Abstract
Large-pore mesoporous silica with 3D wormhole framework structures (denoted MSU-J) are prepared through a supramolecular hydrogen-bonding assembly pathway from low-cost sodium silicate as the silica source and commercially available mono- and triamine Jeffamine and Surfonamine surfactants as structure-directing porogens. The calcined mesostructures exhibit large pore sizes (up to 8.2 nm), surface areas (632–1030 m2/g) and pore volumes (0.5–2.0 cm3/g), depending on the surfactant chain length and synthesis temperature (25–65 °C). The textural properties of these new wormhole mesostructures are comparable to those of hexagonal SBA-15 derivatives and large pore MCM-48. However, unlike the SBA-15 structure type, wherein the 3D pore network is formed by connecting 1D cylindrical mesopores through micropores, MSU-J mesophases have wormhole framework structures containing fully interconnected 3D mesopores that can minimize the diffusion limitations often encountered in adsorption and chemical catalysis. Also, unlike large pore MCM-48, which requires cost-intensive tetraethylorthosilicate as a silica source and the use of a co-surfactant as a pore expander under strong acid conditions, MSU-J mesostructures are assembled from low cost sodium silicate in the presence of a single Jeffamine or Surfonamine porogen at near-neutral pH.
Keywords: mesoporous silica, wormhole mesostructure, amine porogens
1. Introduction
Electrically neutral supramolecular templating routes to mesoporous silica were demonstrated previously based on hydrogen-bonding interactions between primary amine surfactants (S°) and molecular silica precursors (I°) [1]. Unlike the cylindrical 1D mesopore structures characteristic of MCM-41 [2] and SBA-15 [3], mesostructures prepared through an electrically neutral hydrogen bonding pathway typically exhibit 3D wormhole framework structures with intersecting pores of nearly uniform diameters for improved accessibility [4, 5]. In addition, the S°I° templating pathway produces mesoporous silica with thicker framework walls and substantially higher thermal stability [6] in comparison to derivatives made by charge-matching electrostatic pathways. Another benefit of the mesostructures made by S°I° assembly is the added textural mesoporosity [7] that results from the intergrowth of very small elementary particle domains size. The sponge-like textural pores facilitate access to the framework mesopores. In contrast, mesostructures made by electrostatic assembly pathways typically exhibit monolithic particle morphologies that greatly lengthen the diffusion pathway for accessing the mesoporous framework [8, 9]. Yet another advantage of the S°I° pathway is the facile and environmentally benign recovery of the cost-intensive template from the as-made products by simple solvent extraction methods.
Large-pore wormhole silica mesostructures (denoted MSU-J) were recently synthesized using α,ω-diamine polypropylene oxide surfactants as the porogen [10]. Commercial versions of these porogens with average molecular weights of 2000 and 4000, denoted Jeffamine D2000 and D4000, respectively, afforded silica mesostructures with BET surface areas of 408–1127 m2/g and average framework pore sizes of 5.3–14.3 nm. Beyond the potential use of MSU-J as catalysts, adsorbents, and templates for the synthesis of ordered mesoporous carbons [4, 5], these 3D large pore silicas exhibit exceptional promise as reinforcing agents for thermoset polymers. The tensile modulus, strength, and toughness of epoxy - MSU-J mesocomposites, for example, were increased up to 4.8-, 5.7-, and 1.6-fold, respectively, compared to silica-free pristine polymer [11, 12].
We herein report new members of the MSU-J family of mesostructured silicas prepared from amine-terminated Jeffamine and Surfonamine polypropylene oxide surfactants. The resulting pore sizes, BET surface areas, and pore volumes of the new MSU-J mesophases are comparable to those of 2D hexagonal SBA-15 [3] and large pore MCM-48 [13]. However, MSU-J mesostructures offer several synthetic and performance advantages in comparison to SBA-15 and large pore MCM-48.
2. Experimental
Three Jeffamine surfactants M2005, T3000 and T5000 and two Surfonamine surfactants ML300 and MNPA1000 were obtained from Huntsman Chemical and used without further purification. A commercially available sodium silicate (Aldrich, 27% SiO2, 14% NaOH) solution was used as the silica precursor.
