Abstract
Background
Little is known concerning the dietary habits of eating disordered women during pregnancy that may lie in the causal pathway of adverse birth outcomes.
Objective
To examine the nutrient and food group intake of women with bulimia nervosa (BN) and binge eating disorder (BED) during pregnancy and compare their intake to women with no eating disorders.
Design
Data on 30,040 mother-child pairs are from the prospective Norwegian Mother and Child Cohort Study was used in cross-sectional analyses. Dietary information was collected using a food frequency questionnaire during the first half of pregnancy. Statistical testing by eating disorder categories with the non-eating disorder category as the referent group were conducted using log (means) adjusted for confounding and multiple comparisons. Food group differences were conducted using a Wilcoxon two-sided normal approximation test also adjusting for multiple comparisons.
Results
Women with BED before and during pregnancy had higher intakes of total energy, total mono-saturated and saturated fat, and lower intakes of folate, potassium, and vitamin C compared to the referent (p<.02). Women with incident BED during pregnancy had higher total energy and saturated fat intake compared to the referent (p=.01). Several differences emerged in food group consumption between women with and without eating disorders including intakes of artificial sweeteners, sweets, juice, fruits and fats.
Conclusions
Women with BN before and during pregnancy and those with BED before pregnancy exhibit dietary patterns different from women without eating disorders, that are reflective of their symptomatology, and may influence pregnancy outcomes.
Introduction
Eating disorders are prevalent in women of reproductive age with approximately 1.5% of women reporting bulimia nervosa (BN) and 3.5% reporting binge-eating disorder (BED) (1). BN is characterized by eating an unusually large amount of food in a discrete period of time while feeling out of control (i.e., binge eating) coupled with compensatory behaviors such as self-induced vomiting, laxative abuse, excessive exercise or fasting. BED shares the symptoms of binge eating with BN, but in the absence of compensatory behaviors. While eating disorders can have well-documented and potentially severe consequences for a woman’s health, (2, 3) only a moderate amount of literature exists on their effect on the course of pregnancy and birth outcomes. The limited data show an increased risk of miscarriage, hyperemesis, preterm birth, lower birth weight, being small for gestational age, and smaller head circumferences (4–8).
The importance of nutrition during pregnancy has been well documented, with evidence suggesting a protective effect against various adverse birth outcomes for pregnant women following professional recommendations (9–11). For example, during pregnancy women are encouraged to eat an additional 340 kcals per day starting in the second trimester and 452 kcals in the third trimester to meet the demands of the pregnancy state (12). The requirements for many micronutrients are increased as well, such as iron, folate and vitamin C to name a few (13, 14). Given that BN and BED are characterized by erratic eating behaviors, and in BN purging and periods of restriction, one would predict high overall energy intake but low micro-nutrient intakes. However, there have been no prior prospective population-based cohorts of pregnant women in which to study the impact of eating disorders on dietary intake during pregnancy. This analysis was carried out to fill this very important gap in the literature. Using data from the Norwegian Mother and Child Cohort (MoBa) we were able to examine the nutrient and food group intake of women with BN and BED during pregnancy and compare their intake to women who were free of any eating disorder either prior or during pregnancy.
Methods
Design
The data collection was conducted as part of the Norwegian Mother and Child Cohort Study (MoBa) at the Norwegian Institute of Public Health. This is a prospective pregnancy cohort that in the period 1999 to spring 2007 has included approximately 85,000 pregnancies, and that aims to include 100,000 by 2008 (15). Pregnant women are recruited to the study by postal invitation after they have signed up for the routine ultrasound examination in their local hospital. Participants are asked to provide biological samples and to answer questionnaires covering a wide range of information, so far up to age 8 for the child. The cohort database is linked to the Medical Birth Registry of Norway (16). Variables used to describe women in this analysis included maternal age, education, marital status, parity, and region of birth.
The study has been approved by the University of North Carolina, School of Medicine Institutional Review Board, the appropriate regional committees for ethics in medical research and the Norwegian National Data Inspectorate.
Subjects
The number of records available for this analysis was initially 54,714. We excluded MoBa participants who had missing information in the MBR (n=2464), had information from an early test version of Questionnaire 1 (n=2,599), did not have valid values for self-reported age, weight, and height (n=3,078), had returned Questionnaire 1 after delivery (n=152), had multiple births (n=1899) or had a stillbirth (n=232). For those with more than one pregnancy in the study we only included the first pregnancy. Of the 54,714 records available, 75.2% were available at the start for this analysis where each record represents one mother who had one child.
Information for the categorization of women into eating disorders subtypes came from Questionnaire 1 and included items that were previously used for studies of eating disorders in the Norwegian Institute of Public Health Twin Panel (17–20) and were designed in accordance with DSM-IV (21). As previously published (22), diagnostic algorithms were constructed from the questionnaire items to define eating disorder subtypes: broadly defined anorexia nervosa (AN), defined as meeting DSM IV criteria for AN (with the exception of amenorrhea and also endorsing a BMI <19.0 at the time of low weight); broadly defined BN (endorsing at least weekly frequency of binge eating and purging and categorized as BN any type, BN purging type, BN non-purging, type); broadly defined BED (at least weekly frequency of binge eating in the absence of compensatory behaviors), and eating disorders not otherwise specified (EDNOS) with purging (purging at least weekly in the absence of binge eating). Questions for binge eating included both eating an unusually large amount of food and feeling out of control. AN was assessed before pregnancy only due to practical difficulties in determining low weight in the presence of pregnancy-related weight gain. BN, BED, and EDNOS with purging were assessed for both 6 months prior to pregnancy (retrospective assessment) and at the time of survey completion. The respondents were specifically asked to distinguish between pregnancy-related nausea and vomiting and self-induced vomiting as a purging method. Self-reported weight and height were used to calculate pre-pregnancy body mass index (BMI, kg/m2) and BMI at the time of assessment. Respondents completed questionnaire 1 at a median of 18.1 weeks gestation (inter-quartile range 16.7–20.1 weeks).
We created categories of eating disorders that were mutually exclusive representing the status of the women both before and during pregnancy. If a woman was missing information on eating disorders either before or during pregnancy she was excluded (n=3983). We also excluded women with AN and EDNOS with purging before pregnancy due to their small sample sizes, 30 and 38 respectively, and only focused on women with BN and BED n=37,106 (Table 1, column 3). Non-eating disordered women were those who did not report any eating disorder during both time periods.
Table 1.
