Calvi B. R., Marzluff W. F., Duronio R. J. Developmental and cell cycle regulation of the Drosophila histone locus body. Mol. Biol. Cell. 2007;7:2491–2502. doi: 10.1091/mbc.E06-11-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A. J., Royzman I., Orr-Weaver T. L. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 2000a;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]
- Whittaker A. J., Royzman I., Orr-Weaver T. L. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 2000b;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rubin G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zielke N., Querings S., Rottig C., Lehner C., Sprenger F. The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes Dev. 2008;15:1690–1703. doi: 10.1101/gad.469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.