The procedures used for the preparation of the MSU-J silicas in this present study are analogous to those of previously reported MSU-J silicas prepared from Jeffemine D2000 [10]. The surfactant/Si ratios used in the syntheses are given in Table 1. The molar ratio for the other reagents used in the reaction was SiO2 : NaOH : HCl : H2O = 1.0 : 0.83 : 0.83 : 230. In a typical synthesis, a desired amount of a surfactant was added to water and then the formal hydroxide content of the sodium silicate was neutralize by adding an equivalent amount of hydrochloric acid. Sodium silicate was added to the surfactant aqueous solution under vigorous stirring at ambient temperature (pH 8.0–8.5) and aged in a heated water bath at the desired synthesis temperature for 20 h with stirring. The solid product was washed with water and dried overnight in air. The surfactant was then removed by calcination at 600 °C for 4 h in air (heating rate, 2 °C/min).
Table 1.
Physical properties for mesostructured wormhole silicas prepared from sodium silicate and Jeffamine or Surfonamine surfactants as porogens.
| Surfactant | Surf./Si molar ratioa | Synthesis Temp. (°C) | d001b (nm) | SBET (m2/g) | Pore size (nm) | Wall thicknessd (nm) | Pore volume (cm3/g) |
|---|---|---|---|---|---|---|---|
| M2005 | 0.062 | 25 | 6.5 / 5.9 | 632 | 3.5 | 2.4 | 0.5 |
| 45 | 7.9 / 7.0 | 673 | 4.8 | 2.2 | 0.9 | ||
| 65 | 7.9 / 7.1 | 885 | 6.1 | 1.0 | 1.4 | ||
| T3000 | 0.083 | 25 | 6.4 / 5.9 | 936 | 5.0 | 0.9 | 1.3 |
| 45 | 6.6 / 6.4 | 964 | 6.0 | 0.4 | 1.5 | ||
| 65 | 7.2 / 7.2 | 881 | 6.7 | 0.5 | 1.8 | ||
| T5000 | 0.033 | 25 | -c | 1030 | 5.2 | - | 1.5 |
| 45 | -c | 882 | 8.2 | - | 2.0 | ||
| ML300 | 0.25 | 25 | 4.6 / 4.2 | 770 | 2.8 | 1.4 | 1.1 |
| MNPA1000 | 0.12 | 25 | 5.6 / 6.0 | 1012 | 5.2 | 1.5 | 1.4 |
Surfactant to silicon ratio used in the synthesis.
Basal spacings of as-made and calcined MSU-J silicas are separated by a slash (as-made / calcined).
The XRD reflections are not resolved.
The wall thickness was determined by subtracting the BJH pore size from the pore-pore correlation distance (d001).
The powder X-ray diffraction (XRD) patterns were obtained on a Rigaku Rotaflex 200B diffractometer equipped with Cu Kα X-ray radiation and a curved crystal graphite monochromator operating at 45 kV and 100 mA. The N2 adsorption-desorption isotherms for calcined samples were measured on a Micromeritics ASAP 2010 instrument at -196 °C. Before measurement, samples were outgassed under vacuum overnight at 150 °C. Transmission electron microscopy (TEM) images were obtained on a JEOL JEM-100CX II microscope with a CeB6 filament and an accelerating voltage of 120 kV. Sample grids of calcined mesoporous silicas were prepared via sonication of powdered sample in EtOH for 5 min and evaporating 2 drops of the suspension onto a carbon-coated film supported on a 3 mm, 300 mesh copper grid.
3. Results
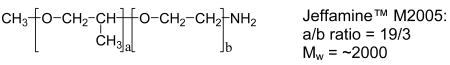
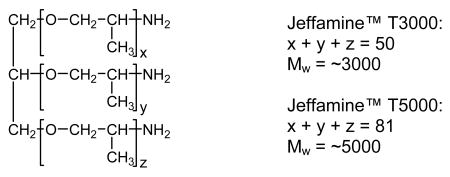
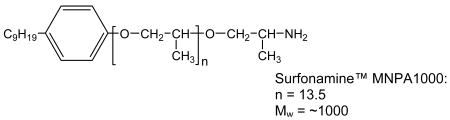
Jeffamine and Surfonamine surfactants in M and T series designations are commercially available from Huntsman Chemical Co. The designations M and T signify the presence of one (mono-) and three (tri-) amine groups, respectively, as represented by the chemical structures 1-4 below. The number designation after the prefix corresponds to the approximate molecular weight of the surfactant.