Categorization of Eating Disorders Before and During Pregnancy Among Women in the Norwegian Mother and Child Cohort (MOBA) Study
| Before Pregnancy | During Pregnancy | Sample Size | Sample Size w/diet | Sample Size excluding outliers |
|---|---|---|---|---|
| Bulimia Nervosa | Bulimia Nervosa | 67 | 59 | 59 |
| Bulimia Nervosa | Binge Eating | 73 | 60 | 56 |
| Bulimia Nervosa | No Eating Disorder | 73 | 58 | 57 |
| Binge Eating | Binge Eating | 779 | 650 | 638 |
| Binge Eating | No Eating Disorder | 488 | 389 | 381 |
| Non-Binge Eating | Binge Eating | 762 | 624 | 615 |
| No Eating Disorder | No Eating Disorder | 34864 | 28200 | 27779 |
| Total | 37,106 | 30,040 | 29,585 |
Assessment of the diet
Dietary intake was assessed using a semi-quantitative food frequency questionnaire (FFQ) including dietary supplements. The FFQ is extensively described in Meltzer et al. (23) while its validation is described in Brantsæter et al. (24–26). The MoBa FFQ was mailed to all participants at gestational weeks 15 – 22. The first version of the questionnaire asked women to reflect on their dietary habits prior to pregnancy; 7066 women in our analytical sample were under this protocol and thus were excluded from the analysis. This left 30,040 (81%) women with complete information on BN and BED and diet that reflected the time period from conception until mid pregnancy (Table 1 column 4). Women answered 255 questions on specific foods and the frequency of use was reported by selecting one out of ten frequencies ranging from never to several times monthly, weekly, or daily. In addition, global questions were asked regarding hot meals and fruit and vegetables and these were used for scaling adjustments. Where portion sizes were not given in the questionnaire, consumption frequencies were converted into food amounts by use of standard Norwegian portion sizes for women. The questionnaires were optically scanned and energy and nutrient intake were calculated with FoodCalc (27) and the Norwegian food composition table (28).
From the total number of food items on the questionnaire (n=255), we aggregated the data into 20 food groups, see Appendix A. For example, all mixed meat products, luncheon meats, liver pate, and organ meats were grouped into one food group called high fat meats and all diet drinks were grouped together. This allowed us to examine food group consumption that was nutritionally meaningful.
Statistics
Data from the MOBA cohort for these analyses are used in a cross-sectional manner. Descriptive statistics including means, medians, and standard errors were calculated by eating disorder categories. Since the nutrient data were not normally distributed, we performed statistical testing by eating disorder categories with the non-ED category as the referent group in all comparisons using a log transformation and adjusting for total energy, maternal education, age, income, and gestational age at the time the questionnaire was completed. For the food group data, certain groups were not commonly consumed by all women, 6 out of 20 food groups had greater than 10% of the population with zero grams consumed. This required us to first estimate if women with eating disorders were more likely to consume from that food group by calculating the odds and 95% CI using logistic regression. We then tested for differences in grams consumed by eating disorder groups among consumers similarly to the commonly eaten foods. For the other 14 food groups that were commonly consumed we tested for food group differences in grams consumed by eating disorders using a Wilcoxon two-sided normal approximation test. We then performed single univariate tests controlling the False Discovery Rate (FDR) with the Benjamini-Hochberg method (29) as a way to control the type I error inflation associated with the multiple testing. In this method of adjustment, there is control of the ratio of false rejections to total number of rejected hypotheses. A P value (<0.05) was considered statistically significant. We assessed the influence of outliers on the robustness of our findings by conducting the analysis both with and without the outliers using cut points of total energy equal to 1076 for the lower and 4777 kcals for the upper bounds (23). SAS® software for Windows and for Solaris (v9.1.3) was used for all the analyses (Cary N. SAS/STAT® Software: Version 9. SAS Institute, Inc; 2004).
Results
The population represented in this analysis (n=30,040) includes pregnant women with a mean gestational age of 20.4 weeks, 49.0% were primiparous, 49.7% reported being married, 95.1% spoke Norwegian, 57.4 % had a combined minimum income of $33–82,000 for the mother and father and 9% <$33,000, and 40% have less than or equal to a high school education, 41.4% 13–16 years of education, and 18.6% >16 years of education. Seventy-six percent reported being employed in either the private or public sector, 9% considered themselves students, and only 6% reported being at home, with 8% indicating “other.” Half of the women reported lifetime smoking, with 10.4% reporting smoking during pregnancy. Using the WHO guidelines to define pregravid weight status (30), 3.0% of the women were classified as underweight, 64.3% as normal, 22.7% as overweight, and 10.0% as obese.
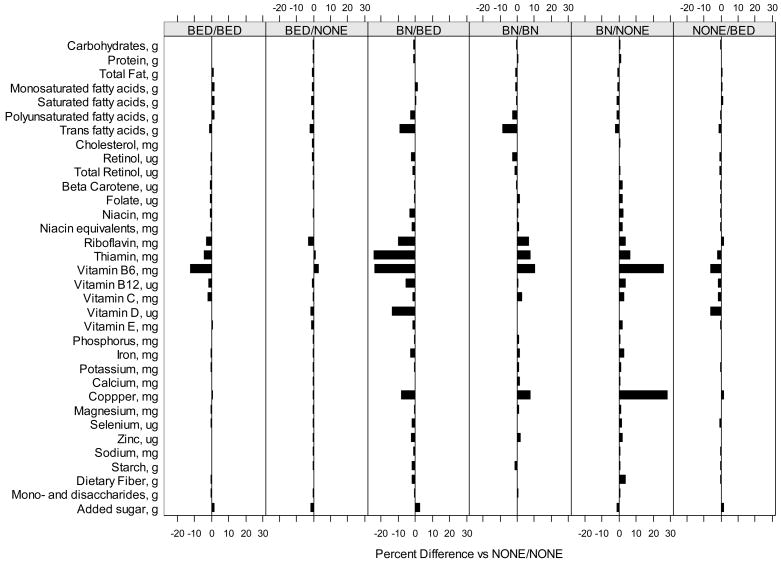
The distribution of nutrient intakes by eating disorder category is presented in Table 2 and percent differences in means adjusted for maternal characteristics and energy intake is illustrated in Figure 1. Women with BED both before and during pregnancy and those with incident BED during pregnancy have higher total energy intake compared to women with no eating disorders (p<0.05). While there appear to be many differences when comparing women with each of the eating disorders to women without eating disorders, after adjusting for total energy, maternal characteristics (age, education, income) and gestational age at the time the questionnaire was completed as well as correcting for multiple comparison testing, few statistically significant differences remained. These differences primarily occurred between women with BED both before and during pregnancy and non-eating disordered women and included: higher intakes of total, mono-saturated and saturated fat and lower intakes of folate, potassium and vitamin C (p<0.02). In addition, women who developed BED during pregnancy had significantly higher saturated fat intake compared to women with no eating disorders (p=0.01). No other significant differences were noted. In sensitivity analysis, excluding women with outliers in energy intake, the results were unchanged with the exception of two additional significant results for women who developed BED during pregnancy with regards to vitamin C and B6 intake (data not shown).
Table 2.
Distribution of Nutrient Intakes by Bulimia Nervosa (BN) and Binge Eating Disorders (BED) of Pregnant Women in the Norwegian Mother and Child Cohort (MOBA) Study n=30,040*
| Nutrients | Eating Disorder Status | |||||||
|---|---|---|---|---|---|---|---|---|
| BN before, BN during | BN before, BED during | BN before, none during | BED before, BED during | BED before, none during | None before, BED during | No eating disorders | ||
| n | 59 | 60 | 58 | 650 | 389 | 624 | 28,200 | |
| Energy, kcal | Mean | 2,253.10 | 2,651.30 | 2,318.70 | 2,459.00* | 2,239.60 | 2,544.10* | 2,348.30 |
| Median | 2,098.00 | 2,302.50 | 2,176.00 | 2,346.00* | 2,143.00 | 2,402.00* | 2,244.00 | |
| SE | 95.9 | 192.1 | 85 | 30.2 | 34 | 41.2 | 4.6 | |
| Energy, kJ | Mean | 9,482.30 | 11,150.90 | 9,751.00 | 10,337.70* | 9,416.70 | 10,699.30* | 9,876.10 |
| Median | 8,820.00 | 9,686.00 | 9,155.50 | 9,883.50* | 9,000.00 | 10,094.50* | 9,437.00 | |
| SE | 404.5 | 809 | 356.7 | 126.9 | 143.2 | 174.3 | 19.2 | |
| Carbohydrates, g | Mean | 307.3 | 360.3 | 316.7 | 327.2 | 302 | 342 | 317 |
| Median | 277.9 | 316.1 | 301.1 | 309.4 | 284.6 | 319.4 | 300.9 | |
| SE | 15.6 | 29.4 | 11.7 | 4.4 | 5.3 | 6.1 | 0.7 | |
| Protein, g | Mean | 86.8 | 93.8 | 88.1 | 89.1 | 83.5 | 92 | 87 |
| Median | 84.4 | 87.5 | 84.6 | 84.3 | 82.5 | 87.9 | 84.2 | |
| SE | 3.2 | 5 | 3.2 | 1 | 1.1 | 1.2 | 0.1 | |
| Total fat, g | Mean | 74.6 | 92 | 77.1 | 87.5* | 76.8 | 89 | 80.8 |
| Median | 72.4 | 83 | 71.7 | 81.8* | 73.4 | 82 | 76.4 | |
| SE | 3.1 | 6.6 | 3.4 | 1.2 | 1.3 | 1.5 | 0.2 | |
| Monounsaturated fatty acids, g | Mean | 23.6 | 30.1 | 24.5 | 27.8* | 24.4 | 28.4 | 25.6 |
| Median | 22.4 | 26 | 23 | 25.9* | 23.2 | 25.9 | 24.1 | |
| SE | 1 | 2.4 | 1.1 | 0.4 | 0.4 | 0.5 | 0.1 | |
| Saturated fatty acids, g | Mean | 29.3 | 36.6 | 29.5 | 34.2* | 29.5 | 34.9* | 31.3 |
| Median | 26.4 | 32.6 | 27.2 | 31.8* | 27.7 | 32.1* | 29.3 | |
| SE | 1.3 | 3 | 1.3 | 0.5 | 0.5 | 0.6 | 0.1 | |
| Polyunsaturated fatty acids | Mean | 13.8 | 16.1 | 14.5 | 16.6 | 14.8 | 16.6 | 15.3 |
| Median | 13.4 | 14.1 | 12.9 | 15.5 | 13.8 | 14.9 | 13.9 | |
| SE | 0.7 | 1 | 0.9 | 0.3 | 0.3 | 0.3 | 0 | |
| Trans fatty acids, g | Mean | 2.1 | 2.5 | 2.3 | 2.5 | 2.2 | 2.5 | 2.4 |
| Median | 2 | 2.3 | 2.1 | 2.2 | 2.1 | 2.3 | 2.1 | |
| SE | 0.1 | 0.2 | 0.1 | 0 | 0.1 | 0.1 | 0 | |
| Cholesterol, mg | Mean | 236.7 | 264.7 | 244.4 | 254.1 | 231.8 | 259.8 | 243.4 |
| Median | 222.4 | 243.4 | 229.0 | 238.3 | 216.7 | 241 | 227.7 | |
| SE | 9.3 | 13.7 | 8.4 | 3.4 | 3.9 | 3.9 | 0.5 | |
| Retinol, ug | Mean | 679.7 | 871.1 | 853.4 | 908.7 | 797.2 | 877.8 | 876.9 |
| Median | 504 | 651 | 652.5 | 681.5 | 605 | 696.5 | 668 | |
| SE | 84 | 83.6 | 82.2 | 28.1 | 30.9 | 27.2 | 4.1 | |
| Total Retinol, ug (1 beta c.=1/6 retinol) | Mean | 1,138.90 | 1,354.60 | 1,363.20 | 1,326.30 | 1,198.10 | 1,324.90 | 1,301.80 |
| Median | 927 | 1,159.00 | 1,126.50 | 1,131.00 | 1,029.00 | 1,128.00 | 1,106.00 | |
| SE | 95 | 86.3 | 106.6 | 31.2 | 35.6 | 31.5 | 4.7 | |
| Beta Carotene, ug | Mean | 2,787.10 | 2,931.70 | 3,101.00 | 2,541.40 | 2,441.50 | 2,720.90 | 2,584.70 |
| Median | 2,156.00 | 2,099.00 | 2,020.00 | 1,950.00 | 1,888.00 | 2,063.50 | 1,999.00 | |
| SE | 287.2 | 268.5 | 328 | 67 | 89.4 | 78.1 | 11.2 | |
| Folate, ug | Mean | 283.5 | 303.8 | 289.2 | 268.9* | 256.2 | 287 | 273.4 |
| Median | 252 | 273 | 256 | 253* | 240 | 265.5 | 257 | |
| SE | 13.2 | 21 | 13.4 | 3.8 | 4.7 | 5.4 | 0.7 | |
| Niacin, mg | Mean | 19.1 | 19.2 | 19.8 | 19.2 | 18.4 | 19.8 | 19 |
| Median | 18.1 | 19 | 18.3 | 18.5 | 17.8 | 19 | 18.5 | |
| SE | 0.7 | 0.7 | 0.7 | 0.2 | 0.3 | 0.3 | 0 | |
| Niacin equivalents, mg | Mean | 30.6 | 31.9 | 31.5 | 30.9 | 29.3 | 31.8 | 30.4 |
| Median | 30 | 29.2 | 29 | 29.8 | 28.9 | 30.7 | 29.6 | |
| SE | 1 | 1.5 | 1.1 | 0.3 | 0.4 | 0.4 | 0 | |
| Riboflavin, mg | Mean | 2 | 2.2 | 1.9 | 2 | 1.8 | 2.1 | 2 |
| Median | 1.9 | 1.9 | 1.8 | 1.9 | 1.8 | 1.9 | 1.8 | |
| SE | 0.1 | 0.2 | 0.1 | 0 | 0 | 0 | 0 | |
| Thiamin, mg | Mean | 1.6 | 1.6 | 1.5 | 1.6 | 1.5 | 1.6 | 1.5 |