 |
(1) |
 |
(2) |
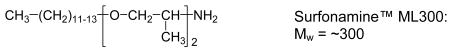 |
(3) |
 |
(4) |
The Jeffamine surfactants with structures 1 and 2 are based on a propylene oxide (PO) or mixed PO/EO (EO = ethylene oxide) backbone. The Surfonamines (Structures 3 and 4) are monofunctional amines similar to the M-series Jeffamines, but they possess long-alkyl groups at the end of the hydrophobic tails. Cost-effective sodium silicate proved to be suitable for use as the silica precursor. Several survey experiments indicated that the optimum surfactant/Si ratios given in Table 1 provided products with the most intense XRD reflections, largest surface areas and highest yields.
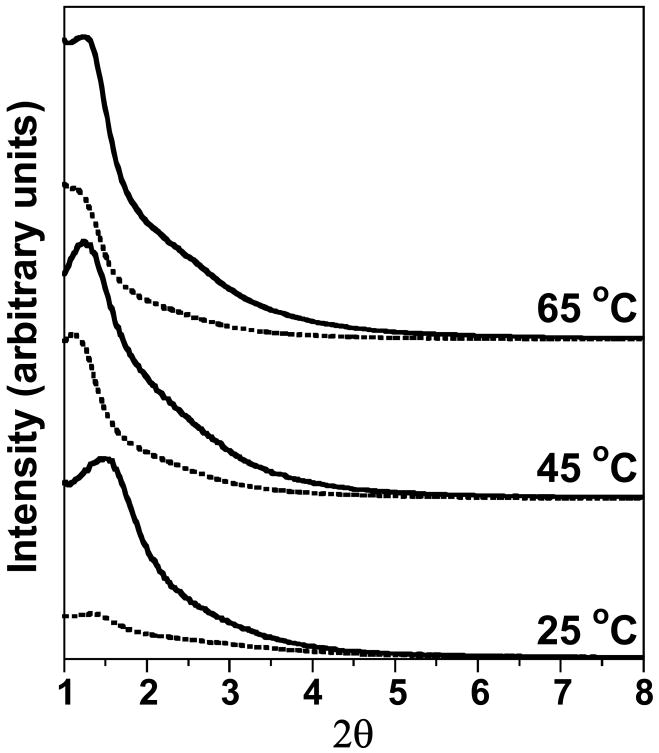
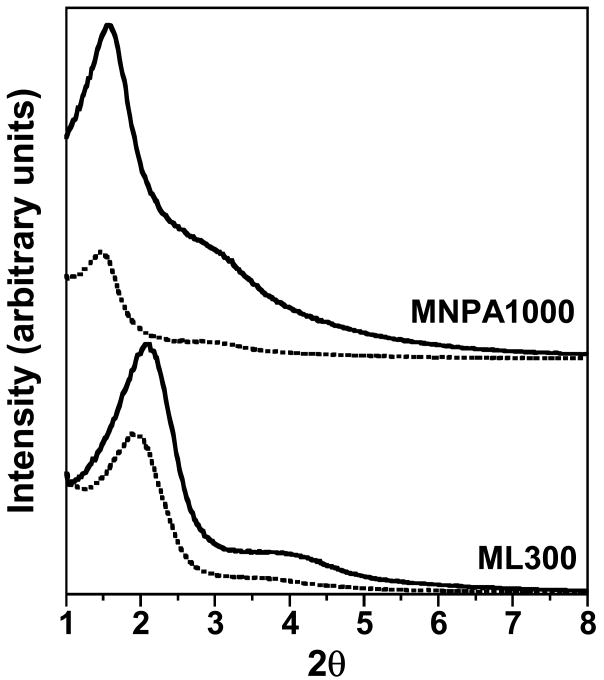
The XRD patterns of as-made and calcined MSU-J silicas prepared at 25, 45, and 65 °C with Jeffamine M2005 as the porogen are illustrated in Fig. 1. Typical of a wormhole framework mesostructure, each sample exhibits a XRD pattern consisting of an intense and relatively broad low angle reflection and a weaker, higher angle shoulder. These patterns signify the presence of a correlated distribution of framework pores but the lack of a regular pore structure. The pore-pore correlation distance of the calcined samples increases from 5.9 to 7.1 nm upon increasing the synthesis temperature from 25 to 65 °C.
Fig. 1.