| Median | 1.4 | 1.6 | 1.4 | 1.5 | 1.4 | 1.5 | 1.5 | |
| SE | 0.1 | 0.1 | 0.1 | 0 | 0 | 0 | 0 | |
| Vitamin B6, mg | Mean | 1.6 | 1.6 | 1.6 | 1.5* | 1.5 | 1.6 | 1.5 |
| Median | 1.5 | 1.5 | 1.5 | 1.4* | 1.4 | 1.5 | 1.5 | |
| SE | 0.1 | 0.1 | 0.1 | 0 | 0 | 0 | 0 | |
| Vitamin B12, ug | Mean | 5.7 | 6.4 | 6.4 | 6.2 | 5.8 | 6.2 | 6.1 |
| Median | 5 | 5.9 | 5.9 | 5.4 | 5.2 | 5.6 | 5.5 | |
| SE | 0.3 | 0.4 | 0.5 | 0.1 | 0.1 | 0.1 | 0 | |
| Vitamin C, mg | Mean | 180.5 | 183.1 | 181.6 | 155.5* | 157 | 166.1 | 167.5 |
| Median | 165 | 165 | 171 | 138* | 131 | 148 | 148 | |
| SE | 13.4 | 16.4 | 11.9 | 3.7 | 5.1 | 4.4 | 0.6 | |
| Vitamin D, ug | Mean | 3.4 | 3.9 | 3.6 | 3.7 | 3.4 | 3.5 | 3.6 |
| Median | 3.1 | 3.2 | 2.8 | 3.4 | 3 | 3.1 | 3.1 | |
| SE | 0.3 | 0.4 | 0.4 | 0.1 | 0.2 | 0.1 | 0 | |
| Vitamin E, mg | Mean | 10.1 | 12 | 10.7 | 11.1 | 10 | 11.4 | 10.6 |
| Median | 9 | 10.5 | 10 | 10 | 9 | 10 | 10 | |
| SE | 0.5 | 0.9 | 0.5 | 0.2 | 0.2 | 0.2 | 0 | |
| Phosphorus, mg | Mean | 1,730.40 | 1,891.90 | 1,730.70 | 1,741.40 | 1,607.60 | 1,816.80 | 1,699.60 |
| Median | 1,666.00 | 1,681.50 | 1,660.50 | 1,629.00 | 1,597.00 | 1,691.00 | 1,623.00 | |
| SE | 71.6 | 128.9 | 67.9 | 21.7 | 25.4 | 28.9 | 3.4 | |
| Iron, mg | Mean | 11.3 | 12 | 11.8 | 11.5 | 10.7 | 12.1 | 11.3 |
| Median | 10.9 | 11.6 | 11.3 | 10.8 | 10.4 | 11.4 | 10.7 | |
| SE | 0.5 | 0.6 | 0.4 | 0.1 | 0.2 | 0.2 | 0 | |
| Potassium, mg | Mean | 4,171.50 | 4,431.30 | 4,207.60 | 4,008.10* | 3,790.00 | 4,220.30 | 4,018.60 |
| Median | 4,024.00 | 3,989.50 | 4,055.50 | 3,846.00* | 3,691.00 | 4,007.50 | 3,847.00 | |
| SE | 165.1 | 304.5 | 147.9 | 49 | 54.3 | 66.2 | 7.6 | |
| Calcium, mg | Mean | 1,095.00 | 1,280.80 | 1,059.10 | 1,081.90 | 991.8 | 1,136.40 | 1,049.30 |
| Median | 1,040.00 | 1,009.00 | 995 | 986.5 | 937 | 997.5 | 968.5 | |
| SE | 61.4 | 132.2 | 52.3 | 18.3 | 21.2 | 23.5 | 2.9 | |
| Copper, mg | Mean | 1.4 | 1.6 | 1.5 | 1.4 | 1.3 | 1.5 | 1.4 |
| Median | 1.3 | 1.5 | 1.4 | 1.4 | 1.3 | 1.4 | 1.3 | |
| SE | 0.1 | 0.1 | 0.1 | 0 | 0 | 0 | 0 | |
| Magnesium, mg | Mean | 408.9 | 443.4 | 412.1 | 406.9 | 377.8 | 427.8 | 399.3 |
| Median | 394 | 402 | 396 | 382.5 | 367 | 403 | 383 | |
| SE | 16.3 | 31.2 | 15.1 | 5.1 | 5.6 | 7.2 | 0.8 | |
| Selenium, ug | Mean | 54.5 | 55.6 | 56.5 | 54.9 | 52.5 | 55.8 | 54.6 |
| Median | 52 | 52.7 | 57.8 | 53.4 | 50.7 | 53.8 | 53 | |
| SE | 2.2 | 2.3 | 2.3 | 0.6 | 0.8 | 0.7 | 0.1 | |
| Zinc, mg | Mean | 11.2 | 12.1 | 11.6 | 11.6 | 10.8 | 12 | 11.3 |
| Median | 10.8 | 11.3 | 11.1 | 11 | 10.6 | 11.5 | 10.9 | |
| SE | 0.4 | 0.6 | 0.4 | 0.1 | 0.2 | 0.2 | 0 | |
| Sodium, mg | Mean | 3,035.40 | 3,207.70 | 3,126.70 | 3,211.90 | 2,978.70 | 3,267.40 | 3,093.90 |
| Median | 2,940.00 | 3,143.00 | 3,011.50 | 3,094.00 | 2,902.00 | 3,142.00 | 3,001.00 | |
| SE | 119.4 | 120.7 | 128.1 | 35.2 | 42.3 | 41.6 | 5.2 | |
| Starch, g | Mean | 139.1 | 151.8 | 146.2 | 154 | 141.8 | 158.5 | 148 |
| Median | 135.7 | 141.9 | 143.3 | 147.7 | 135.2 | 150.9 | 142.7 | |
| SE | 6.7 | 7 | 7.1 | 2 | 2.7 | 2.6 | 0.3 | |
| Dietary fiber, g | Mean | 32 | 32.4 | 33.5 | 30.7 | 29.1 | 32.6 | 30.6 |
| Median | 30.4 | 30.1 | 31.2 | 29.1 | 27.4 | 30.6 | 29.1 | |
| SE | 1.6 | 1.8 | 1.4 | 0.4 | 0.6 | 0.6 | 0.1 | |
| Mono- and disaccharides, g | Mean | 153.6 | 192.7 | 157.2 | 160.1 | 148.1 | 169.9 | 156 |
| Median | 135.5 | 151.5 | 152.9 | 145.8 | 135.8 | 152.4 | 142.9 | |
| SE | 9.5 | 23.4 | 7.4 | 3 | 3.4 | 4 | 0.4 | |
| Added sugar. g | Mean | 62.1 | 97.2 | 63.5 | 75.3 | 64.7 | 78 | 66.8 |
| Median | 50.7 | 64 | 56 | 61.3 | 51.2 | 63.2 | 55.6 | |
| SE | 5.6 | 16.7 | 5 | 2.2 | 2.5 | 2.5 | 0.3 | |
Indicates statistical differences of mean log transformed nutrient values at an alpha level of 0.05 compared to the “no eating disorders” category. For nutrients other than total energy, adjustment was made for total energy intake, maternal age, education, income and gestational age at the time of the questionnaire and False Discovery Rate (FDR) control by the Benjamini-Hochberg (B-H) method (29).
Figure 1.