XRD patterns for MSU-J silicas templated by the monoamine porogen Jeffamine M2005 at different assembly temperatures. The dotted and solid scans are for as-made and calcined MSU-J silicas, respectively.
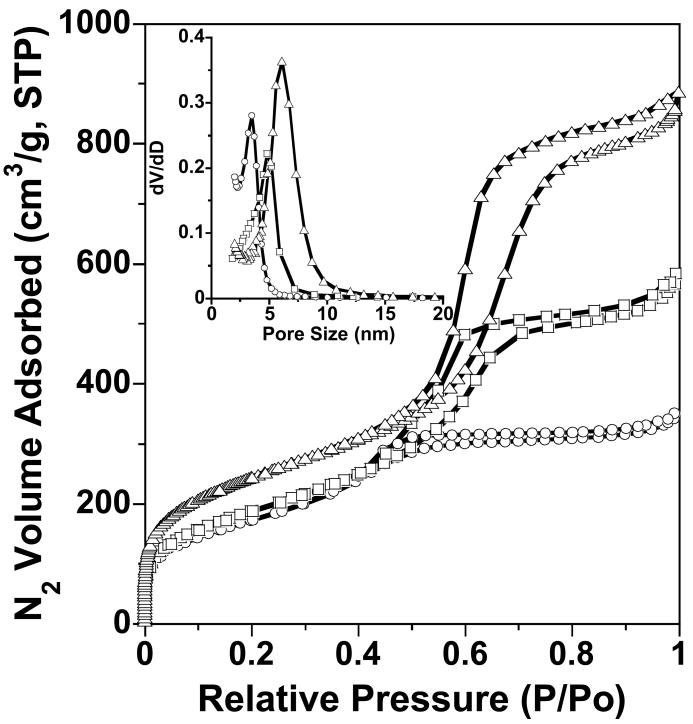
Fig. 2 provides the N2 adsorption-desorption isotherms for MSU-J silicas prepared in the presence of M2005 surfactant and Barrett-Joyner-Halenda (BJH) framework pore size distributions (inset) obtained from adsorption branch of the isotherms. Capillary condensation of liquid nitrogen in the framework-confined mesopores occurred at P/Po = 0.4–0.7. As shown by the BJH pore size distribution plots obtained from the adsorption branches of the nitrogen isotherms (inset), the pore size increases systematically from 3.5 nm at 25 °C to 6.1 nm at 65 °C.
Fig. 2.
N2 sorption isotherms and pore size distributions (inset) for MSU-J silicas prepared from Jeffamine M2005. The isotherms are for calcined products synthesized at 25 (○), 45 (□), and 65 (Δ) °C.
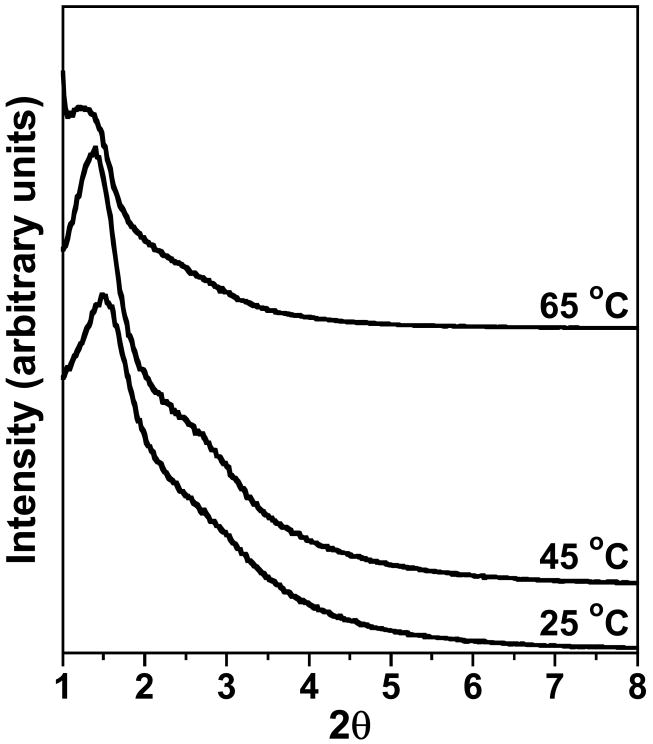
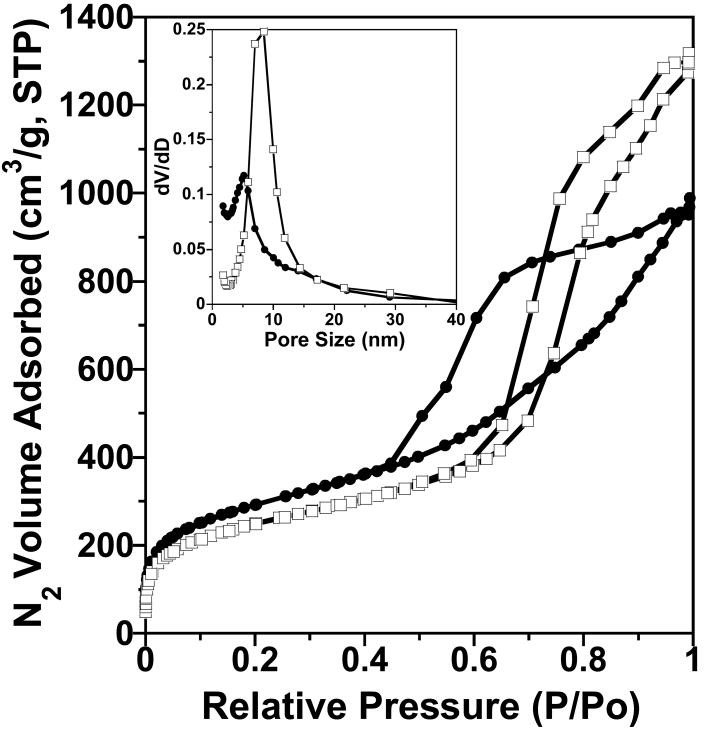
MSU-J silicas assembled from the triamine surfactant Jeffamine T3000 also exhibit typical wormhole mesophases as indicated by the XRD patterns in Fig. 3. The TEM image (Fig. 4c) of the MSU-J silica prepared at 25 °C shows typical wormhole framework morphology with a regular pore size (5.0 nm) consistent with that obtained from the pore size distribution. The pore sizes of the T3000-templated products (5.0 – 6.7 nm depending on assembly temperature) are comparable to those of the MSU-J silicas synthesized in the presence of the diamine surfactant Jeffamine D2000 [10]. The latter surfactant has a block of 33 PO units with a hairpin configuration and provides pore sizes of 5.3 – 6.6 nm under analogous synthesis conditions. T3000 contains 50 PO units distributed over three chains (see structure 2), so that the average length of the hydrophobic segment is comparable to that of D2000 and similar pore sizes are generated.
Fig. 3.
XRD patterns for calcined MSU-J silicas prepared using the triamine surfactant Jeffamine T3000 as the porogen.
Fig. 4.
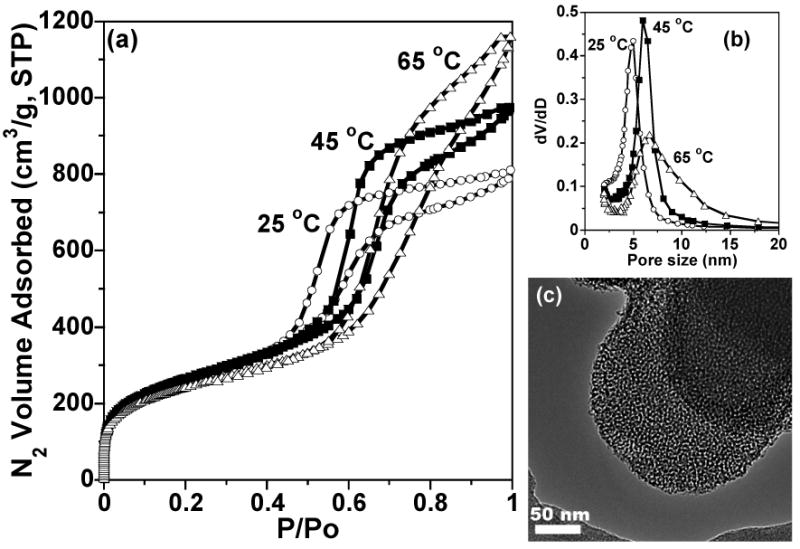
(a) N2 sorption isotherms, (b) pore size distributions, and (c) a TEM image for MSU-J silicas prepared in the presence of Jeffamine T3000. The synthesis temperatures were 25 ° (○), 45 ° (■), and 65 °C (Δ) (a and b).