Percent differences in mean log transformed nutrient values by eating disorder subtype using the “no eating disorders” category as the reference group. Adjusted for total energy, gestational age, mother’s age, education, minimum combined income, and parity.
Food group intake by eating disorder categories are presented in Table 3. For the food groups commonly consumed by all women (fish, high fat meat, eggs, grains, vegetables, fruits, juice, yogurt and cheeses, milk desserts, cakes, sugar-sweetened beverages, and fats) there were no statistical differences between women with BN before or BED during pregnancy compared to women without eating disorders. Women with BN before and during pregnancy had lower intakes of sweetened beverages and high fat meats than women with no eating disorders. Women with BN before who remitted during pregnancy had higher intakes of fruits and lower intakes of fats but these differences were of borderline significance (p=0.05). Women with BED both before and during pregnancy had lower intakes of juice, and fruits but higher intakes of candy, fats and milk desserts compared to women with out eating disorders. Women with BED before pregnancy who remitted during pregnancy had lower intakes of yogurt and cheeses, fish, juices, grains and cakes while women with incident BED during pregnancy had higher intakes of yogurt and cheeses, grains, fats, cakes, candy, and milk desserts and lower intakes of juices compared to women with no eating disorders.
Table 3.
Distribution of Food Group Consumption (gms) by Bulimia Nervosa (BN) and Binge Eating Disorder (BED) Categories for Pregnant Women in the Norwegian Mother and Child Cohort (MOBA) Study, n=30,040*
| Eating Disorder Status | ||||||||
|---|---|---|---|---|---|---|---|---|
| Food Groups | BN before, BN during | BN before, BED during | BN before, none during | BED before, BED during | BED before, none during | None before, BED during | No eating disorders | |
| Milk Drinks | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 325 | 412.9 | 320.6 | 363.8 | 353.8 | 373.1 | 363.5 | |
| Median | 214.8 | 212.2 | 218.5 | 281.4 | 280.9 | 248.5 | 325.9 | |
| SE | 48.3 | 71.8 | 42.7 | 14.3 | 17 | 15.6 | 2.1 | |
| Yogurt and Cheese | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 159* | 154.8 | 114.6 | 105.4 | 82.3* | 129.3* | 108.7 | |
| Median | 104.6* | 88.3 | 78.9 | 60.5 | 47.3* | 70.5* | 63.8 | |
| SE | 22.2 | 33.8 | 14.9 | 5.1 | 5.7 | 7.3 | 0.8 | |
| Milk Desserts | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 19.4 | 24 | 26.6 | 24.3* | 21.3 | 25.7* | 20.7 | |
| Median | 13.7 | 19.1 | 18.4 | 18* | 15.4 | 17.8* | 15.4 | |
| SE | 2.1 | 2.5 | 4.4 | 0.9 | 1.2 | 1.1 | 0.1 | |
| Juices | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 168.3 | 153.5 | 152.9 | 155.3* | 165.9* | 170.6* | 178.2 | |
| Median | 92.6 | 106.8 | 94 | 92.6* | 85.5* | 92.6* | 142.5 | |
| SE | 36.7 | 20.8 | 26.1 | 7.5 | 10.8 | 9.4 | 1.2 | |
| Drinks-Sweetened | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 122.6* | 258.3 | 146.9 | 223 | 194.8 | 210.8 | 200 | |
| Median | 35.6* | 105.8 | 41.4 | 87.7 | 75.1 | 77.8 | 79.5 | |
| SE | 30.2 | 63.2 | 27.1 | 14.1 | 16.8 | 13.7 | 1.9 | |
| Articially Sweetened Drinks† | n | 48 | 40 | 35 | 425 | 257 | 420 | 15,958 |
| Mean | 426.3* | 427.5* | 378.2* | 306.6* | 283.3* | 334.9* | 221.8 | |
| Median | 186.3* | 178.1* | 194.5* | 106.8* | 106.8* | 178.1* | 106.8 | |
| SE | 76.6 | 89.3 | 22.9 | 27.3 | 27.3 | 22 | 2.8 | |
| Fish | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 41.3 | 31.1 | 39.3 | 35.7 | 33.5* | 36.7 | 37.2 | |
| Median | 43.1 | 31.6 | 38.6 | 34 | 30.5* | 34.7 | 35.7 | |
| SE | 3 | 2.7 | 3.1 | 0.9 | 1.1 | 0.9 | 0.1 | |
| Fish-other† | n | 48 | 45 | 47 | 501 | 307 | 464 | 21,480 |
| Mean | 13 | 12 | 16.6 | 11 | 11.9 | 10.3 | 10.8 | |
| Median | 6.1 | 6.6 | 6.3 | 5.9 | 5.3 | 5.1 | 5.5 | |
| SE | 2.6 | 2.2 | 4.2 | 0.7 | 0.1 | 0.7 | 0.1 | |
| Chicken† | n | 54 | 55 | 52 | 569 | 349 | 548 | 25,279 |
| Mean | 20.5 | 17.5 | 21.6 | 18.7* | 19.9 | 19.3 | 19.9 | |