MSU-J made from Jeffamine T5000 (the highest molecular weight porogen used in this study) provides wormhole structures with a pore-pore correlation distance that exceeds the detection limit of our X-ray diffractometer. However, well-resolved BJH pore distributions centered at 5.2 and 8.2 nm were obtained from the nitrogen adsorption isotherms of the products made at 25 and 45 °C (see Fig. 5). Well-defined mesostructures were not obtained at assembly temperatures >45 °C, suggesting that the cloud temperature for the surfactant may lie above this temperature. The larger pore sizes obtained for the products assembled from T5000 are consistent with the higher molecular weight of this surfactant.
Fig. 5.
N2 sorption isotherms and pore size distributions (inset) for MSU-J silicas prepared from Jeffamine T5000 surfactant. The synthesis temperatures were 25 ° (•), and 45 °C (□).
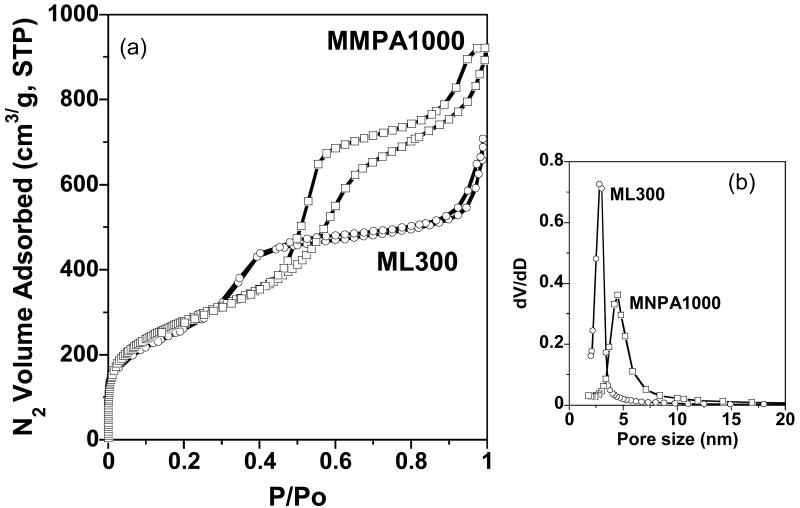
MSU-J silicas assembled from monoamine Surfonamine surfactants ML300 and MNPA1000 also exhibit typical wormhole framework structures as judged by the XRD patterns and the N2 adsorption-desorption isotherms shown in Figures 6 and 7, respectively. These surfactants have alkyl and PPO hydrophobic segments and a single amine as the hydrophilic head group (c.f., Structure 4). Being the surfactant with the lowest molecular weight used in this study, ML300 provides the smallest average pore size (2.8 nm), as expected. But MNPA1000 affords a wormhole mesostructure with an average framework pore size comparable to or larger than the pore sizes of the mesostructures made from M2005, T3000, and T-5000 with molecular weights two- to five-times larger (c.f., Table 1). Apparently, the presence of the aromatic ring MNPA1000 substantially impacts the conformation of the hydrophobic segment and promotes the formation of micelles comparable in size to those formed from surfactants with much higher molecular weights.
Fig. 6.
(a) XRD patterns as-made (dotted line) and calcined (solid line)forms of MSU-J silicas prepared fromML300 and MNPA Surfonamine surfactants.
Fig. 7.
(a) N2 sorption isotherms and (b) pore size distributions for MSU-J silicas prepared from Surfonamine surfactants ML300 (○) and MNPA1000 (□).
4. Discussion
All of the amine structure-directing agents used in this study afford mesoporous silica framework structures through hydrogen-bonding interactions with the silanol groups of the inorganic precursor. Although the surfactants are removable by ethanol extraction, they were removed by calcination for convenience. The calcined mesostructures exhibit large pore sizes (up to 8.2 nm), surface areas (632–1030 m2/g) and pore volumes (0.5–2.0 cm3/g), depending on the surfactant chain length and synthesis temperature (25–65 °C). These textural properties overlap those observed previously for wormhole MSU-J derivatives derived from the α,ω-diamine polypropylene oxide surfactants Jeffamine D2000 and D4000 which afford average pore sizes up to 14.3 nm [10].
The new wormhole mesostructures also are comparable in textural properties to 2D hexagonal SBA-15 and large pore cubic MCM-48 derivatives, which exhibit pore sizes in the range 4.7–10.6 nm [3] and 4-12 nm, respectively. However, unlike SBA-15 silicas, which have a hexagonal framework structure wherein 1D cylindrical mesopores are connected by micropores, MSU-J mesophases have wormhole framework structures containing interconnected 3D mesopores that can minimize diffusion limitations often encountered in adsorption and chemical catalysis. Large pore MCM-48 also has 3D-connected cylindrical mesopores, but the assembly of these materials requires the use of cost-intensive tetraethylorthosilicate as a silica source and a pore expanding co-surfactant under strongly acidic reaction conditions. In contrast, MSU-J wormhole structures are assembled from low-cost sodium silicate in the presence of a single Jeffamine or Surfonamine surfactant at near neutral pH.
For Jeffamine M2005 as a porogen, the surfactant head group contains in addition to an amine group a hydrophilic PEO segment, whereas all the other surfactants used in this study contain a only an amine as the hydrophilic entity. The larger head group of M2005 allows for a relatively thicker pore wall (1.0 – 2.4nm) in comparison to the pore wall thickness (0.9–0.4 nm) of mesostructures assembled at the same synthesis temperature from T3000 and T5000 porogens that contain only an amine head group. The increase in pore size with increasing assembly temperature is accompanied by a decrease in framework wall thickness. An analogous relationship was observed for wormhole MSU-X mesostructures assembled in the presence of alkyl-PEO surfactant porogens [14-17]. The dependence of wall thickness on temperature was attributed mainly to reduced hydrogen-bonding interactions between the head group of the surfactant and silanol groups of the inorganic precursor with increasing temperature. A similar reduction in hydrogen bonding most like accounts for the broadening of the pore size distribution observed at assembly temperatures above 65 °C (cf., Fig 4).
We also observe a decrease in wall thickness for the Jeffamine M2005 surfactant that contains a PEO segment as part of the hydrophilic head group (c.f., Table 1). The surfactants containing only an amine group do not show a dependence of wall thickness on assembly temperature. It is noteworthy that for surfactants containing both PEO and amine groups as part of the hydrophilic head group, an appropriate polarity balance between the hydrophilic and hydrophobic segments is needed to form structure-directing micelles under synthesis conditions. For instance, the PEO-rich Jeffamine M2070 has the same general structure as M2005 but with a much lower PO/EO ratio (5/16 versus 19/3, respectively) Thus, M2005 templates a mesostructure with a uniform pore size, whereas M2070 does not, due to the imbalance in the hydrophobic and hydrophilic segments and the lack of adequate amphiphilic character under synthesis conditions.
The pore diameter and pore volume of MSU-J silicas generally increase with increasing synthesis temperature. The temperature dependence of MSU-J pore sizes is mainly associated with a decrease in the I°S° interfacial curvature resulting from decreased H-bonding at the silica-surfactant interface with increasing temperature [9, 16]. The hydration of the amine group decreases at elevated temperature as the polarity of the interface decreases. Consequently, the decrease in hydrogen bonding between the surfactant and silica precursor at the I°S° interface decreases the effective head group area (a0) and increases the surfactant packing parameter. Thus, the decrease in micelle curvature results in an increase in the pore size.
The above results show that large-pore wormhole silica mesostructures are readily prepared through the supramolecular assembly reactions of PPO amine surfactants (Jeffamines) without the need for an auxiliary co-surfactant pore expander. The calcined versions of these 3D pore structure exhibit relatively large pore sizes (up to 8.2 nm) and high surface areas and pore volumes in comparison to other 3D cylindrical pore systems such as MCM-48. The large interconnected pore systems are ideally suited for the adsorption of large and rigid molecules. For example, our preliminary studies indicate that the 8.2 nm pore derivative is effective as an adsorbent for the removal of 2,3,7,8-tetrachlorodioxin from solution.