| Median | 14.6 | 14.4 | 15.6 | 15.5* | 16 | 16.1 | 16.6 | |
| SE | 2.1 | 1.7 | 2.4 | 0.6 | 0.7 | 0.5 | 0.1 | |
| Red Meats | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 14.4 | 15.3 | 15.9 | 16.9 | 16.7 | 16.8 | 16.6 | |
| Median | 15.1 | 15.9 | 14.6 | 15.5 | 15 | 15 | 15.2 | |
| SE | 1.2 | 1.3 | 1.2 | 0.5 | 0.6 | 0.5 | 0.1 | |
| High-fat-meats | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 26.5* | 33.3 | 30.7 | 35.7 | 34.2 | 35.5 | 33.9 | |
| Median | 22.6* | 32.3 | 28.3 | 31.9 | 31.2 | 31.4 | 30.6 | |
| SE | 2.2 | 2.5 | 2.4 | 0.8 | 1 | 0.9 | 0.1 | |
| Vegetables | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 224.1 | 233 | 231.9 | 198 | 192.9 | 207.1 | 200.3 | |
| Median | 222.8 | 198.4 | 198.8 | 181 | 168 | 183.2 | 182.3 | |
| SE | 13.8 | 20.6 | 17.5 | 4 | 5.4 | 4.9 | 0.6 | |
| Fruits | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 299.7 | 274.4 | 332.2* | 245.2* | 266.1 | 272.5 | 274 | |
| Median | 253.8 | 200.2 | 307.2* | 196.6* | 209.6 | 225.7 | 227.1 | |
| SE | 24.4 | 33 | 25.6 | 7.2 | 11.6 | 9.4 | 1.2 | |
| Grains | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 279.2 | 297.6 | 311.1 | 312.7 | 289* | 323.2* | 304.8 | |
| Median | 266.4 | 288.8 | 300.6 | 297.1 | 276.8* | 304.4* | 292 | |
| SE | 15.3 | 14.7 | 17.6 | 4.6 | 6.3 | 5.9 | 0.7 | |
| Cakes | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 14.5 | 20.6 | 15.3 | 18 | 15.7* | 19.1* | 16.9 | |
| Median | 13.3 | 18.5 | 13.2 | 14.3 | 12.3* | 15.3* | 14.2 | |
| SE | 1.3 | 2.1 | 1.4 | 0.6 | 0.8 | 0.8 | 0.1 | |
| Coffee† | n | 40 | 44 | 33 | 411 | 215 | 395 | 16,642 |
| Mean | 179.9 | 220.3 | 112.6 | 204.3* | 135.7* | 224.1* | 164.8 | |
| Median | 133.1 | 111.8 | 26.3 | 144.7* | 57* | 150* | 98.6 | |
| SE | 32.5 | 42.2 | 26.9 | 11.3 | 14 | 11.7 | 1.5 | |
| Tea† | n | 44 | 44 | 42 | 470 | 277 | 456 | 21,480 |
| Mean | 189.7 | 209.4 | 190.9 | 167.5 | 155.7 | 184.5 | 168.4 | |
| Median | 106.8 | 106.8 | 106.8 | 106.8 | 35.6 | 106.8 | 106.8 | |
| SE | 36.3 | 37.3 | 40.7 | 10 | 12.6 | 10.7 | 1.4 | |
| Herbal Teas† | n | 28 | 29 | 29 | 236 | 145 | 259 | 11,584 |
| Mean | 162.3 | 136.2 | 138.5 | 121.4 | 135.3 | 128.1 | 127.2 | |
| Median | 47.9 | 52.1 | 106.8 | 35.6 | 35.6 | 35.6 | 35.6 | |
| SE | 37.1 | 26.7 | 28.7 | 10.8 | 16.1 | 13.8 | 1.9 | |
| Fats | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 23.5 | 26.7 | 20.8* | 31.8* | 25.5 | 30.3* | 27.6 | |
| Median | 17 | 19.8 | 15.4* | 28.5* | 22.9 | 26.3* | 24.1 | |
| SE | 2.6 | 3 | 2.3 | 0.9 | 0.9 | 0.9 | 0.1 | |
| Candy and Chocolates | n | 59 | 60 | 58 | 650 | 389 | 624 | 28,194 |
| Mean | 81.7 | 123.7 | 80.2 | 88.2* | 73.3 | 86.6* | 68.2 | |
| Median | 53.8 | 63.2 | 57.2 | 63* | 49.6 | 64.5* | 50 | |
| SE | 14.3 | 25.1 | 12.5 | 4.1 | 4.1 | 3.7 | 0.4 | |
Indicates statistically significant location shifts at an alpha level of 0.05 compared to the “no eating disorders” category using a Wilcoxon two-sided normal approximation test. Adjustment was made for total energy intake, maternal age, education, income and gestational age at the time of the questionnaire and False Discovery Rate (FDR) control by the Benjamini-Hochberg (B-H) method (29)
Means, Medians, and Standard Errors are calculated among consumers only.
For the food groups not commonly consumed, artificially sweetened beverages, coffee, tea, herbal teas, other categories of fish, and chicken, women with eating disorders, with the exception of those with BN who remitted during pregnancy, had a higher odds of consuming artificially sweetened beverages compared to women with no eating disorders (data not shown). Among consumers of these food groups, women with any eating disorder reported higher intakes of artificially sweetened beverages. Women with BED during pregnancy reported higher coffee consumption, while women with BED who remitted during pregnancy reported lower coffee consumption than those with no eating disorders. Women with BED both before and during pregnancy also had a significantly lower intake of chicken items compared to women with no eating disorders.
In sensitivity analysis, excluding the women with outliers in energy intake resulted in similar results with few exceptions. The finding related to higher consumption of artificially sweetened beverages among women with BN before and BED during pregnancy was no longer significant. The findings of higher fruit intake and lower fats among women with BN before who remitted during pregnancy were also not significant. Lastly, the higher intakes of grains, yogurt and cheeses and fats among women who developed BED during pregnancy were no longer significant.
Discussion
This study documents differences in nutrient and food group intakes during the first half of pregnancy among women with BN and BED in a large prospective cohort. Our results showed overall higher total energy, total fat, and mono and saturated fat consumption and lower intakes of folate, potassium, and vitamin C among women with BED both before and during pregnancy compared to women without eating disorders. This was reflected in the food group consumption comparison in that they had higher intakes of fats (butter, margarines, oils etc) and milk desserts and lower intakes of fruits and juices. Women who developed BED during pregnancy also had higher total energy and saturated fat intakes compared to women with no eating disorders and this is reflected in their higher consumption of cakes, yogurt and cheeses, milk desserts, and overall fats. No other statistically significant differences were noted for the nutrient comparisons. This lack of difference may be due to our conservative approach of adjusting for covariates and multiple comparisons. The results presented in Table 2 allow for future comparisons with other studies that use a similar approach to assessing dietary intakes.
Compared to the recently published Nordic Nutrition Recommendations (14), the average intake of all nutrients in the eating disorder groups was sufficient, with the exception of potassium and phosphorous being very high and iron and folate intake being much lower than desired. However, the data do not include dietary supplements which were reported to be taken by 80 % of the women in MoBa (23). Thus it is premature to determine whether the low dietary intakes are of concern.
The higher consumption of artificially sweetened beverages among all women with eating disorders is intriguing. Although high rates of use of artificial sweeteners have been reported in non-pregnant women with eating disorders (31), this is the first report of elevated use during pregnancy. This clearly demonstrates a behavior pattern of limiting intakes of added sugars by replacing diet drinks with other sweetened beverages. To date, there is little direct clinical evidence demonstrating a negative long term effect with the use of artificial sweeteners during pregnancy (32). Whether the observed differences are related to sweet taste preference is unclear, indeed, women with BN before and BED during pregnancy as well as those with BED both before and during also had higher intakes of sweetened beverages compared to women with no eating disorders, though the differences were not statistically significant.
Other food group differences were noted for women who had BN before and during pregnancy. The higher intakes of yogurt and cheeses and lower intakes of high-fat-meats and sweetened beverages are counter to what we would expect given the nature of eating disorders, however, since this represents their eating pattern during pregnancy it may well reflect the avoidance of “fattening” foods during an interval when purging episodes may be reduced. Indeed, a substantial number of women who reported BN prior to pregnancy resolved into BED during pregnancy. Subsequent data collection waves will allow us to determine whether the elimination of purging behavior was temporary or whether it returns after birth of the child. Women with BED before who remitted during pregnancy also demonstrated a behavioral pattern of limiting high calorie foods by consuming lower amounts of cakes, yogurt and cheeses, fish, grains, and juices as did women with BN before who remitted during pregnancy with their lower intakes of fats and higher intakes of fruits. This difference could simply reflect the absence of binge eating episodes in these women whose eating normalized during pregnancy. In contrast, women with BED both before and during pregnancy exhibited the opposite pattern by consuming higher amounts of candy and chocolates, fats, and milk desserts. Previous research has shown a high consumption of sweets early in pregnancy is associated with excessive gestational weight gains (33) and another study done among adolescents found a high sugar consumption (>10th percentile) was associated an increased risk of small-for-gestational age infants (34). Thus, further investigation of certain food group consumption on pregnancy outcomes is warranted for the future in this cohort.
Despite the many advantages that the use of the MoBa data provided us for examining nutrient and food group differences among women with eating disorders, we acknowledge several limitations. First, although the diagnostic questions used in the survey had been used in previous epidemiologic studies in Norway, they nonetheless were based on self-report and targeted broadly defined eating disorders. Second, dietary habits were assessed using a semi-quantitative food frequency questionnaire and are subject to the errors inherent in this mode of data collection. Third, generalizability of the findings may be limited to higher educated women who are receiving prenatal care. Comparing the sociodemographic characteristics of women who participated in MoBa to all births it has been found that the MoBa participants may be somewhat more educated than the general Norwegian population with 58% attending some form of college in comparison to 46% of women between 25–29 years and 43% of women between 30–39 years reported to have higher education in 2005 by Statistics Norway (35). Lastly, because of the observational nature of our data, we cannot easily exclude the possibility that important confounders were omitted from the analysis or that adjustment for confounders was incomplete. Nonetheless, this study provides interesting information concerning the dietary habits of women with BN and BED both before and during pregnancy. Whether the observed differences influence the course of pregnancy (ie. weight gain, gestational diabetes) and birth outcomes will be the topic of future analyses with this data set.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participants of MOBA and have no conflict of interest. All the authors were involved in obtaining the funding for the analysis. Ms. Von Holle was responsible for the statistical analysis with guidance from Drs. Siega-Riz, Bulik, and Hamer; Dr. Siega-Riz with Drs. Meltzer and Haugen drafted the manuscript and critical and intellectual feedback was provided by Drs. Bulik, Berg, Torgensen, and Reichborn- Kjennerud.
Funding: This research was supported by the National Institutes of Health Grants (HD047186) to CMB, CNRU (NIDDK56350) for AMSR and the MoBa study is supported by the Norwegian Ministry of Health, NIH/NIEHS (N01 - ES – 85433), NIH/NINDS (1 UO1 NS 047537-01) and Norwegian Research Council/FUGE (151918/S10).