PPO amines are attractive curing agents for thermoset epoxy polymers [18,19]. The diverse chain lengths afford epoxy polymers with a broad range of mechanical properties and glass transition temperatures. As-made MSU-J wormhole mesostructures containing intercalated Jeffamines are promising candidates as in situ reinforcement agents for the corresponding thermoset epoxy polymers [10,11]. Moreover, the mechanical properties of the epoxy polymers can be systematically modified by controlling pore size, pore volume and surface area of the MSU-J silicas. In addition, wormhole mesostructured silicas can be assembled in the presence of Surfonamines (see Table 1). Surfonamines have been used as amino-functional polymeric segments [20] so that the as-made MSU-J silicas synthesized from these surfactants may also be effective agents for improving the mechanical properties of polymeric materials.
Conclusions
The results of the present study, together with those of an earlier study [10] demonstrate that commercially available mono-, di-, and tri-amine surfactants containing PPO hydrophobic segments (ie., Jeffamines and Surfonamines) are effective porogens for the assembly of mesoporous MSU-J silica with wormhole framework structures and three dimensionally connected large mesopores 4 - 14 nm in diameter. Conventional alkyl amine surfactants afford wormhole structures with framework pores only 3 - 4 nm in diameter [21]. These large pore wormhole derivatives are comparable to SBA-15 and large pore MCM-48 with framework pore sizes in the range 6 – 12 nm and 4 -12 nm, respectively [3, 13]. Moreover, the Jeffamine and Surfonamine porogens allow for the assembly of large pore MSU-J silica from cost effective sodium silicate at near neutral pH without the need of a co-surfactant as a pore expander.
Acknowledgments
The partial support of this research by NIEHS grant ES004911 is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanev PT, Pinnavaia TJ. Science. 1995;267:865. doi: 10.1126/science.267.5199.865. [DOI] [PubMed] [Google Scholar]
- 2.Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Nature. 1992;359:710. [Google Scholar]
- 3.Lettow JS, Han YJ, Schmidt-Winkel P, Yang P, Zhao D, Stucky GD, Ying JY. Langmuir. 2000;16:8291. [Google Scholar]
- 4.Lee J, Yoon S, Oh SM, Shin CH, Hyeon T. Adv Mater. 2000;12:359. [Google Scholar]
- 5.Lee J, Han S, Hyeon T. J Mater Chem. 2004;14:478. [Google Scholar]
- 6.Tanev PT, Pinnavaia TJ. Chem Mater. 1996;8:2068. [Google Scholar]
- 7.Zhang WZ, Pauly TR, Pinnavaia TJ. Chem Mater. 1997;9:2491. [Google Scholar]
- 8.Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, Mccullen SB, Higgins JB, Schlenker JL. J Am Chem Soc. 1992;114:10834. [Google Scholar]
- 9.Huo QS, Leon R, Petroff PM, Stucky GD. Science. 1995;268:1324. doi: 10.1126/science.268.5215.1324. [DOI] [PubMed] [Google Scholar]
- 10.Park I, Wang Z, Pinnavaia TJ. Chem Mater. 2005;17:383. [Google Scholar]
- 11.Park I, Peng HG, Gidley DW, Xue SQ, Pinnavaia TJ. Chem Mater. 2006;18:650. [Google Scholar]
- 12.Jiao J, Sun X, Pinnavaia TJ. Adv Func Mater. 2008;18:1. [Google Scholar]
- 13.Kim TW, Kleitz F, Paul B, Ryoo R. J Amer Chem Soc. 2005;127:7601. doi: 10.1021/ja042601m. [DOI] [PubMed] [Google Scholar]
- 14.Bagshaw SA, Prouzet E, Pinnavaia TJ. Science. 1995;269:1242. doi: 10.1126/science.269.5228.1242. [DOI] [PubMed] [Google Scholar]
- 15.Prouzet E, Pinnavaia TJ. Angew Chem Int Edit. 1997;36:516. [Google Scholar]
- 16.Blin JL, Leonard A, Su BL. J Phys Chem B. 2001;105:6070. [Google Scholar]
- 17.Kunieda H, Ozawa K, Huang KL. J Phys Chem B. 1998;102:831. [Google Scholar]
- 18.Yildirim A, Kiskan B, Demirel AL, Yagci Y. Eur Polym J. 2006;42:3006. [Google Scholar]
- 19.Franco M, Mondragon I, Bucknall CB. J Appl Polym Sci. 1999;72:427. [Google Scholar]
- 20.Wolak TJ, Wollenberg KF, Adams PE. J Heterocyclic Chem. 2000;37:1157. [Google Scholar]
- 21.Pauly TR, Pinnavaia TJ. Chem Mater. 2001;13:987. [Google Scholar]