References
- 1.Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. epub 2006 July 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulik C, Reichborn-Kjennerud T. Medical morbidity in binge eating disorder. Inter J of Eat Disord. 2003;34:S39–46. doi: 10.1002/eat.10204. [DOI] [PubMed] [Google Scholar]
- 3.Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: A systematic review of the literature. Int J Eat Disord. 2007;40:293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- 4.Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190:255–259. doi: 10.1192/bjp.bp.106.020768. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JF, Lacey JH, Chung E. Risk of postnatal depression, miscarriage and preterm birth in bulimia nervosa: retrospective controlled study. Psychosom Med. 2006;68:487–92. doi: 10.1097/01.psy.0000221265.43407.89. [DOI] [PubMed] [Google Scholar]
- 6.Kouba S, Hallstron T, Lindholm C, Hirschberg AL. Pregnancy and neonatal outcomes in women with eating disorders. Obstet Gynecol. 2005;105:255–260. doi: 10.1097/01.AOG.0000148265.90984.c3. [DOI] [PubMed] [Google Scholar]
- 7.Sollid CP, Wisborg K, Hjort J, Secher NJ. Eating disorder that was diagnosed before pregnancy and pregnancy outcome. Am J Obstet Gynecol. 2004;190:206–210. doi: 10.1016/s0002-9378(03)00900-1. [DOI] [PubMed] [Google Scholar]
- 8.Torgersen L, Von Holle A, Knopf Bergh C, Hamer R, Siega-Riz AM, Sullivan PF, Reichborn-Kjennerud T, Bulik CM. Nausea and vomiting of pregnancy in women with bulimia nervosa and EDNOS. doi: 10.1002/eat.20564. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Dietetic Association. Position of the American Dietetic Association: Nutrition and lifestyle for a health pregnancy outcome. JADA. 2002;102:1479–1490. doi: 10.1016/s0002-8223(02)90327-5. [DOI] [PubMed] [Google Scholar]
- 10.Nutrition during pregnancy. ACOG Technical Bulletin Number 179--April 1993. Int J Gynaecol Obstet. 1993;43(1):67–74. [PubMed] [Google Scholar]
- 11.Institute of Medicine, Food and Nutrition Board. Nutrient Supplements. Washington, D.C: National Academy Press; 1990. Nutrition during Pregnancy, Part I. Weight Gain. Part II. [Google Scholar]
- 12.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohyrdates, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, D.C: National Academy Press; 2002. [Google Scholar]
- 13.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 14.Nordic Nutrition Recommendations. Nordic Council of Ministers; Copenhagen: 2004. [Google Scholar]
- 15.Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C, et al. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006 doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 16.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–39. [PubMed] [Google Scholar]
- 17.Harris J, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Res. 2002;5:415–23. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 18.Reichborn-Kjennerud T, Bulik C, Kendler K, Maes H, Roysamb E, Tambs K, Harris J. Gender differences in binge-eating: A population-based twin study. Acta Psychiatr Scand. 2003;108:196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 19.Reichborn-Kjennerud T, Bulik C, Kendler K, Roysamb E, Tambs K, Harris J, Torgeson S. Influence of weight on self-evaluation: A population-based study of gender differences. International Journal of Eating Disorders. 2004;35:123–32. doi: 10.1002/eat.10252. [DOI] [PubMed] [Google Scholar]
- 20.Reichborn-Kjennerud T, Bulik C, Tambs K, Harris J. Genetic and environmental influences on binge eating in the absence of compensatory behaviours: a population-based twin study. International Journal of Eating Disorders. 2004;36:307–314. doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association Press; 1994. [Google Scholar]
- 22.Bulik C, Holle A, Hamer R, Knoph Berg C, Torgerson L, Magnus P, et al. Patterns of remission, continuation, and incidence of eating disorders during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Psychol Med. 2007;37:1109–18. doi: 10.1017/S0033291707000724. Epub 2007 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meltzer HM, Brantsaeter AL, Ydersbond TA, Alexander J, Haugen M the Moba Dietary Support Group. Methodological challenges when monitoring the diet of pregnant women in a large cohort study; experiences from The Norwegian Mother and Child Cohort Study. Maternal and Child Nutrition. doi: 10.1111/j.1740-8709.2007.00104.x. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brantsæter AL, Haugen M, Alexander J, Meltzer HM. Maternal and Child Nutrition. Validity of a new Food Frequency Questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa) (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brantsæter AL, Haugen M, Hagve T-A, Aksnes L, Rasmussen SE, Julshamn K, Alexander A, Meltzer HM. Self-reported dietary supplement use is confirmed by biological markers in the Norwegian Mother and Child Cohort Study (MoBa) Ann Nutr Metab. 2007;51:146–154. doi: 10.1159/000103275. [DOI] [PubMed] [Google Scholar]
- 26.Brantsæter AL, Haugen M, Rasmussen SE, Alexander J, Samuelsen SO, Meltzer HM. Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Public Health Nutr. 2007;10:274–283. doi: 10.1017/S1368980007339037. [DOI] [PubMed] [Google Scholar]
- 27.Lauritsen J. FoodCalc. [accessed 5 July 2005]; Version Current 1 July 2005;Internet: http://www.ibt.ku.dk/jesper/foodcalc.
- 28.Rimestad AH, Borgejordet Å, Vesterhus KN, Sygnestveit K, Løken EB, Trygg K, et al. Den store matvaretabellen/The Norwegian Food Composistion Table. Oslo: Statens råd for ernæring og fysisk aktivitet, Statens næringsmiddeltilsyn, Institutt for ernæringsforskning; 2001. [Google Scholar]
- 29.Benjamini Yoav, Hochberg Yosef. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 30.World Heath Organization. Obesity: preventing and managing the global epidemic. WHO technical report series 894. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 31.Klein DA, Boudreau GS, Devlin MJ, Walsh BT. Artificial sweetener use among individuals with eating disorders. Int J Eat Disord. 2006 May;39(4):341–5. doi: 10.1002/eat.20260. [DOI] [PubMed] [Google Scholar]
- 32.American Dietetic Association. Position of the American Dietetic Association: Use of Nutritive and Nonnutritive Sweeteners. JADA. 2004;104:255–275. doi: 10.1016/j.jada.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Olafsdottir AS, Skuladottir GV, Thorsdottir A, Hauksson A, Steingrimsdottir L. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes. 2006;30:492–499. doi: 10.1038/sj.ijo.0803184. [DOI] [PubMed] [Google Scholar]
- 34.Lenders CM, Hediger ML, Scholl TO, Khoo CS, Slap GB, Stallings VA. Gestational age and infant size at birth are associated with dietary sugar intake among pregnant adolescents. J Nutr. 1997;127:1113–1117. doi: 10.1093/jn/127.6.1113. [DOI] [PubMed] [Google Scholar]
- 35.Statistics Norway available at www.ssb.no
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.